Abstract
Crohn’s disease (CD) is a chronic idiopathic inflammatory bowel disease (IBD) which affects any site of the gastrointestinal tract and occasionally extraintestinal organs. The natural history of CD varies remarkably but a considerable proportion of patients develop complications leading to hospitalizations and surgeries, impaired quality of life, and disability. In these patients, effective medical therapy should aim beyond control of clinical symptoms to include induction and maintenance of steroid-free clinical and serological remission and mucosal healing, as this has shown to reduce complications, hospitalizations and surgeries, and to decrease the risk of colorectal cancer, at least in the short term. This therapeutic goal can be achieved in a considerable proportion of patients with anti-tumor necrosis factor (TNF)-α agents if applied early in the disease course. Clinical recommendations from a panel of Greek IBD experts are herein provided, regarding the clinical profiles and the use of anti-TNF-α therapy in patients with moderate and severe CD, based on literature review and personal experience. The objectives of this advisory workshop were to define the profiles of patients with moderate and severe CD using routine clinical and laboratory parameters, as well as the clinical profiles of patients with moderate CD, severe CD, perianal CD, and/or extra-intestinal manifestations, who are candidates for biologic therapies. Emphasis was given on patients with newly diagnosed CD. The proposed recommendations may provide a useful and practical approach for improving therapeutic strategies with anti-TNF-α in patients with active moderate and severe CD.
Keywords: Crohn’s disease, inflammatory bowel disease, anti-tumor necrosis factor-α agents, extra-intestinal manifestations, expert consensus guidelines
Introduction
Crohn’s disease (CD) is a chronic idiopathic inflammatory bowel disease (IBD) marked by a transmural, asymmetric, discontinuous, and, occasionally granulomatous inflammation of the gastrointestinal tract [1]. CD usually starts with an inflammatory phenotype. Symptoms are heterogeneous, but commonly include chronic diarrhea and abdominal pain. Systemic symptoms of malaise, anorexia, weight loss and/or fever are also common [2]. If inappropriately treated, uncontrolled inflammation may be complicated by intestinal strictures, intestinal perforation, abscesses and/or fistulae which may necessitate hospitalizations and surgical interventions to restore bowel integrity and function. Surgery for CD, however, is not curative and disease often relapses initiating again a relentless vicious cycle of inflammation and complications which may lead to additional surgical interventions. Eventually, this may end up to irreversible bowel damage and intestinal failure [3,4].
Until recently, the main goal of conventional medical therapy in CD was to induce clinical remission, and, if possible, discontinue corticosteroids. Conventional therapy comprises sulfasalazine or mesalazine and systemic corticosteroids followed by immunosuppressive agents, thiopurines [azathioprine (AZA), mercaptopurine (MP)] or methotrexate (MTX) in case of corticosteroid-dependency or -refractoriness. In this approach, known as ‘step-up’ strategy, anti-tumor necrosis factor (TNF)-α agents are offered when adequately dosed conventional therapy cannot achieve and/or maintain corticosteroid-free remission or is associated with adverse events [5-7]. Although highly effective in the short term, corticosteroids lead to low rates of sustained remission and mucosal healing [8] whereas their therapeutic benefit is also frequently offset by adverse effects, occasionally serious and lethal. Immunosupressives may achieve mucosal healing but they are slow-acting and only modestly effective agents. One meta-analysis suggested that the probability of remaining in remission at 1 year with AZA is about 40% [9].
Evidence has accumulated to support the need that medical therapy of CD should be re-targeted towards an earlier and tighter control of inflammation (‘treat-to-target’). Such an approach should aim at achieving and maintaining sustained clinical and serological steroid-free remission and mucosal healing to prevent disease complications, hospitalizations and surgeries, and improve quality of life and disability [10]. Currently, anti-TNF-α agents [infliximab (IFX), adalimumab (ADA), and certolizumab] are the only approved biologic agents for the treatment of moderate CD in patients not responding to or intolerant of conventional therapy [10]. Post hoc analysis of randomized controlled trials and expert reports suggest that early use of anti-TNF-α in moderate-to-severe CD could be more beneficial [11,12]. The updated guidelines of the European Crohn’s and Colitis Organization (ECCO) on CD recommend the use of anti-TNF-α treatment for steroid-refractory, -dependent, and/or -intolerant CD [13]. However, as it is difficult to predict which patient will develop severe course of disease it is essential to identify at diagnosis features associated with a poor prognosis that may help select the appropriate candidates for timely applied anti-TNF-α treatment [14,15]. Studies have shown that young age at disease onset, active smoking, need for corticosteroids at diagnosis, rectal involvement, perianal disease, extensive small bowel and/or upper gastrointestinal involvement may be the more robust factors in predicting a poor outcome of CD and determine the need of biological therapy [16].
