Abstract
Functional and structural alterations in brain connectivity associated with brain ischemia have been extensively studied. However, the mechanism whereby local ischemia in deep brain region affect brain functions is still unknown. Here, we first established a mini-stroke model by infusion of endothelin-1 (ET-1) into the dorsal hippocampus or the lateral amygdala, and then investigated how these mini-infarcts affected brain functions associated with these regions. We found that rats with ET-1 infusion showed deficit in recall of contextual fear memory, but not in learning process and recall of tone fear memory. In novel object task, ET-1 in the hippocampus also eliminated object identity memory. ET-1 in the lateral amygdale affected acquisition of fear conditioning and disrupted retention of tone-conditioned fear, but did not impair retention of contextual fear. These findings suggest that ET-1-induced mini-infarct in deep brain area leads to functional deficits in learning and memory associated with these regions.
Keywords: endothelin-1, dorsal hippocampus, lateral amygdala, fear conditioning
Introduction
Occlusions in the blood supply to the brain lead to ischemic stroke[1]. Focal stroke is associated with abnormal synaptic activity, morphologic plasticity and neurologic impairments[2-3]. Several rodent focal ischemia models are available to study the pathology of stroke[4]. The most commonly used one is middle cerebral artery occlusion (MCAO)[5] by insertion of an intraluminal suture[6-7]. Mini-ischemia can be produced by coagulation of the MCA, embolism or photothrombosis. In photothrombosis, a photosensitive dye (i.e., Rose Bengal) is systemically injected into animals where part of the skull has been thinned[8]. It can be used to produce small infarcts in any cortical region[9], but it seemed unable to induce deep infarct. In MCAO or MCA embolism animal models, clots can undergo spontaneous thrombolysis, thereby inducing multiple infarcts and high mortality[4,10]. To study memory and emotion linked neural circuit remodeling after stroke, it is desirable to establish ischemia models with infarct in deep brain regions (i.e., the hippocampus and the amygdala). Endothelin-1 (ET-1), a potent vasoconstrictor[11], occludes local blood flow to levels that cause ischemia injury[12]. ET-1 can be stereotaxically injected into brain regions of interest to constrict local vessels. Lesion size can be adjusted by altering the concentration or volume of ET-1 to achieve reproducible or permanent injury. It has been reported that ET-1 injections into the hippocampus caused significant loss of hippocampal tissues[13-14]. However, it is still unclear that if ET-1 injections cause deficits in function associated with these brain regions.
In the present study, we infused ET-1 to dorsal hippocampus and lateral amygdala to produce focal deep brain ischemia and then test whether ET-1 can create reliable learning and memory deficits. In earlier studies, lesions caused by pharmacological method (ibotenic acid\APV) or adeno-associated virus injection (expression of tetanus neurotoxin) have confirmed the corresponding dysfunction of the hippocampus[15-20] or lateral amygdala[21-24]. Our results demonstrated that mini-stroke in these regions caused deficits in learning and memory.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats weighing approximately 300 g at the time of surgery used in this study were housed in environmentally controlled conditions (23 °C±1 °C, a 12 hours light/dark cycle with the light cycle from 6:00 to 18:00 and the dark cycle from 18:00 to 6:00). The rats were randomly divided into groups. Before testing, rats were handled for about 4-5 minutes daily for 4 consecutive days. The study protocol was approved by the local institutional review board at the authors’ affiliated institutions and animal studies were carried out in accordance with the established institutional guidelines regarding animal care and use. Animal welfare and the experimental procedures were carried out strictly in accordance with the Guide for Care and Use of Laboratory Animals.
