Abstract
Background
The risk of developing pancreatic cancer is uncertain in patients with clinically suspected branch duct intraductal papillary mucinous neoplasm (BD-IPMN) based on the “high-risk stigmata” or “worrisome features” criteria proposed in the 2012 international consensus guidelines (“Fukuoka criteria”).
Methods
Retrospective case series involving patients referred for endoscopic ultrasound (EUS) of indeterminate pancreatic cysts with clinical and EUS features consistent with BD-IPMN. Rates of pancreatic cancer occurring at any location in the pancreas were compared between groups of patients with one or more Fukuoka criteria (“Highest-Risk Group”, HRG) and those without these criteria (“Lowest-Risk Group”, LRG).
Results
After exclusions, 661 patients comprised the final cohort (250 HRG and 411 LRG patients), 62% female with an average age of 67 years and 4 years of follow up. Pancreatic cancer, primarily adenocarcinoma, occurred in 60 patients (59 HRG, 1 LRG). Prevalent cancers diagnosed during EUS, immediate surgery, or first year of follow up were found in 48/661 (7.3%) of cohort and exclusively in HRG (33/77, 42.3%). Using Kaplan-Meier method, the cumulative incidence of cancer at 7 years was 28% in HRG and 1.2% in LRG patients (P<0.001).
Conclusions
This study supports using Fukuoka criteria to stratify the immediate and long-term risks of pancreatic cancer in presumptive BD-IPMN. The risk of pancreatic cancer was highest during the first year and occurred exclusively in those with “high-risk stigmata” or “worrisome features” criteria. After the first year all BD-IPMN continued to have a low but persistent cancer risk.
Keywords: Pancreatic cyst, pancreatic cancer, natural history, endosonography, intraductal papillary mucinous neoplasm
Introduction
Pancreatic cancer is usually discovered at advanced stage and there are no screening or surveillance strategies proven to reduce risk. Pancreatic cysts have been targeted for intervention since mucin-producing cysts are associated with a risk of progression to adenocarcinoma over time. Though pancreatic cysts are easily identified on cross-sectional imaging, most types pose no risk of malignant degeneration. With trending use and improvements in imaging, incidental pancreatic cysts have been increasingly detected, occurring in 2.4-14.4% of the general population [1-3]. The minority of these cysts are easily and accurately distinguished using clinical and radiographic features such as main duct intraductal papillary mucinous neoplasms (MD-IPMN) and high-risk (≥3 cm in size) mucinous cystic neoplasms (MCN). However, computed tomography (CT) and magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) perform poorly in discerning cyst type for the majority of cysts, with accuracy of diagnosis <50% [4]. Many are presumably neoplastic cysts such as branch duct (BD)-IPMN or small MCN whose natural history and risk for progression to adenocarcinoma are poorly understood [5,6]. Resection is reserved for symptomatic or probable malignant cysts with features as suggested per Fukuoka criteria and the 2015 AGA guidelines on diagnosis and management of asymptomatic neoplastic pancreatic cysts [7]. Surgical resection of pancreatic cysts carries a morbidity of 35% and mortality of 1% [8-13]. Thus, non-operative management in most cases is reasonable as transformation of pancreatic cysts into adenocarcinoma appears to be a rare event (33.2 events per 100,000) [14]. The challenge is discerning BD-IPMN with highest malignant potential that warrant operative management from those that can be safely entered into a surveillance program, thereby minimizing risks to this patient population.
In 2006 international consensus guidelines for the management of pancreatic BD-IPMN and MCN were released by the International Association of Pancreatology (IAP) [15] and in 2012 were updated [16]. The most notable changes in the revised Fukuoka guidelines were refinements in criteria used to stratify the malignant potential of BD-IPMN (“Fukuoka criteria”), and de-emphasis of cyst size ≥30 mm solely as an indication for surgery. The Fukuoka criteria are separated into “high-risk stigmata” [obstructive jaundice with cystic lesion located in the head of the pancreas, cyst containing an enhancing solid component, and main pancreatic duct (PD) diameter ≥10 mm] and “worrisome features” (pancreatitis, cyst size ≥30 mm, thickened/enhancing cyst walls, PD size 5-9 mm, cyst with non-enhancing mural nodule, abrupt change in caliber of PD with distal pancreatic atrophy, and lymphadenopathy). Consideration of immediate resection is recommended for surgically fit patients with “high-risk stigmata”, “worrisome features” and concerning endoscopic ultrasound (EUS) findings (definite mural nodule, PD abnormalities and fine-needle aspiration (FNA) cytology with either high-grade dysplasia or malignant cell) or otherwise benign-appearing cyst with high-grade epithelial atypia. In the absence of Fukuoka criteria and/or high-risk EUS-FNA features, BD-IPMN are thought to confer low risk for malignancy and are suitable for non-operative management and surveillance.
