Abstract
Initial studies about ablation therapies of the pancreas were associated with significant morbidity and mortality, which limited widespread adoption. Development of techniques with high quality imaging used as guidance improve outcomes reducing complications. Moreover, only few experiences of percutaneous pancreatic ablations are reported. They are performed by very skilled operators in highly specialized centers. This review presents the current status of percutaneous local ablative therapies in the treatment of advanced pancreatic cancer.
Keywords: Percutaneous ablation, inoperable pancreatic cancer, review
Introduction
Pancreatic cancer is one of the most aggressive human malignancies. Because of the insidious course and rapid growth, tumors are diagnosed at an advanced stage and therefore only the minority of these patients, about 20%, can benefit from surgical resection [1]. Nearly half of the patients are diagnosed with stage III disease [2,3]. Typically, involvement of the celiac axis, hepatic artery, or superior mesenteric artery (SMA) (>180° of the circumference of the vessel wall) is a contraindication to surgical resection. Gastroduodenal artery encasement up to the hepatic artery with either short segment encasement or direct abutment of the hepatic artery, without extension to the celiac axis, is considered a borderline factor for resection. Involvement of the portal vein or superior mesenteric vein (SMV) is not a contraindication nor does it preclude negative-margin resection, but may alter the sequence of multimodal treatment [4]. An additional 20% of patients have localized disease but it is unresectable [4].
Surgical procedures are sometimes excluded based on general considerations, such as advanced age, comorbidities, or patient refusal [4,5]. In patients with unresectable or locally advanced pancreatic cancer several palliative therapeutic modalities have been applied for tumor regression, local control, slowing of growth, and relief of pain/symptoms, such as chemoradiation, intraoperative electron beam irradiation, interstitial or intraluminal brachytherapy [6-8].
Over the last decade, image-guided tumor ablation with both thermal and nonthermal sources has received substantial attention for the treatment of many focal malignancies, in particular liver tumors [9]. Increasing interest has been accompanied by continual advances in energy delivery, application technique, and therapeutic combinations with the intent to improve the efficacy and/or specificity of ablative therapies [10]. However, in contrast to the liver, where tumor-bearing tissue is surrounded by normal hepatic parenchyma, the pancreas is surrounded by structures such as the duodenum and common bile duct; the risk of thermal injury to these structures has limited the application of thermal ablation to pancreatic tumors [9]. Moreover, locally advanced pancreatic cancer often encases the vessels and extends retroperitoneally or proximally, making direct ablation of the entire tumor impossible [11]. However, recently there has been a growing interest in the use of ablation also for pancreatic tumors; inoperable cases of pancreatic adenocarcinomas, and also some cases of neuroendocrine symptomatic tumors and metastases, have been treated by various ablation techniques with promising results [12-14].
Pancreatic neuroendocrine tumors (P-NETs) are comparatively rare neoplasms, and account for only 1-2% of all pancreatic neoplasms [15]. P-NETs include benign neoplasms without metastasis or invasion, as well as high-grade malignant neoplasms [15]. The latter may be staged using the AJCC staging system for pancreatic adenocarcinoma [15,16]. To date, however, there have been only sporadic reports of percutaneous thermal ablation to treat P-NETs in humans [14].
Image-guided minimally invasive ablative therapies delivered by using needlelike applicators include both thermal (i.e., radiofrequency [RF], microwave [MW], laser and cryoablation) and nonthermal (i.e., chemical ablation and irreversible electroporation [IRE]) techniques [10]. High intensity focused ultrasound (HIFU), conversely, does not require percutaneous placement of needles [11].
Percutaneous thermal therapies are limited, however, by the quality of imaging guidance and, in some cases, by complex anatomy and difficult access [10]. Sometimes, palliative treatment of unresectable pancreatic cancers has the only purpose to improve quality of life by reducing pain [17]. Pain management for patients with local advanced pancreatic cancer is an ongoing challenge. Pain can be both neuropathic and inflammatory, resulting from both tumor expansion and tumor invasion of the celiac and mesenteric plexus [18]. At disease progression, however, nonsteroidal anti-inflammatory drugs and narcotic analgesics may not be sufficient. Therefore, anesthetic blocking of the celiac plexus was used to palliate pain, but the duration of pain relief is limited [18].
Use of percutaneous ablation in the pancreas is related to well-founded fear of adverse effects, including thermal injury induced pancreatitis and thermal damage to structures in and around the pancreas (e.g. the duodenal wall, nearby viscera, and blood vessels), and to technical limitations of the real-time imaging modalities used to visualize target lesions and needles/electrodes used to ablate them [5]. Thermal ablation has been used only sporadically to treat P-NETs, because the Whipple procedure and other types of pancreatic resection are considered the treatment of choice of these tumors throughout the world, even though it is associated with high rates of major complications, including death [15]. Local control of the disease not suitable for surgery is often the first indication for ablation. Unfortunately, locally advanced pancreatic tumors usually bear a dismal prognosis and pain relief may be another aim of the ablation. Nowadays, very few P-NETS were treated percutaneously with ablation. At the moment it is difficult to establish inclusion criteria for ablation [5].
To date, treatment of the pancreas with ablative techniques has been mostly performed in the operating room by surgeons. The most important advantage of the ablative therapies performed percutaneously is to be mini-invasive, permitting to treat a lot of patients with comorbidities that contraindicate surgical approach [13,19]. The purpose of this review is to present the current status of local ablative therapies in the treatment of pancreatic tumors with a percutaneous approach.
