Abstract
The present study was undertaken to investigate the effect of diacerein on the histopathology of articular cartilage and subchondral bone of the femorotibial joint in rats. Osteoarthritis was induced in rats after single intra-articular injection of sodium iodoacetate. Rats were sacrificed 1, 2, 4, and 8 weeks post intra-articular injection to evaluate the progression of histopathogenesis of osteoarthritis. Diacerein was orally administered (15 mg/kg) once daily post 1 and 2 weeks of iodoacetate injection in two groups, respectively, for up to 12 weeks. Articular cartilage and subchondral bone of the rats of both groups were examined after 8 and 12 weeks, respectively. Quantitative histological analyses were performed by scoring these sections as per the OARSI system. Chondroitin sulfate was also estimated in articular cartilage by decrease in absorbance of methylene blue on complexation with chondroitin sulfate using a spectrophotometer. Intra-articular injection of iodoacetate induced loss of articular cartilage with progressive subchondral bone sclerosis and degeneration. Based on histopathological and biochemical findings, diacerein treatment showed chondroprotective effect. Furthermore, the chondroprotective effect of diacerein was found to be more pronounced after 12 weeks as compared to 8 weeks in both cases (i.e., post 1 and 2 weeks of iodoacetate injection). Similar results were observed by investigation of chondroitin sulfate during biochemical study, showing the chondroprotective effect. In conclusion, diacerein exhibits chondroprotective effect in rats with late onset of action.
Keywords: diacerein, osteoarthritis, articular cartilage, chondroitin sulfate, histopathology
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease characterized by destruction of the articular cartilage, subchondral bone alterations and synovitis. Though the hip, ankle, shoulder and small joints of the hands and feet may be involved, the most common joints to be affected are the knee joints. The WHO estimates that 10% of the world population over 55-60 years of age has symptomatic OA[1-3]. According to the Center for Disease Control and Prevention, about 27 million U.S. adults suffer from OA; this may increase to up to 35 million by 2030[4]. In India, it is the second most common joint problem with a prevalence of 22% to 39%[5-6].
The hallmark of the disease is progressive degeneration of articular cartilage and subsequent joint space narrowing. OA is a major therapeutic challenge because of the lack of effective therapies which may alter the natural course of OA progression. Pharmacological treatment strategies for OA usually include acetaminophen which is a poorly effective drug and non-steroidal anti-inflammatory drugs which are more effective than acetaminophen but have a higher risk profile[7]. Thus, current treatment recommendations for OA are intended to provide patients with symptomatic relief only. There is no generally accepted drug or structure modifying agent for OA.
Diacerein (4, 5-diacetoxy-9, 10-dihydro-9, 10-dioxo-2-anthracenecarboxylic acid) is a novel chondroprotective agent intended for the treatment of OA. It is a semi-synthetic anthraquinone derivative. Diacerein is converted into rhein before reaching the systemic circulation. Rhein selectively inhibits the synthesis of interleukin-1 (IL-1) beta and downregulates the production of nitric oxide (NO), both of which are responsible for cartilage degeneration. Rhein does not inhibit the production of PGE2; therefore, no gastrointestinal toxicity is observed[8-12].
Very few experimental rat models are available to study the course and outcome of OA. One of the best characterized rat models for analyzing the effects of drugs on the pathology of OA involves the injection of the metabolic inhibitor monosodium iodoacetate into the femorotibial joint of rats. This inhibits the activity of glyceraldehyde-3-phosphate dehydrogenase in chondrocytes, resulting in disruption of glycolysis and eventual cell death. The progressive loss of chondrocytes results in histological and morphological changes of the articular cartilage that closely resemble the degeneration seen in OA patients[13-15]. OA involves a complex cascade interaction of biochemical and biomechanical factors which alters pathology of all articular tissues[16]. Therefore, for assessment of a structure modifying agent for the treatment of OA, histopathology of articular cartilage, subchondral bone and other structures of an affected joint becomes important.
Chondroitin sulfate is a type of glycosaminoglycan (GAG) which forms a major component of articular cartilage. Chondroitin sulfate is widely distributed to the extracellular matrix of body tissue. It is reported to modify the chondrocyte death process and to maintain the metabolic process of cartilage matrix[17-18]. Several studies have shown that the concentration of Chondroitin sulfate is decreased at the site of osteoarthritic lesions, which is normally characterized by softening and erosion of cartilage[19-20]. Thus, the estimation of chondroitin sulfate in articular cartilage along with histopathology could be an important investigation for supporting the chondroprotective effect of structure modifying agent in treatment of OA.
