Highlight
This research successfully used image-based spectral indices acquired in the field to assess variability of response to drought in a tree mapping population and to detect the related genetic determinisms.
Keywords: Malus×domestica, multispectral imagery, quantitative trait locus (QTL), surface temperature, thermal infrared, vegetation index.
Abstract
Genetic studies of response to water deficit in adult trees are limited by low throughput of the usual phenotyping methods in the field. Here, we aimed at overcoming this bottleneck, applying a new methodology using airborne multispectral imagery and in planta measurements to compare a high number of individuals.
An apple tree population, grafted on the same rootstock, was submitted to contrasting summer water regimes over two years. Aerial images acquired in visible, near- and thermal-infrared at three dates each year allowed calculation of vegetation and water stress indices. Tree vigour and fruit production were also assessed. Linear mixed models were built accounting for date and year effects on several variables and including the differential response of genotypes between control and drought conditions.
Broad-sense heritability of most variables was high and 18 quantitative trait loci (QTLs) independent of the dates were detected on nine linkage groups of the consensus apple genetic map. For vegetation and stress indices, QTLs were related to the means, the intra-crown heterogeneity, and differences induced by water regimes. Most QTLs explained 15−20% of variance.
Airborne multispectral imaging proved relevant to acquire simultaneous information on a whole tree population and to decipher genetic determinisms involved in response to water deficit.
Introduction
According to current climate change models for the 21st century, an increase in global mean temperatures is expected, with longer or more frequent episodes of extreme temperatures and drought, notably in the Mediterranean basin (IPCC, 2014). Climate change will lead to reconsideration of bree ding programmes for many crops, and optimization of water use by improving the plant water use efficiency and/or the tolerance to drought will become an increasingly important issue (Hamdy et al., 2003; Condon et al., 2004). Although plant behaviour in response to drought can be analysed in terms of survival (McDowell et al., 2008), it more usually refers to the ability of one genotype to yield better than another under more or less severe water deficit. However, while breeding programmes in fruit species have not yet included drought tolerance among the targeted traits, some authors consider that tree response to water scarcity will become a crucial character to consider (Bassett, 2013).
Plants have developed various mechanisms to cope with drought that depend on the duration and intensity of the water deficit, and their responses occur at different temporal and spatial scales, from cell to whole tree level (Jones et al., 2002). One first response to soil drought is stomatal closure, an avoidance mechanism mediated by the hormone abscisic acid (Pantin et al., 2013). A main consequence of stomatal closure is the decrease in CO2 influx and assimilation, which can lead to carbon depletion. When transpiration is reduced by stomatal closure, the outgoing water vapour flux and the latent heat dissipation are also reduced. Stomatal closure thus induces an increase in leaf temperature with a risk of heat stress (Tardieu, 2005). However, efficiency of stomatal regulation is variable according to species and Tardieu and Simonneau (1998) have shown that plants display contrasting transpiration behaviours (isohydric vs anisohydric) in response to drought. At the intra-specific level, genetic variability of stomatal regulation has also been highlighted in apple (Massonnet et al., 2007; Liu et al., 2012) and grapevine (Marguerit et al., 2012; Coupel-Ledru et al., 2014).
As leaf or canopy temperature can be used as a proxy for stomatal conductance, thermal infrared (TIR) imagery appears as a powerful tool to reveal genetic variability of stomatal behaviour (Jones et al., 2009). Numerous indices have been developed to assess crop water stress from canopy surface temperature (T s) with data acquired in signal or imagery mode, from aerial platforms (satellites, aircrafts, unmanned aerial vehicles) or sensors installed directly in the field to observe crop canopies (White et al., 2012). T s minus air temperature (T a) is a raw variable that is easy to extract from images, but it is sensitive to rapid changes in environmental conditions such as radiative conditions, wind speed and air vapour pressure deficit (Maes and Steppe, 2012).
The presence of mixed soil/plant pixels is a recurring problem when TIR imagery is applied to phenotyping of heterogeneous covers (Hackl et al., 2012; Jones and Sirault, 2014). It is generally considered that using the vegetation surface temperature directly is risky, because the weight of mixed or soil image pixels yielded in porous plant covers can create a shift towards the soil surface temperature (Jackson et al., 1981). To overcome the limitations of environmental and soil influence on T s, Moran et al. (1994) developed a Water Deficit Index (WDI) based on the Vegetation Index−Temperature (VIT) trapezoid concept, which is applicable to field crops with varying contributions of bare soil in the aggregated thermal pixels. This index is particularly suitable for estimation of transpiration rates on heterogeneous vegetation cover. It has been successfully related to the soil moisture and to the plant midday stem water potential (Köksal, 2008; Virlet et al., 2014). Different authors indicated that the use of aerial vectors (ultralight aircraft or unmanned aerial vehicle) coupled with high resolution sensors enables to distinguish the individual trees within a plant grove, even in the TIR waveband where image resolution is low (Berni et al., 2009; Stagakis et al., 2012). Moreover, the intra-crown T s variability has also been used in tree crops as complementary indicator of moderate water stress effect (González-Dugo et al., 2012), confirming previous work that considered leaf temperature distribution as better indicator of stress than its average (Fuchs, 1990).
Apart from TIR imagery, plant cover can be characterized by different vegetation indices based on the combination of spectral reflectances measured in visible and near-infrared (NIR) wavebands (Zarco-Tejada et al., 2005). These indices can possibly be acquired by broadband commercial sensors (Lebourgeois et al., 2008). In the remotely-sensed image, reflectance in the Red band is affected by light absorption of leaf pigments (mainly chlorophyll a), while the NIR waveband is affected by the scattering in the medium (Zarco-Tejada et al., 2005). Therefore, vegetation indices computed from Red and NIR, such as the normalized difference vegetation index (NDVI), can be related to canopy structure and biomass production (Zarco-Tejada et al., 2005) and also considered as indicators of tree vigour. However, NDVI is sensitive to low chlorophyll concentration (Peng and Gitelson, 2011) and it also tends to saturation when leaf area index (LAI) is higher than 3 or 4. Two other indices only retrieved from visible bands were used: the visible atmospherically resistant index, VARI, which shows a better sensitivity to higher values of vegetation cover fraction (Gitelson et al., 2002) and the simple ratio pigment index, SRPI, which enables characterization of the crop nitrogen status, being sensitive to change in the pigment relative content (chlorophyll vs carotenoids) (Peñuelas et al., 1994, 1995).
Recent studies on field crops, e.g. wheat (Babar et al., 2006; Comar et al., 2012), maize (Cairns et al., 2012) and cotton (Andrade-Sanchez et al., 2014) assessed potentiality of vegetation indices to be used for large-scale phenotyping. More generally, plant phenotyping based on multispectral or hyperspectral imagery shows promise as a non-invasive method adapted for screening a wide range of individuals in a short period of time. Connecting genotype to phenotype on large datasets currently sustains the development of pheno mics (Furbank and Tester, 2011; Fiorani and Schurr, 2013).
To date, quantitative genetic analyses of tree features in fruit crops have mostly concerned disease resistance, yield and production regularity (Guitton et al., 2012; Celton et al., 2014), and plant architecture (Segura et al., 2008). Owing to low-throughput techniques, few studies on genetic determi ni sms of traits related to water use have been undertaken in these crops except recently in grapevine (Marguerit et al., 2012). Other perennials like forest trees have been compared in natural environments (Brendel et al., 2008) and controlled environments (e.g. Salix: Rönnberg-Wästljung et al., 2005; Populus: Street et al., 2006) to distinguish well-irrigated and water deficit conditions and to study the genetic/genomic bases of responses to drought and/or water use efficiency.
In this study, we assumed that a genetic analysis could be performed on an apple segregating population submitted to contrasting water regimes, considering different traits mainly issued from airborne multispectral imagery. An experiment was conducted in two successive growing seasons, during which image-based phenotypic variables and agronomic traits such as fruit production or trunk diameter (a proxy for tree vigour) were analysed for both well-watered and water-stress conditions, as well as the difference between the two for a given genotype. For each image-based variable, we considered the mean value of a representative tree crown zone and the variation within this zone, on which mean broad-sense heri tability was computed from genetic linear models. Using a genetic map, quantitative trait loci were detected. Altogether these results demonstrate the relevance of airborne imagery for high-throughput phenotyping of individual trees in the field for their response to water stress and provide the first demonstration that QTL detection could result from such methodology and plant descriptors.