Materials and methods
A systematic literature search was performed using the PubMed databases for studies published in the English language up to October 2014, with the following keywords; RF ablation (RFA), cryoablation, MW ablation (MWA), irreversible electroporation (IRE), high intensity focused ultrasound (HIFU) and (pancreas OR pancreatic). Only articles which described percutaneous ablation in pancreatic tumor were included. In cryoablation and HIFU, many of the largest case-series are published in non-English language medical journals and were excluded from this systematic review. All references were screened for potentially relevant studies not identified in the initial literature search.
The following variables were extracted for each report when available: number of patients, demographic data (sex and age); tumor histology diameter of the lesion; disease extent; device used and settings; distance of probe from surrounding structures; duration of therapy and number of ablations applied; complications; mean follow up and survival; and treatment response.
All patients underwent radiologic investigations (ultrasound, contrast-enhanced CT and/or MRI) which revealed lesions suspicious of pancreatic tumors, confirmed by histological examination in almost all studies.
RFA
Five studies (Table 1) involving 11 patients with pancreatic tumor treated with percutaneous RFA were analyzed [5,12 14,20]. There were 3 case reports [12-14], 1 prospective study [20], and 1 pilot study [5]. All patients were treated percutaneously with CT (4 pts) or US (7 pts) guidance. The mean age of patients was 70 years old. Specifically, there were 4 non secretory neuroendocrine tumors, 3 insulinomas, 2 gastrinomas, 1 metastasis from renal cell carcinoma, and 1 locally advanced adenocarcinoma. The tumor size ranged from 13 to 40 mm, and in one study it was not described. Tumor was located in the pancreatic body-tail in 5 patients and in another 5 it was located in the head. Singh et al [20] did not specify the location of the tumor. Complications related to procedure included: diffuse abdominal pain, self-limiting form of pancreatitis associated with thermal injury, minimal peripancreatic inflammatory reaction, slight and transient increase in serum amylase and lipase, and a fluid peripancreatic collection (completely disappeared at CT scan at 1 year follow up [12]). There were no deaths related to the procedure. In all 5 studies a significant level of pain relief was observed in the patients.
Table 1.
Use of percutaneous radiofrequency ablation (RFA) in the treatment of advanced pancreatic tumor (articles; number; histology; dimension and location of the tumor; complications; survival and follow up and response)
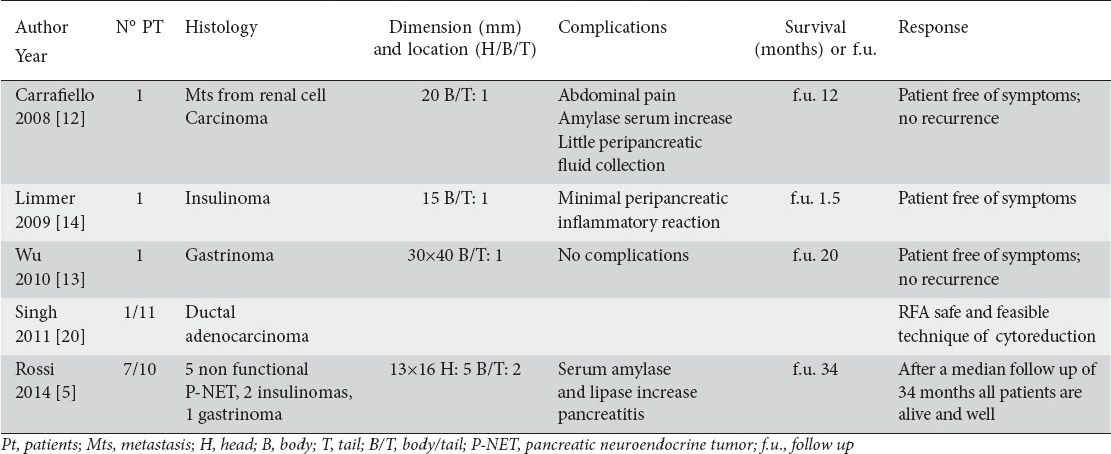
Data regarding the follow up and survival were not available in all the studies. There was a mean follow-up of 17 months.
MWA
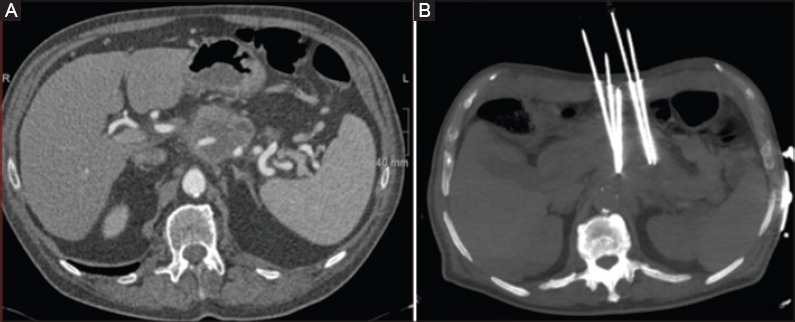
Although there are no pre-clinical studies published about MWA of pancreatic lesions and only two articles about the use of MWA in pancreatic cancer are available, this technique is considered an emerging option for the treatment of a variety of tumors and, when compared with RFA, offers several advantages. The largest case series of MWA in locally advanced pancreatic cancer reported in the literature includes 15 patients, but the ablation was performed intraoperatively at the time of palliative bypass surgery [21]. Carrafiello et al [17] (Table 2) evaluated the safety and efficacy of percutaneous MWA treatment in locally advanced, non resectable, non metastatic pancreatic head cancer [17]. Ten patients were treated, with percutaneous (n=5) or laparotomic (n=5) approach. In all patients treated with the percutaneous approach, MWA was performed under moderate sedation. The path of the antenna was carefully evaluated on the basis of a preliminary US examination; a path was chosen such that the vessels, stomach, and bowel were far from the antenna (Fig. 1 A, B). The most important evaluation involved the prediction of the ablation area on the basis of the position of the antenna; in some cases, cone-beam CT acquisition provided a correct and safe position for the antenna with respect to the adjacent structures [17].