The aim of this study was to evaluate the performance of Fukuoka criteria in stratifying risk of pancreatic cancer of clinically suspected BD-IPMN, and identifying low-risk cysts that can be safely managed non-operatively following EUS evaluation.
Patients and methods
This is a retrospective study involving consecutive patients who underwent pancreaticobiliary EUS evaluation of indeterminate pancreatic cysts between December 2000 and July 2012 at four referral centers in the same geographic region. The primary end points of this study were development of pancreatic cancer and survival in the HRG and LRG. Each center obtained Institutional Review Board approval. Included in the study were patients ≥18 years of age referred for EUS evaluation of one or more newly discovered pancreatic cysts with EUS features consistent with BD-IPMN. Patients were referred by their primary care provider, surgeon, or gastroenterologist after discovery of one or more pancreatic cysts on CT, MRI or transcutaneous ultrasound. After EUS evaluation, the management of each patient was individualized based upon cyst characteristics, patient age and comorbidity. EUS procedure reports and electronic medical records (EMR) were reviewed for patient demographics and medical history, associated symptoms at time of referral, and EUS findings (i.e. Fukuoka criteria, location in pancreas, number, size of largest cyst if multifocal or of conglomeration of abutting cysts, and morphology), FNA cytology, cyst fluid analyses and clinical follow up. Patients or close family members were contacted by telephone or online death registries were reviewed if follow-up information was not obtainable through review of the EMR. Diagnosis of pancreatic malignancy was based on FNA cytology, surgical pathology, or surveillance CT or MRI showing an obvious, locally invasive pancreatic mass. When patient was deceased, telephone interview of a close family member was utilized to confirm pancreatic malignancy. Prevalent cancers were defined as those that were diagnosed at the time of EUS-FNA, early surgery or during first year of follow up. Date and cause of death were determined by reviewing patients’ electronic medical records, contacting family members, or from online registries.
A single unifocal, septated or “honeycombed” pancreatic cyst was classified as “unilocular”, whereas multiple, discrete cysts were classified as either “abutting” (including oligocystic and multilobular cysts) or “separate” depending on whether or not cysts were contiguous. The size of the largest discrete cyst or conglomeration of abutting cysts in each patient was used for analyses. Pancreatic cysts were categorized into one of six suspected cyst types using clinical and EUS features as follows. MD-IPMN - isolated segmental or diffuse dilation of PD ≥5 mm. MCN - unilocular cyst ≥30 mm in size located within the body or tail of female patients. Serous cystic neoplasm (SCN) - unilocular cyst with honeycomb appearance with normal caliber PD. BD-IPMN - unilocular cyst <30 mm in size or multifocal cysts located anywhere within the pancreas associated with normal PD (i.e. <5 mm). Suspected BD-IPMNs were classified as main duct or mixed-IPMN when there was an associated dilated PD (i.e. ≥5 mm). Cysts were classified as suspected pseudocysts if discovered after an episode of acute pancreatitis or located predominantly outside the pancreas parenchyma. EUS documentation of clear communication between cysts and the PD was reported at the discretion of each endosonographer, and if present these were considered BD-IPMN, but if no mention in the report of whether the cyst communicated with the PD then the cysts were still considered as suspected BD-IPMN if were multiple cysts and did not have an appearance or clinical characteristics consistent with other types of pancreatic cystic lesion. FNA was not routinely performed but rather performed at the discretion of each endoscopist, thus neither cytology nor carcinoembryonic antigen (CEA) was used in this study to discern cyst types. All sites used rapid on-site cytological evaluation of FNA samples. DNA and molecular analysis was not employed for any patients.
Following chart review patients were excluded if: cysts were not consistent with BD-IPMN, patient had history of pancreatic cancer, chronic pancreatitis (imaging demonstrating calcifications or history or recurrent episodes of pancreatitis), suspected pseudocyst (prior history of pancreatitis with new pancreatic cyst) or von Hippel-Lindau disease; or, patient did not have at least 1 year of clinical follow up in the absence of a diagnosis of pancreatic cancer, cyst resection, or death.
