Abstract
Idiopathic epilepsies have frequently been linked to mutations in voltage-gated channels (channelopathies); recently, mutations in several genes encoding presynaptic proteins have been shown to cause epilepsy in humans and mice, indicating that epilepsy can also be considered a synaptopathy. However, the functional mechanisms by which presynaptic dysfunctions lead to hyperexcitability and seizures are not well understood. We show that deletion of synapsin II (Syn II), a presynaptic protein contributing to epilepsy predisposition in humans, leads to a loss of tonic inhibition in mouse hippocampal slices due to a dramatic decrease in presynaptic asynchronous GABA release. We also show that the asynchronous GABA release reduces postsynaptic cell firing, and the parallel impairment of asynchronous GABA release and tonic inhibition results in an increased excitability at both single-neuron and network levels. Restoring tonic inhibition with THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol; gaboxadol), a selective agonist of δ subunit-containing GABAA receptors, fully rescues the SynII−/− epileptic phenotype both ex vivo and in vivo. The results demonstrate a causal relationship between the dynamics of GABA release and the generation of tonic inhibition, and identify a novel mechanism of epileptogenesis generated by dysfunctions in the dynamics of release that can be effectively targeted by novel antiepileptic strategies.
Keywords: asynchronous, epilepsy, GABA, synapsin, tonic
Introduction
Epilepsy is a network phenomenon in which changes in excitability, inhibitory tone, and/or excitation/inhibition balance play a major role. Accordingly, monogenic epilepsies have widely been associated with mutations in voltage- and ligand-gated channels that cause changes in neuronal network excitability (Poduri and Lowenstein 2011). In addition, several genes encoding presynaptic proteins are associated with epilepsy in both humans and mice. STXBP1, a gene encoding Munc18-1, a presynaptic protein that modulates neurotransmitter release, is mutated in Ohtahara syndrome—early infantile epileptic encephalopathy (IEEE; Saitsu et al. 2008; Milh et al. 2011). STXBP1 is also linked with the West syndrome (Otsuka et al. 2010) and non-syndromic epilepsy with mental retardation (Hamdan et al. 2009). Compound mutations of NRXN1, encoding the presynaptic protein neurexin, are associated with severe early-onset epilepsy (Harrison et al. 2011). SV2A, a synaptic vesicle (SV) protein that regulates presynaptic Ca2+ and SV exocytosis (Xu and Bajjalieh 2001), binds the antiepileptic drug levetiracetam (Lynch et al. 2004). Epileptic patients show reduced levels of SV2A in their brain (Feng et al. 2009), and SV2A−/− mice experience severe seizures and die within 3 weeks (Crowder et al. 1999).
Recently, mutations in human SYN1/2 genes encoding synapsins (Syns) I and II, members of a family of SV phosphoproteins that regulate synaptic transmission and plasticity at inhibitory and excitatory synapses (Cesca et al. 2010), have been implicated in epilepsy. Non- and missense mutations in SYN1 were identified as the causes of epilepsy and/or autism in several studies (Garcia et al. 2004; Fassio et al. 2011; Lignani et al. 2013). In addition, genetic mapping analysis identified variations or non-/missense mutations in SYN2 as significantly contributing to epilepsy and autism predisposition (Cavalleri et al. 2007; Lakhan et al. 2010; Corradi et al. 2014). Mice lacking Syn I, Syn II, Syn I/II, or Syn I/II/III develop seizures starting at 2—3 months of age (Li et al. 1995; Rosahl et al. 1995; Gitler et al. 2004), with Syn II deletion producing the most severe phenotype (Corradi et al. 2008; Etholm et al. 2012; Greco et al. 2013). Alterations in inhibition underlie many animal models of epilepsy (Avoli and de Curtis 2011; Pavlov and Walker 2013) and the deletion of Syns in mice severely impairs inhibitory transmission (Gitler et al. 2004; Baldelli et al. 2007; Cesca et al. 2010).
We recently showed that the deletion of Syn II is associated with a specific loss of asynchronous GABA release at inhibitory synapses, and that the desynchronizing action of Syn II is mediated by an interaction with P-/Q-type Ca2+ channels (Medrihan et al. 2013). Here, we investigate how changes in the dynamics of GABA release may lead to hyperexcitability and how this state can be targeted by specific therapeutic strategies. We demonstrate that: (1) the lack of asynchronous GABA release in SynII−/− mice causes a reduction in tonic inhibition that results in hyperexcitability and epileptogenesis and (2) rescuing tonic inhibition by agonists of extrasynaptic GABAA receptors reverts the epileptic phenotype both in vitro and in vivo.
Materials and Methods
Experimental Animals
Syn II knockout (Syn II−/−) mice were generated by homologous recombination and extensively backcrossed on a C57BL/6J background (Charles River, Calco, Italy) for over 10 generations. Experiments were performed on 4- to 8-month-old epileptic Syn II−/− male mice and age-matched C57BL/6J wild-type (WT) animals. All experiments were carried out in accordance with the guidelines established by the European Communities Council (Directive 2010/63/EU of 22 September 2010) and were approved by the Italian Ministry of Health.
Preparation of Slices
After anesthesia with isofluorane, horizontal hippocampal slices (400-μm thickness) from WT and Syn II−/− mice were cut using a Microm HM 650V microtome equipped with a Microm CU 65 cooling unit (Thermo Fisher Scientific, Waltham, MA, USA) at 2–4 °C in a solution containing (in mM): 87 NaCl, 25 NaHCO3, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 25 glucose, 75 sucrose, and saturated with 95% O2 and 5% CO2. After cutting, we let the slices recover for 1 h at 35 °C and for another 2 h at room temperature in recording solution.
Patch-Clamp Recordings
Whole-cell, patch-clamp recordings from dentate gyrus (DG) granule neurons in acute hippocampal mice slices were performed as previously described (Medrihan et al. 2013). Recordings were performed with a Multiclamp 700B/Digidata1440A system (Molecular Devices, Sunnyvale, CA, USA) on visually identified DG cells using an upright BX51WI microscope (Olympus, Tokyo, Japan). We recorded mature DG neurons in which Rm < 300 MΩ. The extracellular solution used for the recordings contained (in mM): 125 NaCl, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2 (bubbled with 95% O2 and 5% CO2). In some experiments, CaCl2 was replaced with an equimolar concentration of SrCl2. For tonic inhibition and spontaneous inhibitory postsynaptic current (sIPSC) recordings, experiments were performed at a holding potential (Vh) of −80 mV in the presence of 50 μM D-(2R)-amino-5-phosphonovaleric acid (D-APV), 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 5 μM CGP 55845 (all from Tocris Bioscience, Ellisville, MO, USA) using a high-chloride intracellular solution containing (in mM): 126 KCl, 4 NaCl, 1 MgSO4, 0.02 CaCl2, 0.1 BAPTA, 15 glucose, 5 HEPES, 3 ATP, and 0.1 GTP in which the pH was adjusted to 7.3 with KOH and osmolarity was adjusted to 290 mOsmol/L with sucrose. For current-clamp experiments, the internal solution contained (in mM): 126 K gluconate, 4 NaCl, 1 MgSO4, 0.02 CaCl2, 0.1 BAPTA, 15 glucose, 5 HEPES, 3 ATP, 0.1 GTP, and pH 7.3. Only cells with resting membrane potential between −70 and −85 mV were considered for analysis. Somatic access resistance was monitored continuously, and cells with unstable access resistance (20% changes) or with values >15 MΩ were excluded from analysis.
All patch-clamp data were acquired with Clampex 10.2 and analyzed offline with Clampfit 10.2 (pClamp, Molecular Devices) and MiniAnalysis (Synaptosoft, Decatur, GA, USA). The analysis of sIPSCs, Aps, and delayed asynchronous release was performed as previously described (Medrihan et al. 2013). For the calculation of the tonic current, 0.5-ms bin histograms of the baseline before and after its shift were constructed and fitted with a single Gaussian. From the fit value, the total tonic current was considered as the reduction in the baseline current after bath addition of 30 μM bicuculline (Glykys and Mody 2006). Neurons were voltage-clamped at −80 mV, and 1 μM (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol; gaboxadol) THIP or 10 μM NO-711 (Tocris Bioscience) was added via bath perfusion.
High-Density Active-Pixel Sensor Recordings
The Active Pixel Sensor Multielectrode Array (APS-MEA) system was extensively characterized in our previous paper (Ferrea et al. 2012). The chip integrates amplification and analog multiplexing circuits designed to provide simultaneous extracellular recordings from 4096 electrodes at a sampling rate of 7.7 kHz per channel. Each square pixel measures 21 × 21 μm, and the array is integrated with an electrode pitch (center-to-center) of 42 μm. Pixels are arranged in a 64 × 64 array configuration, yielding an active area of 7.22 mm2 with a pixel density of 567 pixel/mm2. Three on-chip amplification stages provide a global gain of 60 dB, with a 0.1- to 5-kHz band-pass filter. This bandwidth is adapted to record both slow local field potential (LFP) signals and fast action potentials (APs). Acquisition was controlled using the BrainWave software (3Brain GmbH, Switzerland).
