Abstract
To determine the significance of germination on phytochemical constituents and non-enzymatic antioxidant activities of Ceiba pentandra seed extracts. Phytochemicals and antioxidant activities of raw and germinating seeds of Ceiba pentandra were estimated by different methods. The levels of phytochemical constituents were influenced by germination and increased except alkaloids and tannins, which were decreased significantly during germination. Among non-enzymatic antioxidants like DPPH, FRAP, reducing assay and hydroxyl radical scavenging activity all showed improved activity compared with non-germinating seeds. This may be due to various reactive oxygen species (ROS) that were generated as by-products of metabolism during germination. This group of ROS included superoxide radicals (O2), hydrogen peroxide radicals (H2O2) and hydroxyl radicals (OH). The formation of these oxygen radicals resulted in the accumulation of lipid hydroperoxides by radical chain oxidation via phospholipids peroxy radicals within membranes. Therefore, it was hypothesized that this could be related to the increase of antioxidant activity in large unilamellar vesicles observed in germinated seeds. The implication of this study is that the Ceiba pentandra seeds as natural antioxidant agents and put forward the possibility of employing for therapeutic potential.
Keywords: antioxidants, butylated hydroxy toluene, phytochemicals, rutin
Introduction
Seed germination is a process by which a seed embryo develops into a seedling. It involves the reactivation of metabolic pathways that lead to growth and the emergence of the radicle or seed root and plumule or shoot. The emergence of the seedling above the soil surface is the next phase of the plant's growth and is called seedling establishment[1]. Since seeds are the primary stage of plant life cycle, they have strong defence mechanism, possibly due to the presence of phytoconstituents, contributing to antioxidant and antimicrobial activities. Many fruits have inedible seeds which are not part of human diet. However, such seeds are a part of ayurvedic preparations against many diseases[2].
Numerous reports concerned the presence of bioactive substances in plant seeds. Seeds from the genus Lupinus are known to accumulate large quantities of poisonous quinolizidine alkaloids. Lignans in seeds either help defend against various pathogens or act as antioxidants. Sesamin, from the sesame seed, has in vitro antioxidant properties that prevent sesame oil from turning rancid during commercial storage. Red sorghum produces proanthocyanidin antifeedant compounds, condensed tannins, which deter birds from feeding on the seed. Coumarins in inhibitory coats appear to possess antimicrobial, antifeedant and germination inhibiting properties[3]. Prunus armeniaca kernels were reported to possess antioxidant and antimicrobial activities[4].
Ceiba (C.) pentandra is a tropical tree of the order Malvales and the family Malvaceae, native to Mexico, Central America and the Caribbean, northern South America, and (as the variety C. pentandra var. guineensis) tropical west Africa. The seeds, leaves, bark and resin from C. pentandra are used to treat fever, asthma, dysentery and kidney disease[5]. The seed oil which has antimicrobial activity is used for making soap, and the residue is used as fertilizer and cattle feed[6]. In the present study, we sought to assess the phytochemicals and antioxidant activities in the buffer extracts of germinating and non-germinating seeds of C. pentandra.
Materials And Methods
Chemicals
Chemicals and reagents used for antioxidant estimations were purchased from Merck (Darmstadt, Germany). All additional chemicals used were of analytical grade. All experiments were performed at room temperature unless otherwise stated.
Collection of seeds
Mature dried fruits of Ceiba pentandra were obtained from the area in and around Andhra University, Visakhapatnam, India. Healthy seeds were selected and washed thoroughly with running tap water and with 5% (w/v) teepol for 10 minutes followed by treatment with bavistin, a commercial fungicide for 5 minutes. The seeds were subsequently surface sterilized with 0.1% (w/v) mercuric chloride (HgCl2) for 5 minutes and then washed with sterile distilled water. The seeds were soaked for 24 hours before they were kept for germination[7] in sterile Petri-plates with double layered moistened filter paper. The germination was carried out at 30°C with 16 hours of light and 8 hours of dark[8]. Radicle emergence of 1 cm was used as a reference to consider seed germination. The 4 days germinated seeds were used for biochemical and antioxidant analyses.