At present, there is no “gold standard” for the measurement of the activity of CD. The Crohn’s Disease Activity Index (CDAI) and the Harvey-Bradshaw Index (HBI) are the most widely used grading systems. To facilitate patient classification in the setting of clinical trials ECCO has suggested that moderate CD be defined as a CDAI of 220-450 whereas severe CD as a CDAI >450; [17] this ensures enrollment of patients in more or less homogeneous groups and assesses response to treatment. However, these activity indices have proven insensitive and impractical in routine clinical practice. Serological markers, such as C-reactive protein (CRP) [18], or stool markers of inflammation, such as fecal calprotectin and lactoferrin [19], endoscopic indices and assessments of quality of life are being increasingly used in routine clinical practice because they represent more objective measures of intestinal inflammation.
The objectives of this advisory workshop were to define the profiles of patients with moderately and severely active CD, moderately or severely active CD, active perianal disease, and/or extra-intestinal manifestations (EIMs) who are appropriate candidates for biologic therapy.
Materials and methods
A steering committee of Greek IBD experts on the use of biological therapies in IBD performed an extensive search and critical review of the available literature and developed questions related to the clinical profiles and management of CD patients according to the severity of their disease based on clinical, laboratory, imaging, and endoscopic characteristics. There were also questions on the management of perianal CD and EIMs of CD, as well as on the indications of anti-TNF-α therapy for these conditions. Then, a panel of 10 Greek gastroenterologists selected on the basis of their expertise especially in using biologic therapy for CD patients, participated in a scientific workshop held on 31st March 2012. This workshop was chaired by two members of the Steering Committee. During this workshop the panel of experts after extensive discussion answered the questions selected by the Steering Committee and expressed a broad spectrum of opinions and/or viewpoints. Consequently, the proposed recommendations derived from critical evaluation of the available literature and published guidelines, as well as from the clinical experience of the participants in the panel. These recommendations would be intended for use by healthcare professionals in managing patients with moderate and/or severe CD. To further fortify the scientific evidence of these recommendations, a workshop of 6 invited key opinion leaders (KOLs) was held. During this workshop, the final recommendations of the March 2012 workshop were amended, to confirm agreement with the literature, and voted. Participants were asked to discuss and then score each recommendation separately on a 5-point scale, as follows:
1 = strongly disagree
2 = disagree
3 = neither agree nor disagree
4 = agree
5 = strongly agree
Consensus amongst the KOLs was defined a priori as agreement in 80% of the participants.
Definition of newly diagnosed moderately active CD
The panel agreed that a CDAI and HBI scores are appropriately used in the context of clinical trials [17] but are impractical to apply in routine clinical practice to measure disease activity. The panel also agreed that the American College of Physicians working definitions [20] are consistent with the ECCO grading of disease activity and provide useful definitions of the disease status because they also include patients who failed to respond to initial treatment and/or have more prominent symptoms [20]. Therefore, as newly diagnosed CD patients with moderately active disease were considered patients with a recent first diagnosis of moderately active CD but also patients with a recent diagnosis of mild CD which worsened despite adequately dosed first-line treatment in the absence of conditions unrelated to the activity of CD per se, such as infection(s), bacterial overgrowth, irritable bowel syndrome, etc. Presenting symptoms may be either abdominal or extra-intestinal. Panelists agreed that the definition of moderate CD should mainly depend on patient clinical characteristics but the impact of disease on patient quality of life should also be seriously considered in determining disease severity (Clinical statement 1).
Clinical statement 1.
Definition of newly diagnosed patients with moderate Crohn’s disease (CD)
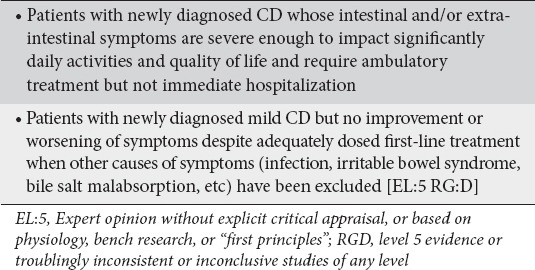
Factors that determine moderate disease severity in newly diagnosed patients
Since there is no single standard for the diagnosis of CD, the final diagnosis was made on the basis of a constellation of clinical, laboratory, imaging, endoscopic, and histological findings as well as the long-term course of disease [17].
