Abstract
Background
The prevalence of resistance to clarithromycin and metronidazole has considerably increased, with a corresponding decrease in the eradication rate for Helicobacter pylori (H. pylori) infection. Primary resistance to amoxicillin is extremely low, and esomeprazole was found to exert a noteworthy antimicrobial activity in vitro against H. pylori. A dual therapy with high-dose of esomeprazole coupled with high-dose amoxicillin might be therefore an ideal first-line treatment for H. pylori eradication. We aimed to assess the efficacy of a first-line 10-day, high-dose dual therapy consisting of amoxicillin and esomeprazole to eradicate H. pylori infection.
Methods
Consecutive naïve H. pylori-infected patients, who underwent an upper endoscopy in 4 Italian hospitals due to dyspeptic symptoms and found to be infected at routine histological assessment, were invited to participate. Patients enrolled received a 10-day, high-dose dual therapy comprising esomeprazole (40 mg t.i.d) and amoxicillin (1 g t.i.d.). At least 4 weeks after the end of the treatment a 13C-urea breath test was performed to evaluate the eradication.
Results
A total of 56 patients agreed to participate in the study and were all followed-up. The overall eradication was 87.5% (95% CI=78.8•96.2), without a statistically significant difference among centres. Overall, 5 (8.9%; 1.5•16.4%) patients complained of side-effects.
Conclusions
The 10-day, high-dose dual therapy with esomeprazole and amoxicillin might be an effective and safe first-line regimen. The efficacy of a longer 14-day regimen should be tested.
Keywords: Helicobacter pylori infection, dual therapy, esomeprazole, amoxicillin
Introduction
Helicobacter pylori (H. pylori) infection causes peptic ulcers, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer [1,2]. Standard treatments for H. pylori infection endorsed by the U.S. as well as European scientific societies and by regulatory authorities rely on clarithromycin, metronidazole, or amoxicillin in conjunction with gastric acid inhibitors [3-6]. Disappointingly, the prevalence of clarithromycin and metronidazole resistance has increased substantially in recent years, and a corresponding decrease has occurred in the eradication rate for H. pylori infection [7,8]. Indeed, first-line therapy success rate has declined to unacceptable levels in most Western countries [9]. In addition, the primary resistance to levofloxacin – an antibiotic generally used for second-line therapy – is also increasing in several countries [7,8]. Consequently, to treat H. pylori eradication failure patients is progressively more difficult, suggesting that a highly successful first-line regimen is the key to contrast the phenomenon.
Considering that the availability of new antibiotics against H. pylori is uncertain in the next few years [10], the identification of new regimens, including the currently available molecules, able to achieve a >90% eradication rate is urgently needed. Such a regimen would need to overcome the increasing prevalence of strains of H. pylori resistant to clarithromycin and/or metronidazole. Amoxicillin is a β-lactam antibiotic included in all current therapeutic regimens for H. pylori eradication [11]. Indeed, minimum inhibitor concentration values against H. pylori strains are ranging from 0.06 to 0.25 mg/L [11]. Although amoxicillin resistance and tolerance have been reported in H. pylori isolates [12,13], the primary resistance to amoxicillin is extremely low in several countries, with a prevalence rate as low as <1% (95% CI: 0.06-1.06) and 3% in Europe and the U.S., respectively [7,8]. However, amoxicillin is largely inactivated by low pH values present in the stomach [14,15], so that a simultaneous proton pump inhibitor (PPI) therapy is mandatory [16]. In addition, a deep suppression of gastric acid secretion, allowing to achieve pH value >6, is expected to favor antibacterial activity of amoxicillin in the gastric juice [14]. However, such a condition is rarely achieved in Caucasian subjects with standard dose of PPIs, due to the genetic polymorphism of hepatic P450 cytochrome responsible of PPI metabolism. Indeed, as many as >95% of Caucasians are rapid or intermediate metabolizers of PPIs [17], suggesting that an increased dose is needed in the majority of Caucasian subjects. On the other hand, among PPIs, esomeprazole was found to exert a greater antimicrobial activity in vitro against H. pylori compared to omeprazole, which could help improve the success rate of eradication regimens [18]. Based on these considerations, a dual therapy with high-dose esomeprazole, which increases intragastric pH and exerts a direct anti-bacterial activity, coupled with high-dose amoxicillin, against which primary resistance is extremely low, would be an ideal first-line treatment for H. pylori eradication.
We therefore designed this proof of concept study to assess the efficacy of such a high-dose dual therapy as first-line treatment in H. pylori-infected patients.