Materials and methods
Field experiments and meteorological measurements
The apple tree population studied consisted of progeny derived from a ‘Starkrimson’×’Granny Smith’ cross, characterized by variability in tree vigour, architectural traits (Segura et al., 2008), biennial bearing (Guitton et al., 2012), hydraulic traits (Lauri et al., 2011) and stomatal regulation in response to vapour pressure deficit (Regnard et al., 2009). In February 2007, four replicates of 122 F1-hybrids and their two parents were grafted onto M9 rootstock and randomly planted in an experimental field at the INRA Melgueil experimental station (Diaphen platform, southeast of France, 43°36ʹ N, 03°58ʹ E). Plantation consisted of 10 rows oriented northwest–southeast, with 5×2 m planting distances. The orchard management was performed consistently with professional practices, throughout the study. Automated soil resistivity profiling conducted in March 2009 showed that the soil of the trial plot (at depths of 0−50cm and 50−100cm) could be considered spatially homogeneous for water-holding capacity, and this was confirmed by soil profile descriptions. The field plot was irrigated using a system of microsprayers located in the rows, with one emitter per tree. During summer, contrasting hydric regimes were established. Full irrigation was ensured in half of trees [two replicates per genotype, well-watered trees (WW)], while irrigation was withheld in the other half, resulting in progressive summer soil drought [two replicates per genotype, water-stressed trees (WS)] since the summer rainfall was negligible. Trees submitted to water deficit during summer were the same during the 2010 and 2011 seasons, and three dates per year were studied, representing various water regimes in order to disentangle genotypic and environmental effects in the tree response. Water regimes developed in WW and WS treatments are illustrated by the soil hydric potential mean values (Ψ soil, Table 1A). Micrometeorological data acquired at field included global radiation (R g), air temperature (T a), air relative humidity (HR), air vapour pressure deficit (VPD), wind speed (u) and rainfall (Table 1A).
Table 1.
(A) Environmental conditions in the field in apple experimental field during image acquisitions in 2010 and 2011: mean values (and SDs) for six dates (see text for detail)
R g, global radiation; T a, air temperature; HR, air relative humidity; VPD, air vapour pressure deficit; u, wind speed. Soil hydric potential (Ψ soil): average for six representative well-watered (WW) trees and water-stressed (WS) trees at 30 and 60cm depths. (B) Ultralight aircraft image acquisition system, cameras used and image settings, and original image resolution for each date of experiment.
| A | Variables | Units | Date 1 | Date 2 | Date 3 | Date 4 | Date 5 | Date 6 |
|---|---|---|---|---|---|---|---|---|
| Solar time | hh:mm | 11:40 | 10:40 | 09:50 | 09:50 | 10:00 | 09:20 | |
| R g | W m-2 | - | 782.20 (114.23) | 472.83 (33.89) | 770.67 (3.27) | 599.27 (102.85) | 705.00 (0.00) | |
| T° air | °C | 29.72 (0.12) | 28.08 (0.42) | 23.78 (0.30) | 26.91 (0.19) | 26.58 (0.33) | 26.85 (0.49) | |
| HR | % | 44.06 (1.44) | 32.97 (1.03) | 37.88 (2.57) | 58.72 (0.75) | 27.96 (0.33) | 31.80 (−1.86) | |
| VPD | kPa | 2.34 (0.04) | 2.55 (0.10) | 1.83 (0.11) | 1.47 (0.04) | 2.51 (0.06) | 2.41 (0.14) | |
| U | m s-1 | 2.01 (0.07) | 2.72 (0.26) | 1.86 (0.10) | 1.99 (0.36) | 1.73 (0.28) | 0.78 (0.32) | |
| Ψ soil WW | MPa | −0.065 (0.054) | −0.053 (0.028) | −0.066 (0.036) | −0.022 (0.012) | −0.046 (0.039) | −0.024 (0.036) | |
| Ψ soil WS | −0.099 (0.035) | −0.133 (0.017) | −0.172 (0.022) | −0.031 (0.021) | −0.078 (0.037) | −0.130 (0.048) | ||
| B | Date 1 | Date 2 | Date 3 | Date 4 | Date 5 | Date 6 | ||
| Flight altitude | 350 m | 330 m | 480 m | 300 m | 300 m | 300 m | ||
| Sensor | ||||||||
| RGB | Canon 400D | Canon 500 D | Canon 500 D | Canon 500 D | Canon 500 D | Canon 500 D | ||
| NIR | Canon 400D (+745nm filter) | Canon 500 D (+745nm filter) | Canon 500 D (+745nm filter) | Canon 500 D (+745nm filter) | Canon 500 D (+745nm filter) | Canon 500 D (+745nm filter) | ||
| TIR | FLIR B20HSV | FLIR B20HSV | FLIR B20HSV | FLIR B20HSV | FLIR B20HSV | FLIR B20HSV | ||
| Setting | ||||||||
| RGB | Sensibility | ISO 100 | ISO 100 | ISO 100 | ISO 100 | ISO 100 | ISO 100 | |
| Shutter speed | 1/1250 | 1/2000 | 1/2000 | 1/2000 | 1/2000 | 1/2000 | ||
| Aperture | F5 | F2.8 | F2.8 | F3.5 | F3.5 | F3.5 | ||
| NIR | Sensibility | 100 ASA | 100 ASA | 100 ASA | ISO 100 | ISO 100 | ISO 100 | |
| Shutter speed | 1/1250 | 1/2000 | 1/2000 | 1/2500 | 1/2000 | 1/2000 | ||
| Aperture | F5 | F2.8 | F2.8 | F3.5 | F3.5 | F3.5 | ||
| Initial pixel size (cm) | ||||||||
| RGB | 5*5 | 3*3 | 5*5 | 3*3 | 3*3 | 3*3 | ||
| NIR | 5*5 | 3*3 | 5*5 | 3*3 | 3*3 | 3*3 | ||
| TIR | 30*30 | 35*35 | 53*53 | 30*30 | 30*30 | 30*30 | ||
| Atmospherical correction for TIR image | No | No | No | Yes | Yes | Yes | ||
Image acquisitions
The image acquisition system from the ultra-light aircraft consisted of two commercial digital cameras (either Canon EOS 400D or 500D, with 10.1 and 15.1 Megapixel CMOS sensors, respectively, Table 1B) equipped with 35-mm lenses, and one FLIR B20HSV (FLIR Systems Inc., Wilsonville, USA) thermal infrared camera (320*240 matrix) (for details, see: Lebourgeois et al., 2008, 2012; Virlet et al., 2014). One camera acquired visible images in red, green and blue bands (RGB). The second was modified according to Lebourgeois et al. (2008, 2012) to obtain images in near-infrared (NIR). Three flights per year were performed during the summers of 2010 and 2011 (Table 1A, 1B). In 2010, flights were realized for low, intermediate and severe water constraints, respectively 8, 27 and 41d after the beginning of drought (Dates 1, 2 and 3). In 2011, the first flight (Date 4) occurred 17 d before the beginning of the drought period, before WW and WS differentiation, while the second and third flights (Dates 5 and 6) were performed respectively 14 and 34d after the beginning of the drought treatment. During the period of water deprivation (i.e. at Dates 1, 2, 3, 5 and 6) WS trees were not irrigated.
Spectral image preprocessing and indices computation
Image preprocessing was performed with Erdas Imagine 9.3 software (Intergraph Corporation, Huntsville, USA). Procedure of ortho-rectification for RGB and NIR images and radiometric normalization on invariant field targets between dates are fully described in Lebourgeois et al. (2008, 2012) and Virlet et al. (2014), as well as image geolocation. Thermal infrared images issued from the six acquisition dates were ortho-rectified on the base of both RGB and NIR images and geo-located as well. For each of the six dates, the difference between the surface and air temperature (hereafter referred to as TsTa) was obtained by subtracting from each pixel value of the TIR images the air temperature acquired at ground level. Spatial resolution of RGB and NIR images was lowered from initial resolution (c. 3−5cm) to that of TIR image (30cm). From RGB and NIR bands, three vegetation indices were computed: NDVI, VARI and SRPI (Table 2). NDVI and TsTa were combined to compute the water deficit index (WDI) as described in Virlet et al. (2014).
Table 2.
List of phenotypic variables and equations used
| Variables | Descriptions | Equations | Related to | References | |
|---|---|---|---|---|---|
| NDVI | Normalized Difference Vegetation Index | (NIR−R)/(NIR+R) | Cover fraction, vegetation density | Rouse et al., 1973
Zarco-Tejada et al., 2005 |
|
| VARI | Visible Atmospherical Resistant Index | (G–R)/(G+R) | Cover fraction, biomass production | Peng and Gitelson, 2011 | |
| SRPI | Simple Ratio Pigment Index | B/R | Nitrogen content, ratio carotenoid/chlorophyll total | Peñuelas et al., 1994, 1995
Lebourgeois et al., 2012 |
|
| TsTa | Air-surface temperature difference |
|
Transpiration rate | ||
| WDI | Water Deficit Index | Evapotranspiration | Moran et al., 1994
Virlet et al., 2014 |
||
| TCSA | Trunk Cross Sectional Area (mm2) | TC2/4π | Vigour, growth | ||
| NbFr | Fruit number per tree | Fruit biomass production | |||
| BmFr | Fruit biomass per tree (kg) | Fruit biomass production |
NIR, near infrared; R, red; B, blue; G, green; T max and T min, maximum and minimum surface temperatures; T s, surface temperature; T a, air temperature; TC, trunk circumference, mm.
