Abstract
Purpose
The neurohormones melatonin and dopamine mediate clock-dependent/circadian regulation of inner retinal neurons and photoreceptor cells and in this way promote their functional adaptation to time of day and their survival. To fulfill this function they act on melatonin receptor type 1 (MT1 receptors) and dopamine D4 receptors (D4 receptors), respectively. The aim of the present study was to screen transcriptional regulators important for retinal physiology and/or pathology (Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ) for circadian regulation and dependence on melatonin signaling/MT1 receptors or dopamine signaling/D4 receptors.
Methods
This was done by gene profiling using quantitative polymerase chain reaction in mice deficient in MT1 or D4 receptors.
Results
The data obtained determined Pgc-1α and Nr4a1 as transcriptional targets of circadian melatonin and dopamine signaling, respectively.
Conclusions
The results suggest that Pgc-1α and Nr4a1 represent candidate genes for linking circadian neurohormone release with functional adaptation and healthiness of retina and photoreceptor cells.
Keywords: retina, circadian regulation, melatonin receptor type 1, dopamine D4 receptor, Nr4a1, Pgc-1α
The present data suggest that the genes Pgc-1α and Nr4a1 are target genes of circadian melatonin and dopamine release in the retina of mice.
The mammalian retina has the ability to adapt to the marked daily changes in the environment. This facilitates the retina, and in particular photoreceptor cells (PRCs), to comply with daily changes in metabolic and functional demands and on a long-term basis may contribute to their survival. Daily adaptation of retinal physiology1–3 is driven by retinal clocks4 partly through the rhythmic release of the hormones melatonin from PRCs and dopamine from amacrine cells (for review, see Ref. 5). Melatonin and dopamine play opposing roles in the control of retinal adaptation (for reviews, see Refs. 6, 7). Melatonin release occurs at night and mediates dark adaptation of retina through G-protein receptors named melatonin type 1 (MT1) and type 2 (MT2) receptors (for review, see Ref. 8) that appear to function as MT1/MT2 heteromers.9 In this process it modulates visual processing and viability of PRCs.10–12 On the other hand, dopamine release is higher during daytime13,14 and promotes adaptation of visual function to light (for reviews, see Refs. 6, 15, 16). Dopamine exerts its effects on various types of dopamine receptors widespread through the retina, but in particular on dopamine D4 (D4) receptors expressed primarily on PRCs to entrain light-adapted vision including contrast sensitivity function,12,16,17 the photoreceptor circadian clock in terms of protein phosphorylation,18 melatonin release,19 and adenylyl cyclase 1/cAMP signaling.20
In the mammalian retina, transcriptional regulators are critical for visual function,21,22 cell fate determination (for reviews, see Refs. 23, 24), and homeostasis.25–28 They are also important for retinal cell health and viability. Thus, transcriptional regulators (1) underlie different forms of inherited retinal degeneration in humans, such as Nr2e329–31 and Rorβ,32 and (2) are protective against retinal dystrophy, such as Nr1d133 and Pgc-1α,27 or activate immune defense pathways in response to damage of the retina, such as Egr-1 (Krox24; Ngfi-a; Zif268; Zenk28,34,35; Fos34,36 and Nr4a1 (Ngfi-b, Nur7734). Remarkably, numerous transcriptional regulators display a daily rhythm of expression in the retina (Dbp37; Egr-138; Fos39; Nr1d140; Nr2e341; Nr4a142; Pgc-1α43; Rorβ44). Therefore, their positive role in retinal health may be based on their ability to promote daily adjustment of the retina to comply with ambient demands.
The aim of the present study was to determine transcriptional regulators that may mediate retinal responses to circadian neurotransmitter release in retina of melatonin-proficient mice not carrying rd mutations. For this, daily regulation of the transcriptional regulators Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ was seen to be driven by a circadian clock, to take place in PRCs, and, most importantly, to depend on melatonin signaling/MT1 receptors or dopamine signaling/D4 receptors. As a result, Pgc-1α and Nr4a1 were identified as putative transcriptional targets of the circadian melatonin/dopamine system in murine retina.
Materials and Methods
Animals
Adult male and female mice with intact PRCs not carrying rd mutation were used in this study. As a rule the mice used were melatonin proficient (C3H/He (rd++), C3H/f+/+MT1+/+, C3H/f+/+MT1−/−, C3H/f+/+Drd4+/+, and C3H/f+/+Drd4−/−) and where indicated melatonin deficient (C57BL/6Jb). Mice were genotyped by PCR analysis of genomic DNA. The mice were kept under standard laboratory conditions (illumination with fluorescent strip lights, 200 lux at cage level during the day and dim red light during the night; 20 ± 1°C; water and food ad libitum) under light/dark 12:12 (LD 12:12) for 3 weeks. When indicated, the animals were then kept for one cycle under dim red light and killed during the next cycle. Animals (two for each time point) were killed at the indicated time points by decapitation following anesthesia with carbon dioxide. All dissections during the dark phase were carried out under dim red light. Retinas (four for each time point) were quickly removed, pooled, and immediately frozen or processed for laser microdissection and pressure catapulting (LMPC). Each experiment was carried out four times independently. Animal experimentation was carried out in accordance with the National Institutes of Health Guide on the Care and Use of Laboratory Animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Institutional Animal Care and Use Committees of Morehouse School of Medicine, Emory University, and the European Communities Council Directive (86/609/EEC).
Laser Microdissection and Pressure Catapulting
To prepare the retinas for LMPC, the HOPE technique (HOPE, Hepes-glutamic acid buffer-mediated organic solvent protection effect; DCS, Hamburg, Germany) was applied for the fixation.45 Photoreceptor cells were isolated from the stained sections in a contact- and contamination-free manner by using the LMPC technique as described previously.46 The purity grades of the preparations obtained were verified by using a specific gene marker of PRCs, namely, Nrl (as markers for rods47), and of inner retinal neurons, namely, Th (as a marker for amacrine cells48). In comparison to whole-retina preparations, in PRCs collected by LMPC, the ratio of Nrl to Th was increased 84-fold.