Surgical procedures
Rats were initially anesthetized with 3% chloral hydrate, and then 1% chloral hydrate was used to maintain anesthesia. Temperature was maintained from 35.5°C to 37.5°C throughout the surgery. SD rats were placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). A gauge syringe was targeted for placement directly into the hippocampus (anteroposterior: 4.50 mm relative to the bregma; mediolateral: ±4.0 mm; dorsoventral: 3.5 mm from skull) and lateral amygdala (anteroposterior: 3.14 mm relative to bregma; mediolateral: ± 6.2 mm; dorsoventral: 5.4 mm from skull). Rats in the experimental group received unilateral (for magnetic resonance imaging, MRI) or bilateral (for behavior test) infusion of ET-1 (15 pmoL, 0.8 µL dissolved in sterile saline; Sigma-Aldrich, St Louis, MO, USA). Rats in the sham group received bilateral infusion of saline with the same surgical procedure. The volume and flow rates (10 nl/s, 0.8 µL) were controlled by a microsyringe pump controller (WPI, Florida, USA). The syringe was left in place for 5 minutes after injection. Rats were returned to their home cage after surgery.
Magnetic resonance imaging
Animals underwent focal ischemia using ET-1 and were examined 24 hours (for sample image, but 48 hours for behavior testing of animals' injected locations) after ET-1 administration. MRI was performed using 7.0-T MR imager (Eclipse, Philips Medical Systems, Best, the Netherlands) equipped with a 21-cm bore magnet as previously studied[25]. MRI sequences were T2-weighted spin-echo sequences [repetition time (msec)/echo time (msec), 500/17.9]. The rats were anesthetized with 1.5% halothane in a 70:30 mixture of N2O:O2. The imaging slice was placed 3 mm anterior to the bregma using as landmark the anterior commissure obtained at the midline. Images were obtained with a matrix size of 128×128, two measurements acquired.
Open field task
The apparatus for the open-field consisted of a 100-cm-square open translucent plastic box with 50 cm high. The box was illuminated by ambient fluorescent ceiling lights. We monitored it by an automated video motility system including a camera and tracing software Any-Maze (Stoelting Company, Wood Dale, IL, USA). In the open-field test, rats were placed individually near the center of the box, and their movements were recorded by video for 5 minutes. The imaginary central zone was defined as a 50×50 cm square in the middle of the observation area. We quantified the time spent in center squares and surrounding area, total moving distance, average speed, and number of times that rats reared on their hind paws.
Fear conditioning
Rats were handled for 3 minutes on each of 5 consecutive days before experiments. Training chambers was positioned inside a sound-attenuating isolation box and included a square chamber with 2 plexiglas sides and 2 aluminum sides and a removable controlled electrifiable grid floor (Coulbourn Instruments, Whitehall, PA, USA). We conditioned rats in a previously optimized standard, combined context and tone conditioned fear protocol in which the rat received 6 shocks as described previously[26].
On day 1, rats were habituated to training background for 12 minutes with no additional operation. On day 2, rats were allowed adapt to the chamber for 4 minutes before the onset of training blocks. Then, rats were presented with 6 consecutive training blocks, each consisting of a 20-second baseline, followed by a 20-second, 1KHz and 80 dB tone (conditioned stimulus, CS), followed by a 2-second scrambled 1.2 mA foot shock (unconditioned stimulus, US), followed by a 38-second inter-trial interval (ITI). Rats were returned to their home cage immediately after training.
Two hours later, rats were tested for contextual and tone fear conditioning. Rats were firstly placed in the training chamber for 3 minutes to evaluate contextual fear conditioning, after which they were returned to home cage for 30 minutes. Testing for tone fear conditioning took place in a novel chamber, which differed from the training chamber in texture (solid plastic floor rather than metal grid floor), visual cues (solid walls with corrugated board on each side opposed to 2 clear Plexiglas and 2 plain aluminum sides) and odor. Rats were allowed to acclimate to the novel chamber for 3 minutes prior to tone presentation. To assess the percentage of freezing in the novel chamber, rats that showed indiscriminate freezing (>50%) in the novel chamber by the end of the adaption period were excluded from further tone fear conditioning analysis[27]. After 3-minute acclimation period, rats were tested with 4 blocks consisting of a 20-second baseline followed by a 20-second, 1 KHz and 80 dB tone, and then followed by a 40-second inter-trial interval.