For each tree, multispectral-based index values were extracted from a 60cm radius buffer zone containing the central upper part of the tree crown. From each buffer (12−16 pixels), mean and standard deviation, SD, were retrieved and considered as two complementary variables characterizing the vegetation response of individuals. As SD characterized the variation occurring inside the buffer zone, it indicated the degree of heterogeneity of the crown structure for the vegetation index and the variability of transpiration rates for the stress indices.
In planta measurements
Trunk circumference (TC) of each tree was tape-measured 15cm above the grafting point each year in February. On that basis, the trunk cross-sectional area (TCSA) considered as representative of tree vigour was calculated (Table 2). Fruits were harvested each year between 22 August and 2 September before the resumption of irrigation, irrespective of the real maturity picking date (September). The number of fruits per tree and the harvest fresh mass (kg per tree) were determined.
Data analyses
Statistical analyses were performed using R software v.2.13.2. (R Development Core Team, 2011). For each variable, phenotypic means were computed from each tree for (i) WW and WS conditions confounded, (ii) WW and WS considered separately and (iii) the difference between them, hereafter referred to as the differential index (DI). For example, the phenotypic mean value of NDVI indepen dent of water treatment is referred to as NDVI, while sdNDVI_WS refers to the phenotypic mean value of the SD in the WS condition.
For each variable, two mixed linear models were built. The first one was used to analyse the response of variables in both WW and WS trees. The second one was used to analyse the drought response of each genotype, through the DI obtained. The first mixed linear models included the irrigation modality (M), date (D) or year (Y), which were considered as fixed effects, while the genotype (G), the interactions between genotype and irrigation modality (G×M), and genotype and date or year (G×D or G×Y) were considered as random effects. For each variable, a selection of the best model was performed through minimization of the Bayesian Information Criterion (BIC). For the DI, effects considered in the mixed linear model were the same as mentioned above, but without M and G×M. For each trait, best linear unbiased predictors (Blups) were extracted for estimation of the G effect, which was considered as independent from the irrigation modality and the date (or year) of experimentation and is hereafter referred to as G-Blup. The Blups corresponding to G×M and G×D effects were computed for each irrigation regime (WW- or WS-Blup), and date (or year) considered separately.
For each variable, when G and interaction effects were included, broad-sense heritability of the mean (h 2 b) was estimated as follows:
where n is the number of trees per genotype (two in the present case), and n M the number of irrigation modalities (two in the present case). When a G×D (or G×Y) effect was included in the model, the denominator also integrated G×D (or G×Y) variances and was divided by the number of dates (six) or years (two). The residual variance was divided by the product of the number of trees per genotype and per irrigation modality and the number of dates (or years). This led us to estimate the broad-sense heritability value of the mean of phenotypic values which accounts for the repetitions of each genotype that were present in the experimental plot, according to Gallais (1989). Phenotypic variables were considered heritable if h 2 b values were greater than 0.2.
QTL mapping
The QTL analysis was performed using means and Blups extracted per genotype (G-means, G-Blups) for each variable. A consensus genetic map of STK and GS, which integrated 177 SSR and SNP genetic markers, was used for QTL mapping (Guitton et al., 2012). QTL analyses were carried out using MapQTL®6.0 (Van Ooijen, 2009). First, a permutation test was performed to determine the loga rithm of the odds (LOD) threshold at which a QTL was declared significant, using a genome-wide error rate of 0.05 with 1000 permutations of the data (Van Ooijen, 2009). In a second step, an interval mapping analysis was carried out with a step size of 1 cM, with a LOD score higher than the threshold. Finally, the nearest marker to each QTL peak was selected as a cofactor to perform a multiple QTL mapping (MQM) (Van Ooijen, 2009). Each significant QTL was characterized by its LOD score, its percentage of explained phenotypic variation, and its confidence interval (in cM) corresponding to a LOD score drop of 1 or 2 on either side of the likelihood peak. QTLs that showed clearly overlapping confidence intervals, close LOD peaks and similar allelic effects, were considered to co-localize.
When a QTL was detected with at least two cofactors, models considering markers and their interactions as cofactors were cons tructed using a backward procedure under R software v2.13.2. Models were selected based on the minimum Akaike Information Criterion values (AIC). In the selected model, the global percentage of phenotypic variation (global R2) was then estimated. When one marker was derived from only one of the parents, the nearest maker included in the QTL and deriving from both parents was chosen. The location of QTLs on the genetic was finally illustrated using MapChart® (Voorrips, 2001).
Results
Variance analysis and heritability
Models selected for vegetation indices were similar whether means or SDs were considered (Table 3A). All vegetation indices were significantly impacted by G, D and M effects. For NDVI, the model included only the G×M interaction, whereas for VARI, SRPI, TsTa and WDI variables G×M and G×D interactions were also taken into account. Concerning tree vigour and fruit production, the models selected included G and Y effects. For TCSA only the G×M interaction was retained in the mixed linear model, while the G×Y interaction was retained for fruit number (NbFr) and both G×M and G×Y interactions were retained for fruit yield biomass (BmFr). For all DI variables (Table 3B) the models selected included G and D (or Y) effects. G×D was also included for sdVARI_DI, sdSRPI_DI, sdTsTa_DI while G×Y was included for NbFr_DI only. It is noticeable that the random interaction effects (G×M and/or G×D) were generally lower than the G effects.
Table 3.
Description of fixed (M, modality; D, date; Y, year) and random (G, genotype) effects used in selected mixed linear models
For each variable, models related to phenotypic values in (A) WW and WS, and (B) models related to DI (differential index: difference of the variable between WS and WW trees) were built. Percentage variances of each random effect and of the residuals (Res), and broad-sense heritability values (h 2 b) are indicated.
| A | Fixed effect | Random effect | % variances | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | D | Y | G | G×M | G×D | G×Y | G | G×M | G×D | G×Y | Res | h 2 b | ||
| NDVI | x | x | - | x | x | - | - | 35 | 19 | - | - | 46 | 0.62 | |
| sdNDVI | x | x | - | x | x | - | - | 21 | 8 | - | - | 71 | 0.50 | |
| VARI | x | x | - | x | x | x | - | 23 | 6 | 9 | - | 61 | 0.77 | |
| sdVARI | x | x | - | x | x | x | - | 9 | 5 | 10 | - | 76 | 0.56 | |
| SRPI | x | x | - | x | x | x | - | 19 | 18 | 4 | - | 59 | 0.60 | |
| sdSRPI | x | x | - | x | x | x | - | 17 | 8 | 5 | - | 70 | 0.69 | |
| TsTa | x | x | - | x | x | x | - | 15 | 18 | 5 | - | 62 | 0.55 | |
| sdTsTa | x | x | - | x | x | x | - | 7 | 9 | 4 | - | 79 | 0.49 | |
| WDI | x | x | - | x | x | x | - | 11 | 7 | 5 | - | 76 | 0.59 | |
| sdWDI | x | x | - | x | x | x | - | 3 | 6 | 6 | - | 85 | 0.31 | |
| TCSA | x | - | x | x | x | - | - | 51 | 15 | - | - | 34 | 0.76 | |
| NbFr | x | - | x | x | - | - | x | 25 | - | - | 37 | 37 | 0.52 | |
| BmFr | x | - | x | x | x | - | x | 12 | 8 | - | 32 | 49 | 0.31 | |
| B | Fixed effect | Random effect | % variances` | |||||||||||
| M | D | Y | G | G×M | G×D | G×Y | G | G×M | G×D | G×Y | Res | h 2 b | ||
| NDVI_DI | - | x | - | x | - | - | - | 44 | - | - | - | 56 | 0.61 | |
| sdNDVI_DI | - | x | - | x | - | - | - | 19 | - | - | - | 81 | 0.32 | |
| VARI_DI | - | x | - | x | - | - | - | 19 | - | - | - | 81 | 0.33 | |
| sdVARI_DI | - | x | - | x | - | x | - | 13 | - | 8 | - | 79 | 0.62 | |
| SRPI_DI | - | x | - | x | - | - | - | 35 | - | - | - | 65 | 0.52 | |
| sdSRPI_DI | - | x | - | x | - | x | - | 17 | - | 7 | - | 75 | 0.70 | |
| TsTa_DI | - | x | - | x | - | - | - | 34 | - | - | - | 66 | 0.50 | |
| sdTsTa_DI | - | x | - | x | - | x | - | 19 | - | 14 | - | 67 | 0.70 | |
| WDI_DI | - | x | - | x | - | - | - | 17 | - | - | - | 83 | 0.29 | |
| sdWDI_DI | - | x | - | x | - | - | - | 9 | - | - | - | 91 | 0.17 | |
| TCSA_DI | - | - | x | x | - | - | - | 35 | - | - | - | 65 | 0.52 | |
| NbFr_DI | - | - | x | x | - | - | x | 13 | - | - | 1 | 86 | 0.38 | |
| BmFr_DI | - | - | x | x | - | - | - | 17 | - | - | - | 83 | 0.29 | |
Broad-sense heritability h 2 b for both WW and WS (Table 3A) showed medium to high values (0.49 to 0.77) except for sdWDI and BmFr, whose heritability was low (0.31). For DI variables (Table 3B), fairly high h 2 b values (0.50 to 0.70) were found for NDVI, sdVARI, SRPI, sdSRPI, TsTa, sdTsTa and TCSA. In contrary, h 2 b for the other variables, including WDI_DI, was much lower (0.17 to 0.38) than that found for WW and WS. Moreover, higher h 2 b were found in VARI_DI, SRPI_DI, and TsTa_DI for SD values than for mean values.
