Abstract
Pattern vision deprivation (BD) can induce permanent deficits in global motion perception. The impact of timing and duration of BD on the maturation of the central and peripheral visual field representations in cat primary visual areas 17 and 18 remains unknown. We compared early BD, from eye opening for 2, 4, or 6 months, with late onset BD, after 2 months of normal vision, using the expression pattern of the visually driven activity reporter gene zif268 as readout. Decreasing zif268 mRNA levels between months 2 and 4 characterized the normal maturation of the (supra)granular layers of the central and peripheral visual field representations in areas 17 and 18. In general, all BD conditions had higher than normal zif268 levels. In area 17, early BD induced a delayed decrease, beginning later in peripheral than in central area 17. In contrast, the decrease occurred between months 2 and 4 throughout area 18. Lack of pattern vision stimulation during the first 4 months of life therefore has a different impact on the development of areas 17 and 18. A high zif268 expression level at a time when normal vision is restored seems to predict the capacity of a visual area to compensate for BD.
Keywords: area 17, area 18, binocular deprivation, central visual field representation, peripheral visual field representation
Introduction
Several events that drive the development of the visual cortex of mammals have been intensely investigated since the inspiring work by Hubel and Wiesel in the cat (Wiesel 1982). The anatomical and functional formation of ocular dominance columns and the establishment of fine acuity vision have been described in detail for the central visual field representation in the primary visual cortex (V1, area 17, for review, see Hensch 2005). However, in the peripheral visual field representation in area 17 the formation of ocular dominance columns most likely starts later. Early monocular deprivation from eye opening (P8-10) induces ocular dominance plasticity in central area 17, yet in far peripheral area 17 this only occurs when the monocular deprivation starts after P35 (Hata et al. 2000; Schmidt et al. 2002). Two other indicators of a slower development of peripheral area 17 are the greater developmental synapse elimination in central than in peripheral area 17 between the age of 2 and 7 months (O'Kusky 1985) and the centro-peripheral difference in N-methyl-d-aspartate receptor subunit expression between the age of 6 and 12 weeks (Beston et al. 2010). These findings are not surprising when considering the development of the retina, the first stage of visual processing. In its central region, all ganglion cells are already present in a newborn kitten and reach their adult size by P20, while in the peripheral retina neurogenesis continues up to postnatal week 3 and the ganglion cells reach their adult size only by P80 (Johns et al. 1979; Rapaport and Stone 1983a,b; Walsh et al. 1983; Walsh and Polley 1985). This developmental central-to-peripheral gradient is thus well characterized for retina yet far less often described at the cortical level, where most of the investigations were focused on the central cortical region including the area centralis representation (e.g., Sherk and Stryker 1976; Mullikin et al. 1984; Jin et al. 2008; Liu et al. 2013; Martin and Schroder 2013; Xu et al. 2013). A cortical central-to-peripheral maturation gradient during normal development has been observed in marmoset monkey (Bourne et al. 2005). In adult marmoset, V1 neurons representing the peripheral visual field were also shown to be specialized more for motion processing than neurons in the central visual field representation that process high acuity vision (Yu et al. 2010; Yu and Rosa 2013). A dominating population of neurons located within the peripheral visual field representation in cat area 17 is also motion sensitive, similar to the neurons of the neighboring motion-sensitive area 18 (Stone and Dreher 1973; Dreher et al. 1980, 1992; Orban et al. 1981; Humphrey et al. 1985; Pasternak and Maunsell 1992; Ribot et al. 2013).
We recently observed that in cats, timing and duration of binocular deprivation from pattern vision (BD) are 2 determinants of the severity of the behavioral outcome, a motion-perception impairment (Burnat et al. 2002; Zapasnik and Burnat 2013). Early long-term BD resembles human congenital cataract, which in contrast to delayed onset cataract, results in a severe impairment in global perception if left untreated (Burnat et al. 2002; Ellemberg et al. 2002). In our cat model for congenital cataract, these behavioral impairments occur together with permanent changes in the number and dendritic tree stratification of Y-type alpha retinal ganglion cells (Burnat et al. 2012). Altogether these observations made us hypothesize that BD early in life might particularly disrupt the normal development of the motion-sensitive primary cortical areas, area 18 and peripheral area 17.
We applied in situ hybridization for measuring changes in the visually driven expression of the activity reporter gene zif268, to visualize possible differences in activation of the central and peripheral region of primary visual cortices 17 and 18 as a function of age and visual manipulation. The immediate early gene (IEG) zif268 has been used frequently as a marker of neuronal activity in the visual cortex (for review, see Sheng and Greenberg 1990; Worley et al. 1991; Zhang et al. 1994, 1995; Kaczmarek and Chaudhuri 1997; Herdegen and Leah 1998; Arckens et al. 2000; Hu et al. 2009; Takahata et al. 2009). To assess the timing of the maturation of normal activation patterns we first compared the visual cortex of 1, 2, 4, and 6-month-old and adult cats reared under normal visual conditions. To scrutinize the outcome of BD, 4 different deprivation strategies were compared, including early onset BD from birth and lasting for 2, 4, or 6 months, and late onset BD for 2 months upon 2 months of normal vision, as animal models of congenital and delayed onset cataract, respectively.
Normal visual experience was characterized by an age-dependent decrease in zif268 expression in layers 2/3 and 4 of areas 17 and 18, which lasted until the fourth month of age, with no explicit central–peripheral differences in developing an adult-like zif268 expression pattern. In general, early BD animals did display higher than normal zif268 levels in the (supra)granular layers of area 17 and 18 thereby best resembling younger controls. Early BD delayed the developmental decrease in zif268 expression in both area 17 regions, and this delay was greater in peripheral than in central area 17. In contrast, in area 18 the course of the delayed decrease of the zif268 signal was the same in both regions and finished within 4 months of age, just like in normal subjects.
Methods
Animals and Visual Deprivation Paradigm
All experiments were carried out in accordance with the European Parliament and the Council Directive of 22 September 2010 (2010/63/EU). The cats were raised under a daily photoperiod of 12 h light and 12 h darkness with water and food ad libitum (Nencki Institute, Warsaw, Poland). All efforts were made to minimize animal discomfort.
To investigate development-related zif268 expression profiles, cats with normal visual experience were analyzed (n = 15): kittens of 1 (1N, n = 3), 2 (2N, n = 3), 4 (4N, n = 3), or 6 months (6N, n = 3), and adult cats of 1–2 years (n = 3). Binocular deprivation from pattern vision (n = 12) was always achieved by having the cats wearing double thickness linen masks covering their eyes. This procedure reduces retinal illumination to a similar level as lid suturing, but is less traumatic (Kossut et al. 1978). For deprivation from birth, the early onset groups, the masks were put on from eyelid opening (P8) and removed at the age of 2 (2BD, n = 2), 4 (4BD, n = 3), or 6 months (6BD, n = 3). Animals from the delayed onset group were deprived for the third and fourth month of life after 2 initial months of normal visual input (2N2BD, n = 3). The masks were replaced daily in a normally lit animal facility room where the kittens lived. The changing procedure lasted no longer than 1 min per day for each cat, which is not sufficient to maintain normal vision (Schwarzkopf et al. 2007; Mitchell et al. 2011) and allowed constant adjustment of the size of the masks to the growing head. The kittens remained with their mothers until the age of 7 weeks, and then they were moved to large cages (3 × 2.6 × 2.35 m) where they played and interacted freely.
IEG Expression Induction
All animals were maintained overnight in total darkness followed by 1-h light stimulation upon removal of the hoods to induce maximal visually driven IEG expression in the visual cortex (Arckens et al. 2000; Van der Gucht et al. 2002). During the 1-h light stimulation cats were placed in a well-lit room. After an overdose of sodium pentobarbital (Nembutal, 60 mg/kg) brains were dissected, instantly frozen by immersion in dry cooled isopentane (Merck Eurolab) and stored at −70°C. Twenty-five micrometer-thick coronal sections were made with a cryostat (Microm HM 500 OM, Walldorf, Germany) and collected on baked and 0.1% poly-l-lysine (Sigma-Aldrich, St. Louis, MO) coated glass slides, 1 section per slide, and stored at −20°C.