Table 2.
Percutaneous microwave ablation of advanced pancreatic tumor (article; number; histology; dimension and location; complications, survival or follow up; and response)
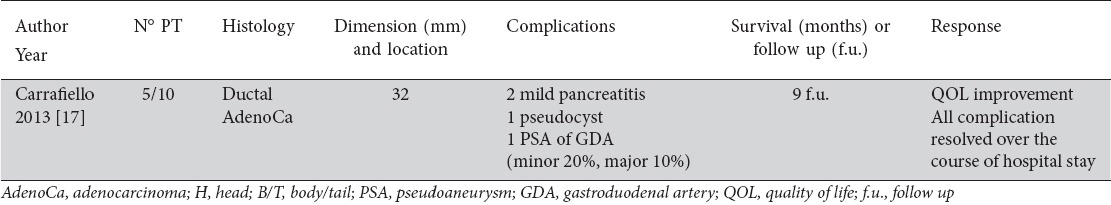
Figure 1.
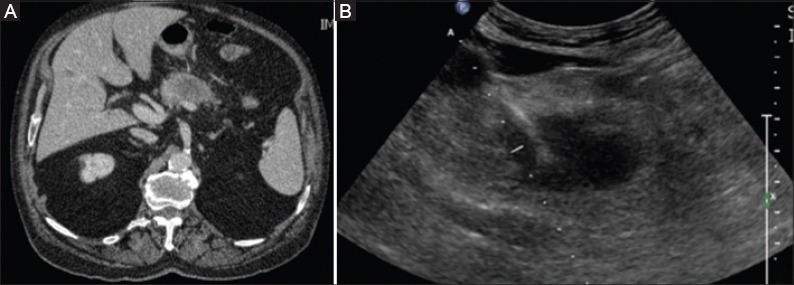
(A) Contrast-enhanced computed tomography reveals the presence of the pancreatic tumor. (B) Antenna for microwave ablation within the lesion, positioned under ultrasound guidance
The procedure was feasible in all patients (100%). One late major complication was observed in one patient, a pseudoaneurysm of the gastroduodenal artery, treated with endovascular approach. Two patients presented with mild pancreatitis after 1 month; one case resulted in a pseudocyst that was managed with a drain [22]. No patients had further surgery, and all minor complications resolved during the hospital stay. An improvement in the quality of life was observed in all patients. No repeat treatment was performed. Despite the small number of patients, Carrafiello et al concluded that MWA is a feasible approach in the palliative treatment of pancreatic tumors [17].
Cryoablation
There are few reports about the use of cryoablation alone or in combination with other therapies for the treatment of pancreatic cancer, but many of the largest case series are published in non-English language medical journals [23]. Three studies involving a total of 105 patients with pancreatic lesions treated with percutaneous cryoablation fulfilled the eligibility criteria of this review (Table 3) [24-26]. All patients were treated percutaneously under CT or US guidance. The mean age of patients was 55 years old.
Table 3.
Percutaneous cryoablation of advanced pancreatic tumor (articles; number; histology; dimension and location; complications; survival or follow up; and response)
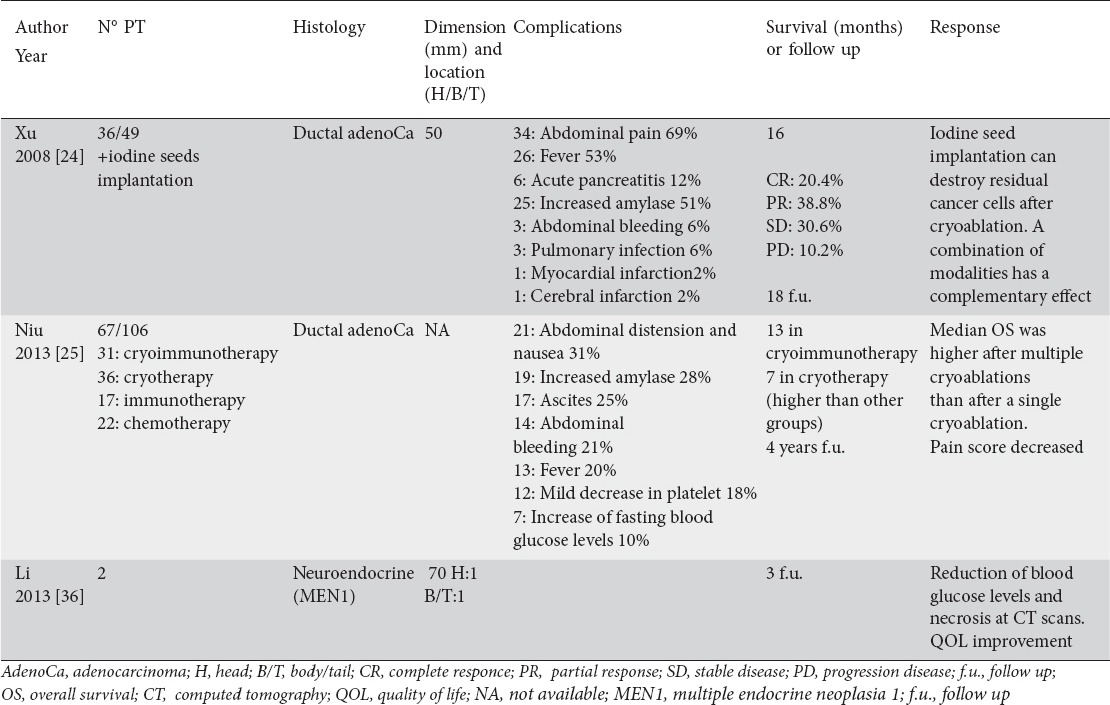
Specifically, there were 103 adenocarcinomas and 2 neuroendocrine tumors in multiple endocrine neoplasia type 1 (MEN1) syndrome (insulinomas). The tumor size ranged from 50 to 70 mm. Complications related to the procedure included: abdominal pain and distension, transient increase of amylases, fever, ascites, abdominal bleeding, mild decrease in platelet (in patients that underwent also immunotherapy), delayed gastric emptying, acute pancreatitis, increase in fasting blood glucose levels, pulmonary infection, myocardial infarction, and cerebral infarction. There were no deaths related to the procedure. In all studies, a significant level of pain relief was observed after procedure. Average survival after cryoablation ranged from 3 to 48 months.