Epileptiform activity was recorded in acute cortico-hippocampal slices in 20 min sessions (once activity had stabilized for at least 30 min). Spontaneous LFPs, in the form of interictal (I-IC) and ictal (IC) in vitro events, were elicited by bath application of 100 μM 4-aminopyridine (4-AP). IC and I-IC events were detected with a previously described Precision Timing Spike Detection (PTSD) algorithm (Maccione et al. 2009). The algorithm, originally tailored to detect fast-spiking activity generated by a few neuronal units, has been adapted to detect slower field potential events. To this purpose, the threshold was set to 5-fold the standard deviation of the noise, whereas the refractory period and the peak lifetime period were set to 50 and 40 ms, respectively. The number of I-IC events was then manually extracted by looking at the raster plot from the whole array.
Activated areas during IC activity for all the experimental conditions were calculated by counting the number of electrodes that, upon detection of the oscillation in the IC events, showed a firing rate within 0.005 and 3 Hz (to eliminate noisy channels). All the analysis algorithms were developed as Matlab scripts (MathWorks, http://www.mathworks.it/), with the exception of the I-IC and IC event detection that was implemented in C# language under Visual Studio (http://www.microsoft.com/visualstudio/en-gb).
Treatment and Seizure Experiments
ALZET-1002 osmotic minipumps, filled with THIP (2.5 mg/kg) or vehicle (artificial cerebrospinal fluid (ACSF)), were implanted subcutaneously to achieve a 1-week continuous treatment with THIP. The THIP dose was equivalent to an initial cerebral concentration of 0.5–1 μM (Cremers and Ebert 2007). Systematic induction of seizures was performed as previously described (Etholm et al. 2012) before the treatment (pre-treatment trial), at the second and seventh day of treatment and 6 weeks after. Seizure provocations consisted of moving the animal from its cage to an adjacent cage. All provocations were recorded on tape with a digital video camera (Panasonic NV-GS180, Tokyo, Japan) angled 45° from above to optimize animal behavior classification. Filmed provocations were later streamed to a personal computer (PC) for digital storage and visually analyzed. Seizure activity was scored using a previously described seizure rating scale for mice (Morrison et al. 1996). Briefly, behavioral feature characteristics of seizures were assessed based on the video recordings as follows: 0 normal behavior; 1 immobility; 2 rigid posture; 3 repetitive scratching, circling, or head bobbing; 4 forelimb clonus, rearing, and falling; 5 level-4 behavior repeated; 6 severe tonic–clonic seizures; and 7 death.
Open Field
Exploratory activity in a novel environment was assessed by a 10-min session in an open field chamber (44 cm L × 44 cm W × 44 cm H) constructed of gray Plexiglas. Locomotor activity in the central and external part of open field was measured by a video camera and analyzed using the ANYmaze program (Ugo Basile, Varese, Italy).
Statistical Analysis
All data are expressed as mean ± SEM. For comparison between WT and Syn II−/− experiments, either the unpaired/paired Student's t-test or the Mann–Whitney U-test was used (whenever the distribution of data fell from normality, as tested with an F-test to compare variances). For multiple comparisons, one-/two-way analysis of variance (ANOVA) was used, followed by the appropriate post hoc test. The level of significance was set at P < 0.05.
Results
Loss of Asynchronous GABA Release Leads to Cellular Hyperexcitability
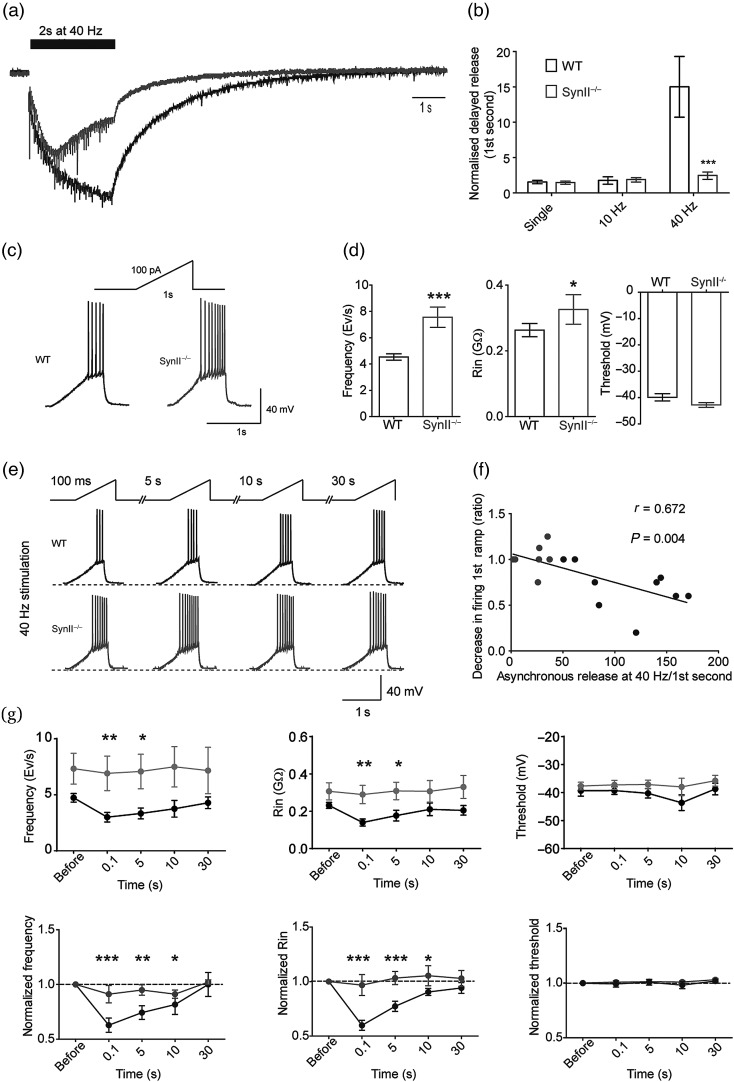
We have recently shown that the Syn II deletion in mice leads to an increase in synchronous release and an almost complete loss of asynchronous GABA release in the DG granule neurons (Medrihan et al. 2013). We further investigated the frequency-dependence and the consequences of this defect in Syn II−/− acute hippocampal slices. We found that, in WT slices, the proportion of delayed asynchronous release is relatively small in response to single stimuli or to low stimulation frequencies (e.g., 10 Hz, not shown), while it strongly increases at higher frequencies (e.g., 40 Hz; Fig. 1a,b). This effect is due to the fact that the stimulus-locked phasic asynchronous GABA release replaces synchronous release due to the intraterminal Ca2+ build-up (Pang and Sudhof 2010) when inhibitory synapses are repeatedly activated. On the contrary, in inhibitory synapses on granule neurons from Syn II−/− adult symptomatic mice, the delayed asynchronous release was almost completely missing and virtually unresponsive to the stimulation frequency (Fig. 1a,b).
Figure 1.
Asynchronous GABA release induced by high-frequency stimulation is lacking in the DG of Syn II−/− mice. (a and b) Example of a delayed asynchronous inhibitory response in DG granule neurons after a 40-Hz train (a) and (b) its mean values at various stimulation frequencies (0.1 Hz, 10 and 40 Hz for 2 s). WT slices respond to the highest stimulation frequency with a marked increase in asynchronous GABA release, while Syn II−/− slices are virtually unresponsive. ***P < 0.001, two-tailed unpaired Student's t-test. (c) Representative responses of WT and Syn II−/− DG neurons to 100 pA/1 s injected current ramps and (d) histograms of mean firing rate, input resistance, and firing threshold of WT (black) and Syn II−/− (gray) DG neurons. WT: n = 10 neurons; Syn II−/−: n = 5 neurons. *P < 0.05; ***P < 0.001, two-tailed Mann–Whitney U-test. (e–g) Effects of 40 Hz stimulation. Representative traces (e) and correlation plot (f) between the delayed asynchronous inhibitory response and the reduction in firing observed after the 40-Hz stimulation (WT: black; Syn II−/−: gray). (g) The delayed asynchronous inhibitory release induced by the train reduced AP firing (left) and Rin (middle) in WT slices (black), but not in Syn II−/− slices (gray) in response to 100 pA injected current, in the absence of significant effects on AP threshold (right). Upper row, absolute values; lower row, normalized values. *P < 0.05; **P < 0.01; ***P < 0.001, one-way ANOVA for repeated measures followed by the Dunnett's post hoc test.