Clinical factors
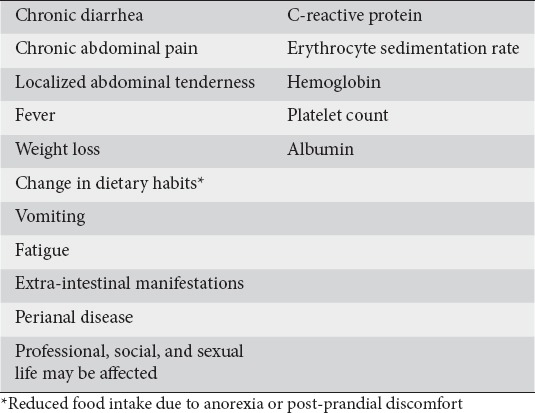
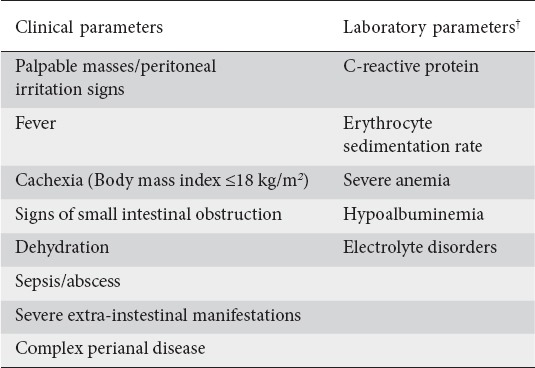
The heterogeneous nature of CD and the overlapping characteristics with other IBDs can make the diagnosis of CD difficult. Symptoms of chronic diarrhea and abdominal pain, weight loss and fever reflect the inflammatory process [21,22]. Additional features that can be present in patients with newly diagnosed CD of moderate activity include localized abdominal tenderness, anorexia, postprandial abdominal pain, vomiting, and fatigue. EIMs and perianal disease can also be present at diagnosis of moderately active CD in some patients (Table 1).
Table 1.
Clinical and laboratory features relevant to moderate disease severity in newly diagnosed patients
Laboratory factors
Laboratory parameters were also considered highly relevant in determining disease activity. The expert panel agreed (87.5%) that typical laboratory parameters (Table 1), such as CRP and erythrocyte sedimentation rate (ESR), lack sufficient specificity but need to be taken into consideration in determining moderate disease activity because they can discriminate satisfactorily moderate from mild disease activity since the latter may be associated with normal levels of these inflammatory indices [23]. Measurement of ferritin, or nutrients, such as folate and vitamin B12, that may be deficient due to anorexia, malnutrition, malabsorption, and/or excessive loss may also be useful, as these may indicate pre-existing chronic active CD at the time of diagnosis [1].
Endoscopic findings
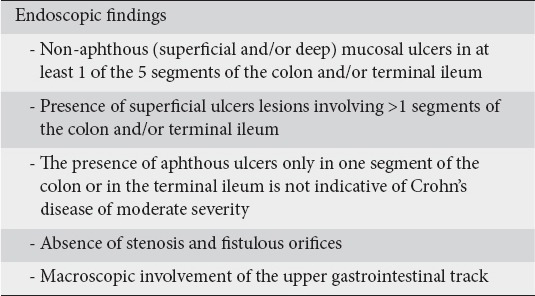
Endoscopy is an essential tool for the diagnosis of CD [17]. Allez et al, previously defined moderate endoscopic lesions as ulcerations covering less than 10% of the mucosal area on one or more segments of the colon [24]. Most of the panel agreed (87.5%) that the presence of non-aphthous ulcers in at least one of the five segments of the colon and/or terminal ileum indicates moderate severity of CD. Other endoscopic features suggestive of moderate CD include skip lesions, a variety of ulcerations involving >10% of the colon and/or terminal ileum, segmental colitis and involvement of the upper gastrointestinal tract (Table 2).
Table 2.
Endoscopic findings highly relevant to moderate disease severity
The panel also agreed that the severity of endoscopic lesions may correlate poorly with clinical and/or biological activity as has been suggested by GETAID publications [25].
Imaging findings
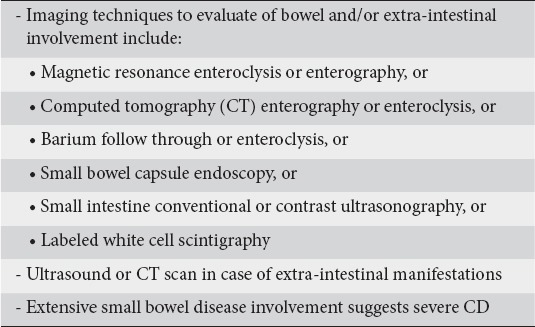
Endoscopic and imaging techniques are complementary in order to assess the extent and severity of CD [17]. Studies have shown that small bowel lesions beyond the reach of a routine ileocolonoscopy are common in CD [26]. The panel agreed (100% agreement) that imaging findings are highly relevant in the classification of newly diagnosed patients as suffering from moderately active CD.
The panel agreed that available imaging techniques for the study of small bowel include magnetic resonance imaging (MRI)- or computed tomography (CT)-enterography/enteroclysis, small bowel capsule endoscopy, small bowel enema (enteroclysis), and small intestine ultrasonography (SICUS) with or without contrast agents. These techniques, used according to their indications, have a high diagnostic accuracy for the detection of small bowel involvement [17]. However, MRI enterography should be the gold standard as it lacks ionizing radiation and allows anatomical and functional assessment of the small bowel. Abdominal CT and conventional US or SICUS should be considered as readily available techniques to exclude complications, such as abscesses. Extensive small bowel involvement in newly diagnosed CD was considered as highly relevant (100% agreement) to grade CD as severely active. It was also agreed that performing a SICUS (or conventional US) or a CT or MRI scan may be relevant for patients presenting with or expressing concurrently EIMs (Table 3).
Table 3.