RNA Extraction, Reverse Transcription (RT), and Quantitative Polymerase Chain Reaction (qPCR)
RNA was isolated and reverse transcribed as described previously.43 In brief, PCR amplification and quantification were performed in an i-Cycler (BioRad, Munich, Germany) according to the following protocol: denaturation for 3 minutes at 95°C, followed by 40 cycles of 30 seconds at 95°C, 20 seconds at 60°C, and 20 seconds at 72°C. All amplifications were carried out in duplicate. By using agarose gel electrophoresis, the generated amplicons for all genes under examination were shown to possess the predicted sizes (Table 1). The amount of RNA was calculated from the measured threshold cycles (Ct) using an internal standard curve with 10-fold serial dilutions (101–108 copies/μL). The values were normalized with respect to the amount of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA present.
Table 1.
Primer Sequences Used for qPCR
Statistical Analysis
All PCR data are expressed as the mean ± standard error of the mean (SEM) of four independent experiments including eight time points. Transcript levels were calculated relative to average expression of each dataset throughout 24 hours to plot temporal expression. Significance of daily regulation was defined by showing a P < 0.05 in ANOVA (one-way analysis of variance). Cosinor analysis was used to fit sine wave curves to the circadian data to mathematically estimate the time of peak gene expression (acrophase) and to assess the amplitude.49,50 The model can be expressed according to the equation f(t) = A + B cos [2π (t + C) / T] with the f(t) indicating relative expression levels of target genes, t specifying the time of sampling (h), A representing the mean value of the cosine curve (MESOR; midline estimating statistic of rhythm), B indicating the amplitude of the curve (half of the sinusoid), and C indicating the acrophase (point of time when the function f(t) is maximum). T gives the time of the period, which is 24 hours for this experimental setting.
Results
Daily Profiling of the Transcriptional Regulators in Whole Retina of Melatonin-Proficient Mouse
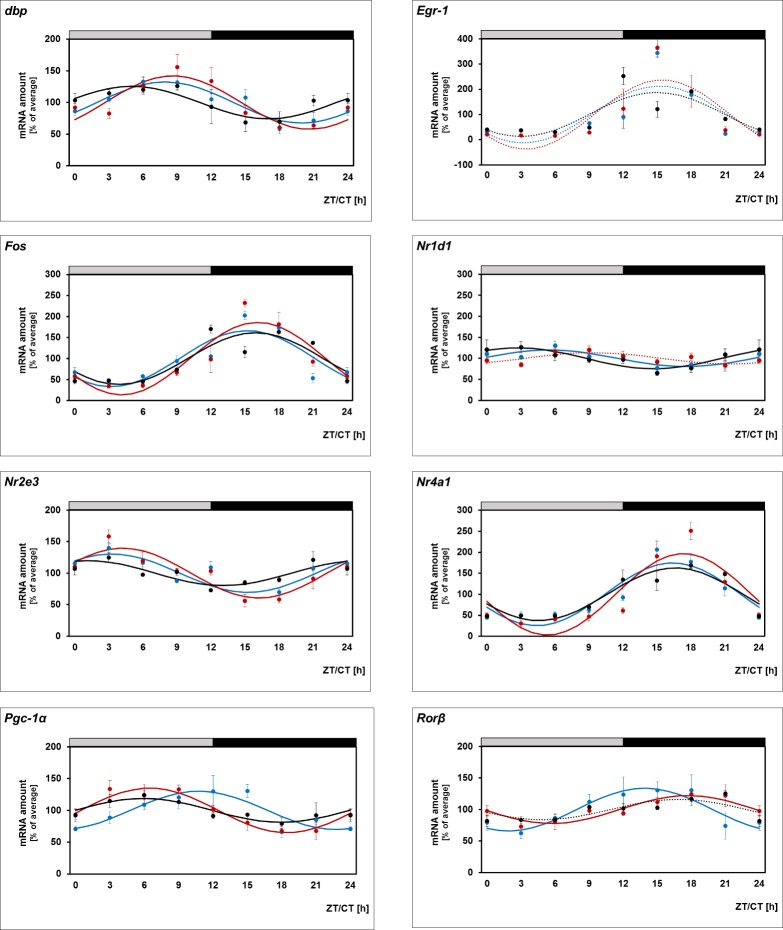
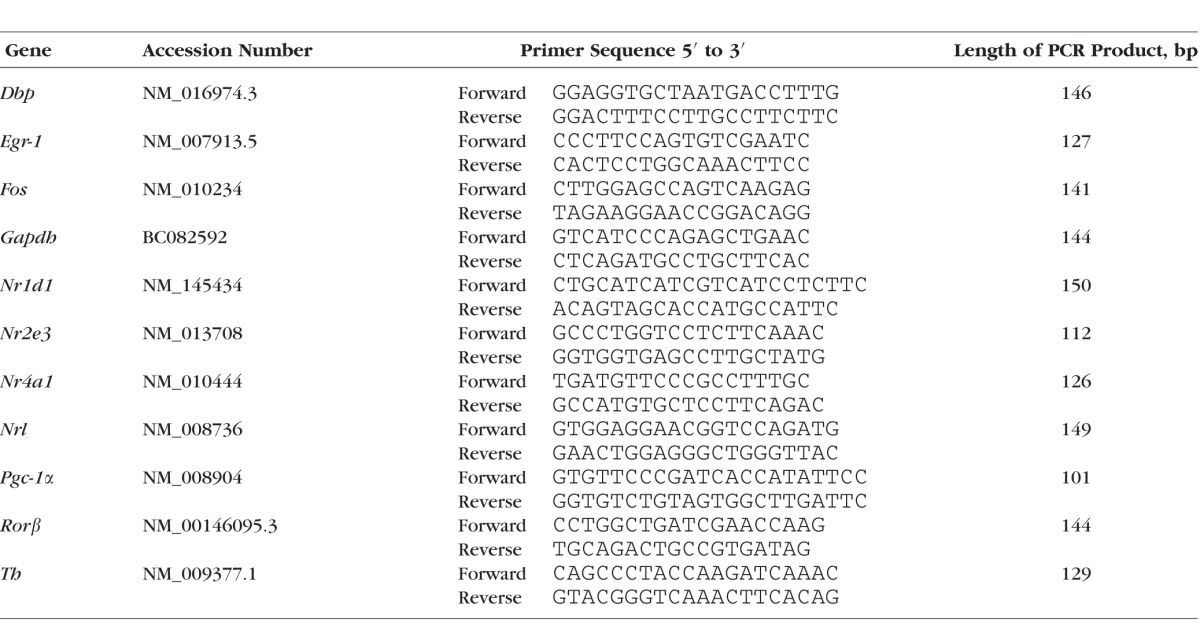
With the exception of a recently published study,37 daily profiling of gene expression in murine retina has so far been performed in mice strains that either are deficient for melatonin or carry rd mutation (resulting in damaged PRCs).51–55 The objective of this study—to investigate melatonin-dependent regulation of genes in the retina including PRCs—requires melatonin-proficient mice with intact PRCs (C3H/He (rd++)). It was seen that in these mice all transcriptional regulators under investigation display daily changes with peak expression during the day (Dbp, Nr1d1, Nr2e3, Pgc-1α) or at night (Egr-1, Fos, Nr4a1, Rorβ) (Fig. 1, blue lines; for statistical analysis, see Table 2).