To show the physiological importance of asynchronous GABA release in the regulation of excitability of target cells, we performed single and repetitive stimulations of the perforant path and analyzed the firing patterns of postsynaptic granule neurons in the current-clamp, whole-cell configuration. Granule neurons were depolarized by a 100-pA ramp lasting 1 s before and 100 ms after the extracellular stimulation of the perforant path axons with a single stimulus or at various times (0.1, 5, 10, and 30 s) after a 2-s stimulation train at 10 or 40 Hz (Fig. 1c–e). The initial firing frequency evoked by the depolarizing ramp before the perforant path stimulation was significantly higher in the DG neurons from Syn II−/− slices (4.53 ± 0.24 Hz, n = 9 in WT slices vs. 7.56 ± 0.77 Hz, n = 5 in Syn II−/− slices; P = 0.003, two-tailed Mann–Whitney U-test) and was accompanied by an increase in the input resistance (Rin), with no changes in the AP voltage threshold (Fig. 1c,d). Neither single stimuli, nor a 10-Hz train, did significantly modify the ramp-evoked firing frequency, Rin and AP voltage threshold in WT or Syn II−/− neurons consistent with the minimal amount of asynchronous GABA release induced by the stimulation (data not shown). However, stimulation with the 40-Hz train reduced the AP frequency in the ramp immediately following the train by 37.22 ± 6.51% in WT neurons, while it was totally ineffective in Syn II−/− neurons (Fig. 1e). The reduction in AP frequency observed after the 40-Hz train in WT slices persisted also during the ramps administered at 5 s (25.71 ± 6.22%) and 10 s (18.33 ± 8.9%), but not during the ramp injected at 30 s (0.1 ± 10.1%) after the stimulation train (Fig. 1e,g), a time frame in close agreement with the decay of the delayed asynchronous release induced by the train (Fig. 1). Moreover, the amount of asynchronous GABA release following the 40-Hz train in WT slices was significantly correlated with the observed reduction in the firing frequency (Pearson's r = 0.672, P = 0.004; Fig. 1f). Interestingly, the sustained decrease in AP frequency observed in WT slices was paralleled by a similar reduction in the Rin that was virtually absent in Syn II−/− slices (Fig. 1g). No changes in AP voltage threshold were observed in either genotype and under all experimental conditions (Fig. 1e,g). The data suggest that asynchronous GABA release has a protective role on the postsynaptic cell firing by increasing the duration of inhibition in a manner directly correlated with the presynaptic stimulation frequency.
The Strong Impairment of Asynchronous GABA Release in Syn II−/− Mice Leads to a Complete Loss of Tonic Inhibition
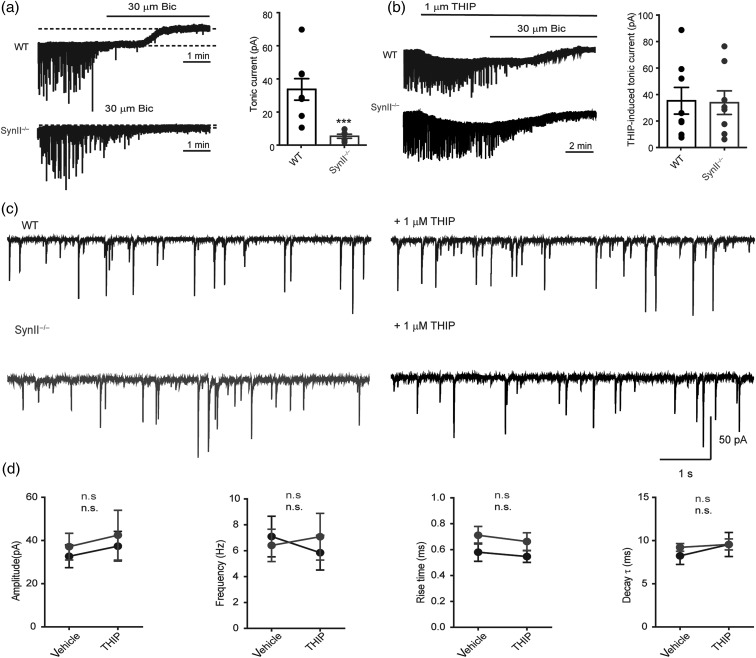
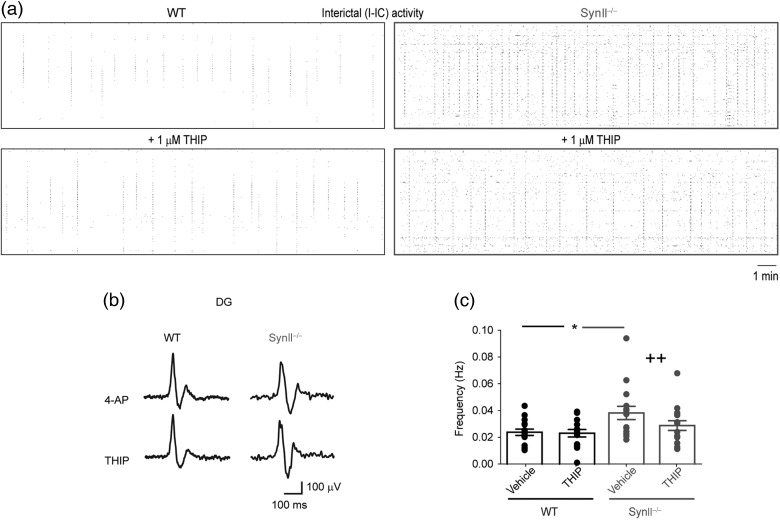
Inhibition mediated by GABA release can be phasic or tonic depending on the postsynaptic receptors that are activated. Our next question was to directly investigate both components of inhibition in the DG neurons of SynII−/− mice. The tonic inhibitory current, measured as the baseline offset following the addition of 30 μM bicuculline, was virtually absent in Syn II−/− DG granule neurons (34.22 ± 6.76 pA, n = 8 in WT slices vs. 4.82 ± 1.27 pA, n = 6 in Syn II−/− slices; P = 0.0007, two-tailed Mann–Whitney U-test) (Fig. 2a), while no difference was detected between genotypes on phasic inhibitory current (Fig. 2c,d). The current hypothesis behind tonic inhibition proposes that the spillover of GABA from the synaptic cleft results in the activation of extrasynaptic GABAA receptors localized a few micrometers away from the synapse and displaying high affinity for GABA (Nusser and Mody 2002; Farrant and Nusser 2005). In the DG granule cells, tonic inhibition is exclusively mediated by extrasynaptic GABA receptors containing the δ subunit (Nusser and Mody 2002). To investigate whether the expression of these receptors was altered in Syn II−/− mice, we used gaboxadol (THIP), a selective agonist for δ subunit-containing extrasynaptic GABAA receptors that induce a tonic conductance in DG granule neurons (Wei et al. 2003). Indeed, addition of THIP (1 μM) induced an inhibitory tonic current in the Syn II−/− neurons equal to the one induced in WT slices (36.00 ± 10.53 pA, n = 8 in WT slices vs. 34.57 ± 9.27 pA, n = 8 in Syn II−/− slices; P = 0.200, two-tailed Mann–Whitney U-test) (Fig. 2b), in the absence of detectable effects on the phasic inhibitory current (Fig. 2c,d). These results suggest that: (1) the loss of tonic inhibition in Syn II−/− mice is not due to impairments of the postsynaptic site, but to dysfunctions of presynaptic GABA release and (2) tonic inhibition seems to be predominantly caused by the asynchronous component of GABA release.
Figure 2.
Tonic inhibition is specifically lost in the DG of Syn II−/− mice and can be rescued upon stimulation of extrasynaptic GABAA receptors. (a) Representative traces of tonic inhibitory currents in WT and Syn II−/− DG granule neurons (left) and the respective histogram showing the complete loss of tonic inhibition in acute slices from Syn II−/− mice (WT: black; Syn II−/−: gray). (b) Rescue of the tonic inhibitory current in the Syn II−/− DG to WT levels by the δ-subunit GABAA agonist THIP (1 μM). Representative traces of tonic inhibitory currents in WT and Syn II−/− DG granule neurons treated with THIP (left) and the respective histogram are shown. Each dot represents an individual neuron. (c) Representative traces of spontaneous inhibitory PSCs recorded in DG granule neurons from WT (black) and Syn II−/− (gray) mice before and after bath application of 1 μM THIP. (d) The results of paired experiments show that no changes in the main sIPSC parameters such as amplitude, frequency, rise time, and decay constant occur after the addition of THIP in both genotypes, consistent with the exclusive extrasynaptic expression of GABAA receptors containing the δ-subunit for which THIP is an agonist. ***P < 0.001, Two-tailed paired Student's t-test was used for statistics. n = 6 neurons/genotype.