In Situ Hybridization
Radioactive in situ hybridization was performed with a degenerated oligonucleotide probe complementary to the cat zif268 gene (5′-ccgttgctcagcagcatcatctcctccagyttrgggtagttgtcc-3′). The in situ hybridization experiments were performed as described previously (Arckens et al. 2000; Hu et al. 2009). Briefly, after postfixation with 4% paraformaldehyde in phosphate buffered saline (0.1 M, pH 7.4) slide-mounted sections were dehydrated and delipidated. The sections were then incubated overnight at 38°C with hybridization solution containing the 3′-end terminal transferase 33P-dATP labeled probes specific for zif268. The next day, sections were thoroughly washed with 1× standard saline citrate buffer at 43°C, dehydrated and exposed to an autoradiographic Bio Max film (Kodak, Zaventem, Belgium). Several in situ hybridization experiments were run to label all necessary sections. Sections were assigned randomly to the different experiments to exclude interexperimental variation as a confounding factor. After 3 weeks the films were developed following standard procedures. The images were scanned with a HP Precision scan Scanjet 5300C at a resolution of 1200 dpi.
Image Analysis
The level of zif268 mRNA signal was quantified in regions of interest by ImageJ software v1.45s and calibrated by means of an autoradiographic [14C] microscale standard apposed to each of the autoradiographic films (Amersham Biosciences UK Limited, Little Chalfont, UK) according to manufacturer's instructions to calculate relative optical density (OD) values. The autoradiographic [14C] microscale standard was used to allow straightforward comparison of data from autoradiograms across the different experiments as relative OD values. Zif268 in situ hybridization labels all layers of areas 17 and 18, except layer 1 (Figs 1–3). The borders between the cortical layers were determined based on Nissl stainings performed on the very same sections, which previously underwent in situ hybridization (Fig. 1B and C; Peters and Regidor 1981; Beaulieu and Colonnier 1983). We chose not to correct the relative OD values from the in situ hybridization images for cell density since Nissl-staining does not enable to differentiate between excitatory and inhibitory cells and zif268 is selectively expressed in excitatory neurons, as shown by Chaudhuri (1995). Furthermore, Beaulieu and Colonnier (1983) showed that there is no statistical significant difference in numerical densities of neurons (the number of neurons per unit volume of tissue) between layers 2, 3, 4, and 6 in the binocular region of adult cat area 17, substantiating that the observed interlaminar zif268 expression pattern changes do not reflect changes in cell density.
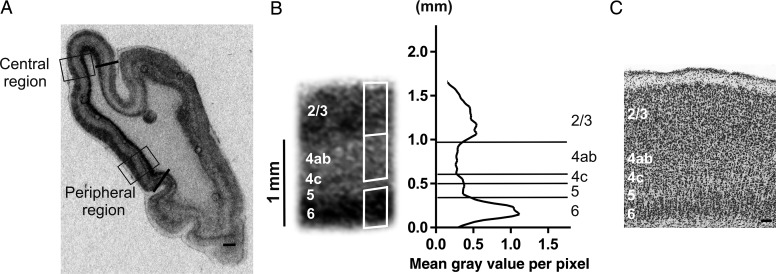
Figure 1.
Illustration of the quantitative analysis of the zif268 in situ hybridization results for cat area 17. (A) Coronal brain section at Horsley–Clarke level posterior 6 (P6, Rosenquist 1985). Areal borders of area 17 are marked by black lines, boxes indicate the regions of interest: the central (C) and peripheral (P) visual field representation in area 17, which are placed within the binocular zone (up to 10° and 15–20°, respectively). (B) Example image of the zif268 mRNA signal from the central visual field representation accompanied by a line graph in which the mean gray value per pixel is plotted in function of cortical depth. Cortical layers (indicated on the left) are based on Nissl counterstaining. White rectangles demarcate lamina-specific zif268 OD quantification regions. Cortical layers are separated by horizontal lines, the Y-axis represents the distance from the underlying white matter in millimeter. (C) Nissl staining of the central region of area 17 with cortical layers 2/3, 4, 5, and 6 enumerated, scale bars (100 μm).
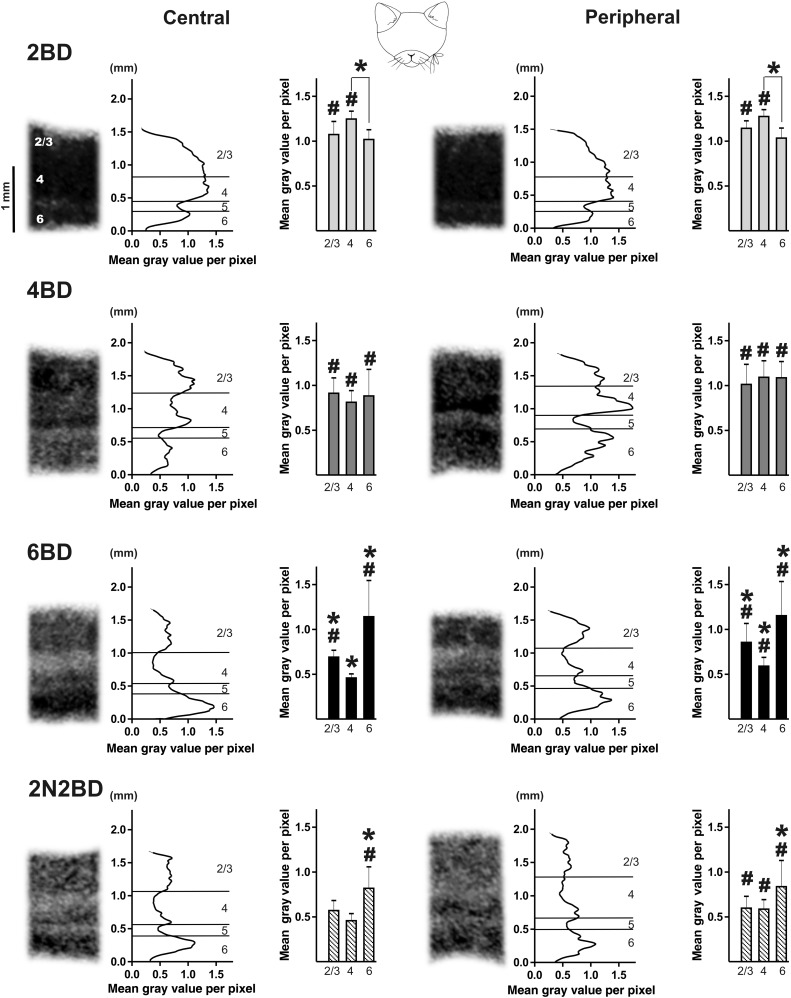
Figure 3.
Illustration of the zif268 mRNA expression patterns, optical density line plots and the average interlaminar zif268 mRNA expression pattern of area 17 for each group of binocularly deprived cats. Zif268 mRNA levels in the cortical layers of central and peripheral area 17, acquired at Horsley–Clarke level posterior 6 (P6, Rosenquist 1985). Line graphs represent the relative zif268 mRNA levels in each of the pictures as the mean gray value per pixel was measured on calibrated section images. Y-axes represent the distance from the underlying white matter in millimeter. Bar graphs represent the average interlaminar zif268 mRNA expression pattern for each group. Note the adult-like interlaminar pattern of zif268 expression is visible in 6BD and 2N2BD cats. *P < 0.01; differences between BD groups and age-matched controls are indicated by #P < 0.01. Results are means with ±SD.
Two sections were taken from each cat at Horsley–Clarke level posterior 6 (P6, Rosenquist 1985) for measurements in area 17. Central and peripheral area 18 were analyzed on sections at Horsley–Clarke level posterior 5.6–7.8 (P5.6–P7.8, Rosenquist 1985). Regions of interest in areas 17 and 18 included the central and peripheral visual field representations within the binocular zone (up to 10 and 15–20°, respectively) as illustrated by rectangles in Figures 1A and 4C.
Figure 4.