Figure 1.
Twenty-four-hour profiling in whole retina and photoreceptor cells. 24-hour profiling of the transcriptional regulators Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ in preparations of the whole retina under light/dark (LD) 12:12 (blue lines), in microdissected photoreceptor cells under light/dark (LD) 12:12 (red lines), and in preparations of the whole retina of mice under constant darkness (DD) (black lines). The mRNA levels are plotted as a function of Zeitgeber time (ZT), and the lines represent the periodic sinusoidal functions (solid and dotted lines for P < 0.05 and P > 0.05 in cosinor analysis). The solid bars indicate the dark period. Data represent a percentage of the average value of transcript amount during the 24-hour period. The value of ZT0 was plotted twice at both ZT0 and ZT24. Each value represents mean ± SEM (n = 4). Statistical analysis of transcriptional profiling is provided in Table 2.
Table 2.
Daily Profiling of the Transcriptional Regulators in PRCs of Melatonin-Proficient Mouse
In order to compare daily regulation of the transcriptional regulators between preparations of the whole retina and PRCs, the LMPC technique was applied.46 The transcript levels of the genes were observed to exhibit daily rhythms in PRCs (Fig. 1, red lines; for statistical analysis, see Table 2) with profiles resembling those obtained from preparations of the whole retina (Fig. 1, blue lines; for statistical analysis, see Table 2). The findings obtained suggest that daily rhythmicity of the transcriptional regulators also occurs in PRCs. Interestingly, the relative strength of cycling of the genes (indicated in terms of the height of the amplitude) was different in PRCs (Nr4a1 > Fos > Dbp > Nr2e3 > Pgc-1α > Rorβ) and whole retina (Nr4a1 > Fos > Rorβ > Dbp > Nr2e3 > Pgc-1α > Rorβ). This might reflect different circadian output in outer and inner retina.
Daily Profiling of the Transcriptional Regulators in Retina of Melatonin-Proficient Mice in Constant Darkness
To gain a view of transcriptional control of the retina by a circadian clock, light input, or both, mice (C3H/He (rd++)) adapted to LD 12:12 were kept in constant darkness (DD) for one cycle and monitored during the subsequent cycle (Fig. 1, black lines; for statistical analysis, see Table 2). Under these conditions, the daily rhythm in mRNA amount continued for all transcriptional regulators investigated (Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ). This observation suggests that daily rhythmicity of the transcriptional regulators investigated requires a circadian clock. As far as assessable (P < 0.05 in cosinor analysis), the retained rhythmicity of the transcriptional regulators occurred with reduced amplitude under DD conditions when compared with LD conditions with the exception of Nr1d1. Therefore clock-dependent rhythmicity of the transcriptional regulators appears to be enhanced by light/dark transitions.
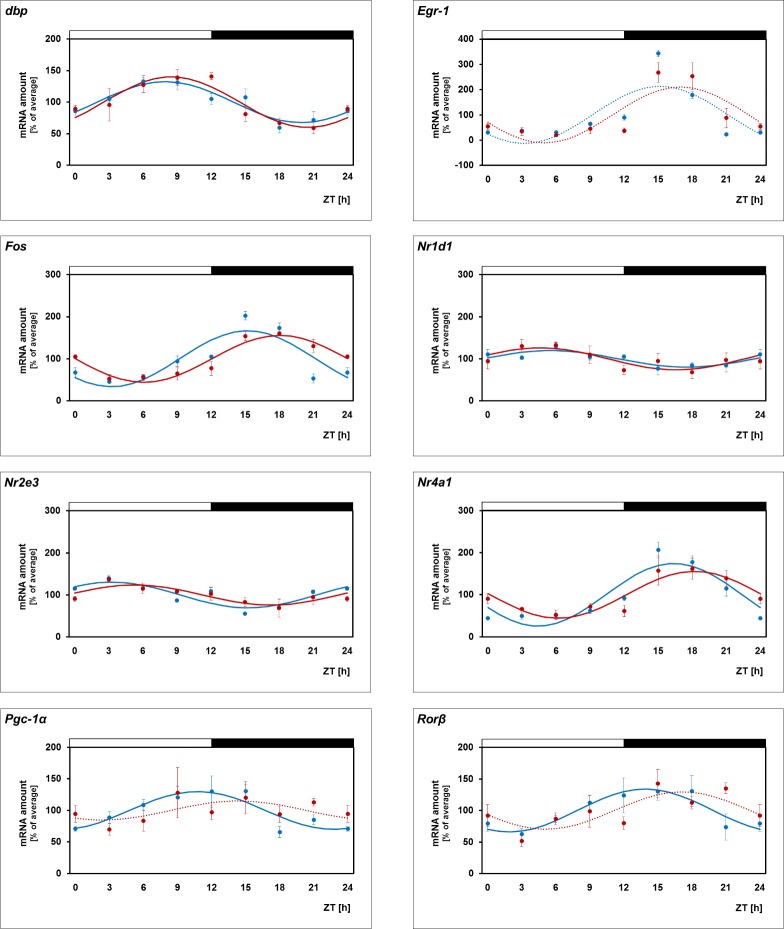
Daily Profiling of the Transcriptional Regulators in Melatonin-Deficient Mice
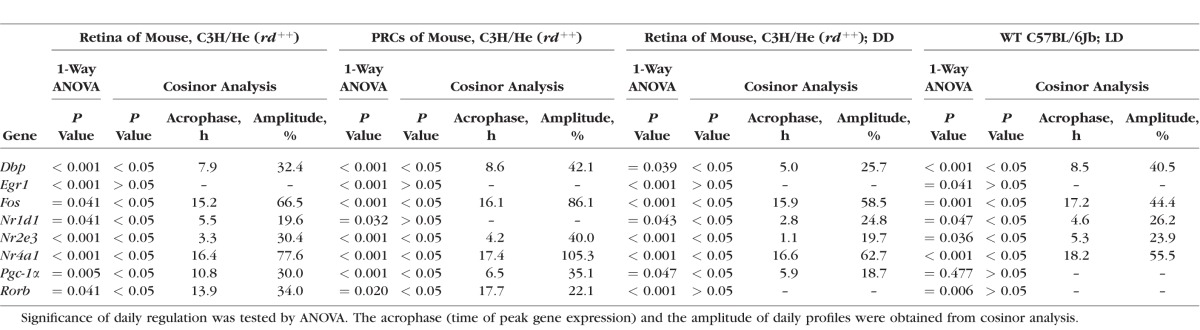
The retinal clock system—probably in terms of a clock within PRCs40,46—evokes a daily rhythm in melatonin release (for review, see Ref. 5). Therefore clock-driven rhythmicity of the transcriptional regulators may be conveyed by melatonin signaling. To test this assumption, daily profiling of the transcriptional regulators in melatonin-proficient mice C3H/He (rd++) was compared with that of melatonin-deficient C57BL/6Jb mice (Fig. 2, blue versus red lines; for statistical analysis, see Table 2). This revealed that Pgc-1α rhythmicity was prevented in melatonin-deficient mice. Conversely, the other transcriptional regulators under investigation (Dbp, Egr1, Fos, Nr1d1, Nr2e3, Nr4a1, Rorβ) were found to display daily patterns similar to those being observed in melatonin-proficient mice. These observations suggest that melatonin signaling is a prerequisite for the Pgc-1α rhythmicity.