Preparation of extract
One gram of non-germinating (soaked overnight) and germinating Ceiba pentandra seeds were homogenized separately with 20 mL of pre-chilled 0.2 M Tris HCl Buffer, pH 7.2, containing 0.1 mM EDTA in chilled pestle and mortar. The homogenates were squeezed through double layered cheese cloth and centrifuged at 16,000 rpm for 15 minutes at 4°C and the supernatant was used for the assessment of phytochemical and non-enzymatic antioxidants.
Assessment of phytochemicals
Total phenolics content in extracts was determined by the Folin-ciocalteu procedure[9]. Samples (200 μL) were introduced into test tubes. One milliliter of Folin-ciocalteu reagent and 0.8 mL of sodium carbonate (7.5%) were added into each tube. The tubes were mixed and allowed to stand for 30 minutes. Absorption at 765 nm was measured. The total phenolics content was expressed as gallic acid equivalents (GAE) in micrograms per gram of extract, as calculated from standard gallic acid graph.
Total flavonoid content of the extract was determined by a modified colorimetric method[10]. Seed extract (1.0 mL) was mixed with 1 mL of distilled water and 75 μL of 5% sodium nitrite (NaNO2) solution. After 5 minutes, 75 μL of 10% AlCl3.H2O solution was added. Next 5 minutes later, 0.5 mL of 1 M sodium hydroxide was added. The solution was mixed well and kept for 15 minutes. The increase in absorbance was measured at 510 nm by using a UV-Visible spectrophotometer. The total flavonoid content was calculated by using a standard quercetin calibration curve. The results were expressed as micrograms of quercetin equivalents (QE) per gram of extract.
Total tannins were determined using the Folins-ciocalteau method[11]. Briefly, 0.1 mL of seed extract, 6.5 mL of water, 0.5 mL of Folins-ciocalteau reagent and 1.5 mL of 20% sodium carbonate at overnight standard solution were added and incubated for 1 hour. The absorbance was measured at 725 nm and the results were expressed as micrograms of tannic acid equivalents per gram of extract.
Total alkaloid content was estimated by the method of Sreevidya and Mehrotra[12]. A standard solution was prepared by dissolving 5 mg of boldine and seed extract separately in 5 mL of warm distilled water each. Five mL of boldine solution/sample extract was adjusted to pH 2-2.5 (with 0.01 mol/L HCl), and 2 mL of DR (Dragendorff's reagent) was added to form an orange precipitate that was centrifuged at 1956.5 g for 15 minutes. Then, DR was added to the supernatant to test for complete precipitation. The mixture containing 2 mL amount of 1% sodium sulfide was added to the residue to form a brownish black precipitate which was centrifuged at 1,956.5 g for 15 minutes. Complete precipitation was checked by further adding 1% sodium sulfide. The resulting residue was dissolved in 2 mL of nitric acid with warming and sonication and then added up to 10 mL with distilled water. The mixture containing 5 mL of 3% thiourea was added to 1 mL of the resulting solution to form a yellow bismuth complex, of which the absorbance was measured at 435 nm. All assays were performed in triplicate. The amount of bismuth from the boldine solution/extract was determined from the calibration curve of bismuth nitrate. The results were expressed as boldine; considering that is a monobasic alkaloid, the complex formed with bismuth follows a 1:1 stoichiometry.
Assessment Of Non-Enzymatic Antioxidants
Diphenyl picryl hydrazyl radical scavenging assay
A mixture containing 3 mL of seed extract was added to 1 mL of 0.1 mM solution of DPPH in methanol. After 30 minutes of incubation at 37°C, absorbance was measured at 517 nm against control by using a spectrophotometer[13]. Rutin and BHT were used as the reference materials. The percentage of inhibition was calculated by comparing the absorbance values of the test samples with those of the controls. The percentage of inhibition (I) was calculated as radical scavenging activity as follows:
Percentage of inhibition (I) = (Absorbance of control-Absorbance of test/Absorbance of control)×100
Ferric reducing or antioxidant power assay
Total antioxidant power of the sample was assayed by the method of Benzie and Strain[14]. Three mL of FRAP working reagent was taken in a test tube, then 100 µL of plant extract was added. Absorbance was read at 593 nm against a reagent blank at a predetermined time after sample-reagent mixture. The results are expressed as ascorbic acid equivalents (µ moles/mL) or FRAP units.