Imaging techniques relevant to moderate disease severity
Risk factors for poor outcome of disease
Not all patients have the same disease course, neither do they respond in the same way to standard therapy [27]. Prognostic factors for poor disease outcome would be of value to select the optimal therapy for the individual patient. Specific markers are required to help identify patients with moderate disease we are currently lacking in well-powered studies [20,27,28].
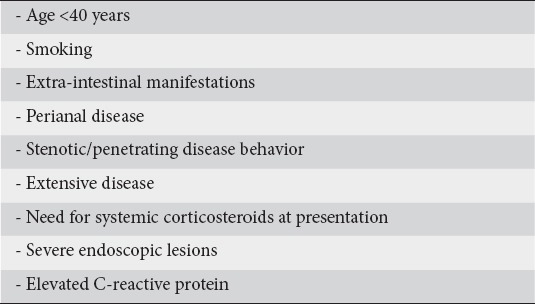
Based on literature and clinical experience, the panelists reached a consensus (100% agreement) on the risk factors associated with a poor outcome of disease in patients with moderate CD (Table 4). The panel agreed that, among these additional risk factors, the need for corticosteroids, extensive disease and perianal lesions at diagnosis, are more robust predictors of a worse disease outcome and these patients require early intervention [27].
Table 4.
Risk factors for poor outcome of newly diagnosed moderately active Crohn’s disease
Profile of newly diagnosed patients with moderate CD who are candidates for biologic therapy
Selection of appropriate patients for early introduction of biological agents depends on clinical characteristics, patient phenotype and previous response to conventional therapy. Clinical trials have shown that timely applied biological therapy may induce rapid and sustained clinical remission and mucosal healing and prevent complications at least in the short term. It was therefore agreed that initiating biological agents early could increase the likelihood of achieving these treatment goals. Panelists agreed that they would recommend biologic therapy for steroid-dependent and steroid-refractory patients as well as in patients who have relapsed following optimal conventional treatment (Clinical statement 2).
Clinical statement 2.
Profile of newly diagnosed patients with moderate Crohn’s disease who are candidates for biologic therapy

Early introduction of biological therapy in patients with steroid-refractory moderate CD
The SONIC trial has demonstrated superiority of IFX-based strategies (monotherapy or combined with AZA) over AZA monotherapy in moderate-to-severe CD patients naïve both to thiopurines and biological agents [15]. The London position statement of the 2009 World Congress of Gastroenterology also suggested that early introduction of biologic therapy may benefit patients with moderately active CD when steroids cannot be tolerated [29]. Based on these treatment recommendations, the panel strongly agreed (100% agreement) that steroid-refractory and immunosuppressive-naïve patients should start biologic agents early with or without immunosuppressives (Table 5).
Table 5.
Clinical and laboratory features of newly diagnosed patients with severe Crohn’s disease
Early introduction of biological therapy in immunosuppressive naïve patients with moderately active steroid-dependent CD
Controlled clinical trials [30-32] and a meta-analysis [9] have shown that AZA is effective in maintaining remission in patients with active steroid-dependent CD. A study conducted by Lemann et al in 113 patients with active, steroid-dependent CD showed that IFX induction treatment followed by AZA maintenance therapy was more effective than AZA monotherapy in achieving and maintaining remission of steroid-dependent patients [33]. The ECCO guidelines recommend anti-TNF-α agents as an alternative for patients with active disease who have previously been steroid-dependent [11]. The panel took into consideration these recommendations and reached consensus (100%) on the early introduction of biologic therapy for steroid-dependent patients with moderately active CD.
The panelists agreed that there are three therapeutic options in steroid-dependent and naïve to immunosuppressives patients: AZA monotherapy; monotherapy with an anti-TNF-α biologic agent; or their combination. The choice between adding AZA as monotherapy or combination therapy should depend on the presence of prognostic factors for a poor long-term outcome of CD (see previously). Furthermore, the panelists pointed out that the duration of combination therapy may be decided on an individual basis but safety issues should be of concern, especially in young males (as the majority of hepatosplenic T cell lymphoma cases occur in young males) and elderly patients (higher risk of lymphoma with thiopurines and opportunistic infections with anti-TNF agents).
Management of relapse under optimal therapy with immunosuppressives
The appropriate choice of treatment of a particular flare of CD is influenced by previous response to this treatment. This is especially true when patients relapse on optimal therapy to which patients strictly adhere. The panelists strongly endorsed the concept that biological agents should be initiated when there is relapse of CD in patients who adhere to optimal therapy with conventional immunosuppressives (100% agreement). The expert panel recommended that patients who have a severe relapse of CD within 6 months after a course of steroids should be considered for biologic therapy, because early administration of anti-TNF-α therapy is more effective than AZA monotherapy [15]. However, it was also recommended that if a patient experiences a relapse of moderate severity, after long-term remission on immunosuppressives, restarting steroids may be appropriate; the response should be further assessed and, if the patient relapses again, initiation of biologic therapy should be considered [1,3] (Clinical statement 3).
Clinical statement 3.