Percentage of freezing was quantified by automated motion detection software (FreezeFrame, coulbourn instruments, Whitehall, PA, USA). A single set of motion detection parameters was optimized for rats in this study and used for all analyses.
Novel object recognition task
An open-field box (50×50×40 cm) was constructed from high density polyethylene board. The box was illuminated as described in the method of open field testing. Before training, rats were individually habituated by driving them to explore the open-field box for 5 minutes per session for 2 sessions. During training stage, 2 novel objects (red regular tetrahedron made by corrugated paper) were positioned in the open-field 30 cm away from each other and rats was allowed to explore for 5 minutes, respectively. By tracing the head of rat, exploring behavior was identified when the head of rats facing the object within 3 cm away or any part of the rats excluding the tail touch the object. The time spent for exploring each object was recorded. Rats were returned to their home cages immediately after training. During retention test (2 hours later), the rats were positioned back into the same open-field box again. However, one of the above used objects during training was replaced by a novel object (blue regular hexahedron made by polypropylene). All objects were balanced and were emotionally neutral. Moreover, the open-field and objects were cleaned by 10% alcohol after each session to avoid possible odorant cues. Discrimination ratio, an index of the time spent to explore the familiar object and the novel object were normalized by the time exploring the familiar and over the total time spent exploring both objects, was used to measure recognition memory.
Data analysis
Data were expressed as mean ± SEM. Differences between groups were compared using independent-sample t-test (two populations) and ANOVA post-hoc comparisons. Two-way ANOVA (treatment×interval) was performed to detect significant differences between groups, and significant differences at some intervals were established with a Bonferroni post-hoc test. In all other cases, a two-tailed Student’s t-test was used. Differences were considered significant when P was <0.05.
Results
ET-1 induces mini-stroke in the dorsal hippocampus
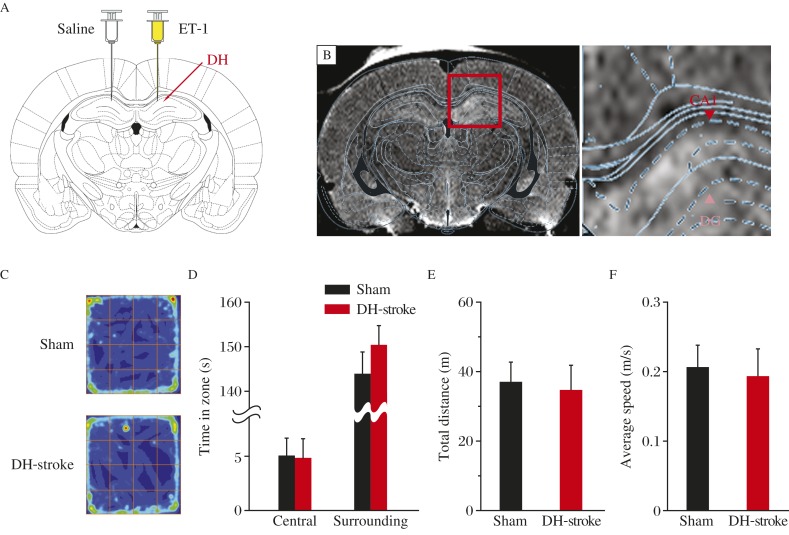
To study ET-1 induced lesion in the hippocampus, we infused ET-1 into the dorsal hippocampus (Fig. 1A)[28].We used MRI to examine and confirm the establishment of the mini-stroke model. Twenty-four hours after injection, we used T2-weighted spin-echo MRI sequences to confirm the generation of mini-stroke (Fig. 1B)[25,29]. The site of local infarct shown by MRI matched ET-1 injection site in the dorsal hippocampus. In contrast to the recession of mobility caused by the damage that results from MCAO), rats receiving ET-1 showed normal spontaneous activity in open field test (Fig. 1C), moving distance (Fig. 1D) and average speed (Fig. 1E) compared to those receiving sham treatment.
Fig. 1. Rats with ET-1-induced DH mini-stroke showed normal spontaneous activity.