Figure 2.
Daily profiling in melatonin-proficient and -deficient mice. Daily profiling of the transcriptional regulators Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ in retina of melatonin-proficient C3H/He (rd++) mice (blue lines, corresponding to those in Fig. 1) and melatonin-deficient C57BL/6Jb mice (red lines). The mRNA levels are plotted as a function of Zeitgeber time (ZT), and the lines represent the periodic sinusoidal functions (solid and dotted lines for P < 0.05 and P > 0.05 in cosinor analysis). The solid bars indicate the dark period. Data represent a percentage of the average value of transcript amount during the 24-hour period. The value of ZT0 was plotted twice at both ZT0 and ZT24. Each value represents mean ± SEM (n = 4). Statistical analysis of transcriptional profiling is provided in Table 2.
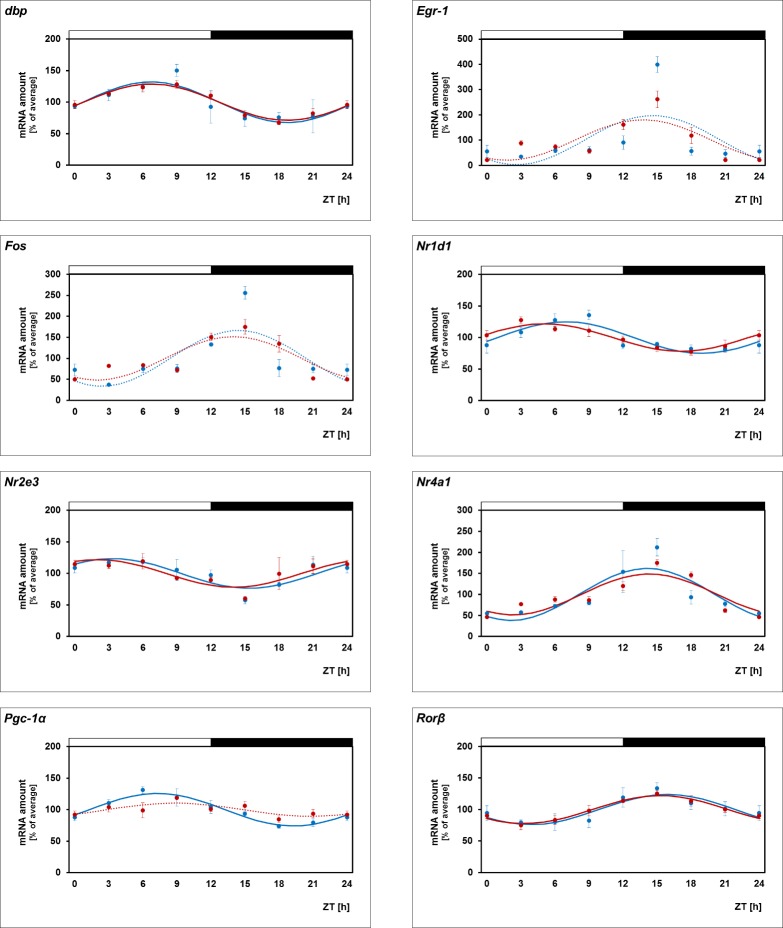
Daily Profiling of the Transcriptional Regulators in Melatonin-Proficient MT1−/− Mice
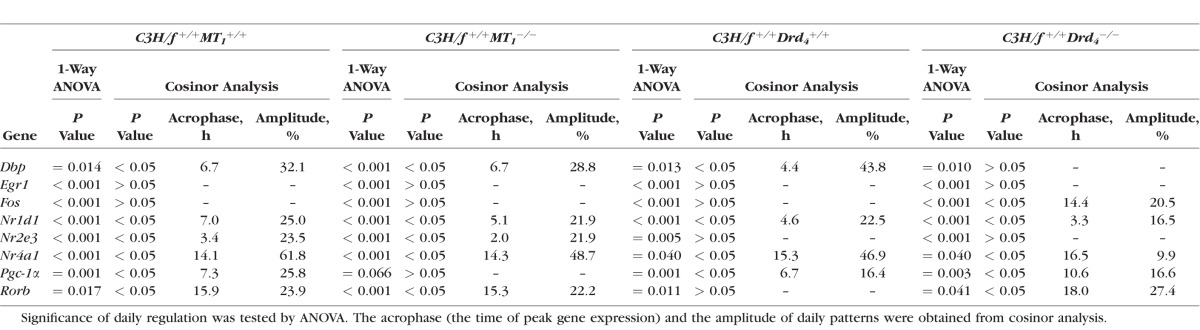
To verify and specify the response of the transcriptional regulators to melatonin signaling, their 24-hour profiles were compared in wild-type (WT) and MT1 receptor-deficient retina (Fig. 3, blue versus red lines; for statistical analysis, see Table 3), with both genotypes deriving from a melatonin-proficient mice strain with intact PRCs (C3H/f+/+11). Consistent with the results obtained from melatonin-deficient mice, the loss of MT1 receptors prevented the cycling of Pgc-1α. As far as assessable (P < 0.05 in cosinor analysis), it was seen to slightly dampen the rhythmicity of the other transcriptional regulators under investigation (Dbp, Nr1d1, Nr2e3, Nr4a1, Rorβ). This observation suggests that MT1 receptors couple the pulsatile melatonin signal to the expression of Pgc-1α and possibly play a general role in amplifying circadian regulation of retinal transcription.