Indications for early administration of biological therapy in newly diagnosed patients with moderate Crohn’s disease
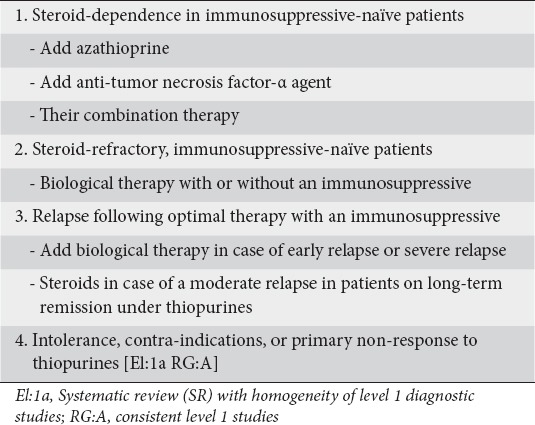
For patients either refractory to or intolerant of thiopurines, available options are to switch to MTX or biologic therapy. The panel reached consensus (100% agreement) regarding the initiation of biologic agents in patients who fail to respond to or are intolerant of or have contraindications to thiopurines. In this case, biological agents may be considered as a treatment option and MTX may be an alternative. Adding MTX to the biologic agent was also recommended to minimize immunogenicity.
The panel agreed that ECCO’s working definition of severe CD as indicated by a CDAI score higher than 450 and persistent symptoms despite treatment with steroids [20] is valid although again it is difficult to apply CDAI in routine clinical practice.
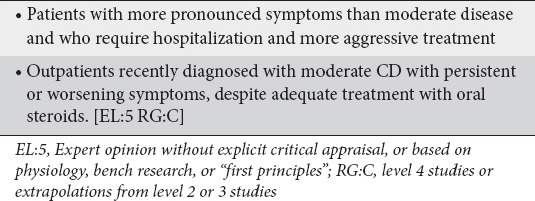
There was general agreement among panelists that the main difference between patients with newly diagnosed moderate or severe CD is that the later have more pronounced symptoms and require immediate hospitalization and more aggressive treatment whereas the former can be treated as outpatients. The panelists also agreed that outpatients recently diagnosed with moderate CD who have symptoms refractory to treatment with adequate doses of oral steroids should also be treated as patients with severe CD (Clinical statement 4).
Clinical statement 4.
Proposed patient profile of newly diagnosed patients with severe Crohn’s disease (CD)
Factors determining severe disease in newly diagnosed patients with CD
Clinical parameters
According to the working definition of the Practice Parameters Committee of the American College of Gastroenterology [20], patients with severe CD are presenting with high fever, persistent vomiting, cachexia, or evidence of intestinal obstruction. ECCO guidelines suggest that severe disease may be manifested by severe cachexia (body mass index <18 kg/m2), evidence of obstruction or abscess, or persistent symptoms despite intensive treatment [17,20]. The panel reached consensus (100% agreement) that the features described in Table 6 may be present in newly diagnosed patients with severe disease.
Table 6.
Endoscopic features that may be present in newly diagnosed patients with severe disease

Laboratory parameters
Regarding laboratory parameters of severe disease, elevated levels of CRP and/or ESR were included with no established cut-offs (100% agreement). Other laboratory parameters suggesting severe CD included: severe anemia (hemoglobin <8 g/dL), thrombocytosis, hypoalbuminemia, and electrolyte disturbances, such as hypokalemia and hypomagnesemia, and dehydration.
Endoscopic parameters
Endoscopic features were considered as highly significant parameters for the classification of newly diagnosed severe CD. The panelists reached consensus that, in severe cases, large and deep ulcers are commonly predominant features with extensive involvement of the colon and/or the small intestine; as in the case of clinical and laboratory parameters, endoscopic findings do not always correlate with clinical parameters. In these severe cases, inflammatory stenotic areas and/or strictures may be already present and lesions in the proximal gastrointestinal tract, if affected, are usually more severe [34].
Imaging findings
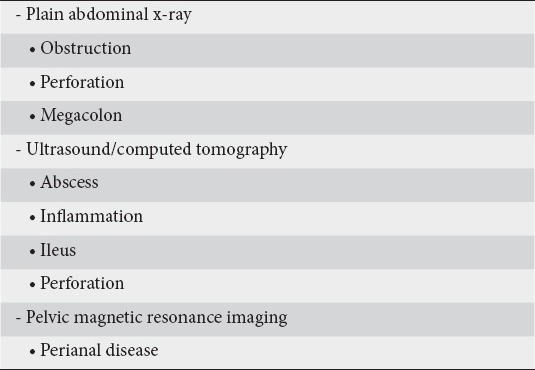
The need for imaging in order to grade the severity of CD and detect any complications was also discussed. The panelists agreed that a plain abdominal x-ray can detect ileus due to intestinal obstruction and megacolon, whereas abdominal ultrasound and CT are readily available imaging techniques that adequately assess bowel integrity and damage in the acute severe stage by revealing features of intestinal inflammation and/or obstruction (ileus), and complications such as perforation and abscess formation. However, despite its cost and limited availability, MRI enteroclysis/enterography should be the first choice imaging modality due to the lack of ionizing irradiation and imaging superiority.