Correlations between variables
High pairwise positive correlations were observed between NDVI, VARI and SRPI for the G-mean, WW, WS and for DI (Pearson’s r from 0.45 to 0.88, Table 4) even though lower r values were found between VARI and SRPI for G-mean and WW (Tables 4A, 4B). These three variables were significantly and negatively correlated with sdNDVI and sdSRPI for the G-mean, WW, WS and for DI (from −0.46 to −0.66, Table 4), despite much lower correlation being found between sdNDVI and these variables for DI (from −0.06 to −0.32, Table 4D). Moreover, sdNDVI, sdVARI and sdSRPI presented pairwise positive correlations for the G-mean and WW (0.33 to 0.88, Table 4A, 4B). For WS and DI, only sdSRPI was significantly correlated with sdNDVI and sdVARI (0.74 and 0.42 respectively, Table 4C, 4D). WDI was highly and positively correlated with sdWDI (0.49 to 0.52). The trunk diameter variable, TCSA, presented generally moderate to high positive correlations with NDVI, VARI and SRPI. The highest correlation was observed with NDVI, either for the G-mean, WW, WS or for DI (0.55 to 0.67). Variables relative to fruit production, NbFr and BmFr, were highly intercorrelated (0.75 to 0.85), and a high positive correlation of these variables with TCSA was also observed, particularly for BmFr (from 0.50 to 0.55). Moreover, BmFr was positively and more highly correlated to NDVI, VARI and SRPI (0.23 to 0.52) than NbFr (0.13 to 0.32) for the G-mean, WW and WS.
Table 4.
Genetic Pearson’s r correlations between NDVI, VARI, SRPI, WDI variables, and TCSA, NbFr and BmFr, (A) for genetic means of two water regimes confounded, (B) for well-watered trees, (C) for water-stressed trees and (D) for differential index DI. r values in bold type were significant for P<0.001
| NDVI | VARI | SRPI | sdNDVI | sdVARI | sdSRPI | WDI | sdWDI | TCSA | NbFr | BmFr | NDVI | VARI | SRPI | sdNDVI | sdVARI | sdSRPI | WDI | sdWDI | TCSA | NbFr | BmFr | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | ||||||||||||||||||||||
| NDVI | 1 | 1 | |||||||||||||||||||||
| VARI | 0.61 | 1 | 0.74 | 1 | |||||||||||||||||||
| SRPI | 0.80 | 0.28 | 1 | 0.64 | 0.26 | 1 | |||||||||||||||||
| sdNDVI | −0.58 | −0.55 | −0.49 | 1 | −0.66 | −0.60 | −0.43 | 1 | |||||||||||||||
| sdVARI | 0.03 | 0.15 | 0.00 | 0.36 | 1 | −0.16 | −0.31 | −0.13 | 0.42 | 1 | |||||||||||||
| sdSRPI | −0.64 | −0.62 | −0.54 | 0.88 | 0.33 | 1 | −0.46 | −0.46 | −0.52 | 0.66 | 0.57 | 1 | |||||||||||
| WDI | 0.15 | 0.06 | 0.02 | 0.16 | −0.16 | 0.12 | 1 | −0.03 | 0.21 | −0.25 | 0.10 | −0.30 | 0.02 | 1 | |||||||||
| sdWDI | 0.03 | 0.06 | −0.14 | 0.39 | 0.21 | 0.27 | 0.52 | 1 | −0.07 | 0.01 | −0.22 | 0.08 | −0.11 | −0.01 | 0.52 | 1 | |||||||
| TCSA | 0.67 | 0.33 | 0.57 | −0.36 | 0.03 | -0.35 | 0.13 | 0.09 | 1 | 0.63 | 0.29 | 0.45 | −0.33 | 0.01 | −0.28 | 0.14 | 0.07 | 1 | |||||
| NbFr | 0.32 | 0.32 | 0.25 | −0.28 | 0.20 | −0.24 | −0.21 | −0.12 | 0.39 | 1 | 0.29 | 0.28 | 0.13 | −0.27 | 0.13 | −0.14 | −0.06 | −0.06 | 0.39 | 1 | |||
| BmFr | 0.40 | 0.35 | 0.32 | −0.27 | 0.22 | −0.23 | −0.20 | −0.04 | 0.50 | 0.85 | 1 | 0.48 | 0.38 | 0.23 | −0.37 | −0.01 | −0.13 | −0.03 | −0.04 | 0.50 | 0.85 | 1 | |
| C | D | ||||||||||||||||||||||
| NDVI | 1 | 1 | |||||||||||||||||||||
| VARI | 0.71 | 1 | 0.74 | 1 | |||||||||||||||||||
| SRPI | 0.88 | 0.51 | 1 | 0.82 | 0.45 | 1 | |||||||||||||||||
| sdNDVI | −0.64 | −0.53 | −0.58 | 1 | −0.19 | −0.06 | −0.32 | 1 | |||||||||||||||
| sdVARI | 0.06 | -0.10 | 0.10 | 0.25 | 1 | −0.05 | −0.29 | 0.02 | −0.12 | 1 | |||||||||||||
| sdSRPI | −0.58 | −0.60 | −0.60 | 0.74 | 0.25 | 1 | −0.51 | −0.52 | −0.57 | 0.25 | 0.42 | 1 | |||||||||||
| WDI | −0.15 | 0.10 | −0.12 | 0.23 | −0.30 | 0.10 | 1 | −0.16 | 0.18 | −0.20 | 0.07 | −0.34 | 0.03 | 1 | |||||||||
| sdWDI | −0.05 | 0.04 | −0.06 | 0.35 | 0.07 | 0.17 | 0.49 | 1 | −0.09 | 0.01 | −0.10 | 0.06 | −0.12 | 0.01 | 0.51 | 1 | |||||||
| TCSA | 0.60 | 0.31 | 0.54 | −0.31 | 0.05 | −0.34 | 0.08 | 0.07 | 1 | 0.55 | 0.48 | 0.30 | 0.13 | −0.06 | −0.26 | −0.13 | −0.10 | 1 | |||||
| NbFr | 0.29 | 0.29 | 0.27 | −0.23 | 0.22 | −0.26 | −0.28 | −0.13 | 0.39 | 1 | 0.55 | 0.49 | 0.33 | 0.22 | −0.08 | −0.19 | −0.03 | −0.04 | 0.52 | 1 | |||
| BmFr | 0.52 | 0.38 | 0.39 | −0.23 | 0.03 | −0.10 | −0.21 | 0.01 | 0.50 | 0.85 | 1 | 0.48 | 0.35 | 0.27 | 0.29 | −0.04 | −0.07 | −0.08 | −0.01 | 0.55 | 0.75 | 1 |
QTL detection
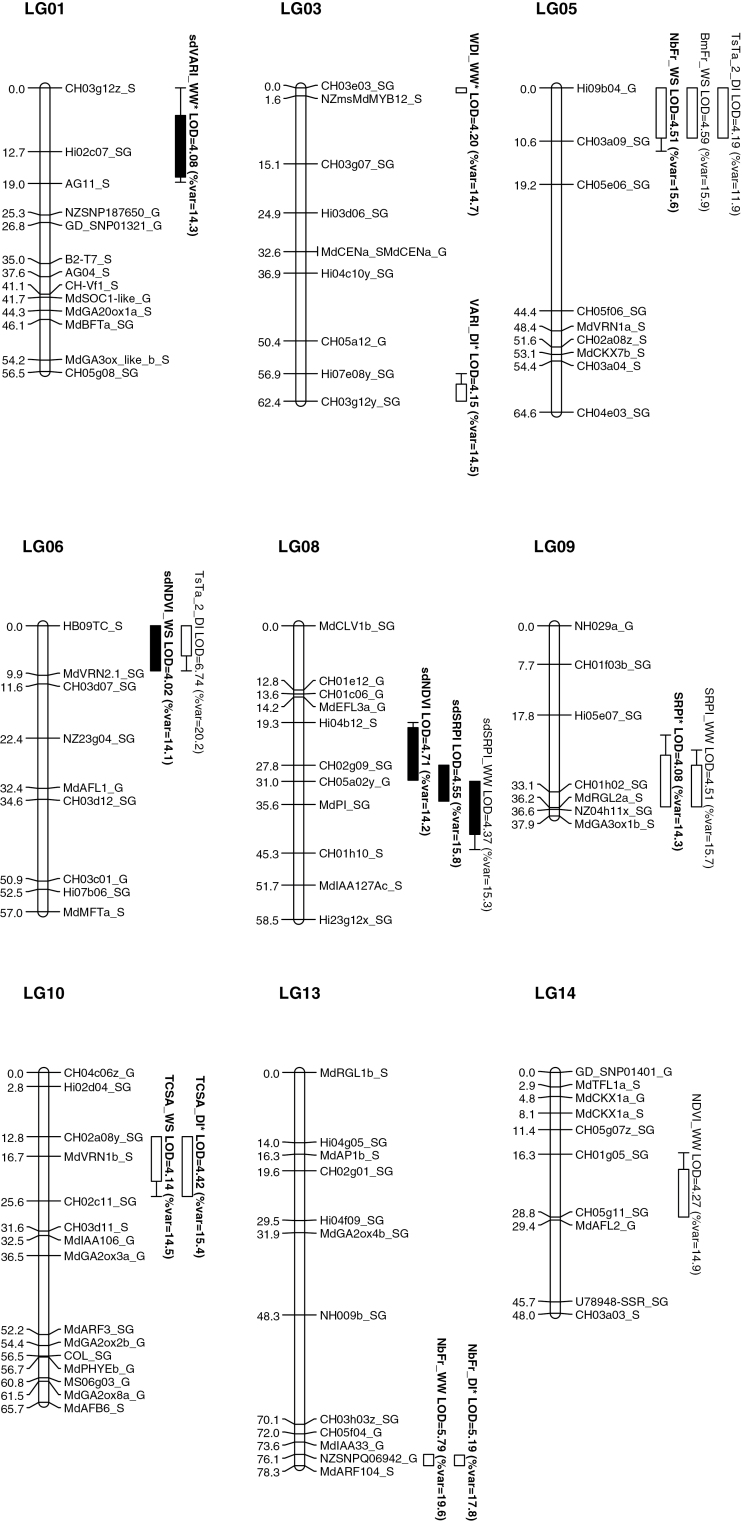