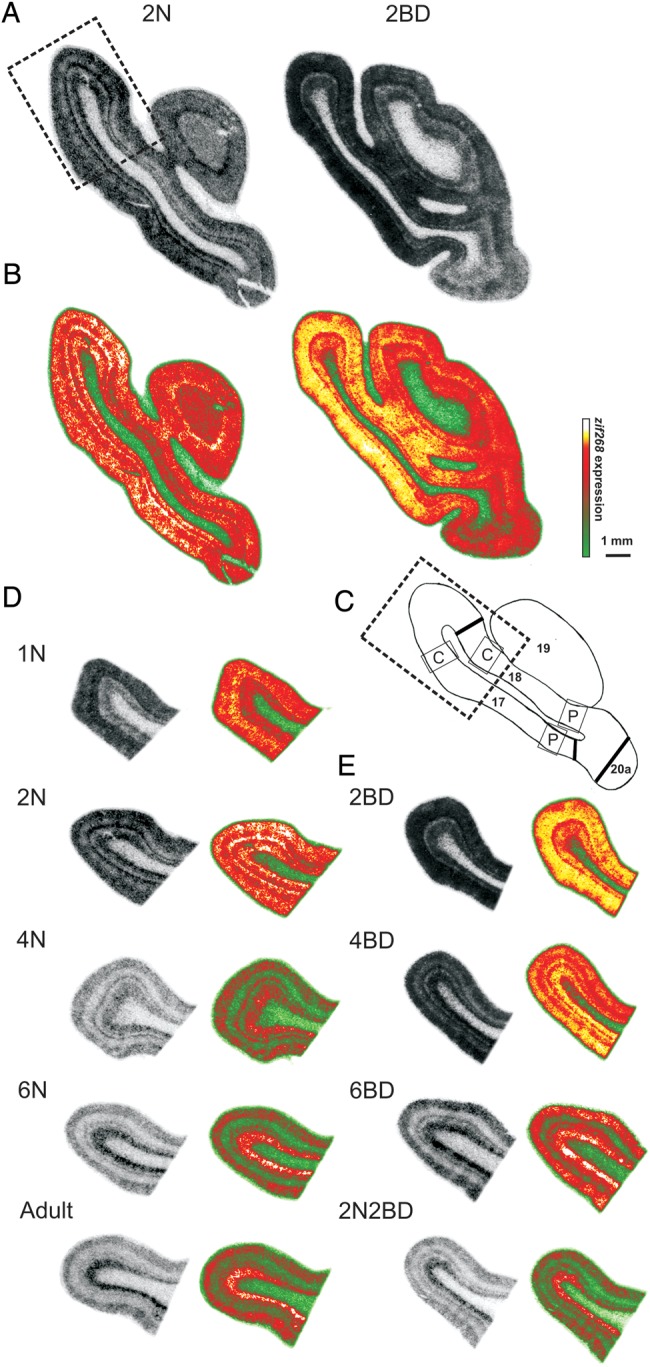
Illustration of the quantitative analysis of the zif268 in situ hybridization results for area 18 as compared with area 17. (A and B) Coronal brain sections of 2N and 2BD kittens at Horsley–Clarke level posterior 7 (P7, Rosenquist 1985) seen as on in situ hybridization autoradiograms (A) and in pseudo-color (B). Note the higher zif268 expression level in 2BD kittens as compared with 2N in area 17 and area 18. (C) Areal borders are marked at Horsley–Clarke level posterior 7.8 (P7.8, Rosenquist 1985) by black lines, boxes indicate the central (C) and peripheral (P) visual field representation in the binocular zone of area 17 and area 18 (up to 10° and 15–20° respectively). The dotted rectangle marks the transition zone between central area 17 and area 18. (D and E) The transition zone between central area 17 and area 18 from a representative section for each group of normal (D) and BD cats (E).
To illustrate interlaminar differences in zif268 expression we plotted profiles of mean gray value changes across the width of the cortex for 1 representative calibrated section for each group of animals by ImageJ software v1.45s (Figs 1B, 2 and 3). In Figures 1B, 2 and 3 the X-axis of each line graph represents the mean gray values across the length of each layer in the corresponding in situ hybridization image. The thickness of the cortex is measured in millimeter and presented in the Y-axis.
Figure 2.
Illustration of the zif268 mRNA expression patterns, optical density line plots and the average interlaminar zif268 mRNA expression pattern of area 17 for each group of normal cats. Zif268 mRNA levels in the cortical layers of central and peripheral area 17, acquired at Horsley–Clarke level posterior 6 (P6, Rosenquist 1985). Line graphs represent the relative zif268 mRNA levels in each of the pictures as the mean gray value per pixel was measured on calibrated section images. Y-axes represent the distance from the underlying white matter in millimeter. Bar graphs represent the average interlaminar zif268 mRNA expression pattern for each group. Note that the interlaminar pattern typical for adult animals was characterized by the highest zif268 mRNA level in layer 6 and was established from the age of 6 months. *P < 0.01; results are means with ± SD.
To quantify lamina-specific zif268 OD in layers 2/3, 4, and 6 inside each region of interest (central and peripheral area 17 and area 18) 3 nonoverlapping rectangles of constant width (0.3 mm) were put along the width of each layer (Fig. 1B), with the height of the rectangles adapted to layer thickness (L2/3 and 4: 0.4–0.8 mm; L6: 0.3–0.6 mm).
To illustrate the differences in zif268 OD values between area 17 and area 18 we present pseudo-color images of the transition zone between central area 17 and 18 at Horsley–Clarke level posterior 7.8 (P7.8, Rosenquist 1985) in Figure 4 (dotted rectangles). Next to in situ hybridization images are corresponding pseudo-color maps, generated by ImageJ software v1.45s, that represent a false coloring of the gray values: a low gray value is represented in green, a high gray value in white/yellow, indicating a low signal response or a high signal response, respectively. This is done in accordance with a gray scale ranging from black (0) to white (255).
Statistics
Statistical analysis of zif268 expression was performed using a nested-design ANOVA model to investigate the effects of group, area, layer, region, and section nested in cat by means of data analysis software system STATISTICA version 10 (Pavlidis 2003). This nested-factorial design was based on the linear model written as:
where m is the overall mean; Cg is the effects of gth cat; Si(g) is the effects of ith section nested in gth cat; Gj is the effects of jth group of animals; Ak is the effects of kth area; Lm is the effects of mth layer; Rn is the effects of nth region; GAjk is interaction effect between the jth group of animals and the kth area; GLjm is interaction effect between the jth group of animals and the mth layer; GRjn is interaction effect between the jth group of animals and the nth region; ALkm is interaction effect between the kth area and the mth layer; ARkn is interaction effect between the kth area and the nth region; LRmn is interaction effect between the mth layer and the nth region; GALjkm is interaction effect between the jth group of animals and the kth area and the mth layer; GARjkn is interaction effect between the jth group of animals and the kth area and the nth region; GLRjmn is interaction effect between the jth group of animals and the mth layer and the nth region; ALRkmn is interaction effect between the kth area and the mth layer and the nth region; GALRjkmn is interaction effect between the jth group of animals and the kth area and the mth layer, and the nth region; egijkmn is the experimental error.
There was a significant effect of interaction between all factors (group × area × layer × region, F = 5.93, df = 53, P < 0.0001). Post hoc test was carried using the Tukey HSD method. Intercondition comparisons for layer 6 are shown in Supplementary Figure 1.
Because the quantification method described above was not applicable to the narrow layers 4c and 5, and for mere completion of the dataset, these layers were analyzed using single line OD measurements for each section (length of 1 mm, ImageJ software v1.45s) with the lines placed parallel to the layers in area 17. Nested-design ANOVA analysis was performed separately on this dataset. A significant effect was only present for group and layer interaction (F = 41.65, df = 8, P < 0.0001, see Supplementary Fig. 2). Therefore, we created 2 separate data sheets for the central and peripheral region, in which we investigated the effect of group and layer interaction separately (central region: F = 17.714, df = 8, P < 0.0001, peripheral region: F = 14.480, df = 8, P < 0.0001). Post hoc Tukey HSD test was also performed separately for data from the central and peripheral region.