Data management and statistical analyses
Data were entered into a password protected de-identified database. Patients were divided into two groups, “Highest-Risk Group” (HRG) and “Lowest-Risk Group” (LRG), based the presence of Fukuoka criteria for analysis [16]. The HRG consisted of BD-IPMN with at least one Fukuoka criterion (obstructive jaundice associated with cyst in head of pancreas, presence of a solid mass component or mural nodule within cyst, PD size ≥5 mm, cyst size ≥30 mm, abrupt change in caliber of PD with distal pancreatic atrophy, lymphadenopathy). The remaining patients with benign-appearing cysts without any Fukuoka criteria comprised the LRG.
Follow-up interval for each patient was calculated as the time between EUS examination and diagnosis of cancer, surgery, the most recent patient contact or death. Associations of individual factors with risk groups were tested using either the chi-square test or the Wilcoxon rank-sum test. The Kaplan-Meier method with the log-rank test was used to compare the incidence of pancreatic cancer. Patients who underwent surgery or died without a cancer diagnosis were censored; however, follow-up information on clinical outcomes of pancreatic cancer and/or death following surgery was obtained for each patient.
Results
A total of 894 patients underwent EUS evaluation of one or more pancreatic cysts identified on CT or MRI during the study period (Fig. 1). Of this number 233 patients were excluded for the following suspected diagnoses: 16 MD-IMPN; 29 MCN; 52 SCN; 40 chronic pancreatitis; 35 pseudocyst; 3 history of pancreatic cancer; 1 with von Hippel-Lindau. 57 were excluded for inadequate follow up. Following these exclusions 661 patients comprised the cohort including 218 patients from Kaiser Permanente, Anaheim Medical Center, California; 148 patients from Kaiser Permanente, San Diego Medical Center/Kaiser Foundation Hospital, California; 235 patients from the University of California, San Diego, California; and 60 patients from the Veterans Administration Medical Center, La Jolla, California. Females comprised 55.8%; average age was 67.4 years; and pancreatic cysts were predominantly asymptomatic (67%). Compared to the LRG, patients in the HRG were more often male (53.6% vs 38.4%, P=0.0001), older (68.7 years vs. 66.5 years, P=0.0051), followed for a shorter period of time (3.7 years vs. 4.1 years, P= 0.0013), more often symptomatic (46.4% vs. 24.8%, P<0.0001) and more likely to undergo surgical resection (14.4% vs. 3.5%, P=0.0001). The cohort was followed for an average of 3.8 years, ranging between 3 days (patients diagnosed with pancreatic cancer at time of EUS) to 12 years.
Figure 1.
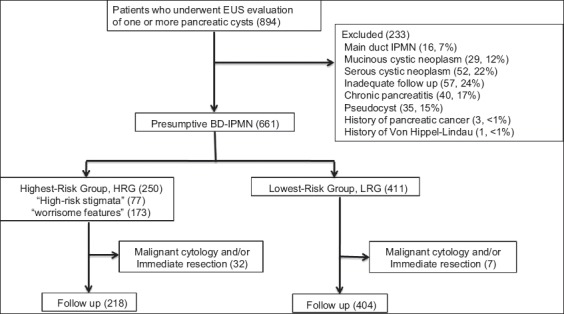
Patient screening, exclusion, and group assignment
BD-IPMN, branch duct-intraductal papillary mucinous neoplasm; EUS, endoscopic ultrasound; HRG, highest-risk group; IPMN, intraductal papillary mucinous neoplasm; LRG, lowest-risk group
EUS findings
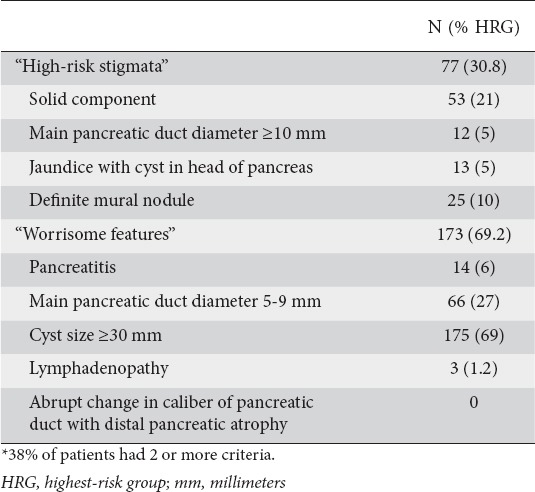
Overall, 250 patients in the cohort (37.8%) had suspected BD-IPMN associated with one or more Fukuoka criteria and were assigned to the HRG (Fig. 1), leaving 411 (62.2%) suspected BD-IPMN that were assigned to the LRG. Of the 250 assigned to the HRG, 95 (38%) had multiple criteria, 173 (69.2%) “worrisome features” and 77 (30.8%) “high-risk stigmata”, most commonly solid component (54) or a mural nodule (25) (Table 1).