IRE
Four studies (Table 4), involving a total of 21 patients with locally advanced ductal adenocarcinoma treated with percutaneous IRE were analyzed [19,27-29] (Fig. 2 A, B). There were 1 prospective study, 2 retrospective studies, and 1 case report. Narayanan [30] added another 4 patients reported in a very recent study which contained data related to vascular patency post IRE in different organs and in particular 18 pancreatic carcinomas (14 published in 2012 [19] and 4 performed in the last years and reported in this recent study). Efficacy was not among the aims of his recent study, and dimensions, locations, histology, complications, follow up, and response were not reported. Recently, Scheffer et al [31] reported a case of percutaneous pancreatic IRE: they reported the feasibility and the safety of the dorsal approach. They did not report data on the follow up and we excluded this case from our study.
Table 4.
Use of percutaneous irreversible electroporation (IRE) for the treatment of locally advanced pancreatic tumor (articles; number; histology; dimension and location of the tumor; complications; survival or follow up; and response)
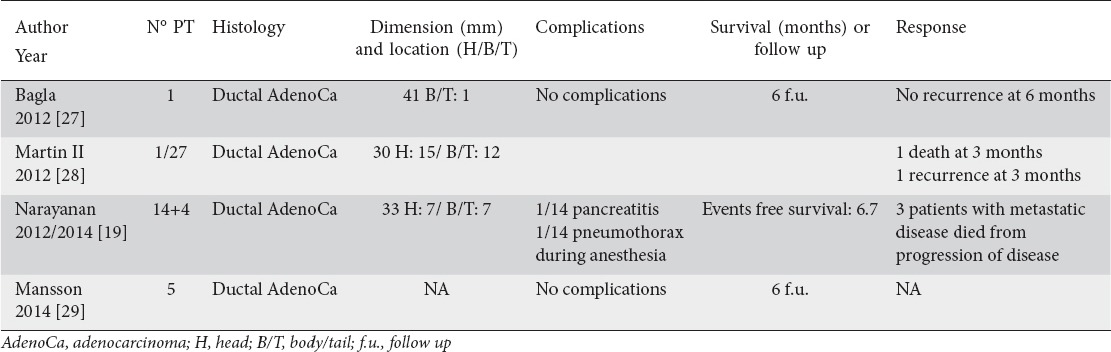
Figure 2.
(A) Contrast-enhanced computed tomography reveals the presence of the pancreatic tumor. (B) maximum intensity projection (MIP) reconstruction demonstrates the presence of 5-needle for irreversible electroporation
We recently described the onset of asymptomatic multiple little splenic perfusion defects after the treatment of a locally advanced body-tail pancreatic cancer with the application of five percutaneous probes for IRE, in a 79-year-old man (Fig. 2 A, B). To the best of our knowledge, until now, no experience concerning percutaneous IRE of pancreatic cancer described that consequence of the treatment. This case report has not been included in the review because it was focused on the description of vascular lock, with few data on the follow up [32]. The mean size of the tumors was 34.5 mm. Mansson et al [29] did not specify the diameter and location of the treated lesions. Tumor was located in the pancreatic head in 7 patients and in the body-tail in 8 patients. Martin II et al [28] treated 26 patients with an open approach and one patient percutaneously because of her multiple earlier surgical procedures unrelated to her disease. Therefore, it was not possible to accurately recover the data about tumor localization and complications.
Complications occurred only in the series of Narayanan and were 1 transient pancreatitis and 1 spontaneous pneumothorax during anesthesia [19]. There were no cases of vessel thrombosis after the procedure and a significant level of pain relief was observed in most of patients. There were no deaths related to the procedure.