As MRI has become the reference imaging modality for the evaluation of anal and perianal disease [33] panelists agreed that a pelvic MRI is required when there is suspicion of any perianal complication(s) (Table 7).
Table 7.
Imaging findings that may be present in newly diagnosed patients with severe disease
Risk factors predictive for severe CD diagnosis
Different views were expressed regarding the implication of additional risk factors in defining severe CD. In fact, the panel argued that severe CD is in itself a risk factor for an adverse outcome of CD and additional risk factors are irrelevant when determining a therapeutic strategy for these patients.
Profile of newly diagnosed patients with severe CD who are candidates for early biologic therapy
Current therapeutic recommendations indicate that patients should be considered for biologic therapy only after immunosuppressives have failed. This approach has raised concern whether many patients receive biologics too late in the course of their disease, i.e. at a time-point when they may have developed irreversible bowel damage such as fibrostenotic or penetrating disease [12]. Accordingly, guidelines of official Organizations as well as experts’ reports have suggested that earlier administration of anti-TNF-α therapy may be considered in selected patients with poor prognostic factors which indicate that their disease evolves rapidly to become disabling, such as patients with extensive ileal disease, severe upper gastrointestinal tract, rectal involvement, or complicated perianal disease, and severe EIMs [11,35]. A considerable body of evidence strongly suggests the early treatment with anti-TNF-α agents may prevent the evolution of these irreversible complications. The panel agreed that all patients with severe CD are eligible for treatment with biologic therapies as soon as septic complications have been excluded or treated appropriately and no other contraindications exist (Clinical statement 5).
Clinical statement 5.
Profile of newly diagnosed patients with severe Crohn’s disease (CD) who are candidates for biologic therapy

Profile of patients with newly diagnosed CD and perianal disease
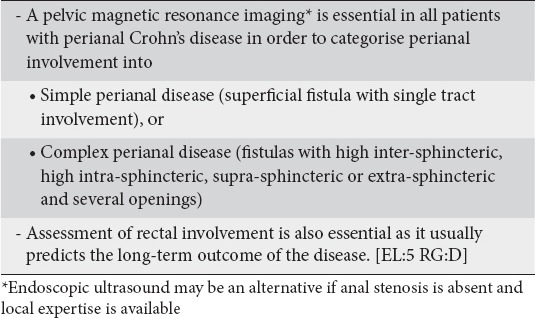
Perianal disease is a distinct phenotype of CD. The reference imaging technique for evaluating perianal CD is pelvic MRI as localization and extent of disease can be demonstrated accurately [36-38]. The panelists, therefore, agreed (100% agreement) that pelvic MRI is important in order to determine the spectrum, severity and inflammatory activity of perianal CD. Classification of perianal CD as simple (superficial fistula with single tract involvement) or complex (fistulas with high inter-sphincteric, high intra-sphincteric, supra-sphincteric or extra-sphincteric and several openings) was considered important in order to define disease severity and design appropriate treatment. The presence or absence and severity of endoscopic lesions in the rectum are also important in determining appropriate treatment and prognosis. Surgical examination under anesthesia guided by the pelvic MRI results is the sine qua non for the appropriate management of perianal disease (Clinical statement 6).
Clinical statement 6.
Determining the severity of perianal disease
Early administration of biologic therapy for perianal disease
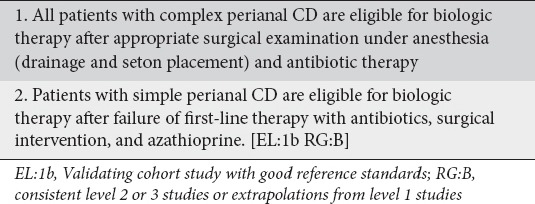
Patients with simple perianal disease should start therapy with antibiotics for 2-4 weeks [12]. However, patients who fail conventional antibiotic therapy or patients with complex perianal CD should start biological therapy with or without immunosuppressives as soon as possible [12,21]. In a study of 35 patients with complex perianal CD, Sciaudone et al showed a better outcome among patients who received a combination of IFX and surgery compared to patients who received either IFX monotherapy or underwent only surgery [39]. The view that biologic therapy along with antibiotics and surgery are important in patients with complex perianal CD was strongly supported (100% agreement) [40,41] (Clinical statement 7).
Clinical statement 7.
Recommendations for the early administration of biologic therapy for perianal Crohn’s disease (CD)
Early administration of biologic therapy in severe EIMs
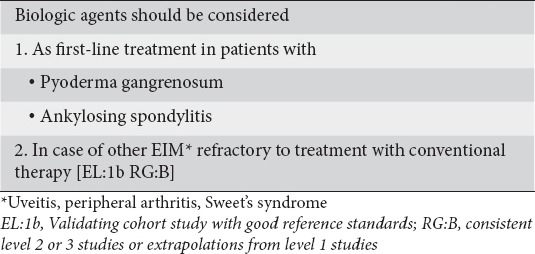
Anti-TNF-α agents are efficacious in treating immune-mediated EIMs of CD. Consensus was reached (100% agreement) on the use of biological therapy for severe EIMs. More specifically, pyoderma gangrenosum extensive or refractory to conventional treatment and ankylosing spondyloarthropathy were considered absolute indications for the use of anti-TNF-α therapy. In addition, severe peripheral arthritis, uveitis, and Sweet’s syndrome (acute febrile neutrophilic dermatosis) refractory to conventional therapy were considered indications for initiation of biological therapy (Table 10) (Clinical statement 8).