Table 1.
The absolute and relative frequency of occurrence of each Fukuoka criterium*
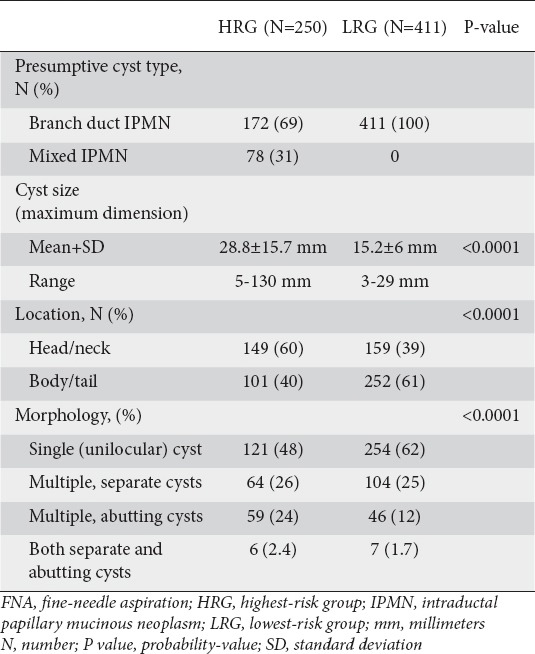
Cyst characteristics are summarized in Table 2. Mixed-IPMN comprised 31% of cysts in the HRG. Compared to the cysts in the LRG, those in the HRG were larger in size (28.8 mm vs. 15.2 mm, P<0.0001), more frequently located in the head of the pancreas (60% vs. 39%, P<0.0001), and more often occurring as multifocal or as multiple abutting cysts rather than as unilocular cysts (52.4% vs. 38.7%, P<0.0001).
Table 2.
Comparison between the highest-risk and lowest-risk groups of the absolute and relative number of presumptive cyst types, mean maximal cyst size, location of dominant cyst within the pancreas, and cyst morphology
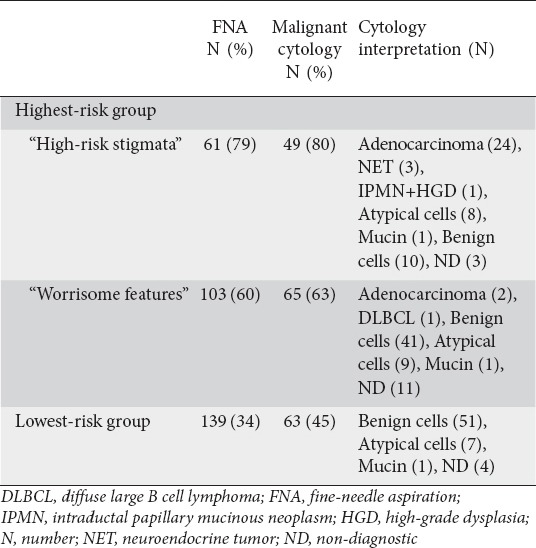
FNA
FNA was performed in 163 patients in the HRG (65.2%) and 139 in the LRG (33.8%) (P<0.0001). Malignant cytology was identified in 30/164 (18.3%) aspirates in the HRG and none in the LRG (P<0.0001) (Table 3). Most aspirates with malignant cytology (27/30, 90%) and the single aspirate with HGD cytology were from cysts with “high-risk stigmata”. CEA was obtained in 19 patients who subsequently underwent resection during follow up.
Table 3.
Frequency of fine-needle aspiration and cytology performance and the associated cytology interpretations within the highest-risk and lowest-risk groups
Immediate surgery
Within 3 months of EUS exam 34 patients underwent surgical resection, 27 from the HRG (10.4%). This includes 16/77 (20.7%) of those with “high-risk stigmata” and 11/173 (6.4%) of those with only “worrisome features”. Overall pathology was malignant in 12 patients, 8 with cysts with “high-risk stigmata” and 3 with “worrisome features”. Out of the 30 patients who had malignant cytology on FNA, 6 proceeded to surgery (20%) and in one case surgical pathology was non-invasive IPMN. In the LRG 7/411 (1.7%) proceeded to surgery within 3 months and pathology was benign.
