Abstract
Fundamental aspects of human behavior operate outside of conscious awareness. Yet, theories of conditioned responses in humans, such as placebo and nocebo effects on pain, have a strong emphasis on conscious recognition of contextual cues that trigger the response. Here, we investigated the neural pathways involved in nonconscious activation of conditioned pain responses, using functional magnetic resonance imaging in healthy participants. Nonconscious compared with conscious activation of conditioned placebo analgesia was associated with increased activation of the orbitofrontal cortex, a structure with direct connections to affective brain regions and basic reward processing. During nonconscious nocebo, there was increased activation of the thalamus, amygdala, and hippocampus. In contrast to previous assumptions about conditioning in humans, our results show that conditioned pain responses can be elicited independently of conscious awareness and our results suggest a hierarchical activation of neural pathways for nonconscious and conscious conditioned responses. Demonstrating that the human brain has a nonconscious mechanism for responding to conditioned cues has major implications for the role of associative learning in behavioral medicine and psychiatry. Our results may also open up for novel approaches to translational animal-to-human research since human consciousness and animal cognition is an inherent paradox in all behavioral science.
Keywords: amygdala, conditioning, nocebo effect, placebo effect, subliminal perception
Introduction
Conditioning is known to play an important role in the formation of treatment expectations and placebo responses (Kirsch et al. 2004). In the case of placebo analgesia, the association between a treatment cue (e.g., morphine shot) and the following pain relief creates predictive knowledge that modulates future pain responses to the same cue, even if the shot contains saline (Buchel et al. 2014). Until recently, theories concerning placebo and nocebo (negative placebo) mechanisms had a strong emphasis on conscious expectations (Kirsch et al. 2004), suggesting that higher-order areas of the brain process the predictive cues associated with pain relief (Wager et al. 2004; Petrovic et al. 2010). However, neuroimaging studies suggest that the human brain can process sensory information before it reaches conscious awareness (Morris et al. 1998; Whalen et al. 1998; Pessiglione et al. 2007, 2008), and thus, predictive cues may be recognized subliminally and mediate nonconscious effects on human cognition and behavior.
The central nervous system can modulate incoming sensory information at different levels of adaptation, including automatic responses with little flexibility, and highly flexible processes modified by associated contexts and consequences (Mesulam 1998). According to this hierarchical theory, reward cues may be processed unconsciously through direct activation of mesolimbic reward areas (Pessiglione et al. 2007, 2008). Similarly, fearful stimuli can be conveyed via direct thalamic projections to the amygdala or indirect, slower, cortical pathways (Mesulam 1998; LeDoux 2000). As placebo analgesia involves reward-related brain processes (Schweinhardt et al. 2009; Scott et al. 2009) and nocebo involves fear-related mechanisms (Benedetti et al. 2006), we hypothesized that placebo and nocebo pain responses could be triggered nonconsciously through rapid pathways for reward and fear processing. In a recent behavioral study, we demonstrated the feasibility of activating conditioned placebo and nocebo pain responses via nonconsciously presented visual cues (Jensen et al. 2012). Here, we investigated the neural circuitry involved in nonconscious activation of conditioned pain responses. We used functional neuroimaging during a within-subject paradigm of conscious and nonconscious activation of conditioned placebo and nocebo responses. We hypothesized that brain regions associated with direct and rapid processing of reward, like the nucleus accumbens (Pessiglione et al. 2008) and orbitofrontal cortex (OFC) (Thorpe et al. 1983; Zhang et al. 1997), would be more activated during nonconsciously compared with consciously activated “placebo.” We also hypothesized that subcortical regions with direct processing of threat and pain signals, such as the amygdala (Whalen et al. 1998; Öhman et al. 2007), hippocampus (Ploghaus et al. 2001; Mobbs et al. 2009), and thalamus (Wager et al. 2004), would be more active during nonconsciously compared with consciously activated “nocebo.”
Materials and Methods
Participants
Participants (n = 24, 10 women; mean age 25 ± 5) were right-handed and had no previous experience with fast image exposures or masking experiments. Participants were considered for the study if they had no chronic health issues, no psychiatric symptoms or ongoing medications (except for hormonal contraception). Participants were not allowed to use any analgesic drugs within 48 h of the study visit. All participants were screened for magnetic resonance imaging (MRI) eligibility and were recruited through posted flyers at several different universities and at information boards in residential buildings. Participants were reimbursed for parking and also received a small monetary compensation for their participation (<$100).