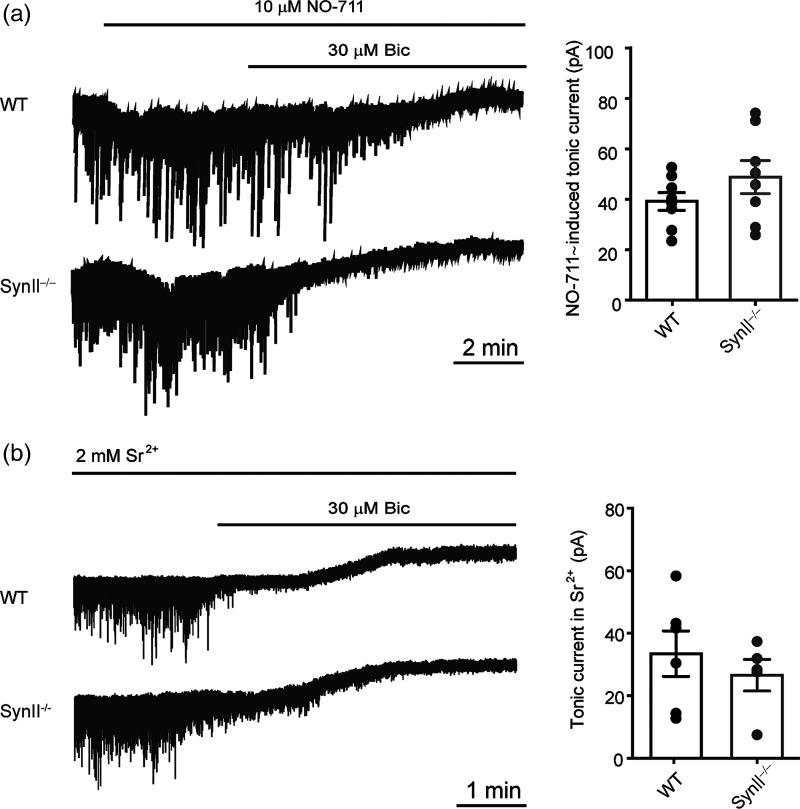
To address the question of whether asynchronous GABA release is indeed the major determinant of the tonic inhibitory current, we used a pharmacological approach to rescue asynchronous release in Syn II−/− mice and test for the parallel recovery of tonic inhibition. First, blockade of the GABA transporter 1 (GAT-1) with NO-711 (10 μM), which inhibits GABA removal from the synaptic cleft, restored the tonic GABA current in Syn II−/− slices to values similar to those observed in WT slices (42.23 ± 3.91 pA, n = 8 in WT slices vs. 52.95 ± 6.95 pA, n = 8 in Syn II−/− slices; P = 0.878, two-tailed Mann–Whitney U-test) (Fig. 3a), strongly suggesting that an impairment of GABA release/spillover is indeed responsible for the loss of tonic inhibition observed in the DG of Syn II−/− mice.
Figure 3.
Tonic inhibition in the DG of Syn II−/− mice is fully recovered by inhibition of GABA uptake or by rescuing asynchronous release. (a) Representative traces (left) of tonic inhibitory currents in WT and Syn II−/− DG granule neurons in the presence of the GAT-1 blocker NO-711 (10 μM) and the respective histograms (right) showing the recovery of tonic inhibition in Syn II−/−. (b) Desynchronization of GABA release by extracellular Sr2+ rescues tonic inhibition in the DG of Syn II−/− mice. Representative traces (left) of tonic inhibitory currents in WT and Syn II−/− DG granule neurons and the respective histograms (right) showing that tonic inhibition is rescued in Syn II−/− slices when extracellular Ca2+ is replaced with an equimolar concentration of Sr2+. Each dot represents an individual neuron.
Next, we attempted to directly demonstrate the functional link between the loss of asynchronous release and the loss of tonic inhibition. To this aim, we desynchronized GABA release by replacing extracellular Ca2+ with an equimolar concentration of Sr2+, which displays a similar affinity for the distinct presynaptic Ca2+ sensors responsible for synchronous and asynchronous release (Goda and Stevens 1994; Atluri and Regehr 1998; Xu-Friedman and Regehr 1999, 2000). This experimental maneuver was previously demonstrated to partially rescue the delayed asynchronous GABA release in SynII−/− DG neurons (Medrihan et al. 2013). In the presence of Sr2+, the tonic inhibitory current measured in Syn II−/− slices, virtually absent under control conditions, returned to levels similar to those in WT slices (36.25 ± 7.96 pA n = 6 in WT slices vs. 27.38 ± 5.37 n = 5 in Syn II−/− slices, P = 0.404, two-tailed Mann–Whitney U-test) (Fig. 3b; cfr. with Fig. 2a). These results demonstrate a direct link between the dynamics of presynaptic GABA release and extrasynaptic tonic inhibition.
Rescue of Tonic Inhibition by THIP Decreases the Hyperexcitability of DG Granule Neurons in Syn II−/− Mice
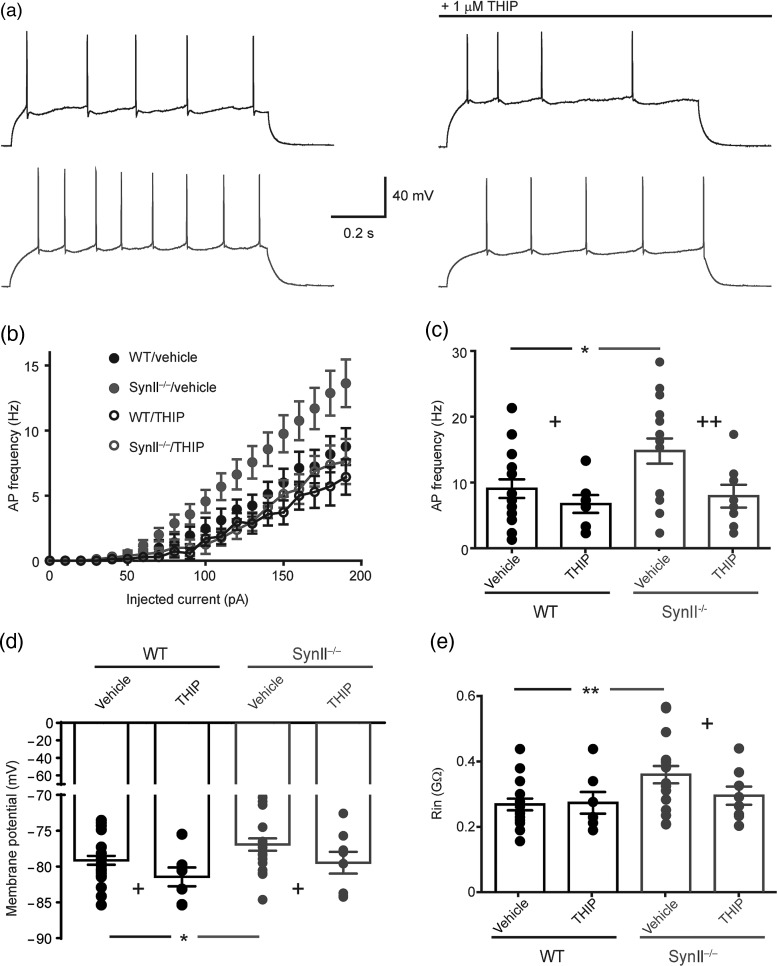
If the hyperexcitability of Syn II−/− mice is indeed due to the specific loss of tonic inhibition due to the altered release dynamics, then boosting tonic inhibition in Syn II−/− cortico-hippocampal slices should rescue hyperexcitablity at both single granule cell and entire network levels. To this aim, we chose THIP that specifically enhances the activity of δ-containing GABAA receptors expressed in granule cells and, partially, in the CA1 pyramidal cells (Semyanov et al. 2004) and is more specific to the hippocampus than NO-711 or Sr2+ that have ubiquitous synaptic action. Current-clamp experiments showed that, consistent with a lack of tonic inhibition (Mitchell and Silver 2003), the AP firing rate of Syn II−/− granule neurons was increased (at 200 pA injected current: WT: 8.76 ± 1.4 Hz; Syn II−/−: 14.47 ± 1.9 Hz; n = 17 slices/genotype; P = 0.011, vehicle across genotype, two-way ANOVA followed by the Bonferroni's post hoc test). Such increased firing was normalized by the addition of THIP (1 μM) that was virtually ineffective in WT neurons (WT + THIP: 6.42 ± 1.3 Hz, P = 0.414 vs. vehicle within genotype; Syn II−/− + THIP: 7.62 ± 1.7 Hz, P = 0.006 vs. vehicle within genotype; P = 0.603, THIP effect across genotype, two-way ANOVA followed by the Bonferroni's post hoc test) (Fig. 4a–c).
Figure 4.