Iron (III) to iron (II) reducing activity assays
The ability of the extracts to reduce iron (III) was assessed by the method of Oyaizu[15]. The mixture containing 1 mL of seed extract was mixed with 2.5 mL of 0.2 mol/L phosphate buffer, pH 6.6, and 2.5 mL of 1% aqueous potassium hexacyanoferrate[K3Fe(CN6)] solution. After 30 minutes of incubation at 50°C, 2.5 mL of 10% trichloroacetic acid was added and the mixture was centrifuged for 10 minutes. Finally, 2.5 mL of the upper layer was mixed with 2.5 mL of water and 0.5 mL of 0.1% aqueous FeCl3, and the absorbance was recorded at 700 nm. Increasing absorbance at 700 nm was interpreted as increasing reducing activity. The results were expressed as micrograms of ascorbic acid equivalents (AscAE) per gm of extract. Butylated hydroxy toluene (BHT) and ascorbic acid were used as positive controls. Ascorbic acid was used as the standard control with concentrations of 10, 20, 40, 60, 80 and 100 μg/mL.
Hydroxyl radical scavenging activity assays
Hydroxyl radical scavenging was administered by measuring the comparison between deoxyribose and the extract for hydroxyl radicals generated from the Fe3+/ascorbate/EDTA/H2O2 system, as described by Gulhan et al.[16]. The mixture containing 0.1 mL of seed extract was added to the reaction mixture containing 0.1 mL of 3.0 mmol/L, deoxyribose, 0.5 mL of 0.1 mmol/L, FeCl3, 0.5 mL of 0.l mmol/L, EDTA, 0.5 mL of 0.1 mmol/L, ascorbic acid, 0.5 mL of 1 mmol/L, H2O2 and 0.8 mL of 20 mmol/L, phosphate buffer, pH 7.4, in a total volume of 3.0 mL. The reaction mixture was incubated at 37°C for 1 hour. The formed thiobarbituric acid reactive substances (TBARS) were measured by adding 1.0 mL of thiobarbituric acid (TBA) and 1.0 mL of trichloroacetic acid (TCA) to the test tubes, and incubated at 100°C for 20 minutes. After the mixtures were cooled, absorbance was measured at 532 nm against a control containing deoxyribose and buffer. A blank was carried out in the similar way as the test except test compound. The percent of inhibition (I) of deoxyribose degradation was calculated as:
I = (Absorbance of control-Absorbance of test/Absorbance of control) ×100.
Statistical analysis
Data were given as mean± standard deviation (SD) obtained from 3 independent experiments, and analyzed with Student's t-test for paired data, and a P value less than 0.05 was considered as significant difference in the analysis.
Results
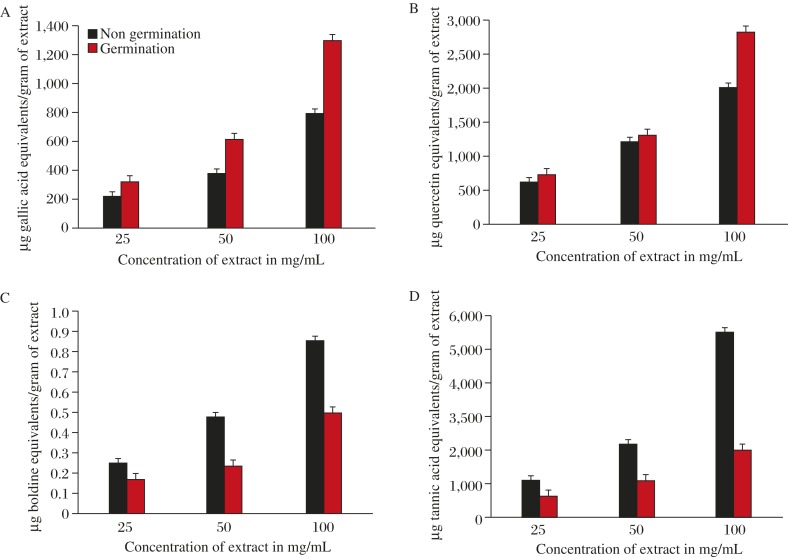
The primary objective of germination was to promote the development of hydrolytic enzymes that were inactive in raw seeds. Even though the success rate of germination was low in our study, the germination percentage of Ceiba pentandra seeds increased with the increase in germination time[17]. The tested levels of phytochemical constituents were influenced by germination and amplified in the increasing concentration from 25 mg/mL to 100 mg/mL except two constituents, including alkaloids and tannins, which were decreased significantly during germination. All the results of analysis mentioned below were at 100 mg/mL. The total phenolic content of germinating seeds was 1,303 µg gallic acid equivalents/g of extract, whereas for raw seeds it was 802.7 µg gallic acid equivalents/g of extract, with t value of 867.757, along with significant P value of 0.000 (2-tailed). The findings are shown in Fig. 1A.