Results
Area 17 and 18: zif268 Expression During Normal Development
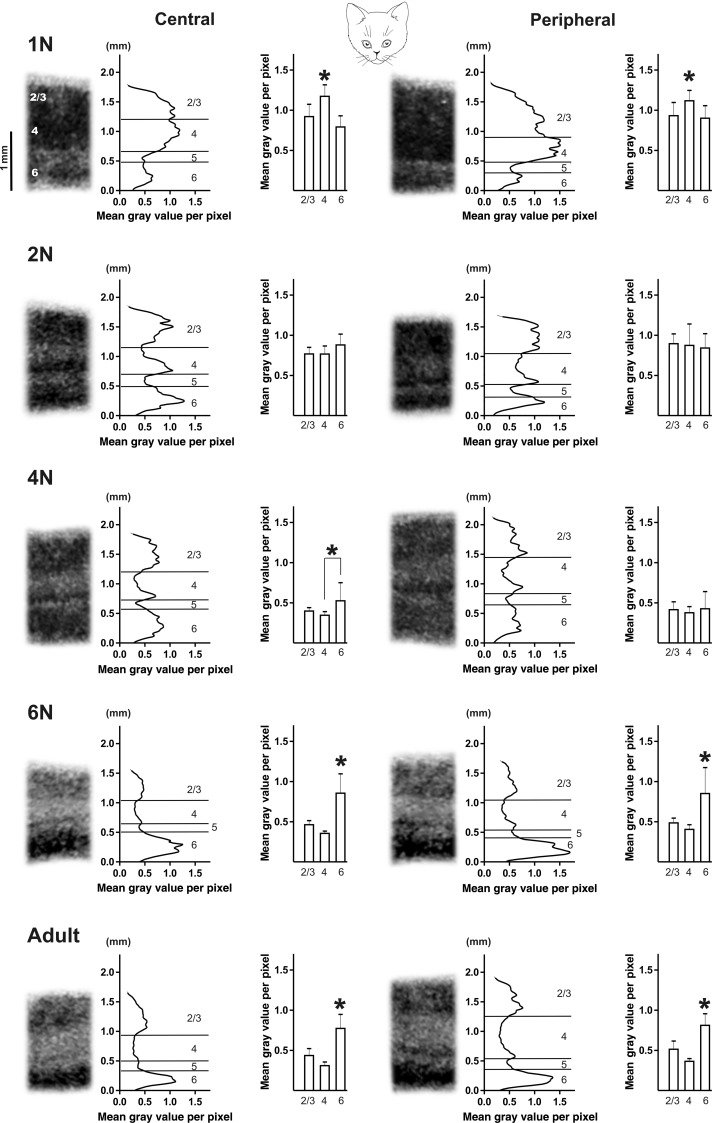
Area 17
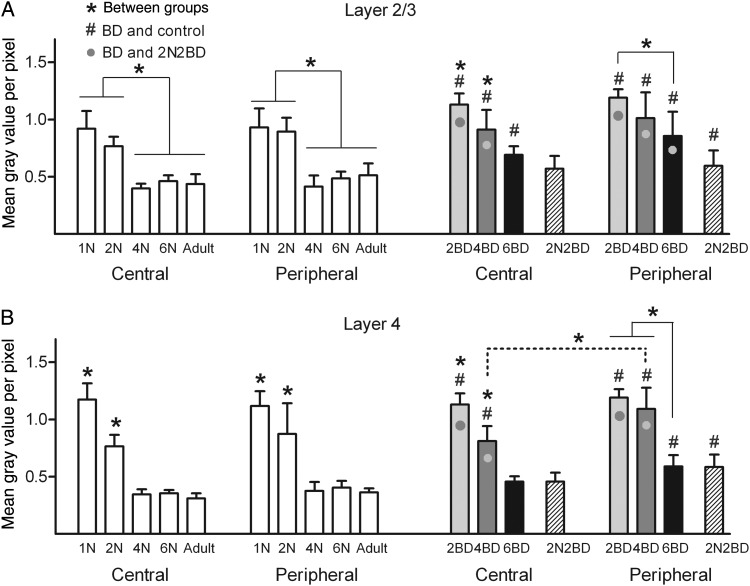
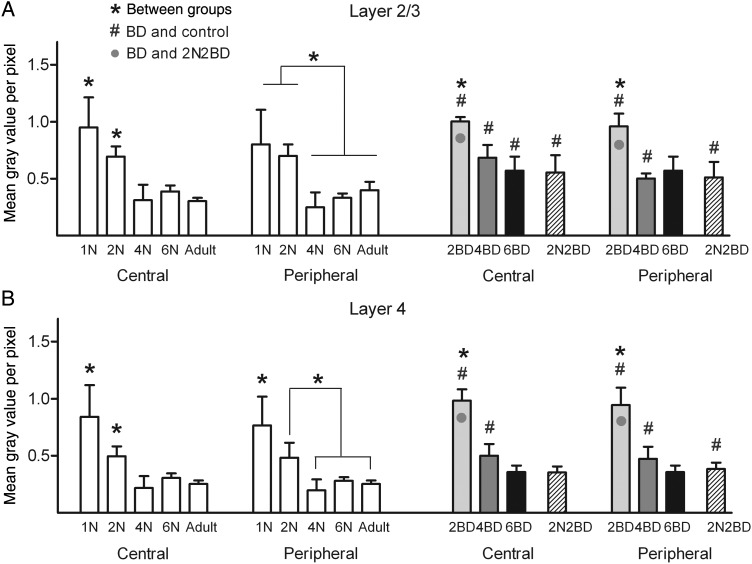
Age had a distinct effect on the intensity of the zif268 mRNA signal of area 17 and this effect was similar for its central and peripheral region under normal rearing conditions (Figs 2 and 5, white bars). In layers 2/3 and 4 maximal signal intensity was observed in 1N and 2N animals (Figs 2 and 5). From 4 months onwards in these layers a similar and lower zif268 mRNA level could be discerned. Central and peripheral layer 4 displayed a significant decrease in zif268 signal already between 1N and 2N (Fig. 5B), whereas for layer 2/3 such a maturation-induced decrease became apparent later, between 2N and 4N (Fig. 5A).
Figure 5.
Age effect on the zif268 mRNA expression levels in layers 2/3 and 4 of area 17 of normal and deprived cats (A and B). In normal cats (white bars) and early BD cats (gray/black bars) both central and peripheral area 17 regions were characterized by a decrease in zif268 expression with age. A common feature for the early BD conditions was the higher zif268 mRNA levels (indicated by #, P < 0.01) as compared with age-matched controls. Only in layer 4 of 4BD animals, the zif268 signal in the peripheral region was significantly higher as compared with the central region as indicated by the dotted line. Note how this is linked to a developmental zif268 signal decrease occurring earlier in the central region than in the peripheral region of area 17 (A and B). Black asterisks above bars denote significant differences (P < 0.01) for a given region and cortical layer between age groups. Gray circles in the BD bar graphs denote a higher zif268 signal as compared with the delayed onset 2N2BD group (P < 0.01, hatched bars). Differences between BD groups and age-matched controls are indicated by #P < 0.01. Results are means with ±SD.
Figure 2 illustrates how age had a clear and comparable effect on the relative interlaminar zif268 expression pattern of central and peripheral area 17. In 1N animals the highest signal was detected in layer 4. The 2N and 4N animals displayed a transitional, still immature interlaminar profile with no interlayer differences, except for the central region of the 4N group which already had a significantly higher zif268 signal in layer 6 as compared with layer 4, possibly indicative for a first transition towards an adult-like interlaminar pattern. The interlaminar pattern typical for adult animals was indeed characterized by the highest zif268 mRNA level in layer 6 and was established in both cortical regions from the age of 6 months.
Area 18
Area 18 displayed a similar developmental decrease in zif268 expression in layers 2/3 and 4 (Figs 4D and 6). A swifter decrease did characterize central layer 2/3, where a decrease was detected already between 1N and 2N (Fig. 6A). Compared with area 17, area 18 displayed a generally lower zif268 level, most pronounced in layer 4 of the youngest 1N and 2N kittens (P < 0.01, Fig. 4D).
Figure 6.
Age effect on the zif268 mRNA expression levels in the layers 2/3 and 4 of area 18 of normal and deprived cats (A and B). In normal cats (white bars) and early BD cats (gray/black bars) both central and peripheral area 17 regions were characterized by a decrease in zif268 expression with age. A common feature for the early BD conditions was the higher zif268 mRNA levels (indicated by #, P < 0.01) as compared with age-matched controls. Note the highest signal in 2BD animals and no difference among 4BD, 2N2BD, and 6BD kittens (A and B). Black asterisks above bars denote significant differences (P < 0.01) for a given region and cortical layer between age groups. Gray circles in the BD bar graphs denote a higher zif268 signal as compared with the delayed onset 2N2BD group (P < 0.01, hatched bars). Differences between BD groups and age-matched controls are indicated by #P < 0.01. Results are means with ±SD.