Follow up
Of the 30 patients with malignant cytology on EUS-FNA 25 died during follow up (83.3%), 15 within the first year (median time to death, 9 months, range 11 days to 102 months). Cause of death was reported in the EMR for 10 patients, 9 as complications of metastatic cancer and 1 as end-stage Parkinson’s disease. Patients who had malignant cytology and were alive during follow up included 3 who did not undergo surgery (2 neuroendocrine tumor, 1 diffuse large B cell lymphoma) and 2 with adenocarcinoma on cytology who proceeded to Whipple resection within 2 months (pathology, 1 pT1N0 adenocarcinoma and 1 IPMN).
The remaining 622 patients (218 in HRG and 404 in LRG) who had neither malignant cytology on EUS-FNA nor resection within 3 months had an average follow up of 49.5 months (range 10 days to 132 months).
Development of pancreatic cancer
During the first year of follow up pancreatic cancer occurred in an additional 10 patients, 8 patients with “worrisome features” and 2 with “high-risk stigmata” based upon repeat EUS-FNA (5), surgery (1), imaging (2) and interview (1) (Table 5). After the first year of follow up 12 additional patients developed pancreatic cancer, 11 in the HRG (8 with “worrisome features” on index EUS and 3 with “high-risk stigmata” on index EUS) and 1 in the LRG based upon repeat FNA (3), surgery (4), imaging (2) and interview (3). The 1 patient in the LRG who developed pancreatic cancer was a female who initially underwent EUS at age of 77 for a unilocular 26 mm cyst in the pancreas neck with pathology revealing BD-IPMN. Over years of follow up multiple cysts accumulated throughout her entire pancreas consistent with multifocal BD-IPMN. At 6 years after presentation, a mass formed in the head of the pancreas, confirmed to be adenocarcinoma on EUS-FNA cytology.
Table 4.
Frequency of pancreatic surgery and associated pathology in the highest-risk and lowest-risk groups
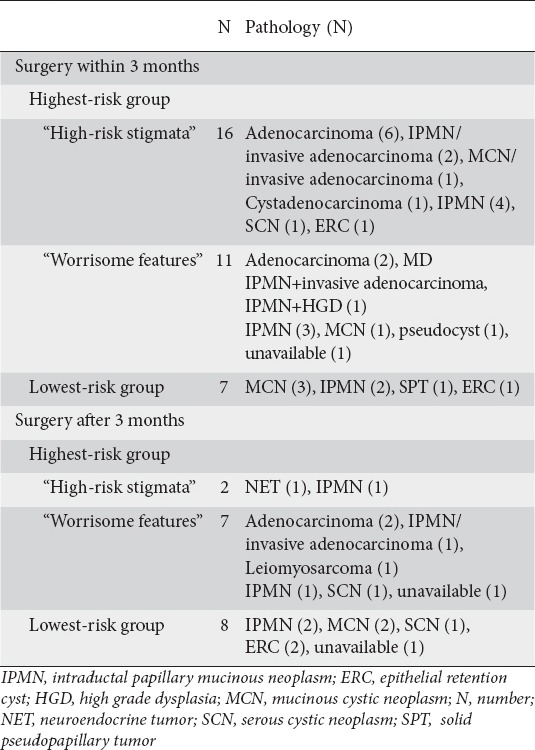
Table 5.
Frequency of pancreatic cancer diagnosed within 3 months, 3 to 12 month, and after 12 months following index endoscopic ultrasound in the highest-risk and lowest-risk groups
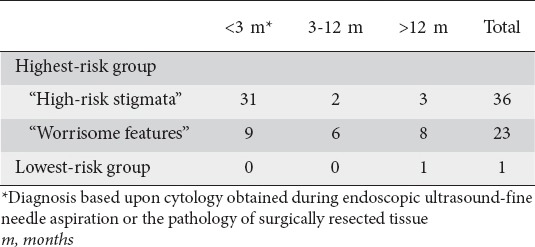
There were 60 cases of pancreatic cancer (59 in HRG and 1 in LRG), 48 of which (80%) were diagnosed at the time of index EUS, immediate surgery or within first year of follow-up (Table 5). These prevalent cancers were diagnosed in 6.4% of the entire cohort, 19.2% of the HRG (42.8% of those with “high-risk stigmata” and 8.7% of those with “worrisome features”) and 0% of those in the LRG. Based upon Kaplan-Meier estimates the cumulative incidence of pancreatic cancer after 7 years of follow up was 28% in the HRG and 1.2% in the LRG (P<0.001) (Fig. 2).