HIFU
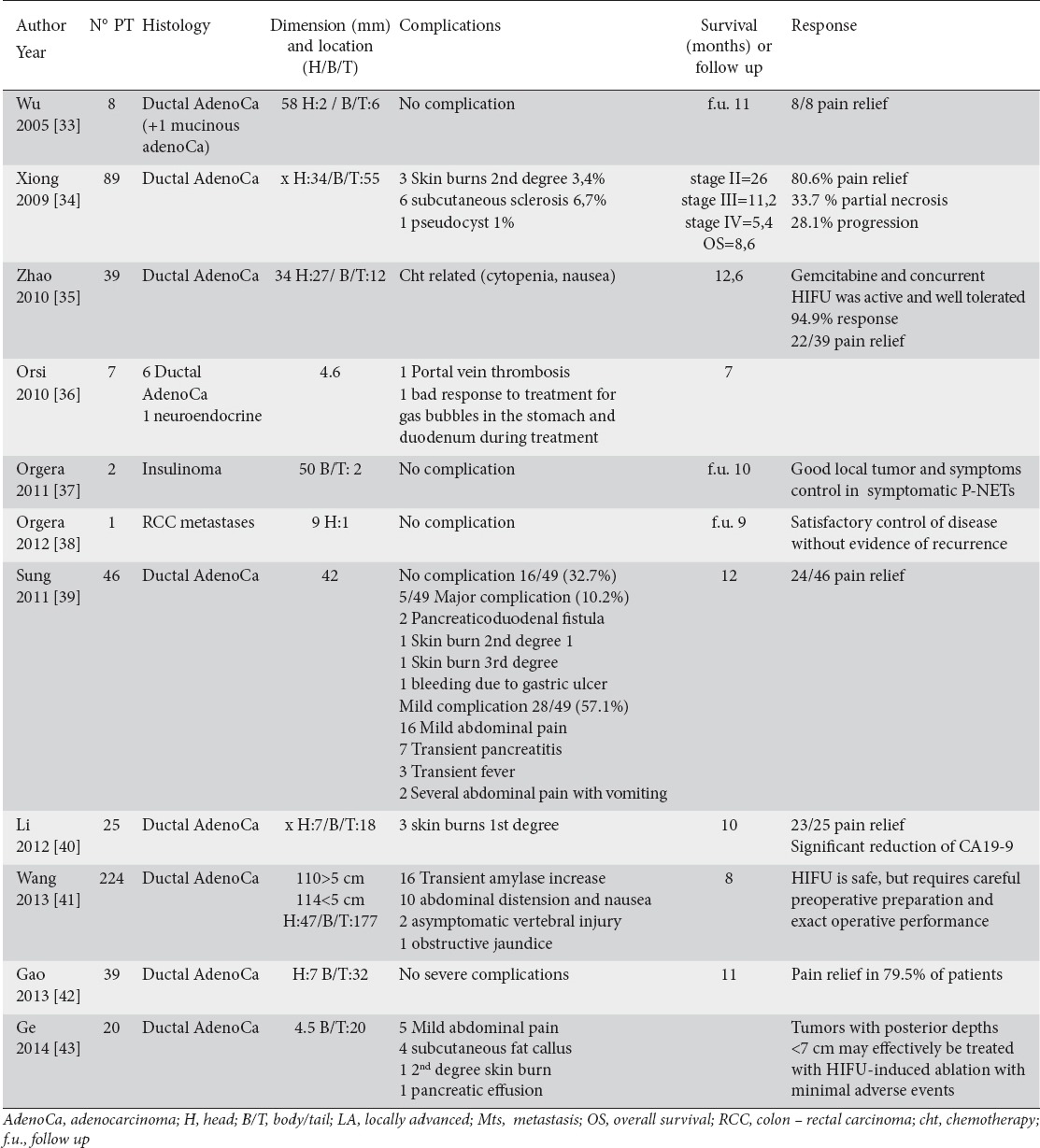
There are many reports about the use of HIFU alone or in combination with other therapies for the treatment of pancreatic tumors. Eleven studies involving a total of 500 patients with pancreatic lesions treated with HIFU fulfilled the eligibility criteria of this review (Table 5) [33-43]. Specifically, they were all advanced pancreatic adenocarcinomas except for 3 neuroendocrine tumors and 1 metastasis from renal cell carcinoma. The tumor size ranged from 4 to 58 mm. Complications related to the procedure included: abdominal pain, transient pancreatitis, subcutaneous sclerosis, skin burns, transient fever, cytopenia (in patients that underwent also chemiotherapy), subcutaneous fat callus, pancreatic-duodenal fistula, portal vein trombosis, pseudocyst, and bleeding due to gastric ulcer. There were no deaths related to the procedure. In almost all studies, a significant level of pain relief was observed after the procedure. The average survival after cryoablation ranged from 5.4 to 26 months.
Table 5.
Use of high intensity focused ultrasound (HIFU) for the treatment of locally advanced pancreatic tumor (articles; number; histology; dimension and location of the tumor; complications; survival or follow up; and response)
Discussion
Over the last decade, RF energy has become increasingly accepted for the ablation of solid parenchymal tumors [12]. There are mainly three different ways that an ablative procedure may be performed: laparotomic or laparoscopic ablation and image-guided percutaneous ablation. Due to the special anatomical location of the pancreas, currently, RFA via laparotomy is mainly used and in literature there are only a few studies referring to the percutaneous approach [13].
The largest series analyzed in this review was the study of Rossi et al that performed RFA on 10 patients with histologically diagnosed P-NETs [5]. Seven of the 10 patients underwent percutaneous RFA. Tumors were potentially resectable but conventional surgery had been refused by patients or contraindicated by the anesthesiology team on the basis of patient’s age and/or comorbidities. Patients treated with the percutaneous approach did not have major complications in a shorter hospital stay period (7 days) compared to those who underwent laparotomy (mean, 22 days) [5]. Rossi et al [5] suggested that only skilled operators with substantial experience in interventional US-guided procedures can overcome the technical difficulties related to the deployment of the electrode very close to arteries of the celiac trunk, venous branches of the portal system, the wall of the duodenum, and the main pancreatic duct. Unfortunately, there are no series in the literature on the use of RFA for treating ductal adenocarcinomas.
Limmer et al [14] and Wu et al [13], like Rossi [5], reported successful data on the use of RFA for the treatment of functioning neuroendocrine tumors; with the advantages of less trauma and pain, rapid recovery, curative effect and repeatability, the image-guided minimally invasive interventional treatment can accurately target tumor, destroy it and improve patients’ quality of life [13,14].