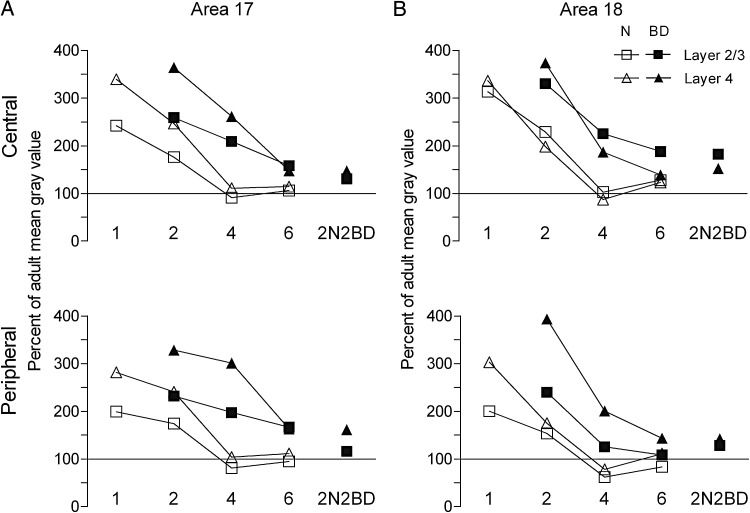
Figure 7 (white symbols) illustrates the comparison of the effect of age between area 17 and 18 for layers 2/3 and 4 and for the central and peripheral representations separately. The zif268 ratios between the mean gray value for each group and the adult mean gray value reveal how age affects both area 17 and area 18 quite similarly.
Figure 7.
Mean zif268 mRNA expression level in area 17 (A) and area 18 (B) in normal and deprived cats expressed as a percentage of the adult mean gray values. BD mainly affects input layer 4 (triangle) and supragranular layers (square). Note that in area 18 the developmental decrease of zif268 signal is faster than in area 17 in BD animals, which may indicate that area 17 is longer sensitive to changes in visual input than area 18. The effect of 2N2BD and 4BD is similar for area 18, which is in line with the similar motion-perception impairments that were observed previously in adult 4BD and 2N2BD cats (Zapasnik and Burnat 2013).
Early BD Delays the Development of Area 17 in an Age- and Region-specific Manner
A common finding for the early and late onset BD cats was the higher zif268 expression levels in both central and peripheral area 17 as compared with age-matched control groups (Figs 3–5, significance to age-matched controls marked with #). Nevertheless, just like in animals with normal visual experience, the overall zif268 expression level in area 17 of BD animals was more dependent on age than on central/peripheral cortical location of the visual field representation. In BD animals, the lowest zif268 levels were reached later in development compared with normal cats, namely at 6 months instead of 4 months (Fig. 5).
In the 2BD group, a higher zif268 signal characterized both central and peripheral area 17 as compared with the age-matched 2N group, thereby resembling more the younger 1N group (Figs 4A, B and 5, #). In layer 2/3 the signal was even higher than in the younger 1N control animals (P = 0.002). Inspection of the interlaminar zif268 pattern of 2BD kittens, revealed a significantly higher signal in layer 4 as compared with layer 6 in both peripheral and central area 17, also indicative of a closer resemblance to the 1N condition, and not to the 2N intermediate layering pattern (Figs. 2 and 3).
In 4BD, layer 2/3 displayed higher zif268 levels in central and peripheral area 17 compared with 4N, thereby resembling more the younger 1N and 2N controls (Fig. 5A). The zif268 signal in central layer 4 was also as high as in the 2N group, while in the peripheral region it was even as high as in the 1N group (Fig. 5B). In 4BD kittens, cortical layer 4 also exhibited a higher signal in peripheral than in central area 17 (Fig. 5B, significance marked by dotted line), indicative for an earlier developmental zif268 signal decrease in the central region than in the peripheral region of area 17. As illustrated in Figure 3, zif268 expression in central and peripheral area 17 of 4BD animals did not show any significant interlaminar differences, thereby best mimicking the 2N condition.
In the 6BD cats both central and peripheral area 17 were characterized by an adult-like interlaminar pattern of zif268 expression (Fig. 3), but all mRNA levels were higher than in age-matched controls, except for central layer 4 (Fig 5, #).
The 2N2BD animals, with delayed onset of deprivation, did not resemble their age-matched 4N in peripheral region, nor the 4BD group, but rather the older 6N and adult animals. None of the cortical layers differed from 6N or adult controls, with the exception of the peripheral layer 4 of 2N2BD that displayed a significantly higher zif268 level (P = 0.005, P = 0.00005 comparison with 6N and adult controls, respectively, Fig. 5). Compared with 4BD, the zif268 signal in layers 2/3 and 4 of the 2N2BD animals was significantly lower in both cortical regions (Fig. 5, gray circles). When judging the interlaminar pattern of zif268 expression in both regions of area 17 of 2N2BD subjects, it best resembles the more adult-like situation like in 6N, 6BD, and adult animals (Figs 2 and 3).
Binocular Deprivation has a Different Impact on Area 18
Just like in area 17, the zif268 expression levels in area 18 were higher as compared with age-matched controls (Figs 4 and 6, #). Only the 6BD group did not have a higher signal, with the exception of a central layer 2/3 (Figs. 4 and 6B). No centro-peripheral differences were detected in area 18 in any of the BD groups (Fig. 6). Similar to 1N and 2N animals, the zif268 mRNA signal in layer 4 of 2BD and 4BD kittens was lower in area 18 as compared with area 17 (P < 0.01, Fig. 4E).
When comparing the different BD conditions, 2BD animals clearly stood out from all other BD subjects due to a high zif268 signal (Fig 4A, B, and E). Moreover, their zif268 expression levels in layers 2/3 and 4 resembled that of younger 1N animals (Fig. 6). In contrast to area 17, the low 4BD zif268 signals in area 18 were indistinguishable from the low levels of 6BD and 2N2BD animals (Fig. 6).
Figure 7 (black symbols) summarizes how BD from birth maintains high zif268 signals in both area 17 and area 18, however, the effect of onset and duration of deprivation is different. The developmental decrease of zif268 expression between the 2BD and 4BD condition is more pronounced in area 18. Clearly, the effect of the 2N2BD and 4BD conditions is similar for area 18, but not for area 17.
Discussion
Developmental Changes in the Laminar zif268 Expression Pattern in cat Primary Visual Cortices
Systematic measurements of zif268 expression levels within areas 17 and 18 as a function of age, cortical layer, and visual field representation allowed us to describe the normal developmental maturation of cat primary visual cortex based on visual stimulation-induced activity reporter gene expression. A significant decrease in zif268 mRNA signal intensity in cortical layers 2/3 and 4, but not in layer 6, characterized the progression of maturation of areas 17 and 18 in animals with normal visual experience. Thus we consider this developmental decrease of zif268 expression as a marker of visual cortex maturation.
Zif268 mRNA levels in cat and monkey V1 are higher in young animals (our results; McCormack et al. 1992; Kaczmarek et al. 1999). In rodents the developmental pattern is different, with the level of zif268 mRNA increasing gradually towards adulthood (mouse: Mataga et al. 2001, rat: Worley et al. 1991; review: Kaczmarek and Chaudhuri 1997). The high zif268 mRNA levels in layers 2/3 and 4 of area 17 of 1- and 2-month-old kittens are consistent with previous immunohistochemical observations. Kaplan et al. (1995, 1996) described in cat a prominent age-dependent decrease in Zif268 immunoreactivity in layer 4, particularly between the age of 5 and 20 weeks (1 and 4 months in our study) and a small decline in supra- and infragranular layers, however, these previous data were not quantitative. Yet in our experiments, particularly at the age of 4 months, all cortical layers showed a remarkable decrease in zif268 mRNA expression in area 17 and 18. This overall activity decline may reflect the GABAergic inhibition-regulated closure of the most plastic period of cortical development, the formation of the ocular dominance columns (Hensch and Stryker 2004). Additional studies will be required for a more complete understanding of the link between these 2 phenomena.
Beaver et al. (2001) described layer-specific differences in ocular-dominance shift in normal cat area 17 aged from 6 weeks till 14 weeks using an electrophysiology approach. Two days of monocular eyelid suturing revealed a difference in the timing of the plasticity decline, with layer 4 being the first to end the critical period for OD plasticity, layers 2/3 next and layer 6 being the last (see also Daw et al. 1992). Likewise, studies on the effect of small retinal lesions on primary visual cortex revealed a different layer-specific response between kittens and adult cats (Waleszczyk et al. 2003).
The adult interlaminar pattern with the higher zif268 expression in layer 6 as compared with low signal in layer 4 has been characterized for cat areas 17 and 18 before (McCormack et al. 1992; Zhang et al. 1994, 1995; Kaplan et al. 1995; Arckens et al. 2000; Hu et al. 2009). Also in adult macaque V1 the greatest density of Zif268-positive neurons was detected in cortical layers 2/3 and 6 and a lower number was observed in layer 4 (Chaudhuri et al. 1995; Okuno et al. 1997). Zif268 expression patterns of adult cat primary visual cortex therefore correspond better to those of adult primates, than to those of rodent V1, where both layer 4 and 6 display high zif268 signals (Schlingensiepen et al. 1991; Worley et al. 1991; Van Brussel et al. 2009, 2011; Nys et al. 2014).