Patients and methods
Patients
This was an open-label, study performed in 4 Italian Hospitals (1 Northern; 2 Central, and 1 Southern Italy). In each participating center, consecutive adult (>18 years) patients, who underwent upper endoscopy due to dyspeptic symptoms and found to be infected with H. pylori at routine histological assessment, were invited to participate. Exclusion criteria were: 1) previous H. pylori eradication therapy; 2) known or suspected allergy to penicillin; 3) use of PPI or antibiotics in the previous 4 weeks; 4) previous surgery of upper gastrointestinal tract; 5) severe diseases (cardiovascular, pulmonary, renal or hepatic); 6) malignant disease during the previous 5 years; 7) alcohol abuse or severe psychiatric or neurologic disorders; 8) pregnancy or lactation; and 9) refusal to consent.
Therapy regimen
All patients received a 10-day, high-dose dual therapy comprising esomeprazole (40 mg t.i.d) and amoxicillin (1 g t.i.d.). The PPI was given half an hour before breakfast, lunch and dinner, whilst amoxicillin just after these meals. At the end of the treatment, compliance to therapy and reported side-effects were assessed by a personal interview. At least 4 weeks after the end of the treatment a 13C urea breath test (UBT) was performed to evaluate H. pylori eradication rate.
Statistical analysis
The eradication rate with 95% confidence intervals was calculated. Before pooling the estimates, a Fisher’s exact test was performed to exclude a significant heterogeneity among the different centers. Based on the study design (pilot study), data of only those patients who took ≥80% of prescribed drugs, and underwent UBT control were considered.
Results
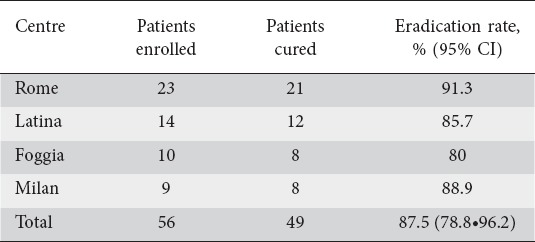
A total of 56 (male/female = 32/24; mean age: 51.3±13.7 years) patients agreed to participate in the study. All patients confirmed having taken all the prescribed drugs, but two patients who performed the therapy for 9 and 8 days, respectively. All these patients underwent the scheduled UBT control. As shown in Table 1, H. pylori infection was successfully cured in 87.5% (95% CI=78.8•96.2), without a statistically significant difference among the participating centers. Overall, 5 (8.9%; 1.5•16.4%) patients complained of side-effects (2 vomiting, 2 nausea, and 1 mild diarrhea), but only the 2 patients with vomiting early interrupted the treatment (at 9 and 8 days). All side-effects were self-limited.
Table 1.
Helicobacter pylori eradication rate achieved in different centers
Discussion
The success rate of standard triple therapies for H. pylori eradication is decreasing worldwide [19], suggesting the need of novel therapy regimens. Since newer agents with elevated activity against such an infection, including resistant strains, are still lacking [10], optimizing the use of available antibiotics would be advantageous. With this purpose, we tested the efficacy of a first-line, high-dose esomeprazole-amoxicillin dual therapy. The rationale of such a regimen consisted in coupling a deep suppression of acid secretion achieved with high-dose esomeprazole which would favor the efficacy of high-dose amoxicillin for which primary resistance in H. pylori isolates is very uncommon. Our study showed an interestingly high efficacy of this regimen, approaching a 90% success rate in our series. Of note, such a high cure rate was achieved using a regimen lasting only 10 days, suggesting that a longer 14-day therapy could perform better, particularly when considering the high tolerability we observed. Indeed, a 14-day high-dose dual therapy regimen with omeprazole 120 mg and amoxicillin 2.25 g achieved an 89% eradication rate in duodenal ulcer patients [20], and 96% in 126 MALT-lymphoma patients [21]. Likewise, a 95.5% eradication rate was achieved with a high-dose lansoprazole and amoxicillin 2 g first-line therapy in Japan [22]. In addition, a recent study performed in Taiwan showed that a high-dose dual therapy with rabeprazole 20 mg and amoxicillin 750 mg, all given q.i.d. for 14 days, achieved a 95.3% cure rate in naïve patients [23]. Interestingly, high-dose dual therapy with omeprazole 20 mg q.i.d and amoxicillin 1 g b.i.d achieved a significantly higher eradication rate than 14-day triple therapy in Turkey [24], where achieving H. pylori eradication is notoriously difficult [25]. On the contrary, a disappointing 53.8% success rate was achieved in 13 patients using dexlansoprazole 120 mg and amoxicillin 1 g, both b.i.d. for 14 days [26]. Moreover, the attempt to improve a high-dose dual therapy with esomeprazole 40 mg b.i.d. and amoxicillin 1 g t.i.d for 10 days by adding metronidazole did not appear to be advantageous, the eradication rates being 82.4% and 88.2% at intention-to-treat and per protocol analysis, respectively [27]. Overall, all these observations would suggest that a study testing our proposed high-dose dual regimen with esomeprazole 40 mg and amoxicillin 1 g, both t.i.d., for 14 days is urged. The usefulness of a study is further supported by the high tolerability of such a regimen, for which the incidence of adverse events was reported to be not significantly superior to those observed in the comparison arms [20,22,28].