Equipment
Measurements of brain activity were performed using a 3 Tesla Siemens MRI System equipped for Echo Planar Imaging (EPI). Thermal pain stimuli were delivered using the Pathway system from Medoc (www.medoc-web.com), with a 30-mm ATS thermode. Inside the scanner, a Sharp XG projector with 1024 × 768 resolution was used for visual presentations, connected to a Lenovo desktop computer. The experiment was programed in Presentation 13.0 (Neurobehavioral Systems, www.neurobs.com). The refresh rate was set to 85 Hz, and the masked stimulus presentations were synchronized with the refresh rate to elicit very fast exposures that prevented visual recognition (12 ms). The images used in the current experiment were taken from The Karolinska Directed Emotional Faces set (www.emotionlab.se/resources/kdef); a set of images specifically developed for use in perception, attention, emotion, memory, and masking experiments. The whole set consists of 70 individuals (35 males, 35 females), mean age 25 years (range 20–30) with 7 different facial expressions per individual. The images used in the present experiment represented men in neutral expressions, that is, no emotional valence. In total, 12 different neutral male faces were used for the purpose of this study.
Procedure
Participants were screened for inclusion and exclusion criteria over the telephone and then scheduled for an experiment. Participants were informed that the study investigated “the influence of implicit and explicit learning on pain perception,” but the full purpose of the study was not revealed until the experiment was over, and all participants were debriefed. All participants gave written informed consent, and the study was approved by the Institutional Review Board at the Massachusetts General Hospital, Boston, MA.
After giving informed consent, the Medoc ATS heat thermode was placed on the participants' volar forearm. Ascending temperatures were applied in order to find a calibrated temperature that would represent each participants “high pain” rating, approximately in the range of ∼15 on a 0–20 Numeric Response Scale (NRS) ranging from “no pain” to “worst imaginable pain,” and a “low pain” rating of ∼5 NRS. The difference between the chosen high and low pain temperature was fixed to 3°C for all subjects, for example, high pain/low pain could be represented by 49°/46°C in 1 individual and 47°/44°C in another. When the calibration of pain temperatures was complete, participants were placed in the scanner. The pain stimulator was placed on the left arm, and participants had a response-device in their right hand that would allow for pain ratings while in the scanner. Participants were given the following instruction before the conditioning run: “You are about to see some pictures on the screen. Each picture is paired with a pain stimulus on your arm. Your task is to focus on the screen at all times and after each picture I would like you to rate how much pain you felt on your arm, using the same 0–20 scale that you used during the calibration.” In order to ensure that subjects maintained high attention, the conditioning sequence was divided in 2 blocks of ∼10 min each. In total, 50 stimuli were presented during the conditioning sequence; 25 for the high pain face and 25 for the low pain face. Between trials, there was a jittered inter stimulus interval (ITI) between 8 and 12 s. After the conditioning cue (duration 100 ms), the painful stimulus was applied for 4 s, followed by a 2 to 6-s jittered wait before the pain rating of 8 s. Depending on the individual temperature used for each participant, the total time for ramp up and down of the heat varied between 2.2 and 3.5 s, with higher temperature corresponding to longer ramp time. For fMRI data analyses, we modeled the pain duration at 6 s to include the ramp up and down of heat and ensure that the disappearance of the heat would not overlap with the rating task.
Immediately after the conditioning runs, subjects were given the following instruction “You are about to see the same pictures on the screen again and each picture will be paired with a pain stimulus on your arm, just like before. The only difference is that this time there will also be pictures of new guys, that you haven't been exposed to before. Your task is to focus on the screen at all times and after each picture I would like you to rate how much pain you felt on your arm using the 0–20 scale. The pictures will sometimes be shown to you much faster than before, and you might not be able to recognize them. This is normal and something that we programmed on purpose. Your only task is to focus on the screen at all times and rate the pain on your arm, even if you can't see the pictures.” The test sequence consisted of 60 stimuli: 20 for the high pain condition, 20 for the low pain condition, and 20 for the neutral condition. Half of these stimuli were conscious, and half were unconsciously presented. The test sequence was divided in 3 10-min runs.
To verify that the masked stimuli would be truly non-recognizable, we performed a forced-choice recognition test immediately after the test sequence. The instructions were as follows: “You are about to see some pictures on the screen again and I would like you to answer if you have seen this face before during the experiment. You can only say ‘yes’ or ‘no’. The pictures will be exposed to you very quickly so you might not be able to tell if you saw it before or not. In any case, you have to guess ‘yes’ or ‘no’ for each exposure.” The recognition test included 12 exposures of the previously used high, low, and control faces and 12 exposures of new faces. Participants were asked to indicate whether the face had been shown before, or not. Our previous studies have verified that the nonconscious image exposures used in this experiment are indeed non-recognizable to the participants (Jensen et al. 2012) and the forced-choice task is commonly used experimental method for assessing participants' ability to recognize masked visual stimuli (Pessiglione et al. 2007).