A: Rats were injected with ET-1 in one side DH and saline in the other side DH. B: Representative T2-weighted MR imaging shows infarct injury of DH as a result of ET-1 injected into this position. C-F: Performance of the open-field task shows similar color-coded time-in-location map (C), the time in the central zone and surrounding zone (D), total moving distance (E) and average speed (F), indicating the same active mode for both treatment groups. In all the animals for behavioral test, ET-1 was injected bilaterally. In (C), same blue-to-red scale is used for each map.
ET-1-induced mini-stroke in the dorsal hippocampus impairs recall of contextual fear memory
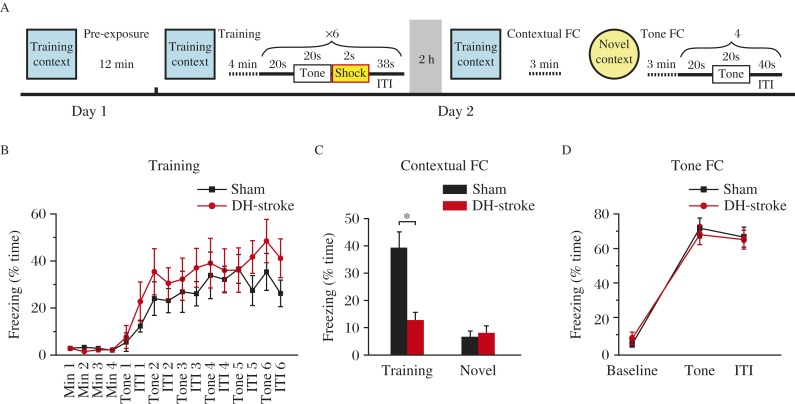
We investigated the acquisition of the association memory between fear and tone. We trained adult male rats (sham and DH-stroke treatment) using a tone fear conditioning protocol (Fig. 2A). Intact hippocampal function is critical for eliciting contextual fear conditioning (freezing in response to training context exposure), but not for tone (freezing in response to tone presentation) fear conditioning in response to this training protocol[24,30-31]. In contrast, rats with ET-1 induced mini-stroke in the dorsal hippocampus rats learned fear memory as well as controls did (Fig. 2B). When tested 2 hours after training, dorsal hippocampus-stroke resulted in a dramatic reduction in retention of contextual fear memory (Fig. 2C) and normal tone fear memory (Fig. 2D). These data provide evidence that ET-1 in the dorsal hippocampus induced hippocampal lesion and impaired hippocampus-dependent fear learning in adult rats.
Fig. 2. ET-1 induced mini-stroke in the dorsal hippocampal (DH) region impairs recall of contextual fear memory.
A: Schematic of fear conditioning protocol. B: Rats with ET-1-induced dorsal hippocampal mini-stroke exhibit normal freezing percentage in a 6-trial training protocol. ITI: inter train interval. C: Freezing during 3 minutes of testing in the training context (*P<0.05, two-tailed Student's t-test) but not in a novel context (P=0.470, two-tailed Student's t-test) is significantly impaired in ET-1 infused rats (sham, n=9, DH-stroke, n=9). D: Tone conditioning is unaffected in ET-1 injected rats 2 hours after training (no significant main effect of treatment, F(1, 42)=1.991, P=0.878).