Many clinical trials, of 6 months to 3 years of duration, studying the safety and efficacy of diacerein in terms of its symptomatic as well as chondromodulating effect have been reported[8,21-23]. Disease modifying characteristics for chondroprotective activity was also reported in a biochemical study conducted in surgically induced OA in dogs[19]. Our literature search suggested that the chondroprotective or structure modifying actions of diacerein have not been supported by a histopathologic study using a suitable rat model based on cartilage destruction. Therefore, the present study was undertaken to evaluate the chondroprotective and structure modifying effect of diacerein on femorotibial joint using IA induced OA rat model along with concurrent estimation of chondroitin sulfate in articular cartilage.
Materials And Methods
The study was performed using adult Charles Foster rats aged 12-14 weeks (200±20 g). Rats were maintained in a controlled room temperature (22±2 °C) with a 12:12 hours light–dark cycle from birth. They had access to food and water ad libitum. Rats used in this study were maintained and treated in accordance with the CPCSEA guidelines for laboratory rat facilities (CPCSEA Guidelines, 2003). The experimental protocol was approved by the Institutional Ethics Committee of Banaras Hindu University, Varanasi, India.
Seventy-two rats of either sex were randomized under ether anesthesia to receive a single intraarticular injection of 40 µL of sodium iodoacetate (2 mg/joint) or an equivalent volume of normal saline through the patellar ligament using a 27-gauge needle, into the left knee of each rat. Then, diacerein [0.3% dispersion in sodium carboxymethyl cellulose (NaCMC)] was orally administered at a daily dose of 15 mg/kg of body weight[24-25] 1 or 2 weeks post the intraarticular injection. The day of iodoacetate injection was counted as day 1.
Histology
Rats were euthanized by high dose of anesthesia and the knee joints of 4 rats at a time were taken. The femorotibial joint of each rat was removed by cutting halfway through the femur and tibia. The joint was opened for gross examination and then subjected for histopathologic sections. The rats were sacrificed at the indicated time points and tissue sections were prepared for light microscopy using standard procedures. Soft tissues around the femorotibial joint were removed from the medial and lateral joint capsule. Tissues were fixed overnight with 10% phosphate-buffered formalin and then decalcified for a maximum of 16 hours in water containing 7% w/v AlCl3.6H2O, 5% formic acid and 8.5% HCl. The decalcified knee joints were washed overnight in 0.1 M phosphate buffer (pH 7.4) and then incubated in 15% sucrose and 0.1 M phosphate buffer for 24 hours. The tissues were dehydrated using gradient alcohol. Paraffin blocks of the tissues were prepared after treating them with xylene. Serial 6 µm thickness sections of the tissue were taken and stained with hematoxylin & eosin (H&E) using standard protocol[26]. Tissue sections were observed under a microscope (Olympus CH20i, Japan)[23,27]. Each of the sections was scored per the Osteoarthritis Research Society International (OARSI) system for quantitative histological analyses. The entire tissue section of each slide was considered in applying the staging parameter of the OARSI system[28].
Biochemical study
The femorotibial joint was removed for biochemical evaluation after the rats were euthanized by a high dose of anesthesia. Articular cartilage was obtained from the joint using a scalpel blade to cut as deeply as possible without removing underlying bone. Samples were removed from the posterior surface of the patella or from the condyles and patellar groove of the femur. Ethanol fractions were prepared from cartilage samples digested in phosphate buffer pH 7 containing trypsin for 16 to 20 hours at 37 °C and then homogenized for 1-2 minutes. Insoluble debris was removed by brief centrifugation. Supernatant was brought to 40%, 50% & 80% ethanol, respectively, by addition of ethanol and aqueous 20% potassium acetate solution. At each step, supernatant was allowed to stand overnight at 4°C and centrifuged. All precipitates were pooled, dissolved in water and used for determination of chondroitin sulfate[19,20]. Chondroitin sulfate was indirectly estimated by decrease in absorbance of methylene blue on complexation with chondroitin sulfate using a spectrophotometer[29].
Statistical analysis
The results were expressed as mean values ± standard deviation. One way analysis of variance (ANOVA) followed by Student-Newman-Keuls test was applied to examine the data of different studies. In all cases, P<0.05 (*) was considered to be significant.
Results
Normal femorotibial joints were compared with the osteoarthritic joints (Fig. 1). Microphotographs of the control femorotibial joints showed normal articular cartilage (Fig. 1A). The articular surface was smooth and without any fissures or debris attached to it. The femoral condyles were separated by meniscus from the tibial plateau. One week post iodoacetate injection, thinning, irregularity, and loss of surface hyaline cartilage of the joints were observed (Fig. 1B). Widening of the intraarticular space was a common finding in the entire joint injected with iodoacetate. Diminished hemopoietic tissue was found in the trabecular space in the subchondral region; the tidemark representing the boundary between calcified and non-calcified region was also lost. Loss of bony spicules and sclerosis of the subcortical bone was also exhibited.