Effect of Early Onset Pattern Deprivation on Area 17
In the first month of a kitten's life, perception of fine detail is still obscured by the eye optics and the hylaoid artery. Therefore, at the initial postnatal stages of development, area 17 of normal and pattern-deprived kittens develops to the same degree (Sherk and Stryker 1976). One-month-old neurons indeed do not yet display sharply tuned orientation selectivity or binocular response properties, but instead still vigorously respond to a variety of stimuli (Pettigrew 1974; Albus and Wolf 1984), reflecting the highest zif268 expression in area 17 of 1-month-old kittens. Orientation-tuned neuronal responses normally appear at the age of 6 weeks, whereas in 2-month-old BD kittens an immature orientation selectivity and binocularity of area 17 neurons has been documented (Frégnac and Imbert 1978; Gödecke and Bonhoeffer 1996; Crair et al. 1998). Under an early BD regime, neurons remain responsive to all presented orientations until 4 months of age (Pettigrew 1974; Crair et al. 1998; for a review, see Sherman and Spear 1982). Consequently, we suggest that a sustained high zif268 signal in area 17 reflects this ongoing unselective responsiveness of BD neurons. Because of the absence of pattern visual input the BD neurons did not develop at a normal rate.
Depending on its timing and duration, the elimination of light input per se by dark rearing can also lead to a delayed maturation of cat visual cortex. Blakemore and Van Sluyters (1975) showed how BD and dark rearing do not induce differential neuronal electrophysiological properties under the age of 2 months. In line, and similar to our BD observations, dark rearing from birth for 5 weeks also induced higher zif268 mRNA levels than those in adult cat visual cortex (Rosen et al. 1992; Mower and Kaplan 2002). Our shortest early onset BD regimes, 2 and 4 months, had the strongest effect on area 17 maturation, showing the highest zif268 expression. Probably, the delay in critical period induced by dark rearing (Mower 1991; Beaver et al. 2001) is comparable to the outcome of early short BD. Also, a delay in the formation of ocular dominance columns has been reported in 5-week-old BD animals (Thompson et al. 1982). Yet periods of dark rearing or BD longer than 4 months do have a different impact on ocular dominance plasticity. Indeed, dark rearing followed by monocular deprivation (MD) leads to a neuronal activity shift where most of the cells are driven only by the open eye, while in BD animals such a delay in critical period for MD-induced reorganization of connections does not occur, leaving a high proportion of unresponsive, unmappable, and monocularly driven cells (Wiesel and Hubel 1965; Chow and Stewart 1972; Cynader et al. 1976; Cynader and Mitchell 1980; Mower et al. 1981). Our observations confirm this developmental delay after short periods of BD, up to 4 months, in which a 1-h light stimulation as a first sign of normal visual input still induces the highest zif268 expression levels. These high zif268 signals appear as indicators of a still immature state of the visual cortex, as similarly high zif268 levels characterized the visual cortex of the youngest, 1-month-old animals reared under normal conditions.
Interrelationship Between Area 17 and 18
Previously, the effect of BD on area 18 development was not extensively investigated making interpretation of the observed zif268 expression changes less straightforward. Zufferey et al. (1999) observed that the regression of local terminal arbors of neurons in areas 17 and 18 only starts to occur when BD is applied at least for the first 2 months of life, however, no difference between normal and BD kittens at the end of the first month was found in any of the areas. This observation together with the high zif268 signal in 2BD kittens in area 17 and 18 suggests that at 2 months of age both areas are still at a similar and early stage of development. Our recent behavioral observations in adult BD cats show that 2BD from birth indeed does not weaken motion sensitivity in adulthood, whereas delayed onset 2N2BD, or 4 months BD do impair motion perception (Zapasnik and Burnat 2013). The good perceptual outcome after 2BD together with the high zif268 expression level in 2BD kittens confirm our assumption that the ability to compensate such a short and early BD period corresponds with this high neuronal activity in motion-sensitive area 18 at the time when normal pattern vision was restored. In contrast, more mature, lower zif268 levels like in area 18 of 4BD and 2N2BD kittens seem to predict the lack of this capacity to compensate, leading to permanent motion-perception impairments in adult life (Zapasnik and Burnat 2013). Six months of BD was previously also shown to be destructive for global motion perception in adulthood (Burnat et al. 2002, 2005), which is most likely also mirrored here by the lack of elevated zif268 expression in area 18 just after the end of the deprivation period in 6BD kittens.
In animals reared under normal visual stimulation conditions, we did not observe a clear difference in the maturation speed of the central and peripheral visual field representations of area 17 or 18. Nevertheless, some indications for a slower development of the peripheral representation of area 17 were detected. In normal 4-month-old kittens the adult-like interlaminar zif268 expression pattern was observed for central area 17 but not yet for its peripheral counterpart. This finding is in line with the well described central-to-peripheral gradient of retinal ganglion cell maturation (Johns et al. 1979; Rapaport and Stone 1983a,b; Walsh et al. 1983; Walsh and Polley 1985) and was also confirmed in marmoset monkey primary visual cortex, where swifter maturation of its central part was visualized by neurofilament immunoreactivity patterns (Bourne et al. 2005). Since in the higher-order area 18 we did not detect at any age or visual manipulation the central–peripheral gradient, we may assume that association projections from area 17 to area 18 which are excitatory and selectively sensitive to slower moving stimuli, thus dominated by X-input (Sherk 1978; Casanova et al. 1992; Dreher et al. 1992) do not have a major impact on zif268 expression levels in area 18.
The slower development of peripheral area 17 under normal visual experience is also in line with behavioral measurements of the expansion of the peripheral visual field in kittens (Sireteanu and Maurer 1982). The size of the visual field increases at the time when growth of area 17 surface takes place, between postnatal weeks 3–6, and coincides with an increase in the number of ocular dominance columns (Sireteanu and Maurer 1982; Rathjen et al. 2003). Interestingly, injections of 3H-proline in 1 eye after 6 months of BD from birth showed that the segregation of geniculate inputs is disrupted in area 17 but not in area 18 (Swindale 1988), indicating that the development of ocular dominance columns is more dependent on visually driven activity in area 17 than in area 18.
The peripheral retina of adult cat is dominated by motion-sensitive Y-type neurons (Cleland and Levick 1974; Walsh and Polley 1985) that project to both the peripheral visual field representation in area 17 and to area 18, an area entirely driven by Y-input (Stone and Dreher 1973; Humphrey et al. 1985; Dreher et al. 1992). Long-lasting BD (from 5 months up to 1 year) interferes with this Y-type peripheral domination at the level of the retina (Burnat et al. 2012) and the dLGN (Sherman et al. 1972; Michalski and Wrobel 1986; Raczkowski et al. 1988) resulting in shrinkage of the peripheral visual field in BD cats (Zablocka 1983). The present observations indicate that 4 months of BD already interferes with the development of the Y-cell input stream to a higher extent than X-cell input, as we observed higher zif268 levels in 4BD peripheral area 17 as compared with its central region.
Conclusion
Lack of pattern vision delays the development of both primary visual areas 17 and 18, depending on the timing and duration of the manipulation. The effect is stronger in area 17, especially in the region representing the contralateral peripheral visual field. In the case of early onset BD, area 17 displayed a high zif268 signal, typical for very young normal animals, for a longer time than area 18, possibly due to a delayed onset or else a prolongation of the critical period. In conclusion, we propose that an immature state of cortical development is characterized by high zif268 signals reflecting high neuronal activity in both areas. If normal vision is restored when zif268 signals are still high in the primary visual cortices, compensation for the effect of early deprivation can occur.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by a ‘European Regional Development Fund’ within the frame of an International PhD Project Programme MPD4-504, the Polish Ministry of Science, a Higher Education Grant N401 041032/1002 to K.B., and N301 654140 to M.Z., the Research Council of the KU Leuven (OT 09/022). Funding to pay the Open Access publication charges for this article was provided by a ‘European Regional Development Fund’ within the frame of an International PhD Project Programme MPD4-504.
Supplementary Material
Notes
We thank Ria Vanlaer for her excellent help with the in situ hybridization experiments, Irena Łapińska for her involvement in animal husbandry, Miłosława Sokół and Marcin Studnicki for their insightful comments on the statistical analyses, and Julie Nys for her valuable input to the discussion. Conflict of Interest: None declared.