Fig. 1. Phenolic (A), flavonoid (B), alkaloid (C) and tannin (D) content of Ceiba pentandra seed extracts.
Flavonoid content in germinating seeds and raw seeds was assessed as 2,805 µg quercetin equivalents g−1 of extract and 2,003.7 µg quercetin equivalents g−1 of extract, respectively, with t value of 908.627 and significant P value of 0.000 (Fig. 1B).
Alkaloid of germinating and raw seeds was 0.5 µg boldine equivalents g−1 of extract and 0.85 µg boldine equivalents g−1 of extract, respectively, with t value of −12.124 and significant P value of 0.007 (Fig. 1C). Tannin content of Ceiba pentandra germinating and raw seeds was 2,006.3 µg tannic acid equivalents g−1 of extract and 5,507.3 µg tannic acid equivalents g−1 of extract, respectively, with t value of −3501.000 and significant P value of 0.000. (Fig. 1D).
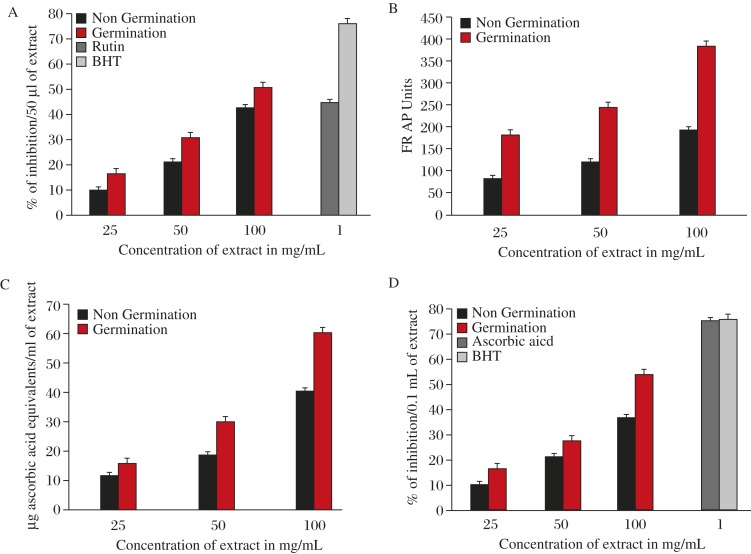
The germination time modified the antioxidant capacity, and all activities of the germinated Ceiba pentandra were increased in a dose dependent manner ranging from 25 to 100 mg/mL. All the outcomes of scrutiny mentioned were at 100 mg/mL, the products for DPPH radical scavenging activity of raw and germinating seeds were 43.5% and 51.3% of inhibition per 50 µL of extract as compared to 45% and 76% of inhibition per 1 mg/mL of Rutin and BHT as positive controls, with t value of 13.440 and significant P value of 0.005 (Fig. 2A).
Fig. 2. DPPH activity (A), FRAP ability (B), reducing activity (C) and hydroxy radical scavenging activity of (D) Ceiba pentandra seed extracts.
For ferric reducing ability power (FRAP), the outcome was observed as 194 and 323 FRAP units for raw and germinating seeds, with t value of 101.657 and significant P value of 0.000. For reducing power assay, the results were observed as 36.6 and 53.6 µg of ascorbic acid equivalents per mL of extract for raw and germinating seeds, respectively, with t value of 48.882 and significant P value of 0.000 (Fig. 2B and Fig. 2C). Finally, hydroxyl radical scavenging activity was inhibited by 40.5% and 60.3% per 0.1 mL of extract for raw and germinating seeds of Ceiba pentandra, respectively, as compared to BHT and ascorbic acid at 1 mg/mL with the percentage of inhibition at 75.6% and 75% in that order, and with t value of 42.439 and significant P value of 0.001 (Fig. 2D).