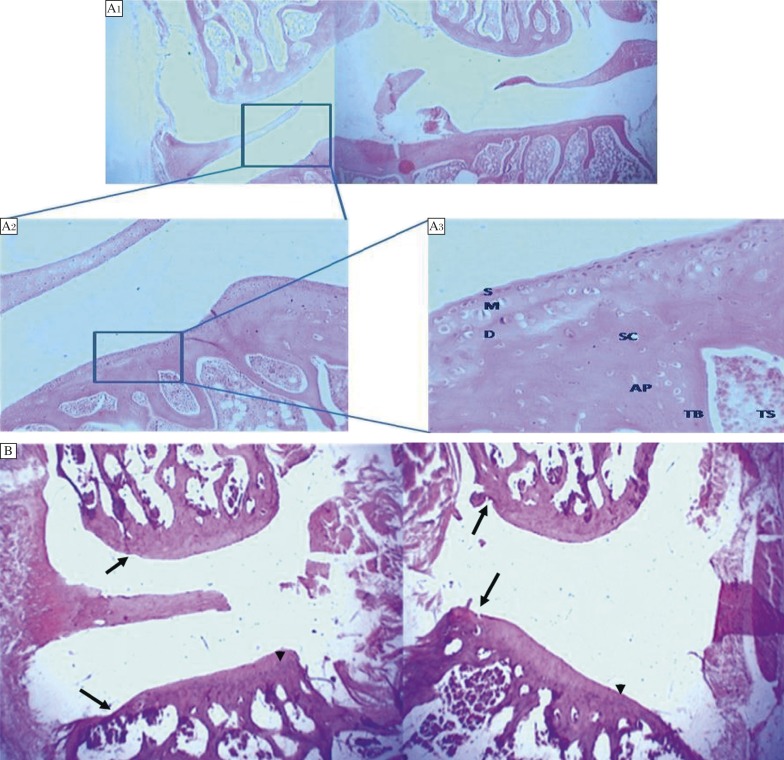
Fig. 1A. Comparison of normal femorotibial joint with osteoarthritic joint.
A: Photomicrograph of a normal (treated with saline as vehicle) Charles Foster rat tibiofemoral joint. The articular surface is smooth. The femoral condyles are separated by meniscus from the tibial plateau. The normal structure of cartilage is organized in three well-ordered zones: the superficial, the mid zone and the deep zone (S, M, D). The subchondral (SC) bone, articular bone plate (AP) and the trabecular bone (TB) are clearly observed. The trabecular space (TS) is filled with hemopoitic tissue. (Grade 0, Stage 0). H & E A1 x20 (panoramic view), A2 x100, A3 x400. B: Photomicrograph of a treated Charles Foster rat femorotibial joint 1 week post IA injection. The articular space between the two ends has widened. The femoral condyles articular surface shows irregularity (arrow). The tibial plateau shows thinning (arrow head) as well as an irregular hyaline cartilage surface (arrow). The subchondral space show diminished hemopoietic tissue with bony spicule loss; (Grade 2-2.5, Stage 2). H&E x20 (panoramic view).
The progression of disease is shown in Fig. 2. Different stages of moderate to advanced OA were seen due to the metabolic inhibitory effect of iodoacetate. Two weeks post iodoacetate treatment, the tissues completely broke down (Fig. 2A, B, and C). Multifocal, denuded cartilage was observed with a large number of necrotic chondrocytes. Deep fissures and cracks were observed, which reached down to the deep calcified zone. Moreover, chondrocyte degeneration was found on the surface layer, which was clearly evidenced by the empty chondrons. Four weeks post iodoacetate treatment, advanced stages of OA were observed (Fig. 2D, E, and F), marked by loss of surface cartilage with subchondral bone collapse and fragmentation. Subchondral hemopoetic tissues were replaced by fibrous tissue. The bony trabeculae collapsed due to extreme thinning and degeneration, which led to an increase in interosseous space, (Fig. 2D, E). Eight weeks post iodoacetate treatment (Fig. 2G, H, and I), there was well-defined subchondral cyst formation within the subchondral bone, demarcated by fibrous tissue. Regenerated chondrocyte cell nests were dispersed without normal arrangements. A large number of degenerative necrotic chondrocytes with pyknotic nuclei and sometimes partly dissolved cytoplasm were present. An increase in interosseous spaces, with a break in the bony spicules in between, resulted in confluence of the interosseous space and large cyst formation.