ET-1-induced mini-stroke in the dorsal hippocampus impairs recognition memory
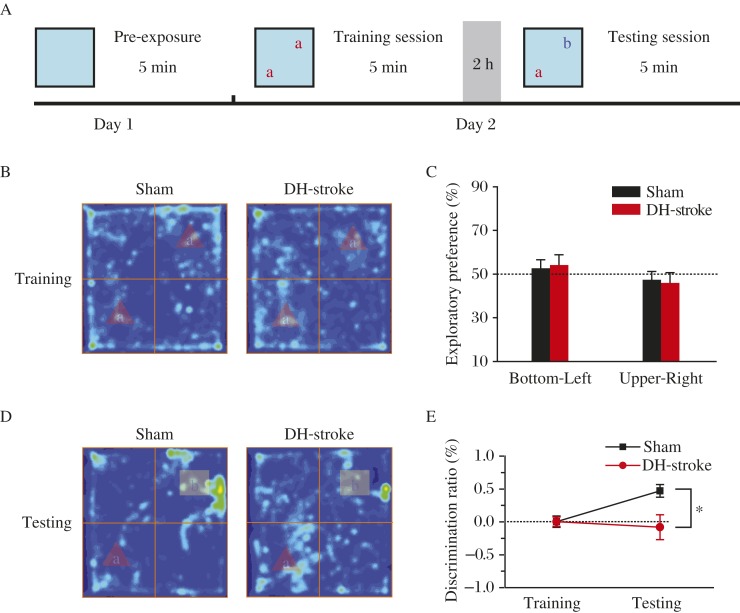
Then, we used the novel-object-recognition task (Fig. 3A) to assess recognition memory, which also requires the hippocampus[32-33], in ET-1-infused animals. During training session, 2 treatments had no significant difference in time that rats spent in exploring the 2 novel objects (as shown by hot map and exploratory preference; Fig. 3B and 3C), indicating that both groups of rats had the same motivation and curiosity to explore the objects. During the testing stage, one of the familiar objects used in the training was replaced by a novel object, and rats were allowed to explore for 5 minutes. At the 2-hour retention test, rats of the ET-1 infusion dorsal hippocampus-stroke groups showed dramatic reduction in time exploring the novel objects compared to the sham group, indicated by the color-coded map (Fig. 3D). In contrast, preference towards the familiar object was similar between the 2 groups (as shown by the discrimination ratio, Fig. 3E). This indicated that ET-1 in the dorsal hippocampus impaired capability of rats to identify and recognize novel objects.
Fig. 3. ET-1 infusion in the dorsal hippocampus impairs recognition memory.
A: Schematic of novel object recognition task. B: Color-coded time-in-location map in the training session. The same objects were positioned bottom-left and upper-right. C: The amount of time spent in exploring the two objects was the same for treatments. Dotted line represents performance at chance (50%). (D) Color-coded map and (E) discrimination ratio in testing session show the impairment of object discriminate memory in the ET-1 infused rats (sham, n=9, DH-stroke, n=9, *P<0.05, two-tailed Student's t-test). The same blue-to-red scale is used for each map. DH: dorsal hippocampus.
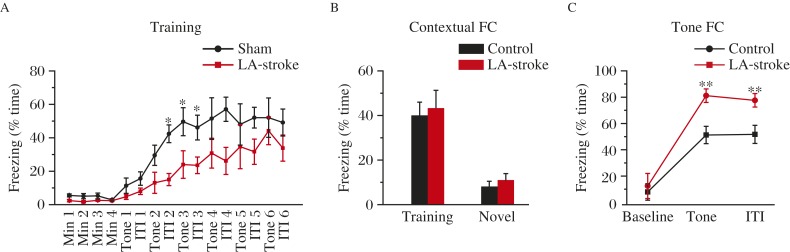
ET-1-induced mini-stroke in the lateral amygdala impairs acquisition and retention of tone-conditioned fear memory
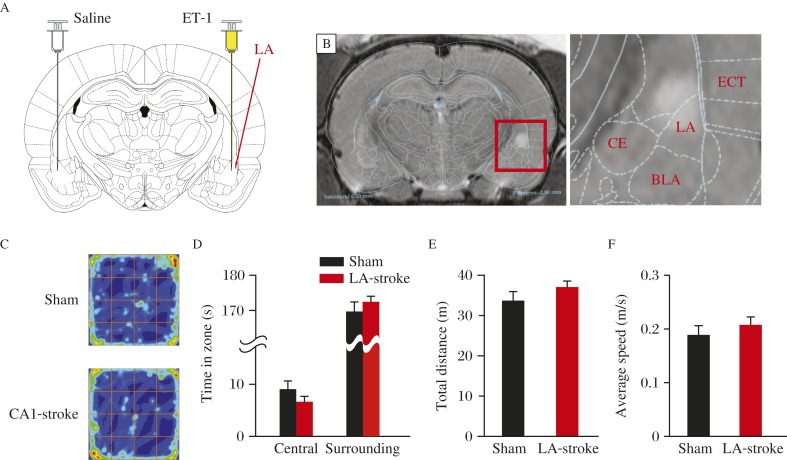
We further studied whether and how ET-1 induced mini-stroke in the lateral amygdala would affect brain functions associated with this subcortical region. Twenty-four hours after ET-1 injection, we used T2-weighted MRI to examine the region of ischemia (Fig. 4A and B). Rats with bilateral lateral amygdala mini-stroke showed normal exercise capacity (Fig. 4C-4F) as the precondition of the following test. The lateral amygdala is imperative for obtaining the association of cued message and emotional memory, and is also critical for tone fear conditioning, but not for contextual fear memory[23-24]. We trained rats of both groups using the protocol as shown in Fig. 2A. Compared to sham rats, lateral amygdala-stroke mice learned the association between shock and tone/context more tardily indicated by a delayed increase in freezing percentage during 6 training trials (Fig. 5A). Two hours later, rats were positioned in training context, neither sham nor lateral amygdala-stroke rats showed significant freezing behavior in the training context (Fig. 5B). Then, rats were placed in a novel context and presented with only the tone. Lateral amygdala -stroke rats showed a dramatic reduction in retention of ton fear memory (Fig. 5C). These results indicated that rats with bilateral lateral amygdala mini-stroke show deficits in tone components of aversive conditioning with contextual fear memory kept intact.