Figure 3.
Twenty-four-hour profiling in WT and MT1−/− mice. 24-hour profiling of the transcriptional regulators Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ in retina of WT (blue lines) and MT1−/− mice (red lines). The mRNA levels are plotted as a function of Zeitgeber time (ZT), and the lines represent the periodic sinusoidal functions (solid and dotted lines for P < 0.05 and P > 0.05 in cosinor analysis). The solid bars indicate the dark period. Data represent a percentage of the average value of transcript amount during the 24-hour period. The value of ZT0 was plotted twice at both ZT0 and ZT24. Each value represents mean ± SEM (n = 4). Statistical analysis of transcriptional profiling is provided in Table 3.
Table 3.
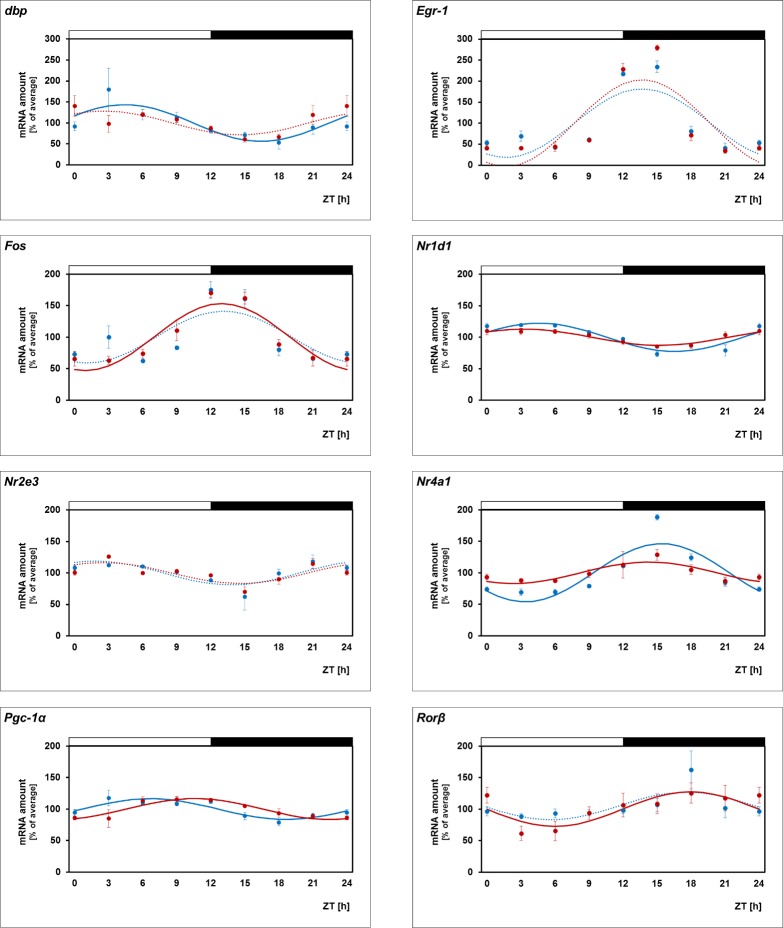
Daily Profiling of the Transcriptional Regulators in Drd4−/− Mice
Clock-dependent regulation of the transcriptional regulators may also involve dopamine signaling via dopamine D4 receptors (for review, see Ref. 5). To investigate this possibility, 24-hour profiling of the transcriptional regulators was performed in Drd4-deficient mice (C3H/f+/+, melatonin-proficient mice with intact PRCs) (Fig. 4, blue versus red lines; for statistical analysis, see Table 3). The rhythmicity of Nr4a1 was seen to be dampened in Drd4−/− mice, with a decrease in amplitude of 84%, whereas the other transcriptional regulators under investigation were seen to display similar daily profiles in both the genotypes. This finding suggests that Nr4a1 links the clock-driven dopamine signaling with retinal gene expression.
Figure 4.
Twenty-four-hour profiling in WT and Drd4−/− mice. 24-hour profiling of the transcriptional regulators Dbp, Egr-1, Fos, Nr1d1, Nr2e3, Nr4a1, Pgc-1α, Rorβ in retina of WT (blue lines) and Drd4−/− mice (red lines). The mRNA levels are plotted as a function of Zeitgeber time (ZT), and the lines represent the periodic sinusoidal functions (solid and dotted lines for P < 0.05 and P > 0.05 in cosinor analysis). The solid bars indicate the dark period. Data represent a percentage of the average value of transcript amount during the 24-hour period. The value of ZT0 was plotted twice at both ZT0 and ZT24. Each value represents mean ± SEM (n = 4). Statistical analysis of transcriptional profiling is provided in Table 3.
Discussion
The retinal clocks exert their influence on visual processing and survival of the retina through the neurohormones melatonin and dopamine and their respective action on MT1 and D4 receptors (for review, see Ref. 5). The main objective of this study was to identify candidate genes for linking clock-dependent neurohormone release with daily adaptation and healthiness of the retina. For this purpose, transcriptional regulators were screened for dependence on melatonin signaling/MT1 receptors or dopamine signaling/D4 receptors that fulfill the requirements to (1) cycle in PCRs (Dbp37: Fig. 1; Egr-1: Fig. 1; Fos, Nr1d140: Fig. 1; Nr2e3: Fig. 1; Nr4a1: Fig. 1; Pgc-1α43: Fig. 1; Rorβ40: Fig. 1), (2) be driven by a circadian clock (Dbp37: Fig. 1; Egr-139: Fig. 1; Fos39: Fig. 1; Nr1d140: Fig. 1; Nr2e3: Fig. 1; Nr4a1: Fig. 1; Pgc-1α: Fig. 1; Rorβ32: Fig. 1), and (3) influence retinal physiology or pathology (Egr-1, Fos34,36; Nr1d133; Nr2e329–31; Nr4a134; Nr1d1: Pgc-1α27; Rorβ32). As our experimental system we used mice with targeted deletion of MT1 receptors and D4 receptors, respectively, that we have crossed onto the C3Hf+/+ background.56,57 Thus the mouse model used in this study combines physiological melatonin formation and intact photoreceptors.