Seventy-four QTLs were detected, mapping over 16 of the 17 linkage groups (LGs) of the consensus STK×GS genetic map. As 56 of these QTLs were found only at specific dates, they are not detailed in the following text. The complete list of QTLs detected is presented in Supplementary Table S1 and Supplementary Fig. S1. The results exposed hereafter (Fig. 1; Table 5) are focusing on the 18 most reliable QTLs that were mapped over nine LGs. These QTLs were detected for G-Blup or for the G-mean, and in some cases for both, whatever the date.
Fig. 1.
Genomic positions of the QTLs detected on the consensus ‘Starkrimson’×’Granny Smith’ (STK×GS) map. QTLs are represented by boxes, in which length represents the LOD-1 confidence interval and extended lines represent the LOD-2 confidence interval. Boxes relative to QTLs for mean values of variables are in white, and those relative to QTLs for standard deviations SD are in black. QTL detected for G-Blups are in bold type and * stand for QTLs detected for G-Blups and G-means.
Table 5.
Main QTLs detected on the consensus STK×GS map by multiple QTL mapping (MQM) for variables NDVI, VARI, SRPI, TsTa, WDI, TCSA, NbFr and BmFr in well-watered (WW) and/or water-stress (WS) conditions and for the differential index DI (WS−WW)
QTLs detected for G-Blups are in bold type and * stand for QTLs detected for G-Blups and G-means.
| Traits | LG a | LOD b | R 2 c | R 2 global d | Position | Cofactor | Allelic effect e | Af | Am | D |
|---|---|---|---|---|---|---|---|---|---|---|
| NDVI_WW | 14 | 4.27 | 0.149 | 26.318 | CH05g11_SG | D, Af, Am | -7.80E-03 | 1.96E-03 | -9.11E-03 | |
| sdNDVI | 08 | 4.71 | 0.142 | 29.763 | CH02g09_SG | Af | 3.15E-03 | 1.60E-04 | -9.93E-04 | |
| sdNDVI_WS | 06 | 4.02 | 0.141 | 1 | HB09TC_S | Af | -1.33E-03 | 3.08E-04 | 9.00E-05 | |
| sdVARI_WW* | 01 | 4.08 | 0.143 | 12.749 | Hi02c07_SG | D | 5.00E-05 | -1.75E-06 | -1.45E-04 | |
| VARI_DI* | 03 | 4.15 | 0.145 | 62.417 | CH03g12y_SG | D, Af, Am | 5.71E-04 | -3.94E-04 | -6.76E-04 | |
| SRPI* | 09 | 4.08 | 0.143 | 34.112 | CH01h02_SG | Af, Am, D | 2.23E-03 | 1.97E-03 | 1.86E-03 | |
| SRPI_WW | 09 | 4.51 | 0.157 | 34.112 | CH01h02_SG | Am, Af, D | 3.14E-03 | 3.32E-03 | 1.32E-03 | |
| sdSRPI | 08 | 4.55 | 0.158 | 29.763 | CH05a02y_G | Af | 1.74E-03 | -9.55E-05 | -9.76E-04 | |
| sdSRPI_WW | 08 | 4.37 | 0.153 | 35.571 | MdPI_SG | Af | 3.51E-03 | 9.96E-04 | 1.49E-04 | |
| TsTa_2_DI | 05 | 4.19 | 0.119 | 0 | Hi09b04_G | Am, Af | -1.48E-01 | -1.64E-01 | -5.78E-02 | |
| 06 | 6.74 | 0.202 | 0.208 | 0 | HB09TC_S | Af, Am | -2.47E-01 | 1.63E-01 | -7.77E-02 | |
| WDI_WW* | 03 | 4.2 | 0.147 | 0 | CH03e03_SG | Am, Af | 3.89E-03 | -4.50E-03 | 3.80E-05 | |
| TCSA_WS | 10 | 4.14 | 0.145 | 15.783 | MdVRN1b_S | Af, D | -7.03E+01 | 4.05E+00 | 2.90E+01 | |
| NbFr_WW | 13 | 5.79 | 0.196 | 77.149 | MdARF104_S | Af, Am | -1.07E+01 | -1.03E+01 | 4.52E+00 | |
| NbFr_WS | 05 | 4.51 | 0.156 | 0 | Hi09b04_G | Af, D | 2.31E+00 | -5.11E-01 | -1.19E+00 | |
| BmFr_WS | 05 | 4.59 | 0.159 | 0 | Hi09b04_G | Af, D | 1.89E+00 | -4.14E-01 | -1.19E+00 | |
| TCSA_DI* | 10 | 4.42 | 0.154 | 16.687 | MdVRN1b_S | Af | -1.57E+02 | -1.17E+01 | 6.40E+01 | |
| NbFr_DI* | 13 | 5.19 | 0.178 | 77.149 | MdARF104_S | Af, Am | 2.65E+01 | 2.55E+01 | -8.87E+00 |
a Linkage group.
b Maximum LOD score value.
c Percentage of phenotypic variation explained by the QTL.
d Percentage of phenotypic variation explained by QTL when it was detected with at least 2 cofactors.
e Female (Af) and male (Am) additive effect estimated as [(μac+μad)–(μbc+μbd)]/4 and [(μac+μbc)–(μad+μbd)]/4 respectively; dominance (D) estimated as = [(μac+μbd)–(μad+μbc)]/4, where μac, μbc, μad, and μbd are the estimated phenotypic means associated with each of the four possible genotypic classes ac, bc, ad and bd, deriving from an <ab×cd> cross.
QTLs for traits related to vegetation indices NDVI, VARI and SRPI.
For G-Blup of NDVI and SRPI variables, three QTLs were detected independently of the environment (water regime or date): two QTLs concerned sdNDVI and sdSRPI and presented a common zone located on LG08. They explained 14.2% and 15.8% of the variability respectively, and were both characterized by female allelic effects. One QTL for SRPI was detected on LG09 and explained 14.3% of the variability. It showed female, male and dominance effects.
Four QTLs were detected for specific G-means of WW on four different LGs. Two of these QTLs, related to NDVI_WW and SRPI_WW, were detected on LG14 and LG09, respectively. They explained 14.9% and 15.7% of the varia bility, and both of them resulted both from female, male and dominance effects. Two other QTLs, related to sdVARI_WW and sdSRPI_WW, were mapped on LG01 and LG08, and resulted from dominance and female effects, respectively. The QTL for sdVARI_WW was also identified for G-Blup.