THIP rescues Syn II−/− DG granule neurons from hyperexcitability. (a) Whole-cell, current-clamp recordings from WT (black) and Syn II−/− (gray) showing AP firing upon injection of 200 pA before and after the addition of 1 μM THIP. (b and c) Mean frequency–current plot (b) and histogram (c) depicting the increased excitability of Syn II−/− DG granule neurons and its normalization in response to the application of 1 μM THIP. (d and e) The analysis of membrane potential and input resistance shows that the addition of 1 μM THIP brings both the depolarized membrane potential (d) and the increased input resistance (e) of Syn II−/− DG granule neurons to WT levels. *P < 0.05, **P < 0.01 across genotype; +P < 0.05, ++P < 0.01, effect of treatment within genotype; two-way ANOVA followed by the Bonferroni's post hoc test.
The analysis of the passive neuronal parameters showed that the increased firing rate of the Syn II−/− granule neurons was due both to a more depolarized membrane potential (WT: −78.83 ± 0.6 mV, n = 26 slices; Syn II−/−: −76.23 ± 0.9 mV, n = 22 slices; P = 0.023, vehicle across genotype; two-way ANOVA followed by the Bonferroni's post hoc test) and to an increased membrane resistance (WT: 0.267 ± 0.01 GΩ, n = 26 slices; Syn II−/−: 0.323 ± 0.01 GΩ, n = 22 slices; P = 0.010, vehicle across genotype; two-way ANOVA followed by the Bonferroni's post hoc test). The addition of THIP normalized both the depolarized membrane potential and the increased input resistance of Syn II−/− granule neurons, while it was ineffective in WT neurons, thus abolishing the difference between genotypes in both parameters (membrane potential: WT + THIP: −81.44 ± 1.3 mV, n = 7 slices, P = 0.111 vs. vehicle within genotype; Syn II−/− + THIP: −79.46 ± 1.5 mV, n = 8 slices, P = 0.047 vs. vehicle within genotype; P = 0.325, THIP across genotype; input resistance: WT + THIP: 0.272 ± 0.01 GΩ, n = 7 slices, P = 0.808 vs. vehicle within genotype; Syn II−/− + THIP: 0.294 ± 0.01 GΩ, n = 8 slices, P = 0.035 vs. vehicle within genotype; P = 0.388, THIP across genotype; two-way ANOVA followed by the Bonferroni's post hoc test; Fig. 4d,e).
Rescue of Tonic Inhibition by THIP Ameliorates Epileptogenic Activity in Cortico-hippocampal Slices From Syn II−/− Mice
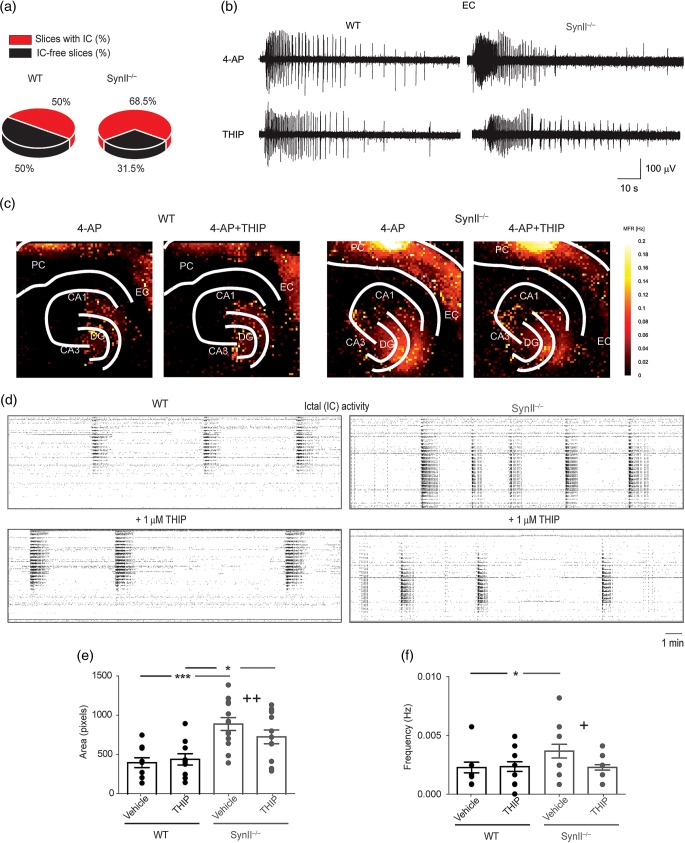
We next investigated the effects of THIP-induced enhancement of tonic inhibition on the epileptiform activity in the cortico-hippocampal network using a high-resolution microelectrode array (Ferrea et al. 2012). Approximately 5% of untreated acute cortico-hippocampal slices from symptomatic Syn II−/− mice showed spontaneous epileptic-like activity that was never observed in WT slices from age-matched mice (not shown). Quantifiable seizure-like activity in vitro was reproducibly obtained by treating slices with the K+ channel blocker 4-AP (100 μM) (Avoli and de Curtis 2011; Figs 5 and 6). In both genotypes, acute treatment with 4-AP led to the generation of I-IC activity consisting of brief (<200 ms) and synchronous field potential discharges (Fig. 5a,b) and long-lasting ictal (IC) activity originating in the rhinal cortices and propagating to the hippocampus (Fig. 6a–d). The percentage of slices exhibiting IC events was significantly higher in Syn II−/− than in WT slices (P = 0.014, χ2 test with Yates correction) (Fig. 6a) and also spread over a wider area in the rhinal cortices (Fig. 6c,e; WT: 407.9 ± 64.03 electrode pixels, n = 10 slices; Syn II−/−: 910.6 ± 83.72 electrode pixels, n = 13; P = 0.0002 across genotype, two-way ANOVA followed by the Bonferroni's post hoc test). The frequency of I-IC events (Fig. 5c; WT: 0.0224 ± 0.002 Hz and Syn II−/−: 0.031 ± 0.002 Hz, n = 16 slices/genotype, P = 0.013 across genotype, two-way ANOVA followed by the Bonferroni's post hoc test) and also the frequency of IC discharges (Fig. 6f; WT: 0.0021 ± 0.0005 Hz, n = 10 slices; Syn II−/−: 0.0040 ± 0.0005 Hz, n = 13; P = 0.040 across genotype, two-way ANOVA followed by the Bonferroni's post hoc test) were all significantly higher in Syn II−/− than in WT slices
Figure 5.
THIP reduces the I-IC epileptiform activity in acute slices from Syn II−/− mice. (a) Raster plots depicting I-IC activities induced by 100 μM 4-AP and recorded over the entire 4096 electrode field in WT (black frame) and Syn II−/− (gray frame) slices before and after THIP treatment. Note the reduction in the frequency of I-IC events induced by THIP in the Syn II−/− networks. (b) Representative traces showing 4-AP-induced I-IC activities in the EC and DG of WT and Syn II−/− slices before and after the administration of 1 μM THIP. Traces are taken from one representative electrode per area. (c) The application of 1 μM THIP reduces the increased frequency of I-IC events in Syn II−/− slices to WT levels. *P < 0.05 across genotype; ++P < 0.01, effect of treatment within genotype; two-way ANOVA followed by the Bonferroni's post hoc test.
Figure 6.
THIP rescues the epileptic-like IC activity in acute brain slices from Syn II−/− mice. (a) Occurrence of 4-AP-induced IC events in WT and Syn II−/− slices (P = 0.014, χ2 test with Yates correction). (b and c) Representative traces of IC activity induced by 100 μM 4-AP in the EC of WT and Syn II−/− slices before and after the administration of 1 μM THIP (b) and its regional distribution in the hippocampus and rhinal cortices (c) calculated from APS-MEA recordings (color-coded mean firing rate recorded from each 40-μm pixel electrode; see Materials and Methods). (d) Raster plots depicting highly synchronized IC activities recorded over the entire 4096 electrode field in WT (black frame) and Syn II−/− (gray frame) slices before and after THIP treatment. Note the reduction in the frequency induced by THIP in the Syn II−/− slices. (e and f) Bath application of 1 μM THIP reduces the epileptic area (e) and the increased frequency of IC events (f) in Syn II−/− slices to WT levels. *P < 0.05; ***P < 0.001 across genotype, +P < 0.05, ++P < 0.01, effect of treatment within genotype; two-way ANOVA followed by Bonferroni's post hoc test. DG, dentate gyrus; CA3, cornu ammonis 3; CA1, cornu ammonis 1; EC, entorhinal cortex; PC, perirhinal cortex.