Clinical statement 8.
Recommendations for administration of biologic therapy for severe extra-intestinal manifestations (EIMs)
Discussion
Anti-TNF-α agents, such as IFX and ADA collectively represent a significant advance in the treatment of active CD, while the roles of mesalazine and systemic corticosteroids as first-line treatments are under discussion. Functional and structural bowel damage as well as patient quality of life are increasingly being taken into account when assessing the effectiveness of treatment. Ultimately, markers and/or predictors that identify those patients who would benefit most from early anti-TNF-α treatment are needed.
Use of systemic steroids is indicated as induction therapy for patients with moderate-to-severe CD, whereas immunosuppressive therapy is reserved for either steroid-dependent or steroid-refractory CD. Biologic therapy is considered only if these agents have failed [6]. However, these guidelines do not consider certain patient-specific parameters such as pre-defined risk and prognostic factors, location and extension of the disease, previous exposure and response to medication [42].
Therefore, one of the main areas of consensus was that accurate identification of candidates for early biologic therapy is essential to prevent progressive tissue damage and steroid-dependency. For this reason, certain clinical, laboratory, endoscopic and imaging characteristics as well as risk factors were identified as critical factors in the decision of early biologic administration in newly diagnosed patients with either moderate or severe disease.
Several studies have provided evidence supporting that the use of biologic agents with or without immunomodulators can reduce inflammation, reduce flares, and promote mucosal healing [12,42], as well as lower the rates of hospitalization and surgery. Treatment with biologic agents early in the disease course could also result in higher rates of remission [43]. In this consensus, we provided guidelines for the early administration of biologics in newly diagnosed patients with moderate CD who are either steroid-dependent or steroid-refractory. D’ Haens et al have shown that early IFX therapy with concomitant AZA is superior to conventional therapy for inducing and maintaining remission in newly diagnosed patients with moderate-to-severe CD. For newly diagnosed patients with moderate CD who have an early relapse within 6 months, we considered the early administration biologic therapy with or without an immunomodulator an appropriate option. For patients with recurring relapses, restarting a cycle of steroids with or without an immunomodulator was recommended. In case of severe disease with poor outcome, we proposed that all patients with severe CD are eligible for early treatment with biological therapies to prevent development of complications such as fibrostenotic or penetrating disease, extensive ileal disease and damage of upper gastrointestinal tract [11,34].
Biologic therapy has also been shown to be effective in severe EIMs of CD, ankylosing spondylitis [44], pyoderma gangrenosum [45] and chronic uveitis [46]. One controlled study evaluating patients with both CD and ankylosing spondylitis have shown the superiority of IFX over conventional therapy (steroids, AZA, and antibiotics) [47]. We therefore proposed that in the presence of severe EIMs initiation of biologic therapy needs to be considered as first-line treatment.
We provided clinical practice guidelines regarding the appropriate use of anti-TNF agents for achieving and maintaining remission of CD. In these guidelines, we aimed at providing a clinical and easier to apply to real life experience definition of CD activity guiding general practitioners and gastroenterologists to therapy. The proposed recommendations may provide a useful and practical approach for improving therapeutic strategies in patients with active CD. Such an approach could also improve patient quality of life by preventing both bowel damage and impaired gastrointestinal function.