Fig. 4. ET-1 induced mini-stroke in lateral amygdala (LA).
A: Rats were injected with ET-1 in one side of LA and saline in the other side of LA. B: Representative T2-weighted MR imaging showing the infarct injury as a result of ET-1 injection into LA. C-F: Performance of the open-field task shows similar color-coded time-in-location map (C), the time in the central zone and surrounding zone (D), total moving distance (E) and average speed (F), indicating the same active mode for both treatment groups.
Fig. 5. ET-1 in the lateral amygdala (LA) impairs acquisition and retention of tone-conditioned fear memory.
A: LA-stroke rats exhibit delayed freezing to a six-trial training protocol as described in Fig. 2A, but comparable freezing levels to the sham group by the end of the training session. A two-way repeated-measures ANOVA revealed a significant treatments × interval interaction, P<0.05, F(1, 132)=2.828 (sham, n=8, LA-stroke, n=8). *P=0.05, Bonferroni post-hoc test, sham vs. LA-stroke. C: Freezing during the training context (P=0.680) and a novel context (P=0.317, two-tailed Student's t-test) is intact in LA-stroke rats. D: Tone conditioning during the four tests block is impaired in LA-stroke rats. A two-way repeated-measures ANOVA revealed a significant treatments × interval interaction, P<0.01, F(1, 42)=0.125 (sham, n=8, LA-stroke, n=8). **P<0.01, Bonferroni post-hoc test, sham vs. LA-stroke.
Discussion
ET-1 is commonly used to produce focal ischemia in rodents[14,29,34-36]. The average lesion size in this study was smaller or larger than the study by Sharkey[37] or Jamshid[14], this may be partly due to the dose of ET-1, stereotaxic coordinates and different rat strain in this study. Previous studies have suggested that MCAO produces a success rate of only 50%[38]. ET-1 induced subcortical nuclei ischemia model showed a lower incidence of sensorimotor deficits and higher percentage of survival compared to MCAO. Photothrombosis as another model of focal ischemia is similar to the cortical ET-1 model in that the site of infarct can be precisely localized. However, as a permanent ischemia model, photothrombosis can only be used for cortical stroke and its mechanisms of injury are complex[4]. Through T2-weight MRI image, the effect of ET-1 stereotaxical injection in dysfunction of the hippocampus and lateral amygdala was confirmed. Our findings also show that ET-1 in the dorsal hippocampus and lateral amygdala produces a significant and enduring dorsal hippocampus and lateral amygdala-related learning and memory deficits.