Figure 2.
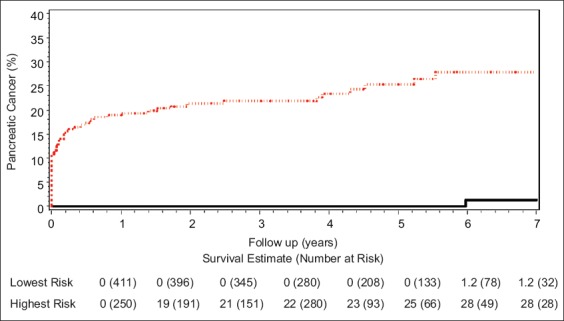
Cumulative incidence of pancreatic cancer in the highest (upper red line) and lowest (lower black line) risk groups
Discussion
This multicenter study of 661 patients with an average of nearly 4 years follow up showed that, collectively, the Fukuoka criteria perform exceptionally well in discriminating presumptive high-risk BD-IPMN from those posing minimal risk or even no risk (such as when patients die from cause other than pancreatic disease). Clinically suspected BD-IPMN with one or more Fukuoka criteria were much more likely to harbor or develop pancreatic cancer compared to those without these features. A total of 59 pancreatic malignancies occurred in the group of 250 patients with at least one Fukuoka criterion. This corresponds to a 28% cumulative risk over a 7-year period of follow up using Kaplan-Meier analysis. There was only one case of pancreatic cancer in the group of 411 patients with benign-appearing lesions (1.2% cumulative risk over 7-year period). These findings support the use of Fukuoka criteria to risk stratify clinically suspected BD-IPMN, as recommended in the 2012 revised international consensus guidelines [16].
Most pancreatic malignancies (80%) were found at the time of index EUS, immediate surgery or during the first year of follow up. This emphasizes the importance of a thorough evaluation of indeterminate pancreatic cysts at time of cyst diagnosis and during the first year of follow up. These prevalent cancers were found in 7.3% of the cohort which is much lower than the estimate of 25.5% derived from surgical data of resected BD-IPMN [5] and likely reflects differences in patient populations and referral patterns. Furthermore, prevalent cancers occurred exclusively in the HRG and were more frequent in patients with “high-risk stigmata” compared to those with only “worrisome features” (42.3% vs. 8.7%). This finding concurs with the surgical literature that has consistently shown higher rates of pancreatic cancer in BD-IPMN with high-risk features compared to those without [17-28]. These findings support the relative weighting of importance of Fukuoka criteria and the recommendation for consideration of immediate surgical resection for cysts with “high-risk stigmata” as outlined in the 2012 IAP guidelines.
The updated 2012 IAP and 2015 AGA guidelines recommend surveillance for suspected low-risk BD-IPMN using EUS, CT or MRI at intervals ranging between 3 months to 2 years depending upon cyst features. Whether the risk of developing pancreatic malignancy is sufficiently low to support non-operative management in benign-appearing lesions remains uncertain given the lack of natural history data of BD-IPMN. Studies that have looked at long-term outcomes of BD-IPMN (i.e. ≥1 year of follow up) have shown good outcomes for those patients entered into surveillance programs. However, small cohort sizes and/or confinement to single institutions limit generalizability of these studies [5,28-34]. In this study, over 600 patients at 4 referral centers were followed long-term for up to 12 years and showed that most patients did well. This is especially true in the LRG, where only one patient developed pancreatic cancer after 6 years of follow up. Thus, patients with presumptive BD-IPMN that appear benign on EUS examination and lack Fukuoka criteria can be observed with little risk of developing cancer. However, the safety of surveillance is less clear in those with Fukuoka “worrisome features” where risk of malignancy is increased.
Establishing the malignant potential of a given cyst using the Fukuoka criteria or AGA guidelines requires discerning the presumed cyst type. Based upon the histology of surgically resected cysts approximately one quarter of presumptive BD-IPMN were likely benign, non-mucinous cyst types (Table 4). Consequently, the calculated risk of pancreatic malignancy in the HRG of this study would likely be slightly higher if benign lesions had been excluded from analyses. Improved accuracy of cyst diagnosis would have also reduced the number of unnecessary surgeries. Selective use of EUS-FNA sampling of cyst fluid for CEA and amylase has been shown to aid in the diagnosis cyst type with an accuracy ranging between 62 and 79% [35-37]. However, cyst fluid analysis requires specialized training, incurs additional expenses for consumables and cytopathology and may carry up to 5% rate of complication [38]. In the current study, less than half of the resected mucinous cysts in which preoperative CEA was available was concentration above the threshold of 192 ng/mL. Though the study was not designed to evaluate the impact of cyst fluid CEA on patient management these results suggest limited effectiveness of CEA in discerning mucinous cysts.