Discussion
Phenolics or phenolic acids, intermediates in phenylpropanoid metabolism, play important roles in plant cells, tissue and organs[18]. They are also known to be involved in the growth regulation and the process of differentiation and organogenesis. Previous studies have revealed that phenolic compounds are involved in plant development during seed germination, plant-microbe recognition and signal transduction[19]. During seed germination, the phenolic content increased corresponding to the increased antioxidant activity. The antioxidant capacity of phenolic compounds is determined by their structure, in particular the ease with which a hydrogen atom from an aromatic hydroxyl group can be donated to the free radicals[20].The increasing phenolic content indicated that the plant produced precursors for the potential synthesis of lignin[21], and the antioxidant activity of the phenolics extract showed that phenolics in seed were also important as antioxidants when oxygen demand was high during germination. The antioxidant might protect the cell from potential oxidation-induced deterioration.
Flavonoids are low molecular weight, ubiquitously distributed and polyphenolic secondary metabolites. These compounds play significant roles in various stages of plant growth, and they exist under environmental stresses. Recent studies have reaffirmed the link between flavonoids and plant architecture by showing that flavonoid-defective mutants display a wide range of alterations to root and shoot development. Flavonoids are remarkable reactive oxygen species (ROS) scavengers and fight continuously against polluted atmosphere. These metabolites are effective in temperature stress, drought situation, freezing injuries of cell membranes and unusual salinity, and act as signal molecules to take preventive measures to save them from pathogenic microbial attack.
A large group of defensive phytochemicals, many of which have amino acid precursors, are alkaloids25, which are an extremely diverse group of low-molecular weight chemicals that are united by the presence of a heterocyclic ring that contains nitrogen. In many plants, alkaloids are most abundant in seeds compared to other plant tissues. As alkaloids are readily transported through the vascular tissues of plants, alkaloids found in seeds are not necessarily synthesized. Furthermore, alkaloid contents of seeds decrease upon germination, suggesting that they may be useful in the early metabolic activities of the plant. It confirmed that tannin content was reduced after germination, and the acquired results agree well with those reported by El-Adawy for germinated chickpea. The decrease in tannin content observed in the 96-hours germinated seeds indicated the degradation of tannins during germination. Studies of Ramakrishna et al. on Indian bean (Dolichos lablab L.) seeds revealed that the level of tannins reduced considerably with germination, concomitant with the results observed by Idris et al., which infers that germination reduces antinutritional content (phytic acid and tannin) of sorghum.
During germination, various ROS were generated as by products of metabolism. This group of ROS included superoxide radicals (O2˙), hydrogen peroxide radicals (H2O2˙) and hydroxyl radicals (OH;). The formation of these oxygen radicals resulted in the accumulation of lipid hydroperoxides by radical chain oxidation via phospholipids, peroxy radicals within membranes. Free-radical scavengers are antioxidants which can provide protection to living organisms from damage caused by uncontrolled production of ROS and subsequent lipid peroxidation, protein damage and DNA strand breaking. Therefore, it was hypothesized that this could be related to the increase of antioxidant activity in large unilamellar vesicles observed in germinated seeds. All these processes showed a trend of change during germination, with the antioxidant capacity increased with germination. The results are similar to that of Velioglu et al.
Nowadays, the search for medicinally potential natural compounds increased primarily due to their affordable and side effects free validation compared with synthetic drugs. Consequently, the present study highlights the importance of germinating Ceiba pentandra seeds in clinical evaluation, in search as alternative to the commercially available drugs. In conclusion, the seeds of Ceiba pentandra that were rich in phytochemical compounds indicating their key role in the growth of embryonic axis. Moreover, our findings strongly support the hypothesis that antioxidant activities were up-regulated against endogenous oxidant radicals generated during seed germination. The whole biological consequences of these alterations, in particular, low molecular mass antioxidants as well as altered antioxidant defence mechanisms during seed germination, are indistinguishable and should be further investigated.