Traditionally well-defined synaptic connectivity in the central nervous system is structured during development and is later sculpted by neural activity. However, it has been reported that neurons that participate in intricate brain functions, such as memory engram or trace, are not contributed by a single brain region but are distributed throughout the whole brain[39]. Supposing that ischemia affects some components of circuitry to route sensate signals to the neural system and motor commands out of it, activity dependent, synapse-based and hebbian-valid learning rules can strengthen and refine these circuits. This extensive connectivity, together with multiformity in neuronal processing, might accelerate recovery from stroke damage.
In physiological conditions, some recent studies indicate distinct appetitive and aversive circuits between the hippocampus and the amygdala[40]. After special emotional shift task, a change probably occurs in the connectivity between hippocampal and amygdalar memory traces[40-41].
In the model used in our present study, it is still unknown if local infarct changes the connectivity involved dorsal hippocampus and/or the lateral amygdala in the process of recovery. It is tempting in the near future to study on the potential circuit plasticity as the result of ischemia in subcortical nuclei to dissect the correlation between activity-dependent plasticity and functional recovery.
Acknowledgments
This work was supported by Major State Basic Research Program of China (Grant No. 2013CB733801).
References
- [1].Hossmann KA. Pathophysiology and therapy of experimental stroke[J] Cell Mol Neurobiol. 2006;26(7-8):1057–1083. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calabresi P, Centonze D, Pisani A, et al. Synaptic plasticity in the ischaemic brain[J] Lancet Neurol. 2003;2(10):622–629. doi: 10.1016/s1474-4422(03)00532-5. [DOI] [PubMed] [Google Scholar]
- [3].Lipton P. Ischemic cell death in brain neurons[J] Physiol Rev. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- [4].Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose[J] NeuroRx. 2005;2(3):396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kanemitsu H, Nakagomi T, Tamura A, et al. Differences in the extent of primary ischemic damage between middle cerebral artery coagulation and intraluminal occlusion models[J] J Cereb Blood Flow Metab. 2002;22(10):1196–1204. doi: 10.1097/01.wcb.0000037992.07114.95. [DOI] [PubMed] [Google Scholar]
- [6].Ginsberg MD, Busto R. Rodent models of cerebral ischemia[J] Stroke. 1989;20(12):1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- [7].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats[J] Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [8].Watson BD, Dietrich WD, Busto R, et al. Induction of reproducible brain infarction by photochemically initiated thrombosis[J] Ann Neurol. 1985;17(5):497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- [9].Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo[J] PLoS Biol. 2007;5(5):e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review[J] J Cereb Blood Flow Metab. 2008;28(12):1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- [11].Yanagisawa M, Kurihara H, Kimura S, et al. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels[J] J Hypertens Suppl. 1988;6(4):S188–191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- [12].Fuxe K, Bjelke B, Andbjer B, et al. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia[J] Neuroreport. 1997;8(11):2623–2629. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- [13].Spanswick SC, Bray D, Zelinski EL, et al. A novel method for reliable nuclear antibody detection in tissue with high levels of pathology-induced autofluorescence[J] J Neurosci Methods. 2009;185(1):45–49. doi: 10.1016/j.jneumeth.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [14].Faraji J, Soltanpour N, Moeeini R, et al. Topographical disorientation after ischemic mini infarct in the dorsal hippocampus: whispers in silence[J] Front Behav Neurosci. 2014;8:261. doi: 10.3389/fnbeh.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murray AJ, Sauer JF, Riedel G, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory[J] Nat Neurosci. 2011;14(3):297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dillon GM, Qu X, Marcus JN, et al. Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice[J] Neurobiol Learn Mem. 2008;90(2):426–433. doi: 10.1016/j.nlm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- [17].Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task[J] Behav Neurosci. 