For WS, one QTL was identified for sdNDVI_WS at the top of LG06. It explained 14.1% of the variability and mainly resulted from female effects. For DI, one QTL was detected for VARI_DI and mapped at the bottom of LG03 for both G-Blup and G-mean. It explained 14.5% of the variability and resulted from female, male and dominance effects.
QTLs for traits related to tree foliage transpiration
One QTL was detected for WDI in WW condition, at the top of LG03 for both G-Blup and G-mean. It explained 14.7% of the variability and was characterized mainly by female and male effects. Finally, two QTLs were detected for TsTa_2_DI (at Date 2) on LG05 and LG06, respectively. The global linear model indicated an interaction between these two QTLs, which together explained 20.8% of the variability. They mainly resulted from female and male effects. The QTL which mapped on LG06 co-localized with the QTL for sdNDVI_WS.
QTLs for traits related to tree vigour and fruit production
Two QTLs were detected in relation to the tree vigour (TCSA), the first one for TCSA_WS, and the second one for TCSA_DI. Both mapped on LG10 and co-localized near the MdVRN1b_S marker. These QTLs explained 14.5% and 15.4% of the variability, respectively, and mostly resulted from female allelic effects. For fruit number, one QTL was detected for WW (NbFr_WW) at the bottom of LG13. It explained 19.6% of the variability. Another QTL was detected for NbFr_DI at the same position, explaining 17.8% of variability. These two QTLs mainly resulted from male and female allelic effects. Finally, two QTLs were found at the top of LG05 for NbFr_WS and BmFr_WS. They explained 15.6% and 15.9% of the variability, respectively, and both resulted from female and dominance effects. Interestingly, these two QTLs co-localized with the QTL identified for TsTa_2_DI.
Discussion
Variability of the phenotypic traits
Due to, on the one hand, the changes in environmental conditions and imagery flight parameters and, on the other, the difficulty to apply comparable water constraints from one year to the next, the analysis of the population behaviour undertaken through linear mixed models did take into account the large variability of phenotypic traits (Supplementary Table S2). Among vegetation indices, NDVI appeared the most stable vegetation index, independent of the environment and acquisition conditions (no G×D interaction) whereas other spectral indices chosen proved to be sensitive to drought, with a G×D interaction revealed for VARI, SRPI, TsTa and WDI. By contrast, the absence of G×D interaction for G-means for the DI variables suggested that the use of the difference between water-stressed and well-irrigated trees somewhat smoothed out the inter-date variations.
To our knowledge, this study is the first one to make use of spectral indices to assess genetic variability in perennial plants in response to drought, and to analyse the related determinisms. As a consequence, comparisons in the ensuing discussion are often referring to results obtained in annual crops. In the present study, the vegetation indices used were extracted from a buffer zone of the same size located in the central zone of each tree crown. As such, the value of these vegetation indices must be considered as more related to the vegetation cover fraction and biomass production rather than to the foliage physiological properties. Whatever the traits considered, either relative to vegetation or to transpiration, moderate to high values of broad-sense heritability were found, indicating an interesting contribution of multispectral imagery for genetic analysis of these traits in a tree population. Concerning the vegetation indices, our results were consistent with those found in annual crops, where high heritability values were also found (e.g. in wheat: Babar et al., 2006; in cotton: Andrade-Sanchez et al., 2014). Heritability values found for agronomic traits in apple, TCSA, NbFr and BmFr, were in the same order of magnitude as those for spectral indices. However, TCSA, which is an integrative variable, exhibited the highest h2b value whereas the lower values for NbFr and BmFr likely resulted from the influence of Y, and Y and M effects, respectively. When heritability was computed for DIs, values of most variables stayed high except for WDI_DI, probably because of the composite nature and complexity of this trait. Concerning TsTa and TsTa_DI, high heritability values were found, consistent with previous results found in wheat (Mason et al., 2013; Rebetzke et al., 2013).
Trait correlations, QTL detection and co-localization
Positive correlations between the vegetation indices, TCSA and fruit production were observed. In particular NDVI exhibited the highest correlation with the trunk diameter, TCSA, and with fruit yield biomass, BmFr. As well as NDVI, TCSA is generally related to plant size, leaf area and light interception (González-Talice et al., 2012), which suggests that NDVI is a good indicator of vigour and development in apple trees. However, QTLs that were found for vegetation indices, fruit yield and TCSA did not co-localize, and this indicated that genetic determinisms controlling these traits likely differ. Moreover, while the three vegetation indices presented high and positive intercorrelations, related QTLs did not co-localize. Indeed, QTLs for NDVI and SRPI were mostly detected on two different LGs: LG14 and LG09, respectively. This could be explained by the spectral bands used: the former vegetation index, making use of NIR for computation, is more related to canopy structure than the second one, only computed from visible bands that are more related to light absorption (Zarco-Tejada et al., 2005; Lebourgeois et al., 2012). Otherwise, the QTL found for VARI_DI, which highlights differences between WS and WW tree responses, suggests that drought could affect the radiation absorption capacity, by lowering the fractional vegetation cover.
Intra-crown variations of vegetation indices, sdNDVI and sdSRPI, presented high and negative correlations with means of NDVI and SRPI, and moderate and negative correlations with TCSA. This indicates that the highest density of vegetation was less variable than the lowest, but also that the vegetation heterogeneity was lower where tree vigour increased. As the intra-crown variability could be an indicator of branching patterns or leaf clumpiness (Da Silva et al., 2014), this needs further investigation. Similarly, as WDI and sdWDI were posi tively correlated, this could be due to spatial heterogeneity in stomatal conductance within the tree crown in response to moderate stress (González-Dugo et al., 2012). The QTL detected for sdNDVI_WS could be attributed to the variation of leaf rolling over genotypes in response to drought, a phenomenon also observed in other species and limiting plant cover fraction (e.g. maize: Lu et al., 2012). Furthermore, sdNDVI and sdSRPI were strongly intercorrelated and QTLs for these variables co-localized at the middle of the LG08 (Table 5; Supplementary Table S2). The location of these QTLs also matched with a QTL zone for traits involved in gas exchange, xylem conductance and fruit production on the STK×GS population (Regnard et al., 2009; Lauri et al., 2011, Guitton et al., 2012). These co-localizations could be explained by an increased capacity of the plant to transport water, carbohydrates and sugar to the growing organs, as suggested by Lauri et al. (2011). Nonetheless, these co-localizations might also be explained by a pleiotropic effect of these QTLs, or by clustering of functionally related genes (Cai and Morishima, 2002). Gene clusters have already been reported in apple for various traits such as resistance to pathogens (Xu and Korban 2002; Baldi et al., 2004). However, discrimina ting between linked and pleiotropic QTLs was not practicable in the present study, considering the limited population size and the density of the genetic map available.
Among QTLs detected for fruit production, two of them, NbFr_WW and NbFr_DI, were located at the bottom of LG13. This zone was adjacent to the one found for biennial bearing on the same STK×GS population (Guitton et al., 2012). Otherwise, a year-specific QTL, NbFr_11, was detected at the same location as NbFr_WW and NbFr_DI (Supplementary Fig. S2), which could confirm the importance of this zone in the control of biennial bearing. In addition, QTLs for fruit production in WS trees (NbFr_WS and BmFr_WS) and for leaf temperature (TsTa_2_DI) co-localized on LG05. Although matching for those traits was only temporary, i.e. when the difference between WW and WS treatments was considered at Date 2 for TsTa, it illustrates the negative relationship between yield and leaf temperature. Indeed, as stated by Naor and Girona (2012), a positive link between plant yield and evapotranspiration is generally observed, and the increase in leaf temperature (here in WS trees) is an indicator of lower transpiration rate and likely of lower carbon assimilation. Reduction of stomatal conductance in WS trees could likely be invoked for limitation of fruit production in this case because water constraint was severe (no irrigation occurred during the summer period). Such a causal relation between water withholding and its effect on yield reduction is nevertheless not straightforward in fruit trees (Bassett, 2013), particularly when a moderate water deficit occurs during stages of low fruit growth (Goodwin and Boland, 2002).
Although one QTL was detected on LG03 for WDI_WW, no co-localization with fruit production variables was observed. Moreover, genetic correlations of WDI with fruit production variables during the two years of study (Table 4) were not significant, whereas significant negative correlations of this variable were observed with NbFr and BmFr (r value of −0.53 and −0.55 respectively, data not shown) when only year 2011 was considered. This negative correlation in a specific year could be attributed to the propensity to biennial fruit-bearing already shown on this progeny (Guitton et al., 2012).