Biography
Evangelismos Hospital, Athens; Attikon University Hospital, Athens; Laikon Hospital, Athens; University Hospital Heraklion, Crete; Hellenic IBD Working Team, Greece
Footnotes
Funding statement: The project was funded and organized by MSD Hellas. The project methods were designed by the authors
Conflict of Interest: The authors have received honoraria from MSD Hellas for participating in the project
References
- 1.Ordas I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn’s disease: time for a change. Gut. 2011;60:1754–1763. doi: 10.1136/gutjnl-2011-300934. [DOI] [PubMed] [Google Scholar]
- 2.Van AG, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Colombel JF, Reinisch W, Rutgeerts PJ. Review article: infliximab for Crohn’s disease treatment-shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther. 2011;33:857–869. doi: 10.1111/j.1365-2036.2011.04598.x. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Lemann M, Befrits R, et al. Recommendations for the treatment of Crohn’s disease with tumor necrosis factor antagonists: an expert consensus report. Inflamm Bowel Dis. 2012;18:152–160. doi: 10.1002/ibd.21870. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940–987. doi: 10.1053/j.gastro.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 7.Travis SP, Stange EF, Lemann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55(Suppl 1):i16–i35. doi: 10.1136/gut.2005.081950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh A, Palmer R, Travis S. Mucosal healing in inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin N Am. 2014;24:367–378. doi: 10.1016/j.giec.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Fidder HH, Hommes DW. Anti-TNF and Crohn’s disease: when should we start? Curr Drug Targets. 2010;11:143–147. doi: 10.2174/138945010790309993. [DOI] [PubMed] [Google Scholar]
- 11.Panaccione R, Rutgeerts P, Sandborn WJ, Feagan B, Schreiber S, Ghosh S. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:674–688. doi: 10.1111/j.1365-2036.2008.03753.x. [DOI] [PubMed] [Google Scholar]
- 12.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 13.Dignass A, van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Ott C, Takses A, Obermeier F, Schnot E, Salzberger B, Muller B. How fast up the ladder? Factors associated with immunosuppressive or anti-TNF therapies in IBD patients at early stages: results from a population-based cohort. Int J Colorectal Dis. 2014;29:1329–1338. doi: 10.1007/s00384-014-2002-z. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 16.Di Palma JA, Farraye FA. Crohn’s disease: the first visit. Gastroenterol Hepatol (NY) 2011;7:163–169. [PMC free article] [PubMed] [Google Scholar]
- 17.Burisch J, Vardi H, Pedersen N, et al. Costs and resource utilization for diagnosis and treatment during the initial year in a European inflammatory bowel disease inception cohort: an ECCO-EpiCom Study. Inflamm Bowel Dis. 2015;21:121–131. doi: 10.1097/MIB.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 18.Chamouard P, Richert Z, Meyer N, Rahmi G, Baumann R. Diagnostic value of C-reactive protein for predicting activity level of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:882–887. doi: 10.1016/j.cgh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 20.Hanauer SB, Sandborn W. Management of Crohn’s disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 21.Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55(Suppl 1):i1–15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 23.Imes S, Pinchbeck BR, Dinwoodie A, Walker K, Thomson AB. Iron, folate, vitamin B-12, zinc, and copper status in outpatients with Crohn’s disease: effect of diet counseling. J Am Diet Assoc. 1987;87:928–930. [PubMed] [Google Scholar]
- 24.Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947–953. doi: 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]
- 25.Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voderholzer WA, Beinhoelzl J, Rogalla P, et al. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369–373. doi: 10.1136/gut.2004.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Bridger S, Lee JC, Bjarnason I, Jones JE, Macpherson AJ. In siblings with similar genetic susceptibility for inflammatory bowel disease, smokers tend to develop Crohn’s disease and non-smokers develop ulcerative colitis. Gut. 2002;51:21–25. doi: 10.1136/gut.51.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212. doi: 10.1038/ajg.2010.392. [DOI] [PubMed] [Google Scholar]
- 30.Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674–678. doi: 10.1136/gut.37.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- 32.O’Donoghue DP, Dawson AM, Powell-Tuck J, Bown RL, Lennard-Jones JE. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet. 1978;2:955–957. doi: 10.1016/s0140-6736(78)92524-2. [DOI] [PubMed] [Google Scholar]
- 33.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–1061. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Wagtmans MJ, van Hogezand RA, Griffioen G, Verspaget HW, Lamers CB. Crohn’s disease of the upper gastrointestinal tract. Neth J Med. 1997;50:S2–S7. doi: 10.1016/s0300-2977(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 35.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlando A, Armuzzi A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: The use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1–20. doi: 10.1016/j.dld.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Sahni VA, Ahmad R, Burling D. Which method is best for imaging of perianal fistula? Abdom Imaging. 2008;33:26–30. doi: 10.1007/s00261-007-9309-y. [DOI] [PubMed] [Google Scholar]
- 38.Caprilli R, Gassull MA, Escher JC, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55(Suppl 1):i36–i58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 40.Sciaudone G, Di SC, Limongelli P, et al. Treatment of complex perianal fistulas in Crohn disease: infliximab, surgery or combined approach. Can J Surg. 2010;53:299–304. [PMC free article] [PubMed] [Google Scholar]
- 41.Panaccione R, Ghosh S. Optimal use of biologics in the management of Crohn’s disease. Therap Adv Gastroenterol. 2010;3:179–189. doi: 10.1177/1756283X09357579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogler G. Top up or step down treatment in Crohn’s disease? Dig Dis. 2013;31:83–90. doi: 10.1159/000347190. [DOI] [PubMed] [Google Scholar]
- 44.Jois RN, Leeder J, Gibb A, et al. Low-dose infliximab treatment for ankylosing spondylitis--clinically- and cost-effective. Rheumatology (Oxford) 2006;45:1566–1569. doi: 10.1093/rheumatology/kel156. [DOI] [PubMed] [Google Scholar]
- 45.Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505–509. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hale S, Lightman S. Anti-TNF therapies in the management of acute and chronic uveitis. Cytokine. 2006;33:231–237. doi: 10.1016/j.cyto.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Generini S, Giacomelli R, Fedi R, et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63:1664–1669. doi: 10.1136/ard.2003.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]