Carrafiello et al [12] confirmed that RFA is feasible for the treatment of metastatic lesion located in the body-tail of the pancreas; as the lesion was located at the body-tail portion of the pancreas, we were quite confident that the risk of a biliary injury was reduced at a minimum.
Improved electrodes for percutaneous approaches could probably solve many of the problems related to difficult encounter during percutaneous RFA of pancreatic tumors; the goal should be to reduce the caliber of the needle while increasing (or at least without decreasing) the volume of the thermal lesions they produce. Electrode tips would also benefit from higher sonographic visibility [5].
MWA appears to have several advantages compared with RFA: at first, considering anatomic relationship of the pancreas with adjacent organs and vessels, MWA can decrease heat-sink effect [44]. MW can guarantee optimal heating of cystic masses and tumors close to the vessels, it takes shorter intraoperative time and, using multiple antennae simultaneously, it reduces the need of multiple treatment sessions decreasing the rate of incomplete treatments of larger tumors [45,46]. Additionally, MWA is associated with a lower amount of intra-procedural pain [44].
Cryosurgery has provided a novel therapeutic approach to the treatment of benign and malignant tumors, especially unresectable tumors [47]. Theories and in vivo and in vitro studies explained the effect of low-temperature exposure on the tissues. Studies showed that the pancreatic tissue may be locally damaged if the temperature is set in the range -80°C to -180°C, which results in aseptic necrosis and apoptosis, local capillary vessel circulation failure, and antiangiogenesis [48]. Niu et al [25] reported the largest retrospective series (67 pts). In this study, combined cryotherapy and immunotherapy were found to significantly improve the treatment of metastatic pancreatic cancer more than conventional chemotherapy. Median overall survival (OS) was higher in the cryoimmunotherapy (13 months) and cryotherapy groups (7 months) than in the chemotherapy group (3.5 months;). In both cryoimmunotherapy and cryotherapy groups, median OS was higher after multiple cryoablations than after a single cryoablation.
Xu et al [24] decided to use the combination of cryosurgery with 125iodine seed implantation that provided γ radiation for a short distance, resulting in the death of the targeted cells. They found that the approach of cryosurgery (intraoperative vs percutaneous), tumor size (≤4 cm vs >4 cm), and location (head vs body or tail) were not independent risk factors affecting prognosis.
Li et al [26] described the efficacy of percutaneous cryoablation for the treatment of neuroendocrine pancreatic tumors in familial MEN1: after treatment, the physical status of patients improved significantly; the preliminary results of blood glucose levels and CT scans have demonstrated the potential curative effects of cryosurgery in a short-term observation period.
Unresectable tumors can be treated with percutaneous cryosurgery, with similar efficacy as intraoperative cryosurgery and much less invasiveness for the patient. During percutaneous cryosurgery, other modalities, such as 125iodine seed implantation, can be used simultaneously and also metastases could be treated simultaneously.
IRE is a relatively new non-thermal modality for ablation of soft-tissue tumors that uses very short pulses of high-voltage, low-energy direct current to induce cellular death by creating cellular membrane disruption [49]. Because of its mechanism of action, IRE is hypothesized to have wider indications than other ablation technologies. Tumors in contact with vessels can be treated with IRE without heat-sink effect [50]. The preservation of vascular and ductal structures within the treatment field of IRE is hypothesized to result from the supporting connective tissue matrix, which is unaffected by this modality as a result of the lack of thermal effects [50]. This makes IRE a very attractive option in patients with local advanced pancreatic cancer (LAPC), as the reason for unresectability is usually vascular encasement. However, IRE is very expensive at the moment and not available in many centers.
One of the most innovative and revolutionary techniques in the field of ablation is HIFU, completely extracorporeal, through the use of nonionizing energy. The first success of HIFU ablation for advanced pancreatic cancer was conducted in Chongqing China in 2000 [33]. It was a phase I-II prospective clinical trial, and both survival benefit and pain control were observed during follow-up period. After HIFU, pre-existing severe back pain of presumed malignant origin disappeared in each patient. Follow-up images showed reduction or absence of tumor blood supply in the treated region with significant shrinkage of the ablated tumor. Subsequently, several clinical studies were performed to investigate the safety and feasibility of HIFU for the treatment of patients with advanced-stage pancreatic cancer [34-43]. The largest clinical experience of using HIFU treatment was reported by Wang et al [41]. A total of 224 patients were enrolled in this study for safety analysis of HIFU treatment. One case developed obstructive jaundice 2 weeks after HIFU treatment. Vertebral injury, identified by MRI, occurred in 2 cases, although no symptoms were reported. No severe complications were observed in all enrolled patients.
Two methods have been developed in HIFU treatment: continuous and pulsed HIFU. They differ in both technical parameters and therapeutic approach [51]. Using high intensities ranging from 5-20 kW/cm2, each continuous HIFU shot can induce coagulation necrosis of a targeted tumor. In contrast, pulsed HIFU uses lower ultrasound intensities, usually <3 kW/cm2. In the first case, the it is a one-session treatment; either sedation or general anesthesia is required for patients during treatment procedure due to discomfort and pain. After treatment, the patients require hospitalization for several days. Pulsed HIFU is a multiple-session treatment, and needs to be repeated for many times (5-10 sessions) if the patients are suitable. Most of the patients do not need sedation performed on an outpatient basis [51].