The data obtained determine the transcriptional coactivator Pgc-1α as a transcriptional target of circadian melatonin/MT1 receptor signaling. This becomes evident from the finding that the daily rhythmicity of Pgc-1α mRNA amount was dampened in both melatonin-deficient (C57BL/6Jb) and MT1−/− mice.
Melatonin/MT1 receptor-dependent control of Pgc-1α may take place in PRCs. This follows from the observation that PRCs combine a prominent rhythmicity of Pgc-1α (this study) with high density of MT1 receptors.11,12 Of interest is that, in PCRs, melatonin appears to act as an autocrine signal.11 Therefore PRCs may regulate their own Pgc-1α expression by melatonin release and its subsequent action on MT1 receptors located at their cell membrane. Recent studies have shown that the action of melatonin in the PRCs is mediated by MT1/MT2 heteromers.9 Therefore, MT1 receptor deficiency may result in nonfunctional MT1/MT2 heterodimers, and MT2 knockout mice would have produced similar results from those obtained in MT1.
Pgc-1α is a master regulator of glucose and energy metabolism in various tissues (for review, see Ref. 58) including retina.27 Therefore the observed melatonin/MT1 receptor-dependent regulation of Pgc-1α may contribute to daily changes in the energy metabolism of the retina and thus to its ability to comply with 24-hour changes in metabolic demands.59
Remarkably, Pgc-1α knockout mice reportedly suffer from increased light damage susceptibility, overexpression of Pgc-1α has antiapoptotic effects, and Pgc-1α expression is reduced in different mouse models of retinitis pigmentosa.27 This suggests that Pgc-1α provides protection against light damage and is therefore a promising candidate gene for transferring the positive effect of melatonin/MT1 receptors on the viability of retinal PRCs and ganglion cells.11 Accordingly, melatonin/MT1 receptor-dependent control of Pgc-1α could contribute to the protection of retinal cells against light damage and on a long-term basis to the survival of retinal cells during aging.
Outside the retina, MT1 receptor-dependent expression of Pgc-1α could play a role in the pathogenesis of type 2 diabetes. This becomes evident from the observations that the removal of MT1 receptors60 or Pgc-1α (for review, see Ref. 61) equally leads to increased insulin resistance in peripheral tissues.
As with Pgc-1α in the melatonergic system, the nuclear orphan receptor Nr4a1 (Nur77, Ngfi-b) was identified to respond to the dopaminergic system. This is evident from the finding that the daily rhythmicity of Nr4a1 is reduced in Drd4−/− mice. Dopaminergic regulation of retinal Nr4a1 probably occurs in PRC since this cell type combines circadian regulation of Nr4a1 (this study) with the abundance of D4 receptors.62,63 In the retina, clock-dependent dopamine release occurs from the unique population of cells in the inner nuclear layer that are either amacrine or interplexiform neurons.15 The dopamine neurons in retina express a full complement of core circadian clock genes.64,65 Therefore circadian/dopaminergic control of Nr4a1 in PRCs appears to be driven by these cell types. Furthermore, Drd4, the gene that encodes the D4 receptor, is itself a circadian clock-controlled gene in retina,3,20,42,66 and this too may contribute to circadian regulation of Nr4a1.
Dopaminergic regulation of Nr4a1 was until now believed to be confined to the motor/motivation system (for reviews, see Refs. 67, 68). Therefore its concurrent occurrence in the visual (this study) and the motor/motivation system suggests that it appears to be a more general feature of different functional brain systems affected by dopamine.
Furthermore, Nr4a1 is a promising candidate gene for being responsible for dysfunction of the retinal dopaminergic system and its subsequent visual defects in type I diabetes mellitus.69 This becomes evident from the observations that Nr4a1 expression on one hand influences the functioning of the dopaminergic system (for reviews, see Refs. 67, 68) and on the other hand depends on glucose metabolism.70–72
Compelling experimental evidence indicates that Nr4a1 not only contributes to dopaminergic responses but also is a decisive factor in dopamine-related neuroadaptation (for reviews, see Refs. 67, 68). If this is also valid in retina, circadian/dopaminergic regulation of Nr4a1 expression (this study) may feed back to the dopaminergic system of the retina to promote its reported circadian plasticity.42
It is important to note that—at variance with previous studies dealing with the role of melatonin in retina51,52—the present investigation was performed in mouse strains/genotypes that combine melatonin proficiency with intact PRCs and thus in mouse models that reflect retinal physiology as closely as possible. On the other hand, a general limitation of the present study is that the observed phenotypes of the mouse models used may reflect not only a direct action of the neurotransmitters/receptors, but also the secondary effects downstream of them. Since melatonin signaling73–75 and dopamine signaling76–78 make a cellular feedback loop in which melatonin inhibits the release of dopamine through melatonin receptors,73–75 whereas dopamine downregulates melatonin formation, probably through dopamine D4 receptors,76,77 this includes a possibility that genetic disturbance of melatonin signaling affects dopamine signaling and vice versa.
In conclusion, the data of the present investigation determined candidate transcriptional regulators for linking the circadian neurohormone systems to adaptation, function, and healthiness of the retina. They could provide a reliable basis for further investigations that may achieve a better understanding of how clock-dependent neurotransmitter release influences visual processing and survival of the retinal neurons.
Acknowledgments
The authors thank Ute Frederiksen and Kristina Schäfer for their excellent technical assistance, Susanne Rometsch and Bettina Wiechers-Schmied for secretarial help, Klaus Wolloscheck for statistical advice, and Russell G. Foster for providing us with C3H/He (rd++) mice. The data contained in this study are included in the thesis of Stefanie Kunst as a partial fulfillment of her doctorate degree at the Johannes Gutenberg University, Mainz. There are no conflicts of interest, financial or otherwise, to be disclosed by the authors.
Supported by the BMBF “HOPE2” (01GM1108D to UW), the Faun-Stiftung Nurnberg (to UW), the European Community FP7/2009/241955 (SYSCILIA to UW), the Naturwissenschaftlich-medizinisches Forschungszentrum of the University Mainz (to UW and RS), the National Institutes of Health (Grants R01 EY004864 to PMI, P30 EY006360 to PMI, T32 EY007092 to PMI; EY-022216 to GT) and an unrestricted grant from Research to Prevent Blindness, Inc. (RPB). PMI was a recipient of a Senior Scientific Investigator Award from RPB.