It has been recently shown that wild germplasm of Malus is exhibiting a certain range of tolerance to drought (Bassett, 2013), but available information on the commercial parental genotypes used in the present study is scarce. González-Talice et al. (2012) suggested that the ‘Granny Smith’ cultivar has a lower hydraulic conductance and/or stomatal conduc tance than that of the ‘Gala’ cultivar, indicating a stronger sensitivity of the former to high evaporative demand and/or soil drought. In contrast, the ‘Starkrimson’ cultivar is well known for its photosynthetic efficiency (Yang and Wang, 1993), while its response to drought is not documented. In the present study, prevailing female allelic effects were found for almost all variables, suggesting a larger polymorphism and allelic contrast in the ‘Starkrimson’ parent than in ‘Granny Smith’. ‘Starkrimson’ could thus provide interesting alleles for adaptation to drought, even though other Malus genetic backgrounds need to be explored in the next future.
Partial homology between LGs that has been described in the ‘Golden Delicious’ apple genome (Velasco et al., 2010) led us to examine homologous regions in which main QTL zones were detected. The median zone on LG08, around CH02g09 in which many QTLs were detected, matches the top of LG15, above CH03b6, where a QTL was detected for SRPI_1_DI. Similarly, the QTL zone on the top of LG03 detected for NDVI_4_WW corresponds to the region on LG11 on which a QTL was detected for sdNDVI_1_DI. By contrast, the QTL zones found in LG05 and LG10 for NbFr_WS and NbFr_11_DI, respectively, were located on chromosomal fragments that are inverted on their respective chromosome and therefore did not matched. Similarly, the QTLs detected on LG06 that were located either above or below CH03d07 were compared to those detected on LG14, without finding evident homology in those regions. These findings suggest that further investigations of QTL zones homologies will be required, along with identification of candidate genes in the zones of highest interest.
To summarize, this work is an important step in the study of tree field phenotyping for response to abiotic stress. It confirmed—if proof was needed—the strong potential of remote sensing tools as a method for screening a large panel of genotypes. Airborne imagery proved relevant to acquire simultaneous information on a tree population, notably for characterizing transpiration behaviour at the individual tree scale as a result of images yielded in the thermal infrared domain. Indices derived from high-resolution airborne field imagery appeared to be highly heritable and enabled detection of a large number of QTLs, for vegetation and water stress indices, and the tree response to water deficit. This study opens future avenues for analysis of candidate genes related to foliage response to drought, and may contribute to future selection of new plant woody plant material bred for its response to drought and/or water use efficiency.
Supplementary data
Supplementary data is available at JXB online.
Supplementary Fig. S1. Total QTLs detected on the consensus ‘Starkrimson’ × ‘Granny Smith’ (STK×GS) genetic map.
Supplementary Table S1. Total list of QTLs detected for all phenotypic variables in well-watered and/or water-stress conditions and for the differential index.
Supplementary Table S2. Values of traits (mean and SD) investigated in well-watered or water-stressed conditions and for the differential index.
Acknowledgments
We thank S. Feral, T. Colonges, R. Marty and A. Jolivot for their contributions to the establishment of the experiment and the field measurements. We also thank the Avion Jaune® Company, V. Lebourgeois (Cirad) and S. Labbé (IRSTEA) for their expertise in airborne imagery. The PhD scholarship of Nicolas Virlet was granted by Montpellier SupAgro and the Languedoc-Roussillon Region.
Glossary
Abbreviations:
- Blup
best linear unbiased predictors
- BmFr
fruit yield biomass per tree per year
- DI
differential index
- G-Blup
Blups extracted per genotype
- G-mean
mean extracted per genotype
- LAI
leaf area index
- NbFr
fruit number per tree per year
- NDVI
normalized difference vegetation index
- NIR
near-infrared
- QTL
quantitative trait locus
- RGB
red, green and blue bands
- SRPI
simple ratio pigment index
- TCSA
trunk cross-sectional area
- TIR
thermal infrared
- VARI
visible atmospherically resistant index
- WDI
water deficit index
- WS
water-stressed
- WW
well-watered.
References
- Andrade-Sanchez P, Gore MA, Heun JT, Thorp KR, Carmo-Silva AE, French AN, Salvucci ME, White JW. 2014. Development and evaluation of a field-based high-throughput phenotyping platform. Functional Plant Biology 41, 68–79. [DOI] [PubMed] [Google Scholar]
- Babar MA, van Ginkel M, Klatt A, Prasad B, Reynolds MP. 2006. The potential of using spectral reflectance indices to estimate yield in wheat grown under reduced irrigation. Euphytica 150, 155–172. [Google Scholar]
- Baldi P, Patocchi A, Zini E, Toller C, Velasco R, Komjanc M. 2004. Cloning and linkage mapping of resistance gene homologues in apple. Theoretical and Applied Genetics 109, 231–239. [DOI] [PubMed] [Google Scholar]
- Bassett C. 2013. Water use and drought response in cultivated and wild apples. In: Vahdati K, Leslie Charles, eds. Abiotic Stress—Plant Responses and Applications in Agriculture . Croatia, Rijeka: InTech, 249–275. [Google Scholar]
- Berni JAJ, Zarco-Tejada PJ, Sepulcre-Cantó G, Fereres E, Villalobos F. 2009. Mapping canopy conductance and CWSI in olive orchards using high resolution thermal remote sensing imagery. Remote Sensing of Environment 113, 2380–2388. [Google Scholar]
- Brendel O, Le Thiec D, Scotti-Saintagne C, Bodénès C, Kremer A, Guehl JM. 2008. Quantitative trait loci controlling water use efficiency and related traits in Quercus robur L. Tree Genetics & Genomes 4, 263–278. [Google Scholar]
- Cai HW, Morishima H. 2002. QTL clusters reflect character associations in wild and cultivated rice. Theoretical and Applied Genetics 104, 1217–1228. [DOI] [PubMed] [Google Scholar]
- Cairns JE, Sanchez C, Vargas M, Ordoñez R, Araus JL. 2012. Dissecting maize productivity: Ideotypes associated with grain yield under drought stress and well-watered conditions. Journal of Integrative Plant Biology 54, 1007–1020. [DOI] [PubMed] [Google Scholar]
- Celton JM, Kelner JJ, Martinez S, Bechti A, Touhami AK, James MJ, Durel CÉ, Laurens F, Costes E. 2014. Fruit self-thinning: A trait to consider for genetic improvement of apple tree. PLoS ONE 9, e91016. doi:10.1371/journal.pone.0091016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comar A, Burger P, de Solan B, Baret F, Daumard F, Hanocq JF. 2012. A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results. Functional Plant Biology 39, 914–924. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55, 2447–2460. [DOI] [PubMed] [Google Scholar]
- Coupel-Ledru A, Lebon É, Christophe A, Doligez A, Cabrera-Bosquet L, Péchier P, Hamard P, This P, Simonneau T. 2014. Genetic variation in a grapevine progeny (Vitis vinifera L. cvs Grenache×Syrah) reveals inconsistencies between maintenance of daytime leaf water potential and response of transpiration rate under drought. Journal of Experimental Botany 65, 6205–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva D, Han LQ, Faivre R, Costes E. 2014. Influence of the variation of geometrical and topological traits on light interception efficiency of apple trees: sensitivity analysis and metamodelling for ideotype definition. Annals of Botany 114, 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Schurr U. 2013. Future scenarios for plant phenotyping. Annual Review of Plant Biology 64, 267–291. [DOI] [PubMed] [Google Scholar]
- Fuchs M. 1990. Infrared measurement of canopy temperature and detection of water stress. Theoretical and Applied Climatology 42, 253–261. [Google Scholar]
- Furbank RT, Tester M. 2011. Phenomics—technologies to relieve the phenotyping bottleneck. Trends in Plant Science 16, 635–644. [DOI] [PubMed] [Google Scholar]
- Gallais A. 1989. Théorie de la sélection en amélioration des plantes . Paris: Masson. [Google Scholar]
- Gitelson AA, Kaufman YJ, Stark R, Rundquist D. 2002. Novel algorithms for remote estimation of vegetation fraction. Remote Sensing of Environment 80, 76–87. [Google Scholar]
- González-Dugo V, Zarco-Tejada P, Berni JAJ, Suárez L, Goldhamer D, Fereres E. 2012. Almond tree canopy temperature reveals intra-crown variability that is water stress-dependent. Agricultural and Forest Meteorology 154, 156–165. [Google Scholar]
- González-Talice J, Yuri JA, Lepe V, Hirzel J, del Pozo A. 2012. Water use in three apple cultivars from the second season to sixth season in a drainage lysimeter. Scientia Horticulturae 146, 131–136. [Google Scholar]