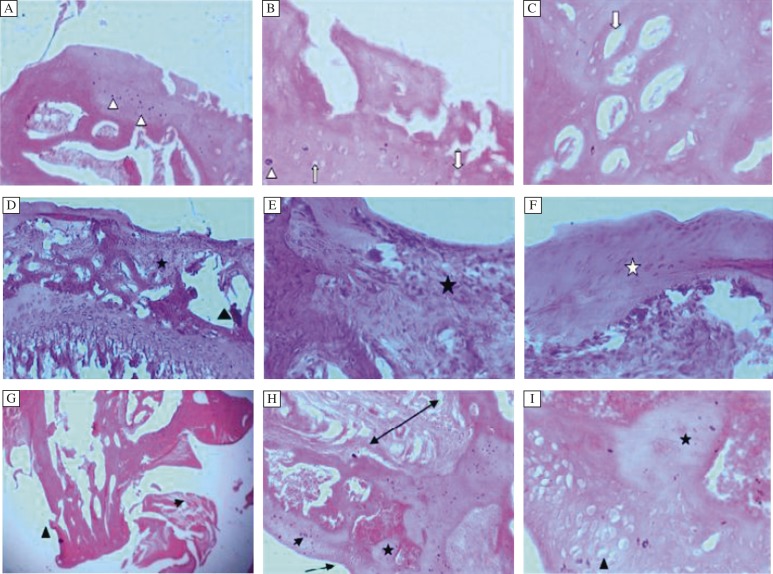
Fig. 2A, Progression of disease (OA) after IA injection.
A and B: Femorotibial joint 2 weeks post IA injection. The articular surface exhibits multifocal denuded cartilage with fissures extending deep into the mid zone. Large numbers of necrotic chondrocytes are seen (White arrow head). C: Chondrocytes degeneration exhibited by empty chondrons in the surface cartilage (White arrow); (Grade 3-3.5, Stage 3). H&E, A x100, B,C x400. D and E: Femoral condyle 4 weeks post IA injection showing marked loss of cartilage from the surface associated with subchondral bone collapse and fragmentation. Large areas of subchondral hemopoietic tissue are replaced by fibrous tissue (*). Marked bony trabeculae loss and collapse leading to formation of large interosseous spaces (black triangle) are seen. F: Irregular margin of the surface cartilage is visualized as well as irregular margin of the matrix of the regenerative fibrocartilage. (White asterisk), (Grade 4-4.5, Stage 3). H & E. D x100.E,F x400. 2G-Tibial condyle 8 weeks post IA injection showing extensive degeneration of the articular cartilage and the subchondral bone (arrow head) H and I: Complete loss of the cartilage and large cysts within the subchondral region (*). Articular cartilage erosion (arrow). Cortical area show fibrous tissue formation (double arrow). Associated large number of degenerative (necrotic) chondrocytes (arrow head). Regeneration of chondrocytes cell nest dispersed without normal arrangement (triangle). Subchondral cyst due to cartilage cell proiferation within the subchodral bone (*), (Garde 5-5.5, Stage 4). H & E.x20, x100, x400.
Diacerein treated rats, when compared with the control rats, had the same degree of OA at 1 and 2 weeks post injection of iodoacetate (Fig. 3 and 4). The femoro-tibial joints exhibited early to moderate levels of OA 1 week post iodoacetate injection (Fig. 3A). Healing was evident by 8 weeks post diacerein treatment and the degree of OA changes regressed at 12 weeks (Fig. 3B and C). Degenerating chondrocytes were not seen in diacereinrats. Similarly, the femorotibial joint, 2 weeks post iodoacetate injection, showed moderate-to-advanced OA and was compared with diacerein treated rats (Fig. 4A). Regeneration of cartilage, as evident by new chondrocyte cell nests and other structure modifications, was evident after diacerein treatment (Fig. 4B, C and D). The degree of regeneration was better at 12 weeks than at 8 weeks. The chondrocyte regeneration was at faster rate than the degeneration, evidenced by pyknotic chondrocytes seen at both 8 and 12 weeks. The chondrocytes exhibited regeneration. Finally, the score values for quantitative histological analyses are summarized in Table 1. Scores for each section were calculated on the basis of individual grading values and staging of respective sections.
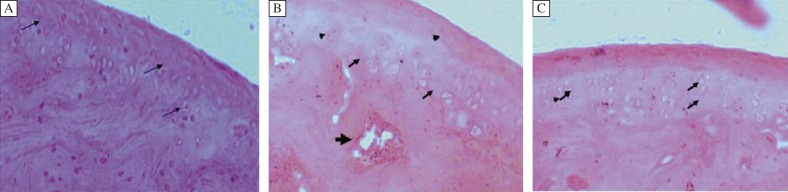
Fig. 3. Effect of treatment of diacerein after post 1 week IA injection.
A: Femorotibial joint 1 week post IA injection showing mild to moderate OA changes. Note the degenerating chondrocyte (Arrow) and loss of the cellular matrix; (Grade 2.5, Stage 2). B: Eight weeks after DC treatment which begun 1 week post IA injection. Regeneration of the cartilage is evident by the cell nest (arrow) within the articular cartilage. Cortical bone formation with microvasculature evident (by bold Arrow head); (Grade 2, Stage 2). C: Twelve weeks after DC treatment which begun 1 week post IA injection. Cell cartilage is well-organized; (Grade 1.5, Stage 2) H & E. x400.