References
- Albus K, Wolf W. 1984. Early post-natal development of neuronal function in the kitten’s visual cortex: a laminar analysis. J Physiol. 348:153–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arckens L, Van Der Gucht E, Eysel UT, Orban GA, Vandesande F. 2000. Investigation of cortical reorganization in area 17 and nine extrastriate visual areas through the detection of changes in immediate early gene expression as induced by retinal lesions. J Comp Neurol. 425:531–544. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Colonnier M. 1983. The number of neurons in the different laminae of the binocular and monocular regions of area 17 in the cat. J Comp Neurol. 217:337–344. [DOI] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Daw NW. 2001. Layer differences in the effect of monocular vision in light- and dark-reared kittens. Vis Neurosci. 18:811–820. [DOI] [PubMed] [Google Scholar]
- Beston BR, Jones DG, Murphy KM. 2010. Experience-dependent changes in excitatory and inhibitory receptor subunit expression in visual cortex. Front Synaptic Neurosci. 2:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. 1975. Innate and environmental factors in the development of the kitten’s visual cortex. J Physiol. 248:663–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JA, Warner CE, Rosa MG. 2005. Topographic and laminar maturation of striate cortex in early postnatal marmoset monkeys, as revealed by neurofilament immunohistochemistry. Cereb Cortex. 15:740–748. [DOI] [PubMed] [Google Scholar]
- Burnat K, Stiers P, Arckens L, Vandenbussche E, Zernicki B. 2005. Global form perception in cats early deprived of pattern vision. Neuroreport. 16:751–754. [DOI] [PubMed] [Google Scholar]
- Burnat K, Vandenbussche E, Zernicki B. 2002. Global motion detection is impaired in cats deprived early of pattern vision. Behav Brain Res. 134:59–65. [DOI] [PubMed] [Google Scholar]
- Burnat K, Van Der Gucht E, Waleszczyk WJ, Kossut M, Arckens L. 2012. Lack of early pattern stimulation prevents normal development of the alpha (Y) retinal ganglion cell population in the cat. J Comp Neurol. 520:2414–2429. [DOI] [PubMed] [Google Scholar]
- Casanova C, Michaud Y, Morin C, McKinley PA, Molotchnikoff S. 1992. Visual responsiveness and direction selectivity of cells in area 18 during local reversible inactivation of area 17 in cats. Vis Neurosci. 9:581–593. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Matsubara JA, Cynader MS. 1995. Neuronal activity in primate visual cortex assessed by immunostaining for the transcription factor Zif268. Vis Neurosci. 12:35–50. [DOI] [PubMed] [Google Scholar]
- Chow KL, Stewart DL. 1972. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 34:409–433. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. 1974. Brisk and sluggish concentrically organized ganglion cells in the cat’s retina. J Physiol. 240:421–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP. 1998. The role of visual experience in the development of columns in cat visual cortex. Science. 279:566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Berman N, Hein A. 1976. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 25:139–156. [DOI] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. 1980. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 43:1026–1040. [DOI] [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. 1992. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 67:197–202. [DOI] [PubMed] [Google Scholar]
- Dreher B, Leventhal AG, Hale PT. 1980. Geniculate input to cat visual cortex: a comparison of area 19 with areas 17 and 18. J Neurophysiol. 44:804–826. [DOI] [PubMed] [Google Scholar]
- Dreher B, Michalski A, Cleland BG, Burke W. 1992. Effects of selective pressure block of Y-type optic nerve fibers on the receptive-field properties of neurons in area 18 of the visual cortex of the cat. Vis Neurosci. 9:65–78. [DOI] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Maurer D, Brar S, Brent HP. 2002. Better perception of global motion after monocular than after binocular deprivation. Vision Res. 42:169–179. [DOI] [PubMed] [Google Scholar]
- Frégnac Y, Imbert M. 1978. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 278:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gödecke I, Bonhoeffer T. 1996. Development of identical orientation maps for two eyes without common visual experience. Nature. 379:251–254. [DOI] [PubMed] [Google Scholar]
- Hata Y, Ohshima M, Ichisaka S, Wakita M, Fukuda M, Tsumoto T. 2000. Brain-derived neurotrophic factor expands ocular dominance columns in visual cortex in monocularly deprived and nondeprived kittens but does not in adult cats. J Neurosci. 20:RC57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. 2005. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 6:877–888. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. 2004. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 303:1678–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. 1998. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 28:370–490. [DOI] [PubMed] [Google Scholar]
- Hu TT, Laeremans A, Eysel UT, Cnops L, Arckens L. 2009. Analysis of c-fos and zif268 expression reveals time-dependent changes in activity inside and outside the lesion projection zone in adult cat area 17 after retinal lesions. Cereb Cortex. 19:2982–2992. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Sur M, Uhlrich DJ, Sherman SM. 1985. Termination patterns of individual X- and Y-cell axons in the visual cortex of the cat: projections to area 18, to the 17/18 border region, and to both areas 17 and 18. J Comp Neurol. 233:190–212. [DOI] [PubMed] [Google Scholar]
- Jin JZ, Weng C, Yeh CI, Gordon JA, Ruthazer ES, Stryker MP, Swadlow HA, Alonso JM. 2008. On and off domains of geniculate afferents in cat primary visual cortex. Nat Neurosci. 11:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Rusoff AC, Dubin MW. 1979. Postnatal neurogenesis in the kitten retina. J Comp Neurol. 187:545–555. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Chaudhuri A. 1997. Sensory regulation of immediate-early gene expression in mammalian visual cortex: implications for functional mapping and neural plasticity. Brain Res Brain Res Rev. 23:237–256. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L, Zangenehpour S, Chaudhuri A. 1999. Sensory regulation of immediate-early genes c-fos and zif268 in monkey visual cortex at birth and throughout the critical period. Cereb Cortex. 9:179–187. [DOI] [PubMed] [Google Scholar]
- Kaplan IV, Guo Y, Mower GD. 1995. Developmental expression of the immediate early gene EGR-1 mirrors the critical period in cat visual cortex. Brain Res Dev Brain Res. 90:174–179. [DOI] [PubMed] [Google Scholar]
- Kaplan IV, Guo Y, Mower GD. 1996. Immediate early gene expression in cat visual cortex during and after the critical period: differences between EGR-1 and Fos proteins. Brain Res Mol Brain Res. 36:12–22. [DOI] [PubMed] [Google Scholar]
- Kossut M, Michalski A, Zernicki B. 1978. The ocular following reflex in cats deprived of pattern vision from birth. Brain Res. 141:77–87. [DOI] [PubMed] [Google Scholar]
- Liu RL, Wang K, Meng JJ, Hua TM, Liang Z, Xi MM. 2013. Adaptation to visual stimulation modifies the burst firing property of V1 neurons. Zool Res. 34:E101–E108. [PubMed] [Google Scholar]
- Martin KA, Schroder S. 2013. Functional heterogeneity in neighboring neurons of cat primary visual cortex in response to both artificial and natural stimuli. J Neurosci. 33:7325–7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Fujishima S, Condie BG, Hensch TK. 2001. Experience-dependent plasticity of mouse visual cortex in the absence of the neuronal activity-dependent marker egr1/zif268. J Neurosci. 21:9724–9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack MA, Rosen KM, Villa-Komaroff L, Mower GD. 1992. Changes in immediate early gene expression during postnatal development of cat cortex and cerebellum. Brain Res Mol Brain Res. 12:215–223. [DOI] [PubMed] [Google Scholar]
- Michalski A, Wrobel A. 1986. Spatiotemporal receptive field structure of neurons in the lateral geniculate nucleus of binocularly deprived cats. Acta Neurobiol Exp (Wars). 46:261–279. [PubMed] [Google Scholar]
- Mitchell DE, Sengpiel F, Hamilton DC, Schwarzkopf DS, Kennie J. 2011. Protection against deprivation amblyopia depends on relative not absolute daily binocular exposure. J Vis. 11:13. [DOI] [PubMed] [Google Scholar]
- Mower GD. 1991. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 58:151–158. [DOI] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. 1981. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 220:255–267. [DOI] [PubMed] [Google Scholar]
- Mower GD, Kaplan IV. 2002. Immediate early gene expression in the visual cortex of normal and dark reared cats: differences between fos and egr-1. Brain Res Mol Brain Res. 105:157–160. [DOI] [PubMed] [Google Scholar]