MRI Parameters
Two functional MRI runs were performed during conditioning, and 3 runs were performed during the test sequence. Thirty axial interleaved slices (4 mm thick with 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were acquired with TR = 2000 ms, TE = 40 ms, flip angle = 90°, and a 3.13 × 3.13-mm in-plane spatial resolution. The number of time points was 293 for each of the functional runs. In addition to the functional MRI scans, participants were also scanned with a high-resolution MPRAGE sequence for anatomical reference. During the entire scan, cushions and earplugs were used to reduce head movement and dampen scanner noise.
Statistical Analyses
All statistical analyses of behavioral data were performed in SPSS Version 20.0. A statistical significance threshold of P < 0.05 was considered, and all tests were two-tailed. The difference in pain ratings between the “low cue,” “high cue,” and “control cue” trials was analyzed using repeated-measures ANOVA. If the overall ANOVA was significant, pairwise comparisons between the different conditions were performed with t-tests. Correlation analyses were performed using Pearson's r.
Preprocessing and analyses of imaging data were performed using the Statistical Parametric Mapping 8 (SPM8) software (SPM8, Wellcome Trust Centre for Neuroimaging) and Matlab 7.4 (Mathworks). All functional brain volumes were realigned to the first volume, spatially normalized to a standard EPI template, and finally smoothed using an 8-mm full-width at half-maximum isotropic Gaussian kernel. High-pass filtering of fMRI data (cutoff: 128 s) and correction for temporal autocorrelations using AR(1) were also done. The univariate data analysis was performed using the general linear model. The individual design matrix for each participant (first-level matrix) included the following regressors for the conditioning run: high cue, low cue, high pain, low pain, high rating, and low rating. The matrix also included the following test-sequence regressors twice, 1 for conscious and 1 for nonconscious trials: high cue, low cue, control cue, high pain, low pain, control pain, high rating, low rating, and control rating. A file containing the movement parameters for each individual (3 translation and 3 rotation axes) was obtained from the realignment step and saved for inclusion in the model. Regression coefficients for all regressors were estimated using least squares within SPM8. Specific effects were tested by creating contrasts of the parameter estimates, resulting in a t-statistic for each voxel. After the individual first-level estimations, a second-level analysis was performed using a within-subject ANOVA. For the main effect of high- minus low-pain stimulation during conditioning, an initial statistical threshold of voxel-wise P < 0.01 was used for all analyses, and all reported clusters were family-wise error corrected over the entire brain. For the evaluation of conscious and nonconscious placebo/nocebo effects, we applied a region of interest (ROI) approach. An initial threshold of P < 0.01 was used, and corrections were based on previous studies on placebo, nocebo, or nonconscious processing. In line with previous placebo neuroimaging studies, the rostral anterior cingulate cortex (ACC) and OFC were corrected using spheres of 12 mm radius and the nucleus accumbens, putamen, amygdala, hippocampus, and thalamus were corrected using spheres of 6 mm radius (Eippert et al. 2009). Extraction of parameter estimates for a specific ROI was performed by extracting a 3-mm sphere around the peak voxel.
Results
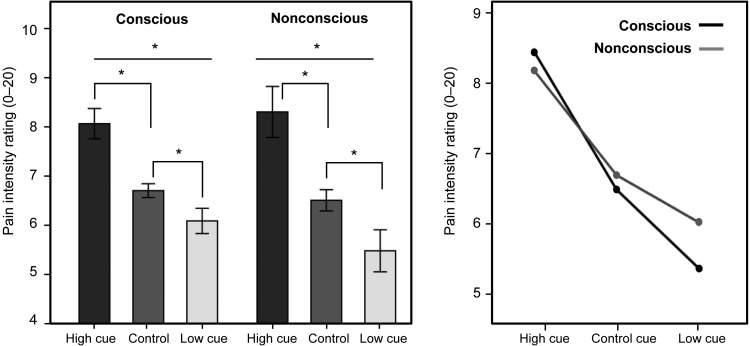
In concert with our previous report (Jensen et al. 2012), both conscious and nonconscious presentation of the conditioned cues led to significant placebo and nocebo responses. A repeated-measures ANOVA showed a main effect for cue type (high/low/control), F2,40 = 53.28, P < 0.001 and no significant main effect for exposure type (conscious/nonconscious) F1,40 = 1.45, P = 0.24. As illustrated in Figure 1, the exposure by cue interaction did not reach significance, F2,40 = 3.07, P = 0.06, but pointed toward a trend of larger “low cue” responses during conscious compared with nonconscious trials. Post hoc comparisons indicated significant placebo and nocebo effects for both conscious and nonconscious stimuli (P < 0.001).
