Abstract
Autophagic lysosome reformation (ALR) is a cellular process in which lysosomes are reformed through scission of proto-lysosomes from tubular structures extruded from autolysosomes. Despite recent progress, the molecular mechanism of ALR is far from clear. A paper in this issue of The EMBO Journal has identified lysosome-localized PI(3)P, which is generated by the VPS34–UVRAG complex in an mTOR-dependent manner, as an important regulator of autolysosome tubule scission (Munson et al, 2015).
See also: MJ Munson et al (September 2015)
During autophagy, autophagosomes fuse with lysosomes to form autolysosomes. At the terminal stage of autophagy, lysosomes are recycled from autolysosomes through an evolutionarily conserved cellular process named autophagic lysosome reformation (ALR) (Yu et al, 2010). During ALR, LAMP1-positive tubular structures are extruded from autolysosomes, and proto-lysosomes are recycled from the tubular structures through a budding/fission process (Yu et al, 2010). Initiation of ALR is dependent on mTOR reactivation, which is triggered by degradation of engulfed autophagic cargo (Yu et al, 2010). ALR is governed by complicated molecular machinery with clathrin and PI(4,5)P2 as its central components (Rong et al, 2011, 2012). Intriguingly, PI(4,5)P2 appears to participate in both formation of reformation tubules and scission of proto-lysosomes from reformation tubules.
Despite major efforts to characterize ALR by various groups, our understanding of this process is far from complete. Does the ALR machinery have more components? How does mTOR regulate ALR? What is the physiological function of ALR?
In this issue of The EMBO Journal, Munson et al (2015) have presented new insights into ALR regulation by revealing the key role of PI(3)P in ALR. They started with the observation that PI(3)P is present on a subset of lysosomes, dependent on the kinase VPS34, which is known to be a component of the autophagy initiating machinery. They further showed that treating cells with a VPS34 inhibitor causes formation of LC3/LAMP1 double-positive tubular structures. The fact that these tubular structures are LC3/LAMP1 double positive, and that they are completely abolished by treatment with an mTOR inhibitor, indicated that the tubular structures are linked to ALR. Next, through a set of elegant biochemistry and cell biology studies, they demonstrated that UVRAG, one of the proteins in the VPS34 complex, is phosphorylated by mTORC1 at S550 and S571. Disrupting these two phosphorylation sites reduced the activity of VPS34 and increased the length of the ALR tubules. The authors concluded that through phosphorylation of UVRAG, mTOR regulates generation of PI(3)P on lysosomes, which in turn regulates the scission of ALR tubules. The author speculates that PI(3)P may function through dynamin 2 which is also involved in the scission process (Schulze et al, 2013). It is worth noting that in this study, the authors confirmed that mTOR is required for the initiation of ALR; thus, like PI(4,5)P2, mTOR appears to regulate both initiation and scission of ALR tubules. Interestingly, cells with persistent ALR tubules are much more sensitive to long-term starvation-induced cell death. Based on this observation, the authors proposed that the successful regeneration of lysosomes following starvation is critical for cell survival under prolonged starvation.
There are several highlights in Munson et al’s paper. First, by showing that PI(3)P is present on lysosomes, and by demonstrating the role of VPS34 and PI(3)P in ALR, this work not only reveals a new mechanism for ALR, but also expands our understanding of the function of VPS34 and PI(3)P. Second, it is well established that when mTOR activity is inhibited, the omegasome pool of PI(3)P increases dramatically due to activation of the VPS34–Beclin1–ATG14L PI(3)K complex and autophagy is initiated. However, when mTOR is reactivated at the termination of autophagy, the lysosome pool of PI(3)P increases due to activation of VPS34–Beclin1–UVRAG and results in scission of ALR tubules. Thus, VPS34, while in different complexes with different localizations, acts as a regulatory node to coordinate the initiation and termination of autophagy. Moreover, the authors further illuminate the role of mTOR reactivation in autophagy, showing that it not only functions in ALR tubule initiation, but also in tubule scission (Fig1).
Figure 1.
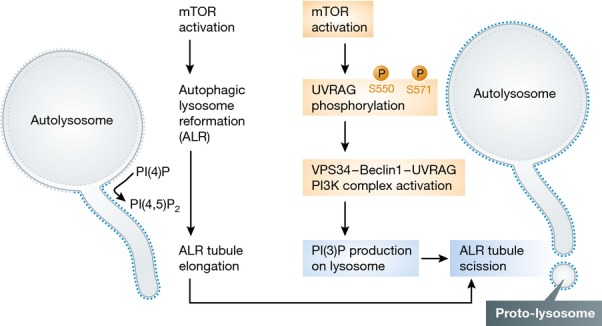
Summary of the regulatory pathway of ALR tubule scission
Two previous studies showed that PI(4)P and PI(4,5)P2 play essential roles in ALR and that the localization of PI(4,5)P2 on reformation tubules is required for tubule scission (Rong et al, 2012; Sridhar et al, 2013). This study adds yet another phosphoinositide, PI(3)P, to the picture, which leads to an intriguing question: Why are so many phosphoinositides required for ALR? Are they working in a synergistic manner? Or are there sequential processes to allow controlled progression of tubulation? Investigating the interplay between different kinases and phosphatases will be very important to answer this question.
As an important mechanism for maintaining lysosome homeostasis, ALR has been speculated to play important pathophysiological roles (Chen & Yu, 2013). Although correlative, the observation that cells with disrupted UVRAG phosphorylation sites have persistent lysosomal tubulation and are much more sensitive to long-term starvation-induced cell death does support the idea that ALR may be an essential cell survival process under cell stress such as prolonged starvation. However, it is very difficult to dissect the physiological role of ALR because most of the known ALR regulators, such as clathrin, also play essential roles in other cellular processes. In this regard, the UVRAG mutant, whose effect on ALR seems to be very specific, looks promising for future studies. Animal models based on this mutant may be highly informative regarding the physiological role of ALR.
References
- Chen Y, Yu L. Autophagic lysosome reformation. Exp Cell Res. 2013;319:142–146. doi: 10.1016/j.yexcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Munson MJ, Allen GF, Toth R, Campbell DG, Lucocq JM, Ganley IG. mTOR activates the VPS34–UVRAG complex to regulate autolysosomal tubulation and cell survival. EMBO J. 2015;34:2272–2290. doi: 10.15252/embj.201590992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, Cao Z, Li Y, Ren H, Zhang C, Li L, Chen S, Xi J, Yu L. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat Cell Biol. 2012;14:924–934. doi: 10.1038/ncb2557. [DOI] [PubMed] [Google Scholar]
- Rong Y, McPhee CK, Deng S, Huang L, Chen L, Liu M, Tracy K, Baehrecke EH, Yu L, Lenardo MJ. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci USA. 2011;108:7826–7831. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze RJ, Weller SG, Schroeder B, Krueger EW, Chi S, Casey CA, McNiven MA. Lipid droplet breakdown requires dynamin 2 for vesiculation of autolysosomal tubules in hepatocytes. J Cell Biol. 2013;203:315–326. doi: 10.1083/jcb.201306140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Patel B, Aphkhazava D, Macian F, Santambrogio L, Shields D, Cuervo AM. The lipid kinase PI4KIIIbeta preserves lysosomal identity. EMBO J. 2013;32:324–339. doi: 10.1038/emboj.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]