An increase in tonic inhibition by acute administration of THIP normalized the epileptic phenotype of Syn II−/− slices treated with 4-AP. In Syn II−/− slices, THIP (1 μM) significantly decreased: (1) The frequency of I-IC events (Fig. 5a–c; WT + THIP: 0.0224 ± 0.002 Hz, P = 0.940 vs. vehicle within genotype; Syn II−/− + THIP: 0.024 ± 0.002 Hz, P = 0.042 vs. vehicle within genotype; P = 0.219, THIP effect across genotype, two-way ANOVA followed by the Bonferroni's post hoc test); (2) the active spread area of IC events (Fig. 6c,e; WT + THIP: 451.2 ± 73.51 electrode pixels, P = 0.727 vs. vehicle within genotype; Syn II−/− + THIP: 744.2 ± 89.76 electrode pixels, P = 0.001 vs. vehicle within genotype; P = 0.024, THIP across genotype, two-way ANOVA followed by the Bonferroni's post hoc test); and (3) the frequency of IC discharges (Fig. 6d,f; WT + THIP: 0.0022 ± 0.0005 Hz, P = 0.798 vs. vehicle within genotype; Syn II−/− + THIP: 0.0020 ± 0.0001 Hz, P = 0.031 vs. vehicle within genotype; P = 0.790, THIP across genotype; two-way ANOVA followed by the Bonferroni's post hoc test). Interestingly, the acute treatment with THIP markedly decreased both I-IC and IC activities in Syn II−/− slices lacking endogenous tonic inhibition by bringing them to WT levels, while it had no detectable effects on I-IC frequency, IC spread, or IC frequency in WT slices bearing physiological levels of tonic inhibition.
In Vivo Rescue of Tonic Inhibition by THIP Reverts the Epileptic Phenotype of Syn II−/− Mice
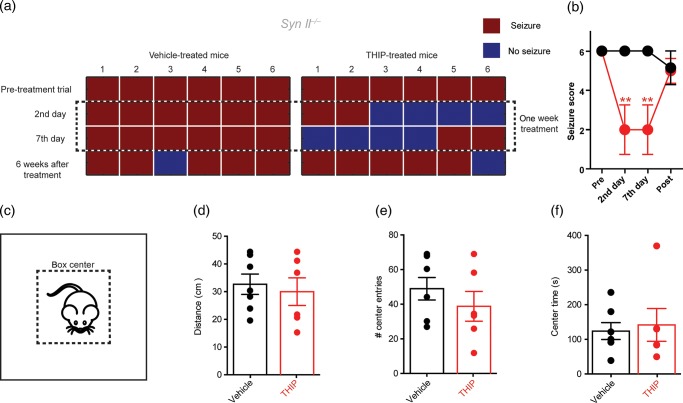
Syn II−/− mice are generally healthy and have a normal life span. However, after 2 months of age, they develop seizures that, rarely spontaneous, are triggered by novel stimuli such as handling of the cage and/or the mouse (Corradi et al. 2008; Etholm et al. 2012). Based on the above results, we tested whether boosting tonic inhibition by in vivo THIP administration had any effects on the propensity and severity of evoked seizures in Syn II−/− mice. Four- to 8-month-old epileptic Syn II−/− mice were subcutaneously implanted with osmotic minipumps filled with either vehicle or THIP to achieve a subchronic treatment for 1 week at dose equivalent to an effective cerebral concentration of 0.5–1 μM (Cremers and Ebert 2007). Seizures were systematically triggered by moving the animal from its cage to an adjacent cage before and at various times after the onset of the treatment and a seizure score was given. We started with all the mice having the highest seizure score (6), which represent a tonic–clonic seizure. Owing to the adaptive characteristic of seizures in Syn KO mice (Etholm et al. 2012), only one provocation for each animal was performed at the stated time points.
Interestingly, 1-week treatment with THIP rescues the evoked seizures in Syn II−/− mice with respect to vehicle-treated littermates at 2nd and 7th day of treatment (n = 6 mice/group, P = 0.006 for THIP-treated and 0.415 for vehicle-treated in both the 2nd and 7th day of treatment; repeated-measures ANOVA followed by the Dunnett's post hoc test; Fig. 7a,b). Virtually, all mice from the initial batch did not present seizure for at least one provocation during the treatment (Fig. 7a). The marked amelioration of the epileptic phenotype of Syn II−/− mice was reversible upon discontinuation of the THIP treatment and their seizure propensity recovered to the pre-treatment level 6 weeks after the treatment. One mouse per each group was still seizure-free after 6 weeks, probably due to the habituation with the handling protocol. No effects of the THIP treatment were detected on the animals' locomotor behavior evaluated in an open field in terms of distance covered, number of entries in the central area, and time spent in the central area (Fig. 7c–f).
Figure 7.
THIP rescues the epileptic seizures of Syn II−/− mice. (a) Experimental protocol of 1-week minipump treatment with THIP (2.5 mg/kg) or vehicle depicting the occurrence (red squares) of evoked seizures in Syn II−/− mice treated with either vehicle or THIP. (b) Seizure score (0–7; see Materials and Methods) of Syn II−/− mice tested before, in the second and seventh day and after the treatment with vehicle (black) or THIP (red). Means ± SEM of the seizure scores are shown. (c) Scheme of the gray Plexiglas open-field chamber with the central area (dotted line). (d–f) Histograms of total covered distance (d), number of entrances in the central part (e), and time spent in the central part (f) by Syn II−/− mice treated with either vehicle (black) or THIP (red). Each dot represents an individual mouse. Open-field experiments were performed on the seventh day of treatment. **P < 0.01; one-way ANOVA for repeated measures followed by the Dunnett's post hoc test.
Discussion
Several mutations in presynaptic proteins have been associated with seizures in human and animal models, but the synaptic mechanisms by which these mutations lead to network hyperexcitability are poorly understood. Our results show that the lack of a presynaptic protein, Syn II, leads to a loss of the tonic inhibitory current, an efficient mean by which networks control their excitability. Tonic inhibition relies on the stimulation of extrasynaptic GABAA receptors by GABA spilled over from inhibitory synapses and diffused through the extracellular volume. Evoked GABA release occurs in two modalities: Time-locked synchronous release mediated by a low-affinity/fast-kinetics Ca2+-sensor and delayed asynchronous release linked to a high-affinity/slow-kinetics Ca2+-sensor. Asynchronous release, already present in the response to single stimuli, becomes predominant at high stimulation frequencies and can last for hundreds of milliseconds (Atluri and Regehr 1998).
To unravel the link between GABA release and the generation of tonic inhibition, we used high-frequency stimulation to boost asynchronous release in WT and SynII−/− mice, a model of genetic epilepsy that was recently shown to specifically lack asynchronous GABA release in the DG of the hippocampus (Medrihan et al. 2013). The results demonstrate a tight and causal link between asynchronous GABA release and tonic inhibition. We found that the dramatic decrease in asynchronous GABA release is responsible for the virtual loss of tonic inhibition in the hippocampus of Syn II−/− mice, resulting in hyperexcitability and epileptogenesis. Boosting asynchronous release in WT slices with a 40-Hz tetanic stimulation significantly decreased the excitability of granule neurons after the train, with a time course compatible with the diffusion of asynchronously released GABA to act at extrasynaptic receptors. Notably, the role of asynchronous GABA release in the generation of the tonic GABA current was further demonstrated in Syn II−/− slices that are unable to respond to the tetanus with asynchronous GABA release and, accordingly, do not show any reduction in excitability after the tetanus. The reduction in the AP frequency induced by the delayed asynchronous GABA release in WT neurons was caused by changes in the input resistance, but not in the threshold of AP initiation of the respective neurons, thus mimicking the functional effects of tonic inhibition on excitability.
Such a link between asynchronous release and tonic inhibition could play an important physiological role in the control of network excitability. Being strongly frequency-dependent, asynchronous GABA release can provide an inhibitory tuning whose efficacy increases with the frequency of presynaptic activity (Hefft and Jonas 2005; Balakrishnan et al. 2009; Volman et al. 2010; Capogna and Pearce 2011). Computational studies have also proposed that the inhibitory asynchronous release is able to change the postsynaptic gain for a relatively prolonged period after presynaptic activity has subsided (Volman et al. 2010), but a direct proof for this functional role has remained elusive. Importantly, recent results have confirmed the existence of asynchronous GABA release in both rat and human fast-spiking inhibitory neurons (Fang and Chen 2012; Jiang et al. 2012) and proposed its involvement in the desynchronization of neural networks (Manseau et al. 2010). In both human epileptic tissue and a rat model of epilepsy, asynchronous release is increased in fast-spiking neurons and appears to play a protective role in the regulation of epileptiform activities (Fang and Chen 2012; Jiang et al. 2012). Why asynchronous, and not synchronous, release provides the major contribution to the tonic inhibitory current? The absence of asynchronous release in the DG of Syn II−/− mice, in which inhibitory transmission is characterized by increased phasic and loss of tonic inhibitory current, unambiguously demonstrates the pivotal role of this GABA release mode in the generation of tonic inhibition. It is possible that asynchronously released GABA is less susceptible of reuptake, more prone to diffusion in the extrasynaptic volume and undergoes temporal summation due to its slow kinetics.