In conclusion, this is the first Italian study showing that a 10-day, high-dose dual therapy with esomeprazole and amoxicillin could achieve high eradication rates, suggesting that the efficacy of a longer 14-day regimen should be tested.
Summary Box.
What is already known:
The success rate of standard triple therapies for Helicobacter pylori (H. pylori) infection has declined to unacceptable levels
The prevalence of primary resistance towards clarithromycin and metronidazole is high, whilst that towards amoxicillin remains extremely low
What the new findings are:
A novel 10-day dual therapy with high-dose of esomeprazole coupled with high-dose amoxicillin was found to be acceptably effective and highly tolerated as a first-line therapy
Biography
Nuovo Regina Margherita Hospital, Rome; Sapienza University of Rome; Polo Pontino Hospital, Latina; Riuniti Hospital, Foggia; Versilia Hospital, Lido di Camaiore; Salvini Hospital, Garbagnate, Milan, Italy
Footnotes
Conflict of Interest: None
References
- 1.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 2.Zullo A, Hassan C, Ridola L, et al. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014;27:27–33. [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection - the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 4.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunt RH, Xiao SD, Megraud F, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointest Liver Dis. 2011;20:299–304. [PubMed] [Google Scholar]
- 6.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 7.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- 8.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 9.De Francesco V, Ierardi E, Hassan C, et al. Helicobacter pylori therapy: present and future. World J Gastrointest Pharmacol Ther. 2012;3:68–73. doi: 10.4292/wjgpt.v3.i4.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorini G, Zullo A, Gatta L, et al. Newer agents for Helicobacter pylori eradication. Clin Exp Gastroenterol. 2012;5:109–112. doi: 10.2147/CEG.S25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Francesco V, Zullo A, Hassan C, et al. Mechanisms of Helicobacter pylori antibiotic resistance: an updated appraisal. World J Gastrointest Pathophysiol. 2011;2:35–41. doi: 10.4291/wjgp.v2.i3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Zwet AA, Vandenbroucke-Grauls CM, Thijs JC, et al. Stable amoxicillin resistance in Helicobacter pylori. Lancet. 1998;352:1595. doi: 10.1016/s0140-6736(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 13.Dore MP, Osato MS, Realdi G, et al. Amoxycillin tolerance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:47–54. doi: 10.1093/jac/43.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Grayson ML, Eliopoulos GM, Ferraro MJ, et al. Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888–889. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JR. Pharmacology of the gastric mucosa: a rational approach to Helicobacter polytherapy. Gastroenterology. 1996;111:521–523. doi: 10.1053/gast.1996.v111.agast961110521. [DOI] [PubMed] [Google Scholar]
- 16.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–367. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935–951. doi: 10.1007/s00228-008-0538-y. [DOI] [PubMed] [Google Scholar]
- 18.Gatta L, Perna F, Figura N, et al. Antimicrobial activity of esomeprazole versus omeprazole against Helicobacter pylori. J Antimicrob Chemother. 2003;51:439–442. doi: 10.1093/jac/dkg085. [DOI] [PubMed] [Google Scholar]
- 19.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. doi: 10.1136/bmj.f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayerdorffer E, Miehlke S, Mannes GA, et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–1417. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 21.Zullo A, Hassan C, Andriani A, et al. Eradication therapy for Helicobacter pylori in patients with gastric MALT-lymphoma: a pooled data analysis. Am J Gastroenterol. 2009;104:1932–1937. doi: 10.1038/ajg.2009.314. [DOI] [PubMed] [Google Scholar]
- 22.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther. 2007;81:521–528. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]
- 23.Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori Infection. Clin Gastroenterol Hepatol. 2015;13:895–905. doi: 10.1016/j.cgh.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ince AT, Tozlu M, Baysal B, Şentürk H, Arıcı S, Özden A. Yields of dual therapy containing high-dose proton pump inhibitor in eradication of H. pylori positive dyspeptic patients. Hepatogastroenterology. 2014;61:1454–1458. [PubMed] [Google Scholar]
- 25.Zullo A, De Francesco V, Hassan C, et al. Modified sequential therapy regimens for Helicobacter pylori eradication: a systematic review. Dig Liver Dis. 2013;45:18–22. doi: 10.1016/j.dld.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Attumi TA, Graham DY. High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter. 2014;19:319–322. doi: 10.1111/hel.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez-Delgado J, García-Iglesias P, Castro-Fernández M, et al. High-dose, ten-day esomeprazole, amoxicillin and metronidazole triple therapy achieves high Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2012;36:190–196. doi: 10.1111/j.1365-2036.2012.05137.x. [DOI] [PubMed] [Google Scholar]
- 28.Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743–749. doi: 10.1007/s00228-007-0302-8. [DOI] [PubMed] [Google Scholar]