Disclosure: S. Kunst, None; T. Wolloscheck, None; D.K. Kelleher, None; U. Wolfrum, None; S.A. Sargsyan, None; P.M. Iuvone, None; K. Baba, None; G. Tosini, None; R. Spessert, None
References
- 1. Cameron MA,, Barnard AR,, Hut RA,, et al. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008; 23: 489–501. [DOI] [PubMed] [Google Scholar]
- 2. Cameron MA,, Barnard AR,, Lucas RJ. The electroretinogram as a method for studying circadian rhythms in the mammalian retina. J Genet. 2008; 87: 459–466. [DOI] [PubMed] [Google Scholar]
- 3. Storch KF,, Paz C,, Signorovitch J,, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007; 130: 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tosini G,, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998; 789: 221–228. [DOI] [PubMed] [Google Scholar]
- 5. McMahon DG,, Iuvone PM,, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014; 39: 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iuvone PM,, Tosini G,, Pozdeyev N,, et al. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005; 24: 433–456. [DOI] [PubMed] [Google Scholar]
- 7. Tosini G,, Pozdeyev N,, Sakamoto K,, Iuvone PM. The circadian clock system in the mammalian retina. Bioessays. 2008; 30: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tosini G,, Owino S,, Guillaume JL,, Jockers R. Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. Bioessays. 2014; 36: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baba K,, Benleulmi-Chaachoua A,, Journe AS,, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013; 6:ra89. [DOI] [PMC free article] [PubMed]
- 10. Baba K,, Mazzoni F,, Owino S,, et al. Age-related changes in the daily rhythm of photoreceptor functioning and circuitry in a melatonin-proficient mouse strain. PloS One. 2012; 7: e37799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baba K,, Pozdeyev N,, Mazzoni F,, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009; 106: 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sengupta A,, Baba K,, Mazzoni F,, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PloS One. 2011; 6: e24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iuvone PM,, Galli CL,, Garrison-Gund CK,, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978; 202: 901–902. [DOI] [PubMed] [Google Scholar]
- 14. Nir I,, Haque R,, Iuvone PM. Diurnal metabolism of dopamine in dystrophic retinas of homozygous and heterozygous retinal degeneration slow (rds) mice. Brain Res. 2000; 884: 13–22. [DOI] [PubMed] [Google Scholar]
- 15. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004; 108: 17–40. [DOI] [PubMed] [Google Scholar]
- 16. Jackson CR,, Ruan GX,, Aseem F,, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012; 32: 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang CK,, Chaurasia SS,, Jackson CR,, et al. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J Neurosci. 2013; 33: 14989–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pozdeyev N,, Tosini G,, Li L,, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008; 27: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cahill GM,, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991; 11: 2959–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson CR,, Chaurasia SS,, Hwang CK,, Iuvone PM. Dopamine D(4) receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur J Neurosci. 2011; 34: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu RT,, Chiang MY,, Tanabe T,, et al. The orphan nuclear receptor Tlx regulates Pax2 and is essential for vision. Proc Natl Acad Sci U S A. 2000; 97: 2621–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haider NB,, Mollema N,, Gaule M,, et al. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res. 2009; 89: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andreazzoli M. Molecular regulation of vertebrate retina cell fate. Birth Defects Res C Embryo Today. 2009; 87: 284–295. [DOI] [PubMed] [Google Scholar]
- 24. Bassett EA,, Wallace VA. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012; 35: 565–573. [DOI] [PubMed] [Google Scholar]
- 25. Zhang CL,, Zou Y,, Yu RT,, Gage FH,, Evans RM. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 2006; 20: 1308–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onishi A,, Peng GH,, Poth EM,, et al. The orphan nuclear hormone receptor ERRbeta controls rod photoreceptor survival. Proc Natl Acad Sci U S A. 2010; 107: 11579–11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Egger A,, Samardzija M,, Sothilingam V,, et al. PGC-1alpha determines light damage susceptibility of the murine retina. PLoS One. 2012; 7: e31272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma YV,, Cojocaru RI,, Ritter LM,, et al. Protective gene expression changes elicited by an inherited defect in photoreceptor structure. PLoS One. 2012; 7: e31371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haider NB,, Jacobson SG,, Cideciyan AV,, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000; 24: 127–131. [DOI] [PubMed] [Google Scholar]
- 30. Sharon D,, Sandberg MA,, Caruso RC,, Berson EL,, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003; 121: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 31. Wright AF,, Reddick AC,, Schwartz SB,, et al. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum Mutat. 2004; 24: 439. [DOI] [PubMed] [Google Scholar]
- 32. Andre E,, Conquet F,, Steinmayr M,, et al. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998; 17: 3867–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cruz NM,, Yuan Y,, Leehy BD,, et al. Modifier genes as therapeutics: the nuclear hormone receptor Rev Erb alpha (Nr1d1) rescues Nr2e3 associated retinal disease. PLoS One. 2014; 9: e87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamphuis W,, Dijk F,, van Soest S,, Bergen AA. Global gene expression profiling of ischemic preconditioning in the rat retina. Mol Vis. 2007; 13: 1020–1030. [PMC free article] [PubMed] [Google Scholar]
- 35. Bakalash S,, Pham M,, Koronyo Y,, et al. Egr1 expression is induced following glatiramer acetate immunotherapy in rodent models of glaucoma and Alzheimer's disease. Invest Ophthalmol Vis Sci. 2011; 52: 9033–9046. [DOI] [PubMed] [Google Scholar]
- 36. Wurm A,, Iandiev I,, Uhlmann S,, et al. Effects of ischemia-reperfusion on physiological properties of Muller glial cells in the porcine retina. Invest Ophthalmol Vis Sci. 2011; 52: 3360–3367. [DOI] [PubMed] [Google Scholar]
- 37. Hiragaki S,, Baba K,, Coulson E,, et al. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014; 9: e106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agarwal N. Diurnal expression of NGF1-A mRNA in retinal degeneration slow (rds) mutant mouse retina. FEBS Lett. 1994; 339: 253–257. [DOI] [PubMed] [Google Scholar]
- 39. Humphries A,, Carter DA. Circadian dependency of nocturnal immediate-early protein induction in rat retina. Biochem Biophys Res Commun. 2004; 320: 551–556. [DOI] [PubMed] [Google Scholar]