We recently reported that a marked excitatory/inhibitory imbalance and loss of tonic inhibition precedes the appearance of the epileptic phenotype and further proceeds during epileptogenesis in the CA1 region of the hippocampus of synapsin triple knockout mice (Syn I,II,III−/−) (Farisello et al. 2013). Such hyperexcitability was associated with I-IC and IC discharges occurring both spontaneously and in response to 4-AP (Boido et al. 2010). In the DG of single Syn II−/− mice, we found a similar hyperexcitability at both single granule cell level and network level that were accompanied by a similar marked loss in tonic inhibition. This indicates that the specific loss of the Syn II isoform recapitulates the decrease in tonic inhibition observed in Syn I,II,III−/− (Farisello et al. 2013). Also, it seems that the action of Syn II on the DG circuitry may be time and cell-dependent. For example, while here we report an increase firing of granule cells, granule cell firing is normal in the presymptomatic phase of SynII−/− mice; moreover, the other principal excitatory neurons of the DG, the hilar mossy cells, present hypoexcitability in both presymptomatic and symptomatic stages (Toader et al. 2013). However, the latter reduced excitability of mossy cells will eventually lead to a decreased feed-forward inhibition onto granule cells (Toader et al. 2013), suggesting that the overall excitation/inhibition imbalance is intimately connected to the circuitry, regardless of the differential effect on the activity of specific neuronal populations. The developmental regulation of the tonic GABAA conductance over the first postnatal weeks may be one other explanation for the lack of seizures in young Syn II−/−. Indeed, some studies reported that the tonic inhibitory current increases with age in cerebellar granule neurons, dentate granule cells, and relay neurons of hypothalamus (see Bright and Smart 2013, for review). Another study reports a decrease in the total tonic current in the adult DG granule cells, but accompanied by an increase in the GABAA δ-subunit-mediated component (Holter et al. 2010), consistent with an increased expression of δ-subunit in the adult hippocampus (Laurie et al. 1992). Moreover, the expression of synapsins is developmentally regulated, and Syn II slowly and steadily increases its expression level after birth to reach a plateau “adult” level only alter 1–2 months (Bogen et al. 2009). We may speculate that both the tonic inhibition and the expression of Syn II in key regions controlling network excitability and seizure development, such as the DG, play a minor role at younger ages than they do in the adulthood, thus explaining the late appearance of the epileptic phenotype of Syn II−/− mice.
Tonic inhibition is often decreased in epilepsy, depending on the cellular targets and the type of epilepsy (Walker and Kullmann 2012). Both tonic inhibition and asynchronous GABA release are known to modulate the input–output behavior of single neurons and have a protective role in epilepsy (Jiang et al. 2012; Walker and Kullmann 2012). Thus, the causal relationship between these 2 trans-synaptic inhibitory mechanisms not only extends our knowledge on the physiological regulation of network excitability, but can also open the path to new therapeutic strategies. The main therapeutic approaches to increase tonic inhibition are either to target the specific GABAA receptor subtypes that mediate it or to increase the extracellular GABA concentrations (e.g., with the antiepileptic drug tiagabine or vigabatrine) (Walker and Kullmann 2012).
As the primary defect in Syn II−/− and Syn I,II,III−/− mice seems to be an impairment of GABA release and tonic inhibition, while synaptic and extrasynaptic GABAA receptors are not affected (Farisello et al. 2013), we attempted to rescue the epileptic phenotype in vitro and in vivo, by selectively enhancing tonic inhibition. So far, it has been impossible to target and specifically correct asynchronous GABA release and the classical way to enhance asynchronous release by replacing extracellular Ca2+ with Sr2+ cannot distinguish between excitatory and inhibitory transmission (Goda and Stevens 1994; Atluri and Regehr 1998; Xu-Friedman and Regehr 1999, 2000). Thus, to specifically enhance tonic inhibition, we used the selective agonist of extrasynaptic GABAA receptors, THIP. Interestingly, a drug similar to THIP acting on the δ subunit-containing GABAA receptors, the synthetic neurosteroid ganaxolone, has recently entered clinical trial for the treatment of epilepsy (Bialer et al. 2010).
The effective rescue of the seizure phenotype in Syn II−/− mice after stimulation of δ subunit-containing GABAA receptors by THIP demonstrates a potential applicability of this treatment to the forms of epilepsy associated with an impaired tonic inhibition. Extrasynaptic GABA receptors are localized a few micrometers away from the synapse and possess an unusually high affinity for GABA (Nusser et al. 1998; Nusser and Mody 2002; Mody and Pearce 2004). Activation of these receptors accounts for an inhibitory charge approximately 4–5 times higher than the charge obtained by the summation of phasic inhibition (Mody and Pearce 2004). The importance of the tonic inhibitory conductance mediated by δ subunit-containing GABAA receptors is emphasized by the hyperexcitability and epileptic phenotype of δ−/− mice (Spigelman et al. 2002) and by the association of polymorphisms in GABAA δ subunits in humans with familial generalized epilepsies (Dibbens et al. 2004). Since the δ subunit of GABAA receptors is regulated by endogenous neurosteroids (Ferando and Mody 2012), this class of substances may also have new potential clinical applications in epilepsy.
An important question is whether the same mechanisms can be found in mice or patients displaying mutations on other presynaptic proteins involved in epilepsy and seizures. Syn II changes the dynamics of GABA release by interacting with presynaptic Ca2+ channels, and all the proteins whose mutations have been found in epileptic patients are connected with the Ca2+-dependent release. Munc 18-1 interacts with syntaxins (Hata and Sudhof 1995), SNARE proteins that regulate SV exocytosis, and the mutants of Munc 18-1 alter the kinetics of neurotransmitter release (Ciufo et al. 2005; Burgoyne et al. 2009). Moreover, an open mutation of Syntaxin-1B leads to generalized seizures and subsequent death at 3 months in mice (Gerber et al. 2008). Neurexin 1 is controlling the coupling of Ca2+ channels with the presynaptic machinery (Missler et al. 2003). SV2A regulates the Ca2+-dependent SV exocytosis (Xu and Bajjalieh 2001) and interacts with synaptotagmins (Schivell et al. 2005), presynaptic Ca2+-sensors involved in changing the ratio between synchronous and asynchronous release (Pang and Sudhof 2010; Wen et al. 2010). Thus, alterations in the release machinery that lead to changes in the kinetics of neurotransmitter release may be connected with network hyperexcitability possibly through a change in tonic inhibition, as we propose here. However, additional studies based on other presynaptic proteins involved in the regulation of release dynamics and whose mutations lead to seizures are required to establish if this is a general mechanisms of regulation of neuronal excitability.
Funding
This study was supported by research grants from the Italian Ministry of University and Research (PRIN to F.B. and P.B.), the Italian Ministry of Health Progetto Giovani (to P.B.), and the Compagnia di San Paolo-Torino (to F.B. and P.B.). The support of EU FP7 Integrating Project “Desire”, Cure Epilepsy “Advanced Innovator Award” (to F.B.), and Telethon-Italy (grants GGP09134 and 13033 to F.B. and GGP09066 to P.B.) is also acknowledged. Funding to pay the Open Access publication charges for this article was provided by Fondazione Telethon Italia.
Notes
We thank Drs Hung-Teh Kao (Brown University, Providence, RI, USA) and Paul Greengard (The Rockefeller University, New York, NY, USA) for providing us with the Syn II mutant mouse strain. We also thank Dr. Luca Berdondini, Andrea Barberis, and Gabriele Lignani for useful discussions. Conflict of interest: The authors declare no competing financial interests.