Though the elderly are disproportionately affected by pancreatic cysts, younger patients are more apt to undergo surgical resection for these cystic lesions. In studies that have included both operatively managed and surveillance, including this study, patients who undergo resection tend to be between 6 and 10 years younger [10,39,40]. This suggests that patient age and preferences may have been decisive factors and that the elderly are less likely to undergo risky surgical procedures.
There are several limitations of this study. This is a retrospective cohort study of patients referred for EUS evaluation of pancreatic cystic lesions using data obtained from review of medical records and EUS reports. Cysts were classified as suspected BD-IPMN based upon available clinical and morphologic features rather than EUS FNA cyst fluid analysis, cytology or histology of resected tissue, and therefore it is possible that some cysts were not BD-IPMN but possibly macrocystic serous cystadenomas, retention cysts, or other benign etiologies. Though cytology, histology or clear cross-sectional imaging confirmed most cases of pancreatic cancer a minority were based upon interview of close family members. Also, distinction was not made between malignancy occurring within IPMN and those arising elsewhere in the pancreas (i.e. concomitant cancer). Patients were medically and surgically managed at the discretion of each endoscopist and other members of their healthcare team in conjunction with patient preference rather than by a standard protocol. Although patients were followed for an average 4 years after initial EUS examination allowing for Kaplan-Meier statistics to 7 years, longer duration of follow up is needed to understand better the natural history of BD-IPMN. Follow-up information on changes in cyst characteristics over time, such as cyst size and development of Fukuoka criteria, was also not evaluated. Fukuoka criteria were not systematically incorporated into report format, thus if criteria not mentioned it was assumed to be absent. Another limitation is that the low rate of cancer development in the low-risk group could be due to the inclusion of cysts that were not BD-IPMN (i.e. macrocystic serous cystadenomas or retention cysts).
The strengths of this study lend to its effective representation of real-life patient care at four separate institutions; thus results are clinically useful and generalizable. Referral bias was minimized since the cohort included patients from a large, integrated healthcare system and a tertiary academic referral center. Cysts types were classified by clinical and EUS information that can be easily attained by most clinicians during diagnostic workup of incidentally discovered pancreatic cystic lesion. The large group of non-operatively managed patients and long-term follow up also allowed for a detailed description of the natural history of presumptive BD-IPMN.
In summary, this study supports the use of the Fukuoka “high-risk stigmata” and “worrisome features” criteria in stratifying the malignant potential of cysts consistent with BD-IPMN. This study supports surveillance of benign appearing cysts that lack Fukuoka criteria, or high-risk features as identified by AGA 2015 guidelines because they pose little to no risk to the patient. Most patients who develop pancreatic cancer in BD-IPMN have at least one Fukuoka criterion and are most often diagnosed with pancreatic cancer within a year of the cyst being detected. However, patients not diagnosed with cancer within the first year, continue to be at risk for developing pancreatic cancer over time, even in those who initially did not have Fukuoka criteria at the time of initial EUS. Thus surveillance should be continued in patients with presumptive BD-IPMN.
Summary Box.