Xiong et al [34] reported the largest retrospective study of using pulsed HIFU treatment for advanced pancreatic cancer. Eighty-nine patients with pancreatic cancer were analyzed after HIFU. The authors concluded that the clinical application of HIFU for pancreatic cancer appeared to be safe and was a promising modality of treatment for palliation of pain related to pancreatic cancer. Recent studies indicated that pulsed HIFU can significantly enhance chemotherapeutic agents against tumor cells, suggesting that pulsed HIFU may be a treatment approach using focused ultrasound for hyperthermia, instead of HIFU for inducing coagulation necrosis: hyperthermia could make some cancer cells more sensitive to radiation and chemotherapy [52,53]. Up to now, HIFU has been largely reported mostly as a palliation option to treat patients with locally advanced pancreatic cancer. Although the mechanism is still unclear, HIFU might be an effective treatment option for pain control, particularly in patients with tumors infiltrating the celiac plexus and in whom conventional pain treatments are not considered an effective option [54]. Orsi and Orgera treated also inoperable patients with P-NET (insulinomas) with HIFU [36,37]. The patients suffered from episodes of severe night hypoglycemia, which was not efficiently controlled by medication. Symptom relief and local disease control were achieved also in these patients, without any complications.
In conclusion, the main advantages of HIFU therapy are: less invasive with no incision, no percutaneous placement of needles, no scarring, less pain, and short recovery time. These results are associated with a reduction in mortality, morbidity, hospital stay, cost, and improved quality of life for cancer patients.
In the future, lower energy treatments may play a significant role in mediating targeted drug and gene delivery for cancer treatment [55].
Concluding remarks
All studies demonstrated that percutaneous ablation of pancreatic tumors is feasible and reproducible. Difficulties related to the choice of the approach can be overcome by accurate selection of patients on the basis of pre-procedural images, and by the experience of the operator. The choice of a percutaneous approach must be carried out by evaluating the possibility of positioning the devices without risking damage to adjacent structures by a multidisciplinary team and with the right equipment. The involvement of vessels is one of the most important factors; some of the techniques considered in our review seem to overcome this limit. But more studies, particularly prospective randomized, with more patients are necessary to define the best treatment for the percutaneous management of locally advanced pancreatic cancer.
Biography
University of Insubria, Varese, Italy; University Hospital of Heraklion, Faculty of Medicine, University of Crete
Footnotes
Conflict of Interest: None
References
- 1.Wagner M, Friess H, Buchler WM. Conservative versus radical resections of the pancreas. In: Neoptolemos JP, Lemoine NR, editors. Pancreatic cancer: molecular and clinical advances, pp 248-270. Oxford: Blackwell Sciences; 1996. pp. 248–270. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Arnoletti JP, Frolov A, Eloubeidi M, et al. A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2011;67:891–897. doi: 10.1007/s00280-010-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 5.Rossi S, Viera FT, Ghittoni G, et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938–945. doi: 10.1097/MPA.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 6.Nori D, Merimsky O, Osian A, et al. Palladium-103: a new radioactive source in the treatment of unresectable carcinoma of the pancreas: a phase I-II study. J Surg Oncol. 1996;61:300–305. doi: 10.1002/(SICI)1096-9098(199604)61:4<300::AID-JSO14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Wanebo HJ, Glicksman AS, Vezerides MP, et al. Preoperative chemotherapy, radiotherapy and surgical resection of locally advanced pancreatic cancer. Arch Surg. 2000;135:81–87. doi: 10.1001/archsurg.135.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Mutignani M, Shah KS, Morganti AG, et al. Treatment of unresectable pancreatic carcinoma by intraluminal brachytherapy in the duct of Wirsung. Endoscopy. 2002;34:555–559. doi: 10.1055/s-2002-33214. [DOI] [PubMed] [Google Scholar]
- 9.Spiliotis J. Commentary on pancreatic carcinoma: the role of radiofrequency ablation in advanced disease. Cancers. 2010;2:2055–2057. doi: 10.3390/cancers2042055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M, Brace CL, Lee FT, Jr, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi M, Orgera G, Hatzidakis A, et al. Minimally invasive ablation treatment for locally advanced pancreatic adenocarcinoma. Cardiovasc Intervent Radiol. 2014;37:586–591. doi: 10.1007/s00270-013-0724-x. [DOI] [PubMed] [Google Scholar]
- 12.Carrafiello G, Lagana` D, Recaldini C, et al. Radiofrequency ablation of a pancreatic metastasis from renal cell carcinoma: case report. Surg Laparosc Endosc Percutan Tech. 2008;18:64–66. doi: 10.1097/SLE.0b013e3181592a13. [DOI] [PubMed] [Google Scholar]
- 13.Wu PH, Pan CC, Huang ZL, et al. Percutaneous radiofrequency ablation approach through the spleen: initial case report for pancreatic tail gastrinoma. Chin J Cancer. 2010;29:836–841. doi: 10.5732/cjc.009.10755. [DOI] [PubMed] [Google Scholar]
- 14.Limmer S, Huppert PE, Juette V, et al. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21:1097–1101. doi: 10.1097/meg.0b013e328323d70e. [DOI] [PubMed] [Google Scholar]
- 15.Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today. 2011;41:1332–1343. doi: 10.1007/s00595-011-4547-6. [DOI] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Bentrem DJ, Merkow RP. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg. 2007;205:558–563. doi: 10.1016/j.jamcollsurg.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Carrafiello G, Ierardi AM, Fontana F, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol. 2013;24:1513–1520. doi: 10.1016/j.jvir.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Molinari M, Helton WS, Espat NJ. Palliative strategies for locally advanced unresectable and metastatic pancreatic cancer. Surg Clin North Am. 2001;81:651–666. doi: 10.1016/s0039-6109(05)70151-1. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Singh V, Varshney S, Sewkani A, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: 10-year experience from single centre. Pancreatology. 2011;11(Suppl 1):1–80. [Google Scholar]