- 40. Sandu C,, Hicks D,, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011; 34: 507–516. [DOI] [PubMed] [Google Scholar]
- 41. Mollema NJ,, Yuan Y,, Jelcick AS,, et al. Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One. 2011; 6: e17494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bai L,, Zimmer S,, Rickes O,, et al. Daily oscillation of gene expression in the retina is phase-advanced with respect to the pineal gland. Brain Res. 2008; 1203: 89–96. [DOI] [PubMed] [Google Scholar]
- 43. Kunst S,, Wolloscheck T,, Holter P,, et al. Transcriptional analysis of rat photoreceptor cells reveals daily regulation of genes important for visual signaling and light damage susceptibility. J Neurochem. 2013; 124: 757–769. [DOI] [PubMed] [Google Scholar]
- 44. Kamphuis W,, Cailotto C,, Dijk F,, Bergen A,, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Biophys Res Commun. 2005; 330: 18–26. [DOI] [PubMed] [Google Scholar]
- 45. Goldmann T,, Burgemeister R,, Sauer U,, et al. Enhanced molecular analyses by combination of the HOPE-technique and laser microdissection. Diagn Pathol. 2006; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schneider K,, Tippmann S,, Spiwoks-Becker I,, et al. Unique clockwork in photoreceptor of rat. J Neurochem. 2010; 115: 585–594. [DOI] [PubMed] [Google Scholar]
- 47. Mears AJ,, Kondo M,, Swain PK,, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001; 29: 447–452. [DOI] [PubMed] [Google Scholar]
- 48. Osborne NN,, Patel S,, Vigny A. Dopaminergic neurones in various retinas and the postnatal development of tyrosine-hydroxylase immunoreactivity in the rabbit retina. Histochemistry. 1984; 80: 389–393. [DOI] [PubMed] [Google Scholar]
- 49. Cornelissen G. Cosinor-based rhythmometry. Theoret Biol Med Model. 2014; 11: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Refinetti R,, Lissen GC,, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007; 38: 275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dinet V,, Ansari N,, Torres-Farfan C,, Korf HW. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2007; 42: 83–91. [DOI] [PubMed] [Google Scholar]
- 52. Dinet V,, Korf HW. Impact of melatonin receptors on pCREB and clock-gene protein levels in the murine retina. Cell Tissue Res. 2007; 330: 29–34. [DOI] [PubMed] [Google Scholar]
- 53. Liu X,, Zhang Z,, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012; 7: e50602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ait-Hmyed O,, Felder-Schmittbuhl MP,, Garcia-Garrido M,, et al. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013; 37: 1048–1060. [DOI] [PubMed] [Google Scholar]
- 55. Dkhissi-Benyahya O,, Coutanson C,, Knoblauch K,, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013; 70: 3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freedman MS,, Lucas RJ,, Soni B,, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999; 284: 502–504. [DOI] [PubMed] [Google Scholar]
- 57. Lupi D,, Cooper HM,, Froehlich A,, et al. Transgenic ablation of rod photoreceptors alters the circadian phenotype of mice. Neuroscience. 1999; 89: 363–374. [DOI] [PubMed] [Google Scholar]
- 58. Liu C,, Lin JD. PGC-1 coactivators in the control of energy metabolism. Acta Biochim Biophys Sin (Shanghai). 2011; 43: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ames A,, III, Li YY. Energy requirements of glutamatergic pathways in rabbit retina. J Neurosci. 1992; 12: 4234–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Contreras-Alcantara S,, Baba K,, Tosini G. Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver Spring). 2010; 18: 1861–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rowe GC,, Arany Z. Genetic models of PGC-1 and glucose metabolism and homeostasis. Rev Endocr Metab Disord. 2014; 15: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen AI,, Todd RD,, Harmon S,, O'Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A. 1992; 89: 12093–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kay JN,, De la Huerta I,, Kim IJ,, et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011; 31: 7753–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruan GX,, Zhang DQ,, Zhou T,, Yamazaki S,, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A. 2006; 103: 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dorenbos R,, Contini M,, Hirasawa H,, Gustincich S,, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007; 24: 573–580. [DOI] [PubMed] [Google Scholar]
- 66. Klitten LL,, Rath MF,, Coon SL,, et al. Localization and regulation of dopamine receptor D4 expression in the adult and developing rat retina. Exp Eye Res. 2008; 87: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Levesque D,, Rouillard C. Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci. 2007; 30: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Campos-Melo D,, Galleguillos D,, Sanchez N,, Gysling K,, Andres ME. Nur transcription factors in stress and addiction. Front Mol Neurosci. 2013; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aung MH,, Park HN,, Han MK,, et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014; 34: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pei L,, Waki H,, Vaitheesvaran B,, et al. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006; 12: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 71. Fu Y,, Luo L,, Luo N,, Zhu X,, Garvey WT. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem. 2007; 282: 31525–31533. [DOI] [PubMed] [Google Scholar]
- 72. Kanzleiter T,, Wilks D,, Preston E,, et al. Regulation of the nuclear hormone receptor nur77 in muscle: influence of exercise-activated pathways in vitro and obesity in vivo. Biochim Biophys Acta. 2009; 1792: 777–782. [DOI] [PubMed] [Google Scholar]
- 73. Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983; 306: 782–784. [DOI] [PubMed] [Google Scholar]
- 74. Boatright JH,, Rubim NM,, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994; 11: 1013–1018. [DOI] [PubMed] [Google Scholar]
- 75. Ribelayga C,, Wang Y,, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004; 554 (pt 2): 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zawilska JB,, Iuvone PM. Melatonin synthesis in chicken retina: effect of kainic acid-induced lesions on the diurnal rhythm and D2-dopamine receptor-mediated regulation of serotonin N-acetyltransferase activity. Neurosci Lett. 1992; 135: 71–74. [DOI] [PubMed] [Google Scholar]
- 77. Nguyen-Legros J,, Chanut E,, Versaux-Botteri C,, Simon A,, Trouvin JH. Dopamine inhibits melatonin synthesis in photoreceptor cells through a D2-like receptor subtype in the rat retina: biochemical and histochemical evidence. J Neurochem. 1996; 67: 2514–2520. [DOI] [PubMed] [Google Scholar]
- 78. Tosini G,, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci Lett. 2000; 286: 119–122. [DOI] [PubMed] [Google Scholar]