References
- [1].Black Michael H, Halmer Peter. Wallingford. UK: CABI; 2006. The encyclopedia of seeds: science, technology and uses[M] p. 224. [Google Scholar]
- [2].Kothari V, Seshadri S. In vitro antibacterial activity in seed extracts of Manilkara zapota, Anona squamosa and Tamarindus indica[J]. Biol Res. 2010;43:165–168. [PubMed] [Google Scholar]
- [3].Croteau R, Kutchan TM, Lewis NG. Biochem Mole Biol Plants[M] In: Buchanan, Gruissem W, Joneas R, editors. American Society of Plant Physiologists. Rockville: 2000. pp. 1250–1318. Natural products (secondary metabolites) [Google Scholar]
- [4].Yigit D, Yigit N, Mavi A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels[J] Braz J Med Biol Res. 2009;42(4):346–352. doi: 10.1590/s0100-879x2009000400006. [DOI] [PubMed] [Google Scholar]
- [5].Olusola L, Ike CO, Mariam SJ. Hypoglycaemic properties of aqueous bark extract of ceiba pentandra in streptozotocin induced diabetic rats[J] Ethnopharmacol. 2003;84:139–142. doi: 10.1016/s0378-8741(02)00321-5. [DOI] [PubMed] [Google Scholar]
- [6].Ravi Kiran Chekuboyina, Koteswara rao Pagolu, BhaskarRao Dadi, et al. Physico-Chemical characterization and antimicrobial activity of ceiba pentandra (kapok) seed oil[J] Alt med studs. 2012;2:e9. [Google Scholar]
- [7].Le Page-Degivry MT, Garello G. Embryo dormancy in Taxus baccata: influence of culture medium on initiation of germination[J] Physiol Plant. 1973;29(11):204–207. [Google Scholar]
- [8].Plummer JA, Bell DT. The effect of temperature, light and gibberellic acid (GA3) on the germination of Australian everlasting daisies (Asteraceae, Tribe Inulae)[J] Aust J Bot. 1995;43(1):93–100. [Google Scholar]
- [9].Javanmardi J, Stushnoff C, locke E, et al. Antioxidant activity and total phenolic content of Iranian Ocimum accessions[J] Food Chem 200383(4547–550. [Google Scholar]
- [10].Bao J, Cay Y, Sun M, et al. Anthocyanins, flavonol and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability[J] Agric and Food Chem. 2005;53:2327–2332. doi: 10.1021/jf048312z. [DOI] [PubMed] [Google Scholar]
- [11].Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins[J] J Biol Chem. 1927;73(2):627–650. [Google Scholar]
- [12].Sreevidya Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials[J] J AOAC Int. 2003;86(6):1124–1127. [PubMed] [Google Scholar]
- [13].Cuendet M, Hostettmann K, Potterat O. Iridoidglucosides with free scavenging properties from Fagracablumei[J] Helv Chim Acta. 1997;80:1144–1152. [Google Scholar]
- [14].Benzie IFF, Strain JJ. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay[J] Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- [15].Oyaizu M. Studies on Product of Browning Reaction Prepared from Glucose Amine[J] Jpn J Nutr. 1986;44(2):6307–6315. [Google Scholar]
- [16].Gulhan VU, ferda C, Atalay S, et al. Antimicrobial and Antioxidant Activity of the Essential Oil and Methanol Extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae)[J] Agric Food Chem. 2003;51:63–67. doi: 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- [17].Chekuboyina Ravi Kiran, Dadi Bhaskar Rao, Nagala Sirisha, et al. Impact of germination on biochemical and antioxidant enzymes of Ceiba pentandra seed (Kapok) seeds[J] Am J Plant Sci. 2012;3:1187–1192. [Google Scholar]
- [18].Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism[J] Plant Cell. 1995;7(7):1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lynn DG, Chang M. Phenolic signals in cohabitation: implications for plant development[J] Annu Rev Plant Physiol Plant Mol Biol. 1990;41:497–526. [Google Scholar]
- [20].Soniya Choudhary, Babeet Singh Tanwer, Rekha Vijayvergia. Total Phenolics, flavonoids and antioxidant activity of Tricosanthes cucumeerena Linn[J] Drug Inven Today. 2012;4(5):368–370. [Google Scholar]
- [21].Lewis NG, Yamamoto E. Lignin: occurrence, biosynthesis and biodegradation[J] Annu. Rev Plant Physiol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]