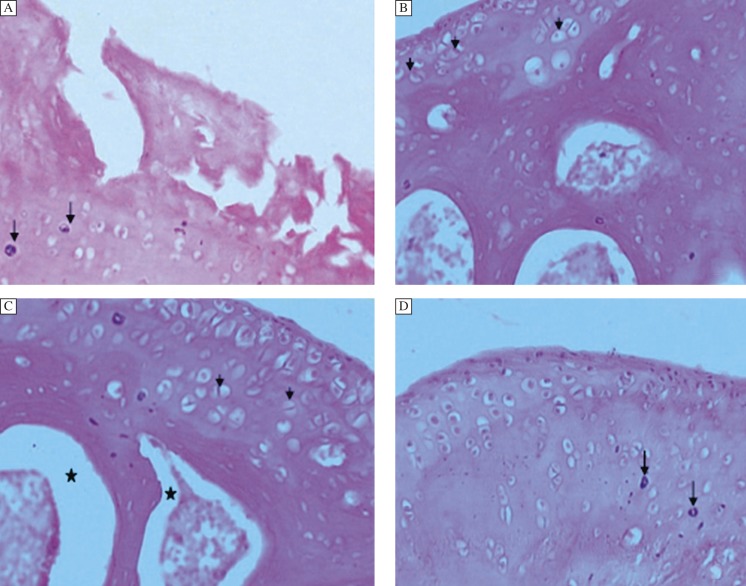
Fig. 4. Effect of treatment of diacerein after post 2 week IA injection.
A: Femorotibial joint 2 weeks post IA injection. The articular surface exhibits multifocal denuded cartilage with fissures extending deep into the deep layer. Large numbers of necrotic chondrocytes were seen (black arrow); (Grade 3.5, Stage 3).x200 B-Eight weeks after DC treatment was begun 2 weeks post IA injection. C, D: Twelve weeks after treatment was begun 2 weeks post IA injection. Regeneration of the cartilage is evident by the cell nest (small black arrow) within the articular cartilage. Note, in the subchondral region, the bone regeneration is poor. The hemopoitic tissue is being pushed to the center (*). Few necrotic chondrocytes are seen (down arrow)); (Grade 1.5-1, Stage 2-1). H & E,x400.
Table 1. OA score of tissue sections for quantitative histological analyses: OARSI system.
| Group | Treatment | Grade | Stage | Score</emph> | Characteristics</emph> |
| Group 1 | Normal saline (without IA)+ Vehicle (0.3% NaCMC) | 0 | 0 | 0 | Normal architecture, smooth surface, cells intact with appropriate orientation |
| Group 2 | Vehicle (0.3% NaCMC); sacrificed 1 week post IA injection | 2.0-2.5 | 2 | 4.0-5.0 | Wavy and irregular surface, thinning of tibial plateau, diminished hemopoietic tissue with loss of bony spicule |
| Group 3 | Vehicle (0.3% NaCMC); sacrificed 2 weeks post IA injection | 3.0-3.5 | 3 | 9.0-10.5 | Fissures and matrix fibrillation extending deep into the mid zone, large number of necrotic chondrocytes |
| Group 4 | Vehicle (0.3% NaCMC); sacrificed 4 weeks post IA injection | 4.0-4.5 | 3 | 12.0-13.5 | Extensive erosion resulting in excavitation, subchondralhemopoietic tissue replaced by fibrous tissue |
| Group 5 | Vehicle (0.3% NaCMC); sacrificed 8 weeks post IA injection | 5.0-5.5 | 4 | 20.0-22.0 | Denudation, complete loss of the cartilage and large cysts within the subchondral region |
| Group 6 | DC dispersion in 0.3% NaCMC 1 week post IA injection; sacrificed after 8 weeks of DC administration | 2.0 | 2 | 4.0 | Surface wavy and discontinued, regeneration of the cartilage as cell nest, cortical bone formation with microvasculature |
| Group 7 | DC dispersion in 0.3% NaCMC 1 week post IA injection; sacrificed after 12 weeks of DC administration | 1.5 | 2 | 3.0 | Fibrillation superficially, organized cartilage cells |
| Group 8 | DC dispersion in 0.3% NaCMC 2 weeks post IA injection; sacrificed after 8 weeks of DC administration | 1.5 | 2 | 3.0 | Few necrotic chondrocytes, few superficial fibrillation |
| Group 9 | DC dispersion in 0.3% NaCMC 2 week post IA injection; sacrificed after 12 weeks of DC administration | 1.0 | 1 | 1.0 | Superficial zone intact, regeneration with cell nest |
IA: Sodium Iodoacetate; DC: Diacerein
Table 2. OA score of tissue sections for quantitative histological analyses: OARSI system.