References
- Atluri PP, Regehr WG. 1998. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 18:8214–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. 2011. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 95:104–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Kuo SP, Roberts PD, Trussell LO. 2009. Slow glycinergic transmission mediated by transmitter pooling. Nat Neurosci. 12:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F. 2007. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci. 27:13520–13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. 2010. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res. 92:89–124. [DOI] [PubMed] [Google Scholar]
- Bogen IL, Jensen V, Hvalby O, Walaas SI. 2009. Synapsin-dependent development of glutamatergic synaptic vesicles and presynaptic plasticity in postnatal mouse brain. Neuroscience. 158(1):231–241. [DOI] [PubMed] [Google Scholar]
- Boido D, Farisello P, Cesca F, Ferrea E, Valtorta F, Benfenati F, Baldelli P. 2010. Cortico-hippocampal hyperexcitability in synapsin I/II/III knockout mice: age-dependency and response to levitiracetam. Neuroscience. 171:268–283. [DOI] [PubMed] [Google Scholar]
- Bright DP, Smart TG. 2013. Methods for recording and measuring tonic GABAA receptor-mediated inhibition. Front Neural Circuits. 7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Barclay JW, Ciufo LF, Graham ME, Handley MT, Morgan A. 2009. The functions of Munc18-1 in regulated exocytosis. Ann N Y Acad Sci. 1152:76–86. [DOI] [PubMed] [Google Scholar]
- Capogna M, Pearce RA. 2011. GABA A, slow: causes and consequences. Trends Neurosci. 34:101–112. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, Grinton B, Szoeke C, Murphy K, Kinirons P, O'Rourke D, et al. 2007. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol. 6:970–980. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. 2010. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 91:313–348. [DOI] [PubMed] [Google Scholar]
- Ciufo LF, Barclay JW, Burgoyne RD, Morgan A. 2005. Munc18-1 regulates early and late stages of exocytosis via syntaxin-independent protein interactions. Mol Biol Cell. 16:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi A, Fadda M, Piton A, Patry L, Marte A, Rossi P, Cadieux-Dion M, Gauthier J, Lapointe L, Mottron L, et al. 2014. SYN2 is an autism predisposing gene: loss-of-function mutations alter synaptic vesicle cycling and axon outgrowth. Hum Mol Genet. 23:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F. 2008. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J Cell Sci. 121:3042–3051. [DOI] [PubMed] [Google Scholar]
- Cremers T, Ebert B. 2007. Plasma and CNS concentrations of gaboxadol in rats following subcutaneous administration. Eur J Pharmacol. 562:47–52. [DOI] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. 1999. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci USA. 96:15268–15273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, et al. 2004. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 13:1315–1319. [DOI] [PubMed] [Google Scholar]
- Etholm L, Bahonjic E, Walaas SI, Kao HT, Heggelund P. 2012. Neuroethologically delineated differences in the seizure behavior of Synapsin 1 and Synapsin 2 knock-out mice. Epilepsy Res. 99:252–259. [DOI] [PubMed] [Google Scholar]
- Fang Q, Chen Z. 2012. Increased asynchronous GABA release causes more inhibition in human epileptic brain? Acta Pharmacol Sin. 33:859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farisello P, Boido D, Nieus T, Medrihan L, Cesca F, Valtorta F, Baldelli P, Benfenati F. 2013. Synaptic and extrasynaptic origin of the excitation/inhibition imbalance in the hippocampus of synapsin I/II/III knockout mice. Cereb Cortex. 23:581–593. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. 2005. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 6:215–229. [DOI] [PubMed] [Google Scholar]
- Fassio A, Patry L, Congia S, Onofri F, Piton A, Gauthier J, Pozzi D, Messa M, Defranchi E, Fadda M, et al. 2011. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet. 20:2297–2307. [DOI] [PubMed] [Google Scholar]
- Feng G, Xiao F, Lu Y, Huang Z, Yuan J, Xiao Z, Xi Z, Wang X. 2009. Down-regulation synaptic vesicle protein 2A in the anterior temporal neocortex of patients with intractable epilepsy. J Mol Neurosci. 39:354–359. [DOI] [PubMed] [Google Scholar]
- Ferando I, Mody I. 2012. GABAA receptor modulation by neurosteroids in models of temporal lobe epilepsies. Epilepsia. 53(Suppl 9):89–101. [DOI] [PubMed] [Google Scholar]
- Ferrea E, Maccione A, Medrihan L, Nieus T, Ghezzi D, Baldelli P, Benfenati F, Berdondini L. 2012. Large-scale, high-resolution electrophysiological imaging of field potentials in brain slices with microelectronic multielectrode arrays. Front Neural Circuits. 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA. 2004. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet. 41:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, et al. 2008. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 321:1507–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. 2004. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 24:11368–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. 2006. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor alpha5 subunit-deficient mice. J Neurophysiol. 95:2796–2807. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. 1994. Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA. 91:12942–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco B, Manago F, Tucci V, Kao HT, Valtorta F, Benfenati F. 2013. Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res. 251:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan FF, Piton A, Gauthier J, Lortie A, Dubeau F, Dobrzeniecka S, Spiegelman D, Noreau A, Pellerin S, Cote M, et al. 2009. De novo STXBP1 mutations in mental retardation and nonsyndromic epilepsy. Ann Neurol. 65:748–753. [DOI] [PubMed] [Google Scholar]
- Harrison V, Connell L, Hayesmoore J, McParland J, Pike MG, Blair E. 2011. Compound heterozygous deletion of NRXN1 causing severe developmental delay with early onset epilepsy in two sisters. Am J Med Genet A. 155A:2826–2831. [DOI] [PubMed] [Google Scholar]
- Hata Y, Sudhof TC. 1995. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J Biol Chem. 270:13022–13028. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. 2005. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 8:1319–1328. [DOI] [PubMed] [Google Scholar]
- Holter NI, Zylla MM, Zuber N, Bruehl C, Draguhn A. 2010. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur J Neurosci. 32:1300–1309. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhu J, Liu Y, Yang M, Tian C, Jiang S, Wang Y, Guo H, Wang K, Shu Y. 2012. Enhancement of asynchronous release from fast-spiking interneuron in human and rat epileptic neocortex. PLoS Biol. 10:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan R, Kalita J, Misra UK, Kumari R, Mittal B. 2010. Association of intronic polymorphism rs3773364 A>G in synapsin-2 gene with idiopathic epilepsy. Synapse. 64:403–408. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. 1992. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 12:4151–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, et al. 1995. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA. 92:9235–9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignani G, Raimondi A, Ferrea E, Rocchi A, Paonessa F, Cesca F, Orlando M, Tkatch T, Valtorta F, Cossette P, et al. 2013. Epileptogenic Q555X SYN1 mutant triggers imbalances in release dynamics and short-term plasticity. Hum Mol Genet. 22:2186–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. 2004. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 101:9861–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccione A, Gandolfo M, Massobrio P, Novellino A, Martinoia S, Chiappalone M. 2009. A novel algorithm for precise identification of spikes in extracellularly recorded neuronal signals. J Neurosci Methods. 177:241–249. [DOI] [PubMed] [Google Scholar]
- Manseau F, Marinelli S, Mendez P, Schwaller B, Prince DA, Huguenard JR, Bacci A. 2010. Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fast-spiking interneurons. PLoS Biol. 8:e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F. 2013. Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun. 4:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milh M, Villeneuve N, Chouchane M, Kaminska A, Laroche C, Barthez MA, Gitiaux C, Bartoli C, Borges-Correia A, Cacciagli P, et al. 2011. Epileptic and nonepileptic features in patients with early onset epileptic encephalopathy and STXBP1 mutations. Epilepsia. 52:1828–1834. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. 2003. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 423:939–948. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. 2003. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 38:433–445. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. 2004. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 27:569–575. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Wenzel HJ, Kinoshita Y, Robbins CA, Donehower LA, Schwartzkroin PA. 1996. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 16:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Mody I. 2002. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 87:2624–2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. 1998. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 18:1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Oguni H, Liang JS, Ikeda H, Imai K, Hirasawa K, Tachikawa E, Shimojima K, Osawa M, Yamamoto T. 2010. STXBP1 mutations cause not only Ohtahara syndrome but also West syndrome—result of Japanese cohort study. Epilepsia. 51:2449–2452. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Sudhof TC. 2010. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 22:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, Walker MC. 2013. Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology. 69:55–61. [DOI] [PubMed] [Google Scholar]
- Poduri A, Lowenstein D. 2011. Epilepsy genetics—past, present, and future. Curr Opin Genet Dev. 21:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. 1995. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 375:488–493. [DOI] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, et al. 2008. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 40:782–788. [DOI] [PubMed] [Google Scholar]
- Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. 2005. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci. 29:56–64. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. 2004. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 27:262–269. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. 2002. Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 43(Suppl 5):3–8. [DOI] [PubMed] [Google Scholar]
- Toader O, Forte N, Orlando M, Ferrea E, Raimondi A, Baldelli P, Benfenati F, Medrihan L. 2013. Dentate gyrus network dysfunctions precede the symptomatic phase in a genetic mouse model of seizures. Front Cell Neurosci. 7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, Levine H, Sejnowski TJ. 2010. Shunting inhibition controls the gain modulation mediated by asynchronous neurotransmitter release in early development. PLoS Comput Biol. 6:e1000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Kullmann DM. 2012. Tonic GABAA receptor-mediated signaling in epilepsy. In: Jasper's basic mechanisms of the epilepsies (Internet). 4th ed. Bethesda (MD): National Center for Biotechnology information (US). [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. 2003. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 23:10650–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Linhoff MW, McGinley MJ, Li GL, Corson GM, Mandel G, Brehm P. 2010. Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proc Natl Acad Sci USA. 107:13906–13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Bajjalieh SM. 2001. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol. 3:691–698. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. 1999. Presynaptic strontium dynamics and synaptic transmission. Biophys J. 76:2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. 2000. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 20:4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]