- 21.Lygidakis NJ, Sharma SK, Papastratis P, et al. Microwave ablation in locally advanced pancreatic carcinoma-a new look. Hepatogastroenterology. 2007;54:1305–1310. [PubMed] [Google Scholar]
- 22.Carrafiello G, Ierardi AM, Piacentino F, et al. Microwave ablation with percutaneous approach for the treatment of pancreatic adenocarcinoma. Cardiovasc Intervent Radiol. 2012;35:439–442. doi: 10.1007/s00270-011-0189-8. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Li JD, Chen XL, et al. Cryosurgery for unresectable pancreatic carcinoma: a report of 44 cases. Zhonghua Gandan Waike Zazhi. 2004;10:523–525. [Google Scholar]
- 24.Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603–1611. doi: 10.3748/wjg.14.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;2:1143–1149. doi: 10.1097/MPA.0b013e3182965dde. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhang C, Chen J, et al. Two case reports of pilot percutaneous cryosurgery in familial multiple endocrine neoplasia type 1. Pancreas. 2013;42:353–357. doi: 10.1097/MPA.0b013e318258f233. [DOI] [PubMed] [Google Scholar]
- 27.Bagla S, Papadouris D. Percutaneous irreversible electroporation of surgically unresectable pancreatic cancer: a case report. J Vasc Interv Radiol. 2012;23:142–145. doi: 10.1016/j.jvir.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Martin RC, 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215:361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Mansson C, Bergenfeldt M, Brahmstaedt R, et al. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res. 2014;34:289–293. [PubMed] [Google Scholar]
- 30.Narayanan G, Bhatia S, Echenique A, et al. Vessel patency post irreversible electroporation. Cardiovasc Intervent Radiol. 2014;6:1523–1529. doi: 10.1007/s00270-014-0988-9. [DOI] [PubMed] [Google Scholar]
- 31.Scheffer HJ, Melenhorst MC, Vogel GA, et al. Percutaneous irreversible electroporation of locally advanced pancreatic carcinoma using dorsal approach: a case report. Cardiovasc Interv Radiol. 2015;38:760–765. doi: 10.1007/s00270-014-0950-x. [DOI] [PubMed] [Google Scholar]
- 32.Ierardi AM1, Lucchina N, Duka E, et al. “Vascular lock” causing splenic perfusion defects during irreversible electroporation of a locally advanced pancreatic tumor. JOP. 2014;15:604–608. doi: 10.6092/1590-8577/2850. [DOI] [PubMed] [Google Scholar]
- 33.Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 34.Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensitàfocused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10:123–129. [PubMed] [Google Scholar]
- 35.Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21:447–452. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 36.Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation: effective and safe therapy for solid tumors in difficult locations. AJR. 2010;195:W245–W252. doi: 10.2214/AJR.09.3321. [DOI] [PubMed] [Google Scholar]
- 37.Orgera G, Krokidis M, Monfardini L, et al. High intensity focused ultrasound ablation of pancreatic neuroendocrine tumours: report of two cases. Cardiovasc Intervent Radiol. 2011;34:419–423. doi: 10.1007/s00270-010-9884-0. [DOI] [PubMed] [Google Scholar]
- 38.Orgera G, Krokidis M, Monfardini L, et al. Ultrasoundguided high intensity focused ultrasound (USgHIFU) ablation in pancreatic metastasis from renal cell carcinoma. Cardiovasc Intervent Radiol. 2012;35:1258–1261. doi: 10.1007/s00270-011-0291-y. [DOI] [PubMed] [Google Scholar]
- 39.Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40:1080–1108. doi: 10.1097/MPA.0b013e31821fde24. [DOI] [PubMed] [Google Scholar]
- 40.Li PZ, Zhu SH, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2012;11:655–660. doi: 10.1016/s1499-3872(12)60241-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Zhu H, Meng Z, et al. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie. 2013;36:88–92. doi: 10.1159/000348530. [DOI] [PubMed] [Google Scholar]
- 42.Gao HF, Wang K, Meng ZQ, Chen, et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60:1906–1910. doi: 10.5754/hge13498. [DOI] [PubMed] [Google Scholar]
- 43.Ge HY, Miao LY, Xiong LL, et al. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablation. Ultrasound Med Biol. 2014;40:947–955. doi: 10.1016/j.ultrasmedbio.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Carrafiello G, Lagana D, Mangini M, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6(Suppl 1):S65–S69. doi: 10.1016/j.ijsu.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 46.Wright SA, Lee FT, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 47.Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463–464. doi: 10.1067/msy.2002.121231. [DOI] [PubMed] [Google Scholar]
- 48.Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59–67. doi: 10.1177/153303460700600202. [DOI] [PubMed] [Google Scholar]
- 49.Martin RC, 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20(Suppl 3):443–449. doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 50.Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–312. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 51.Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:16480–16488. doi: 10.3748/wjg.v20.i44.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–2727. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang RS, Liu LX, Gu YH, et al. The effect of endostatin and gemcitabine combined with HIFU on the animal xenograft model of human pancreatic cancer. Biomed Pharmacother. 2010;64:309–312. doi: 10.1016/j.biopha.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27:101–107. doi: 10.3109/02656736.2010.525588. [DOI] [PubMed] [Google Scholar]
- 55.Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2014;4:1–8. doi: 10.3109/02656736.2014.969789. [DOI] [PubMed] [Google Scholar]