What is already known:
Histologically proven branch duct intraductal papillary mucinous neoplasms (BD-IPMN) with Fukuoka “high risk stigmata” have a high risk for pancreatic cancer and should be considered for immediate surgery
Histologically proven BD-IPMN with “worrisome features” and those without Fukuoka criteria are at intermediate and low risk for pancreatic cancer and may be managed non-operatively, however the safety of this strategy is not completely known
Non-invasive methods of establishing cyst diagnosis and malignant potential are imprecise
What the new findings are:
Fukuoka criteria perform well in stratifying risk of malignancy of clinically suspected BD-IPMN based upon clinical, radiographic and endoscopic ultrasound information
Suspected BD-IPMN without Fukuoka have an extremely low risk of either harboring or transforming into pancreatic cancer over at least first 7 years following diagnosis (1.2% cumulative risk) compared to those with at least 1 criterion (28%)
Biography
Naval Medical Center, San Diego, CA; Kaiser Permanente, San Diego; Kaiser Permanente, Anaheim, CA; Veterans Affairs Medical Center, La Jolla, CA; Kaiser Permanente, Pasadena, CA; Institute of Digestive Disease, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong; Digestive Health Specialists, NC; University of California, La Jolla, CA
Footnotes
Conflict of Interest: None
References
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Sahani DV, Kambadakone A, Macari M, et al. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200:343–354. doi: 10.2214/AJR.12.8862. [DOI] [PubMed] [Google Scholar]
- 5.Farrell JJ, Fernandez-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303–1315. doi: 10.1053/j.gastro.2013.01.073. [DOI] [PubMed] [Google Scholar]
- 6.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–438. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 7.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848. doi: 10.1053/j.gastro.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–154. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 9.de Castro SM, Houwert JT, van der Gaag NA, et al. Evaluation of a selective management strategy of patients with primary cystic neoplasms of the pancreas. Int J Surg. 2011;9:655–658. doi: 10.1016/j.ijsu.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212:590–600. doi: 10.1016/j.jamcollsurg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4–12. doi: 10.1016/j.surg.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–475. doi: 10.1097/SLA.0b013e3182a18f48. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer CM, Jr, Sanchez N, Gondek S, et al. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2012;16:89–102. doi: 10.1007/s11605-011-1753-x. [DOI] [PubMed] [Google Scholar]
- 14.Gardner TB, Glass LM, Smith KD, et al. Pancreatic Cyst Prevalence and the Risk of Mucin-Producing Adenocarcinoma in US Adults. Am J Gastroenterol. 2013;108:1546–1550. doi: 10.1038/ajg.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Hwang DW, Jang JY, Lee SE, et al. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93–102. doi: 10.1007/s00423-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto S, Lawler LP, Horton KM, et al. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186:687–695. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 19.Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952–959. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Park KT, Lee YJ, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg. 2008;15:183–188. doi: 10.1007/s00534-007-1231-8. [DOI] [PubMed] [Google Scholar]
- 21.Aso T, Ohtsuka T, Matsunaga T, et al. “High-risk stigmata” of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014;43:1239–1243. doi: 10.1097/MPA.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 22.Ohtsuka T, Kono H, Nagayoshi Y, et al. An increase in the number of predictive factors augments the likelihood of malignancy in branch duct intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2012;151:76–83. doi: 10.1016/j.surg.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Takahashi S, Gotohda N, Konishi M. Risk factors for malignancy in branched-type intraductal papillary mucinous neoplasms of the pancreas during the follow-up period. World J Surg. 2015;39:244–250. doi: 10.1007/s00268-014-2789-3. [DOI] [PubMed] [Google Scholar]
- 24.Fritz S, Klauss M, Bergmann F, et al. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg. 2014;260:848–855. doi: 10.1097/SLA.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 25.Roch AM, Ceppa EP, DeWitt JM, et al. International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB (Oxford) 2014;16:929–935. doi: 10.1111/hpb.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 28.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 29.Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:198–202. doi: 10.1016/j.pan.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Cauley CE, Waters JA, Dumas RP, et al. Outcomes of primary surveillance for intraductal papillary mucinous neoplasm. J Gastrointest Surg. 2012;16:258–267. doi: 10.1007/s11605-011-1757-6. [DOI] [PubMed] [Google Scholar]
- 31.Bae SY, Lee KT, Lee JH, et al. Proper management and follow-up strategy of branch duct intraductal papillary mucinous neoplasms of the pancreas. Dig Liver Dis. 2012;44:257–260. doi: 10.1016/j.dld.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261–265. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Arlix A, Bournet B, Otal P, et al. Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41:295–301. doi: 10.1097/MPA.0b013e3182285cc8. [DOI] [PubMed] [Google Scholar]
- 34.Baiocchi GL, Portolani N, Grazioli L, et al. Management of pancreatic intraductal papillary mucinous neoplasm in an academic hospital (2005-2010): What follow-up for unoperated patients? Pancreas. 2012;42:696–700. doi: 10.1097/MPA.0b013e318270b98b. [DOI] [PubMed] [Google Scholar]
- 35.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Cizginer S, Turner BG, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024–1028. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- 37.Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis. 2013;45:920–926. doi: 10.1016/j.dld.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Shin CM, Park JK, et al. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci. 2007;52:2653–2659. doi: 10.1007/s10620-006-9634-y. [DOI] [PubMed] [Google Scholar]
- 40.Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144:448–454. doi: 10.1001/archsurg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]