1997;111(1):104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- [18].Bannerman DM, Yee BK, Good MA, et al. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions[J] Behav Neurosci. 1999;113(6):1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- [19].Moser MB, Moser EI, Forrest E, et al. Spatial learning with a minislab in the dorsal hippocampus[J] Proc Natl Acad Sci U S A. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pothuizen HH, Zhang WN, Jongen-Relo AL, et al. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory[J] Eur J Neurosci. 2004;19(3):705–712. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- [21].Ciocchi S, Herry C, Grenier F, et al. Encoding of conditioned fear in central amygdala inhibitory circuits[J] Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- [22].Duvarci S, Pare D. Amygdala microcircuits controlling learned fear[J] Neuron. 2014;82(5):966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johansen JP, Cain CK, Ostroff LE, et al. Molecular mechanisms of fear learning and memory[J] Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology[J] Nat Rev Neurosci. 2013;14(6):417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ju S, Teng G, Zhang Y, et al. In vitro labeling and MRI of mesenchymal stem cells from human umbilical cord blood[J] Magn Reson Imaging. 2006;24(5):611–617. doi: 10.1016/j.mri.2005.12.017. [DOI] [PubMed] [Google Scholar]
- [26].Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats[J] Behav Brain Res. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- [27].Esclassan F, Coutureau E, Di Scala G, et al. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning[J] J Neurosci. 2009;29(25):8087–8093. doi: 10.1523/JNEUROSCI.0543-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Windle V, Szymanska A, Granter-Button S, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat[J] Exp Neurol. 2006;201(2):324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- [29].Biernaskie J, Corbett D, Peeling J, et al. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats[J] Magn Reson Med. 2001;46(4):827–830. doi: 10.1002/mrm.1263. [DOI] [PubMed] [Google Scholar]
- [30].Zelikowsky M, Pham DL, Fanselow MS. Temporal factors control hippocampal contributions to fear renewal after extinction[J] Hippocampus. 2012;22(5):1096–1106. doi: 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ehrlich I, Humeau Y, Grenier F, et al. Amygdala inhibitory circuits and the control of fear memory[J] Neuron. 2009;62(6):757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- [32].Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation[J] Behav Neurosci. 1997;111(4):667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- [33].Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted[J] Behav Neurosci. 1988;102(3):356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- [34].Callaway JK, Lawrence AJ, Jarrott B. AM-36, a novel neuroprotective agent, profoundly reduces reactive oxygen species formation and dopamine release in the striatum of conscious rats after endothelin-1-induced middle cerebral artery occlusion[J] Neuropharmacology. 2003;44(6):787–800. doi: 10.1016/s0028-3908(03)00068-6. [DOI] [PubMed] [Google Scholar]
- [35].Moyanova S, Kirov R, Kortenska L. Multi-unit activity suppression and sensorimotor deficits after endothelin-1-induced middle cerebral artery occlusion in conscious rats[J] J Neurol Sci. 2003;212(1-2):59–67. doi: 10.1016/s0022-510x(03)00102-3. [DOI] [PubMed] [Google Scholar]
- [36].Ploughman M, Granter-Button S, Chernenko G, et al. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia[J] Neuroscience. 2005;136(4):991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- [37].Sharkey J, Butcher SP. Characterisation of an experimental model of stroke produced by intracerebral microinjection of endothelin-1 adjacent to the rat middle cerebral artery[J] J Neurosci Methods. 1995;60(1-2):125–131. doi: 10.1016/0165-0270(95)00003-d. [DOI] [PubMed] [Google Scholar]
- [38].Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury[J] J Neurosci. 2001;21(14):5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lashley KS. In search of the engram[J] Symp Soc Exp Biol. 1950;4:454–482. [Google Scholar]
- [40].Redondo RL, Kim J, Arons AL, et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram[J] Nature. 2014;513(7518):426–430. doi: 10.1038/nature13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Herry C, Ciocchi S, Senn V, et al. Switching on and off fear by distinct neuronal circuits[J] Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]