| No. | Grade | Stage | Score | Characteristics |
| Group 1 | 0 | 0 | 0 | Normal architecture, smooth surface, cells intact with appropriate orientation |
| Group 2 | 2.0-2.5 | 2 | 4.0-5.0 | Wavy and irregular surface, thinning of tibial plateau, diminished hemopoietic tissue with loss of bony spicule |
| Group 3 | 3.0-3.5 | 3 | 9.0-10.5 | Fissures and matrix fibrillation extending deep into the mid zone, large number of necrotic chondrocytes |
| Group 4 | 4.0-4.5 | 3 | 12.0-13.5 | Extensive erosion resulting in excavitation, subchondralhemopoietic tissue replaced by fibrous tissue, |
| Group 5 | 5.0-5.5 | 4 | 20.0-22.0 | Denudation, complete loss of the cartilage and large cysts within the subchondral region |
| Group 6 | 2.0 | 2 | 4.0 | Surface wavy and discontinued, regeneration of the cartilage as cell nest, cortical bone formation with microvasculature |
| Group 7 | 1.5 | 2 | 3.0 | Fibrillation superficially, organized cartilage cells |
| Group 8 | 1.5 | 2 | 3.0 | Few necrotic chondrocytes, few superficial fibrillation |
| Group 9 | 1.0 | 1 | 1.0 | Superficial zone intact, regeneration with cell nest |
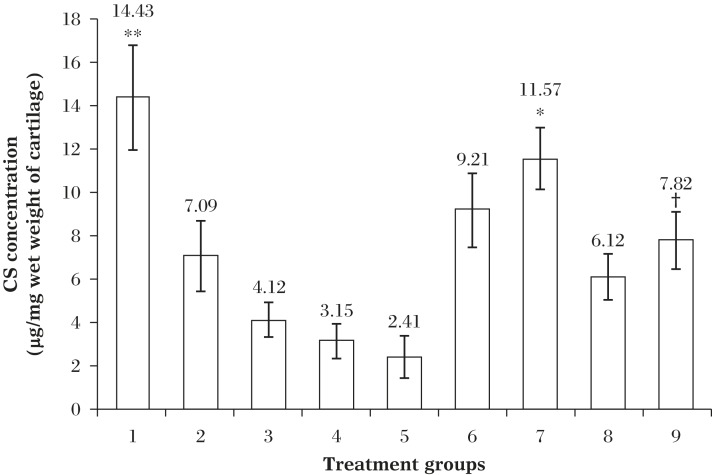
OA induction caused significant reduction in chondroitin sulfate concentration in a time dependent manner. Chondroitin sulfate concentration in the articular cartilages of diacerein treated rats was found to be higher, compared to the control rats (i. Further, chondroitin sulfate concentration was even higher after treatment for 12 weeks, compared to 8 weeks (Fig. 5).
Fig. 5. Concentrations of CS in the articular cartilage of the treatment groups.
All values are reported as Mean±SD (n = 4; ANOVA), *P≤0.01 compared to Group 2 (arthritic control; post 7th day IA injection); †P≤0.05 compared to Group 3 (arthritic control; post 14th day IA injection); **P≤0.001 compared to all other group except Group 7.
Discussion
Iodoacetate induced experimental OA in rats has been reported as a useful rat model of osteoarthritis in terms of similar time dependent degeneration of the articular cartilage and subchondral bone to that in humans[27]. Degeneration was evident as early as 1 week post iodoacetate injection accompanied by osteoclastic and osteoblastic activity in the subchondral region. An intimate relation was exhibited by the degenerating articular cartilage and the subchondral bone, which was similar to earlier findings[1,14]. According to Kraan et al. (2007), progression of OA leads to subchondral bone sclerosis and/or formation of osteophytes[30]. These types of damage were observed 4 weeks post injection of iodoacetate. Rats showed no regenerative changes 1 and 2 weeks post diacerein treatment. Eight weeks post diacerein treatment, regeneration became apparent and further improved at 12 weeks post diacerein treatment. It may be suggested that these regenerative effects of diacerein could be attributed to the slow onset of action of the drug. The difference in chondroprotection and regeneration after 1 and 2 weeks of iodoacetate injection shows that the earlier diacerein treatment commences, the better the regeneration of OA associated lesions[31]. All the above findings were well supported and explained by scoring done for tissue sections for each specimen (Table 2). Pelletier et al. (2010) reported that diacerein decreased the inhibitory effect of IL-1 on the synthesis of collagen and proteoglycans[32]. Loss of these parts of cartilage matrix by induction of OA was observed in the current study and regeneration of these structures was evident due to the chondroprotective effect of diacerein.