- Goodwin I, Boland AM. 2002. Scheduling deficit irrigation of fruit trees for optimizing water use efficiency. In: Deficit Irrigation Practices . Water Reports No 22. Rome: FAO, 67–79. [Google Scholar]
- Guitton B, Kelner JJ, Velasco R, Gardiner SE, Chagné D, Costes E. 2012. Genetic control of biennial bearing in apple. Journal of Experimental Botany 63, 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl H, Baresel JP, Mistele B, Hu Y, Schmidhalter U. 2012. A comparison of plant temperatures as measured by thermal imaging and infrared thermometry. Journal of Agronomy and Crop Science 198, 415–429. [Google Scholar]
- Hamdy A, Ragab R, Scarascia-Mugnozza E. 2003. Coping with water scarcity: water saving and increasing water productivity. Irrigation and Drainage 52, 3–20. [Google Scholar]
- IPCC. 2014. Summary for Policymakers. In: Edenhofer O, Pichs-Madruga R, Sokona Y, et al, eds. Climate Change 2014, Mitigation of Climate Change. Contribution of Working Group III to the fifth assessment report of the Intergovernmental Panel on Climate Change . Cambridge & New York: Cambridge University Press. [Google Scholar]
- Jackson RD, Idso SB, Reginato RJ, Pinter PJ. 1981. Canopy temperature as a crop water stress indicator. Water Resource Research 17, 1133–1138. [Google Scholar]
- Jones HG, Serraj R, Loveys BR, Xiong L, Wheaton A, Price AH. 2009. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Functional Plant Biology 36, 978–989. [DOI] [PubMed] [Google Scholar]
- Jones HG, Sirault XRR. 2014. Scaling of thermal images at different spatial resolution: the mixed pixel problem. Agronomy 4, 380–396. [Google Scholar]
- Jones HG, Stoll M, Santos T, de Sousa C, Chaves MM, Grant OM. 2002. Use of infrared thermography for monitoring stomatal closure in the field: application to grapevine. Journal of Experimental Botany 53, 2249–2260. [DOI] [PubMed] [Google Scholar]
- Köksal ES. 2008. Irrigation water management with water deficit index calculated based on oblique viewed surface temperature. Irrigation Science 27, 41–56. [Google Scholar]
- Lauri PÉ, Gorza O, Cochard H, Martinez S, Celton JM, Ripetti V, Lartaud M, Bry X, Trottier C, Costes E. 2011. Genetic determinism of anatomical and hydraulic traits within an apple progeny. Plant, Cell & Environment 34, 1276–1290. [DOI] [PubMed] [Google Scholar]
- Lebourgeois V, Bégué A, Labbé S, Houlès M, Martiné JF. 2012. A light-weight multi-spectral aerial imaging system for nitrogen crop monitoring. Precision Agriculture 13, 525–541. [Google Scholar]
- Lebourgeois V, Bégué A, Labbé S, Mallavan B, Prévot L, Roux B. 2008. Can commercial digital cameras be used as multispectral sensors? A crop monitoring test. Sensors 8, 7300–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Cheng L, Ma F, Zou Y, Liang D. 2012. Growth, biomass allocation, and water use efficiency of 31 apple cultivars grown under two water regimes. Agroforestry Systems 84, 117–129. [Google Scholar]
- Lu YL, Xu J, Yuan ZM, et al. 2012. Comparative LD mapping using single SNPs and haplotypes identifies QTL for plant height and biomass as secondary traits of drought tolerance in maize. Molecular Breeding 30, 407–418. [Google Scholar]
- Maes WH, Steppe K. 2012. Estimating evapotranspiration and drought stress with ground-based thermal remote sensing in agriculture: a review. Journal of Experimental Botany 63, 4671–4712. [DOI] [PubMed] [Google Scholar]
- Marguerit E, Brendel O, Lebon E, Van Leeuwen C, Ollat N. 2012. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytologist 194, 416–429. [DOI] [PubMed] [Google Scholar]
- Mason RE, Hays DB, Mondal S, Ibrahim AMH, Basnet BR. 2013. QTL for yield, yield components and canopy temperature depression in wheat under late sown field conditions. Euphytica 194, 243–259. [Google Scholar]
- Massonnet C, Costes E, Rambal S, Dreyer E, Regnard JL. 2007. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Annals of Botany 100, 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178, 719–739. [DOI] [PubMed] [Google Scholar]
- Moran MS, Clarke TR, Inoue TR, Vidal A. 1994. Estimating crop water deficit using the relation between surface-air temperature and spectral vegetation index. Remote Sensing of Environment 49, 246–263. [Google Scholar]
- Naor A, Girona J. 2012. Apple. In: Steduto P, Hsiao TC, Fereres E, Raes D, eds. Crop yield response to water . Rome: FAO; (Food and Agriculture Organization of the United Nations), 332–345. [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B. 2013. The dual effect of abscisic acid on stomata. New Phytologist 197, 65–72. [DOI] [PubMed] [Google Scholar]
- Peng Y, Gitelson AA. 2011. Application of chlorophyll-related vegetation indices for remote estimation of maize productivity. Agricultural and Forest Meteorology 151, 1267–1276. [Google Scholar]
- Peñuelas J, Filella I, Lloret P, Muñoz F, Vilajeliu M. 1995. Reflectance assessment of mite effects on apple trees. International Journal of Remote Sensing 16, 2727–2733. [Google Scholar]
- Peñuelas J, Gamon JA, Fredeen AL, Merino J, Field CB. 1994. Reflectance indices associated with physiological changes in nitrogen- and water-limited sunflower leaves. Remote Sensing of Environment 48, 135–146. [Google Scholar]
- Rebetzke GJ, Rattey AR, Farquhar GD, Richards RA, Condon AG. 2013. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Functional Plant Biology 40, 14–33. [DOI] [PubMed] [Google Scholar]
- Regnard JL, Segura V, Merveille N, Durel CÉ, Costes E. 2009. QTL analysis for leaf gas exchange in an apple progeny grown under atmospheric constraints. Acta Horticulturae 814, 369–374. [Google Scholar]
- Rönnberg-Wästljung AC, Glynn C, Weih M. 2005. QTL analyses of drought tolerance and growth for a Salix dasyclados × Salix viminalis hybrid in contrasting water regimes. Theoretical and Applied Genetics 110, 537–549. [DOI] [PubMed] [Google Scholar]
- Rouse JW, Hass RH, Schell JA, Deering DW. 1973. Monitoring vegetation systems in the Great Plains with ERTS . Third ERTS Symposium, Vol. 1: NASA SP-351, 309–317. [Google Scholar]
- Segura V, Cilas C, Costes E. 2008. Dissecting apple tree architecture into genetic, ontogenetic and environmental effects: mixed linear modelling of repeated spatial and temporal measures. New Phytologist 178, 302–314. [DOI] [PubMed] [Google Scholar]
- Stagakis S, González-Dugo V, Cid P, Guillén-Climent ML, Zarco-Tejada PJ. 2012. Monitoring water stress and fruit quality in an orange orchard under regulated deficit irrigation using narrow-band structural and physiological remote sensing indices. ISPRS Journal of Photogrammetry and Remote Sensing 71, 47–61. [Google Scholar]
- Street NR, Skogström O, Sjödin A, Tucker J, Rodríguez-Acosta M, Nilsson P, Jansson S, Taylor G. 2006. The genetics and genomics of the drought response in Populus . The Plant Journal 48, 321–341. [DOI] [PubMed] [Google Scholar]
- Tardieu F. 2005. Plant tolerance to water deficit: physical limits and possibilities for progress. Comptes Rendus Geoscience 337, 57–67. [Google Scholar]
- Tardieu F, Simonneau T. 1998. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. Journal of Experimental Botany 49, 419–432. [Google Scholar]
- Van Ooijen J. 2009. MapQTL 6, Software for the Mapping of Quantitative Traits in Experimental Populations of Diploid Species . The Netherlands, Wageningen: Kyazma BV. [Google Scholar]
- Velasco R, Zharkikh A, Affourtit J, et al. 2010. The genome of the domesticated apple (Malus× domestica Borkh.). Nature Genetics 42, 833–839. [DOI] [PubMed] [Google Scholar]
- Virlet N, Lebourgeois V, Martinez S, Costes E, Labbé S, Regnard JL. 2014. Stress indicators based on airborne thermal imagery for field phenotyping a heterogeneous tree population for response to water constraints. Journal of Experimental Botany 65, 5429–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorrips RE. 2001. MapChart Version 2.0: Windows Software for the Graphical Presentation of Linkage Maps and QTLs. Wageningen: Plant Research International. [DOI] [PubMed] [Google Scholar]
- White JW, Andrade-Sanchez P, Gore MA, et al. 2012. Field-based phenomics for plant genetics research. Field Crops Research 133, 101–112. [Google Scholar]
- Xu ML, Korban SS. 2002. A cluster of four receptor-like genes resides in the Vf locus that confers resistance to apple scab disease. Genetics 162, 1995–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wang Z. 1993. Studies on the photosynthetic properties of young Red Delicious and Starkrimson apple trees. Journal of Fruit Science 10, 1–5. [Google Scholar]
- Zarco-Tejada PJ, Berjón A, López-Lozano R, Miller JR, Martín P, Cachorro V, González MR, de Frutos A. 2005. Assessing vineyard condition with hyperspectral indices: leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sensing of Environment 99, 271–287. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.