- Mullikin WH, Jones JP, Palmer LA. 1984. Receptive-field properties and laminar distribution of X-like and Y-like simple cells in cat area 17. J Neurophysiol. 52:350–371. [DOI] [PubMed] [Google Scholar]
- Nys J, Aerts J, Ytebrouck E, Vreysen S, Laeremans A, Arckens L. 2014. The cross-modal aspect of mouse visual cortex plasticity induced by monocular enucleation is age-dependent. J Comp Neurol. 522:950–970. [DOI] [PubMed] [Google Scholar]
- Okuno H, Kanou S, Tokuyama W, Li YX, Miyashita Y. 1997. Layer-specific differential regulation of transcription factors Zif268 and Jun-D in visual cortex V1 and V2 of macaque monkeys. Neuroscience. 81:653–666. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR. 1985. Synapse elimination in the developing visual cortex: a morphometric analysis in normal and dark-reared cats. Brain Res. 354:81–91. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kennedy H, Maes H. 1981. Response to movement of neurons in areas 17 and 18 of the cat: velocity sensitivity. J Neurophysiol. 45:1043–1058. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Maunsell JH. 1992. Spatiotemporal sensitivity following lesions of area 18 in the cat. J Neurosci. 12:4521–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P. 2003. Using ANOVA for gene selection from microarray studies of the nervous system. Methods. 31:282–289. [DOI] [PubMed] [Google Scholar]
- Peters A, Regidor J. 1981. A reassessment of the forms of nonpyramidal neurons in area 17 of cat visual cortex. J Comp Neurol. 203:685–716. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. 1974. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 237:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczkowski D, Uhlrich DJ, Sherman SM. 1988. Morphology of retinogeniculate X and Y axon arbors in cats raised with binocular lid suture. J Neurophysiol. 60:2152–2167. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Stone J. 1983a. Time course of morphological differentiation of cat retinal ganglion cells: influences on soma size. J Comp Neurol. 221:42–52. [DOI] [PubMed] [Google Scholar]
- Rapaport DH, Stone J. 1983b. The topography of cytogenesis in the developing retina of the cat. J Neurosci. 3:1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen S, Schmidt KE, Lowel S. 2003. Postnatal growth and column spacing in cat primary visual cortex. Exp Brain Res. 149:151–158. [DOI] [PubMed] [Google Scholar]
- Ribot J, Aushana Y, Bui-Quoc E, Milleret C. 2013. Organization and origin of spatial frequency maps in cat visual cortex. J Neurosci. 33:13326–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen KM, McCormack MA, Villa-Komaroff L, Mower GD. 1992. Brief visual experience induces immediate early gene expression in the cat visual cortex. Proc Natl Acad Sci U S A. 89:5437–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist AC. 1985. Connections of visual cortical areas in the cat. In: Peters A, Jones EG, eds. Cerebral Cortex. New York: Plenum; p. 81–117. [Google Scholar]
- Schlingensiepen KH, Luno K, Brysch W. 1991. High basal expression of the zif/268 immediate early gene in cortical layers IV and VI, in CA1 and in the corpus striatum—an in situ hybridization study. Neurosci Lett. 122:67–70. [DOI] [PubMed] [Google Scholar]
- Schmidt KE, Stephan M, Singer W, Lowel S. 2002. Spatial analysis of ocular dominance patterns in monocularly deprived cats. Cereb Cortex. 12:783–796. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf DS, Vorobyov V, Mitchell DE, Sengpiel F. 2007. Brief daily binocular vision prevents monocular deprivation effects in visual cortex. Eur J Neurosci. 25:270–280. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 4:477–485. [DOI] [PubMed] [Google Scholar]
- Sherk H. 1978. Area 18 cell responses in cat during reversible inactivation of area 17. J Neurophysiol. 41:204–215. [DOI] [PubMed] [Google Scholar]
- Sherk H, Stryker MP. 1976. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 39:63–70. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Hoffmann KP, Stone J. 1972. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 35:532–541. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. 1982. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 62:738–855. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Maurer D. 1982. The development of the kitten’s visual field. Vision Res. 22:1105–1111. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher B. 1973. Projection of X- and Y-cells of the cat’s lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 36:551–567. [DOI] [PubMed] [Google Scholar]
- Swindale NV. 1988. Role of visual experience in promoting segregation of eye dominance patches in the visual cortex of the cat. J Comp Neurol. 267:472–488. [DOI] [PubMed] [Google Scholar]
- Takahata T, Higo N, Kaas JH, Yamamori T. 2009. Expression of immediate-early genes reveals functional compartments within ocular dominance columns after brief monocular inactivation. Proc Natl Acad Sci U S A. 106:12151–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I, Kossut M, Blakemore C. 1982. An investigation of the development of ocular dominance and orientation columns in cat striate cortex using 2-deoxyglucose. In: Sharma SC, ed. Organizing Principles of Neural Development. Valhalla (New York): New York Medical College; p. 261–275. [Google Scholar]
- Van Brussel L, Gerits A, Arckens L. 2011. Evidence for cross-modal plasticity in adult mouse visual cortex following monocular enucleation. Cereb Cortex. 21:2133–2146. [DOI] [PubMed] [Google Scholar]
- Van Brussel L, Gerits A, Arckens L. 2009. Identification and localization of functional subdivisions in the visual cortex of the adult mouse. J Comp Neurol. 514:107–116. [DOI] [PubMed] [Google Scholar]
- Van der Gucht E, Clerens S, Cromphout K, Vandesande F, Arckens L. 2002. Differential expression of c-fos in subtypes of GABAergic cells following sensory stimulation in the cat primary visual cortex. Eur J Neurosci. 16:1620–1626. [DOI] [PubMed] [Google Scholar]
- Waleszczyk WJ, Wang C, Young JM, Burke W, Calford MB, Dreher B. 2003. Laminar differences in plasticity in area 17 following retinal lesions in kittens or adult cats. Eur J Neurosci. 17:2351–2368. [DOI] [PubMed] [Google Scholar]
- Walsh C, Polley EH. 1985. The topography of ganglion cell production in the cat’s retina. J Neurosci. 5:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Polley EH, Hickey TL, Guillery RW. 1983. Generation of cat retinal ganglion cells in relation to central pathways. Nature. 302:611–614. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. 1982. Postnatal development of the visual cortex and the influence of environment. Nature. 299:583–591. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. 1965. Extent of recovery from the effects of visual deprivation in kittens. J Neurophysiol. 28:1060–1072. [DOI] [PubMed] [Google Scholar]
- Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. 1991. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci U S A. 88:5106–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang L, Song XM, Li CY. 2013. The detection of orientation continuity and discontinuity by cat V1 neurons. PLoS One. 8:e79723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Rosa MG. 2013. Uniformity and diversity of response properties of neurons in the primary visual cortex: selectivity for orientation, direction of motion, and stimulus size from center to far periphery. Vis Neurosci. 25:1–14. [DOI] [PubMed] [Google Scholar]
- Yu HH, Verma R, Yang Y, Tibballs HA, Lui LL, Reser DH, Rosa MG. 2010. Spatial and temporal frequency tuning in striate cortex: functional uniformity and specializations related to receptive field eccentricity. Eur J Neurosci. 31:1043–1062. [DOI] [PubMed] [Google Scholar]
- Zablocka T. 1983. Visual field measurements in binocularly deprived cats. Acta Neurobiol Exp (Wars). 43:129–133. [PubMed] [Google Scholar]
- Zapasnik M, Burnat K. 2013. Binocular pattern deprivation with delayed onset has impact on motion perception in adulthood. Neuroscience. 255:99–109. [DOI] [PubMed] [Google Scholar]
- Zhang F, Halleux P, Arckens L, Vanduffel W, Van Bree L, Mailleux P, Vandesande F, Orban GA, Vanderhaeghen JJ. 1994. Distribution of immediate early gene zif-268, c-fos, c-jun and jun-D mRNAs in the adult cat with special references to brain region related to vision. Neurosci Lett. 176:137–141. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vanduffel W, Schiffmann SN, Mailleux P, Arckens L, Vandesande F, Orban GA, Vanderhaeghen JJ. 1995. Decrease of zif-268 and c-fos and increase of c-jun mRNA in the cat areas 17, 18 and 19 following complete visual deafferentation. Eur J Neurosci. 7:1292–1296. [DOI] [PubMed] [Google Scholar]
- Zufferey PD, Jin F, Nakamura H, Tettoni L, Innocenti GM. 1999. The role of pattern vision in the development of cortico-cortical connections. Eur J Neurosci. 11:2669–2688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.