In the present study, chondroitin sulfate was estimated as cartilage matrix biomarker along with the histological study to assess the chondroprotective effect of diacerein. Chondroitin sulfate concentration in the articular cartilage of OA induced rats (i.e. group 2 to 5) was found to be significantly reduced in comparison to normal rats. This indicates loss of cartilage matrix due to the destructive action of iodoacetate when used as chemical agent for inducing osteoarthritis. The drug treated rats showed less loss of cartilage matrix, which may be due to the larger amount of chondroitin sulfate in these rats.
Although clinical reports about the chondroprotective effect of diacerein are available, this work has proven that the effect is at the site of chondrocytes. The present study has elucidated the chondroprotective effect of the diacerein on the tibiofemoral joint by histological examination. The study also suggests that the chondroprotective effect of diacerein is evident after prolonged treatment.
In conclusion, injection of iodoacetate induces loss of chondral cartilage with subchondral bone lesions that mimic human OA. A clear relationship between the cartilage damage and the subjacent damage to the underlying subchondral bone was evident. The therapeutic effect of diacerein was better when the treatment was started post one week, rather than 2 week, of intra-articular injection of iodoacetate. The effect of diacerein was more pronounced after 12 weeks of treatment in comparison to eight weeks. This reflects that onset of therapeutic action is delayed. These results may be extrapolated to humans with the suggestion that the treatment efficiency would progress better if diacerein is used during the earlier phase of OA. Studies with direct evidence from the human cartilage showing effect of diacerein need to be explored in the future.
Acknowledgments
First author is thankful to University Grant Commission, New Delhi for the financial support in the form of Senior Research Fellowship. The authors are thankful to Dr. Reddy’s Laboratories Ltd. (Hyderabad, India) for providing diacerein as gift sample.
References
- [1].Lorenz H, Richter W. Osteoarthritis: Cellular and molecular changes in degenerating cartilage[J] Prog Histochem Cytochem. 2006;40:135–163. doi: 10.1016/j.proghi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Alcaraz MJ, Megias J, Arnandis IG, et al. New molecular targets for the treatment of osteoarthritis[J] Biochem Pharmacol. 2010;80:13–21. doi: 10.1016/j.bcp.2010.02.017. [DOI] [PubMed] [Google Scholar]
- [3].Kobayashi K, Imaizumi R, Sumichika H, et al. Sodium Iodoacetate-Induced Experimental Osteoarthritis and Associated Pain Model in Rats[J] J Vet Med Sci. 2003;65(11):1195–1199. doi: 10.1292/jvms.65.1195. [DOI] [PubMed] [Google Scholar]
- [4].Cheng YJ, Hootman JM, Murphy LB, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation -United States, 2007-2009[J] Centers for Disease Control and Prevention MMWR weekly. 2010;59(39):1261–1265. [PubMed] [Google Scholar]
- [5].Mahajan A, Singh K, Tandon VR, et al. Diacerein: A New Symptomatic Slow Acting Drug for Osteoarthritis[J] JK Science. 2006;8(3):173–175. [Google Scholar]
- [6].Mahajan A, Verma S, Tandon VR. Osteoarthritis[J] J Assoc Physicians India. 2005;53:634–641. [PubMed] [Google Scholar]
- [7].Berenbaum F. Targeted therapies in osteoarthritis: a systematic review of the trials on. Best Pract Res Clin Rheumatol. 2010;24:107–109. doi: 10.1016/j.berh.2009.08.007. www.clinicaltrials.gov [DOI] [PubMed] [Google Scholar]
- [8].Dougados M, Nguyen M, Berdah L, et al. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip[J] Arthritis Rheum. 2001;44(11):2539–2547. doi: 10.1002/1529-0131(200111)44:11<2539::aid-art434>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [9].Quintao NL, Medeiros R, Santos AR, et al. The Effects of Diacerhein on Mechanical Allodynia in Inflammatory and Neuropathic Models of Nociception in Mice[J] Anesth Analg. 2005;101:1763–1769. doi: 10.1213/01.ane.0000184182.03203.61. [DOI] [PubMed] [Google Scholar]
- [10].Borsa M, Roberto M. Process for the preparation of esters of diacerein with hyaluronic acid and pharmaceutical compositions containing such esters[J] US patent application. 20090239822. [Google Scholar]
- [11].Gao D, Wu J, Lu W, et al. Pharmaceutical Compositions Containing Diacerein[J] US patent application. 20100104651. [Google Scholar]
- [12].Deepak P, Ehrenpreis ED. Diarrhea[J] Dis Mon. 2011;57(9):490–510. doi: 10.1016/j.disamonth.2011.05.005. [DOI] [PubMed] [Google Scholar]
- [13].Dinser R. Rat models for arthritis[J] Best Pract Res Clin Rheumatol. 2008;22(2):253–267. doi: 10.1016/j.berh.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [14].Pritzker KPH. Rat models for osteoarthritis: processes, problems and prospects[J] Ann Rheum Dis. 1994;53:406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Silva A, Andersen ML, Tufik S. Sleep pattern in an experimental model of osteoarthritis[J] Pain. 2008;140:446–455. doi: 10.1016/j.pain.2008.09.025. [DOI] [PubMed] [Google Scholar]
- [16].Girling SL, Bell SC, Whitelock RG, et al. Use of biochemical markers of osteoarthritis to investigate the potential disease-modifying effect of tibial plateau leveling osteotomy[J] J Small Anim Pract. 2006;47(12):708–714. doi: 10.1111/j.1748-5827.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- [17].Wildi LM, Raynauld JP, Martel-Pelletier J, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI[J] Ann Rheum Dis. 2011;70(6):982–989. doi: 10.1136/ard.2010.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Monfort J, Pelletier JP, Garcia-Giralt N, et al. Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues[J] Ann Rheum Dis. 2008;67(6):735–740. doi: 10.1136/ard.2006.068882. [DOI] [PubMed] [Google Scholar]
- [19].Carney SL. Effect of diacetyl rhein on the development of experimental osteoarthritis. A biochemical investigation[J] Osteoarthr. Cartil. 1996;4:251–261. doi: 10.1016/s1063-4584(05)80103-7. [DOI] [PubMed] [Google Scholar]
- [20].Bollet AJ, Nance JL. Biochemical Findings in Normal and Osteoarthritic Articular Cartilage. II. Chondroitin Sulfate Concentration and Chain Length, Water, and Ash Content[J] JCI. 1966;45(7):1170–1177. doi: 10.1172/JCI105423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nicolas P, Tod M, Padoin C, et al. Clinical pharmacokinetics of Diacerein[J] Clin Pharmacokinet. 1998;35(5):347–359. doi: 10.2165/00003088-199835050-00002. [DOI] [PubMed] [Google Scholar]
- [22].Pelletier JP, Yaron M, Haraoui B, et al. Efficacy and safety of diacerein in osteoarthritis of the knee A Double-Blind, Placebo-Controlled Trial. The Diacerein Study Group[J] Arthritis Rheum. 2000;43(10):2339–2348. doi: 10.1002/1529-0131(200010)43:10<2339::AID-ANR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- [23].Pham T, Henanff AL, Ravaud P, et al. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis[J] Ann Rheum Dis. 2004;63:1611–1617. doi: 10.1136/ard.2003.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jain A, Singh SK, Singh Y, et al. Development of lipid nanoparticles of diacerein, an antiosteoarthritic drug for enhancement in bioavailability and reduction in its side effects[J] J biomed nanotechnol. 2013;9:891–900. doi: 10.1166/jbn.2013.1580. [DOI] [PubMed] [Google Scholar]
- [25].Tamura T, Shirai T, Kosaka N, et al. Pharmacological studies of diacerein in rat models of inflammation, arthritis and bone resorption[J] Eur J Pharmacol. 2002;448:81–87. doi: 10.1016/s0014-2999(02)01898-8. [DOI] [PubMed] [Google Scholar]
- [26].Kaul G, Cucchiarini M, Remberger K, et al. Failed cartilage repair forearly osteoarthritis defects: abiochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques[J] Knee surg sports traumatol arthrosc. 2012;20(11):2315–2324. doi: 10.1007/s00167-011-1853-x. [DOI] [PubMed] [Google Scholar]
- [27].Guzman RE, Evans MG, Bove S, et al. Mono-Iodoacetate-Induced Histologic Changes in Subchondral Bone and Articular Cartilage of Rat Femorotibial Joints: An Rat Model of Osteoarthritis[J]. Toxicol Pathol. 2003;31:619–624. doi: 10.1080/01926230390241800. [DOI] [PubMed] [Google Scholar]
- [28].Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging[J] Osteoarthr. Cartil. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- [29].Somashekar PL, Tripathy AS, Sathish KP, et al. Colorimetric estimation of Chondroitin Sulfate in bulk drug and pharmaceutical formulation using cationic dye Methylene Blue[J] Der Pharma Chemica. 2011;3(1):90–96. [Google Scholar]
- [30].Kraan PM, Berg WB. Osteophytes: relevance and biology[J] Osteoarthr Cartilage. 2007;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [31].Fidelix TSA, Soares B, Trevisani VFM. Diacerein for osteoarthritis[J] Cochrane Database Syst Rev. 2009;1:1–67. doi: 10.1002/14651858.CD005117.pub2. [DOI] [PubMed] [Google Scholar]
- [32].Pelletier MP, Pelletier JP. Effects of diacerein at the molecular level in the osteoarthritis disease process[J] Ther Adv Musculoskel dis. 2010;2(2):95–104. doi: 10.1177/1759720X09359104. [DOI] [PMC free article] [PubMed] [Google Scholar]