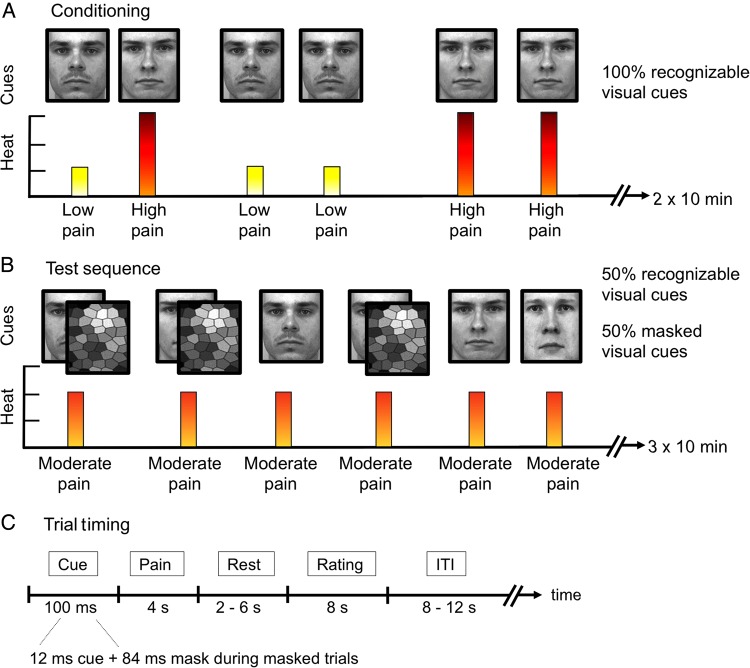
Figure 1.
Experimental procedure. The conditioning procedure (A) included clearly recognizable images of 2 male faces (experimental cues) presented on a computer screen. Each face cue was consistently paired with either a high or low heat pain stimulus on the volar forearm. After conditioning, a test sequence was performed (B) in which the high cue, low cue, and a neutral control cue were paired with identical moderate heat stimuli. During the test sequence, 50% of the cue exposures were exposed long enough for all subjects to clearly recognize them (100 ms), and 50% of the face cues were exposed for only 12 ms and then followed by a mask to prevent conscious recognition (backward-masking). (C) A detailed description of the trial timing during fMRI scans. The ITI lasted between 8 and 12 s.
An initial validation of brain activations in response to pain during the conditioning sequence revealed a comprehensive representation of pain processing regions [high pain > low pain], including the ACC, thalamus, and bilateral insula; see Table 1.
Table 1.
Results from fMRI statistical analyses
| Pain main effect [high pain > low pain] | MNI x | MNI y | MNI z | Cluster size (voxels) | Z-score | FWE P-value |
|---|---|---|---|---|---|---|
| Middle/posterior insula | 36 | 5 | 7 | 473 | 4.08 | P < 0.05 |
| Posterior insula | −33 | −16 | 16 | 590 | 3.60 | P < 0.05 |
| ACC | 0 | 17 | 34 | 592 | 3.41 | P < 0.05 |
| Thalamus | 0 | −16 | 7 | 67 | 3.30 | P < 0.05 |
| Placebo main effect | ||||||
| R. rostral ACC | 9 | 26 | −8 | 20 | 3.07 | P < 0.05 |
| Placebo [nonconscious > conscious] | ||||||
| R. orbitofrontal cortex | 36 | 29 | −2 | 116 | 3.12 | P < 0.05 |
| Placebo [conscious > nonconscious] | ||||||
| L. cuneus visual cortex | −3 | −91 | 28 | 30 | 3.25 | P = 0.07 |
| Nocebo main effect | ||||||
| L. cingulate cortex | 0 | −13 | 31 | 848 | 3.53 | P < 0.05 |
| L. posterior insula | −42 | −4 | 4 | 563 | 3.26 | P < 0.05 |
| R. hippocampus/temporal pole | 42 | 2 | −14 | 146 | 3.05 | P < 0.05 |
| R. posterior insula | 39 | −1 | −2 | 24 | 2.76 | P < 0.05 |
| L. thalamus | −6 | −19 | −5 | 56 | 2.90 | P < 0.05 |
| Nocebo [nonconscious > conscious] | ||||||
| R. brainstem | 9 | −16 | −5 | 1768 | 3.07 | P < 0.002 |
| R. thalamus | 6 | −1 | 10 | 28 | 3.00 | P < 0.05 |
| R. amygdala | 30 | −4 | −14 | 203 | 3.10 | P < 0.05 |
| R. hippocampus | 33 | −10 | −14 | 203 | 3.10 | P < 0.05 |
| Nocebo [conscious > nonconscious] | ||||||
| None | ||||||
Note: The pain main effect refers to the brain activations in response to thermal pain during the conditioning run [high pain > low pain]. Brain activations during placebo and nocebo trials are within-subject comparisons. Coordinates (x, y, z) correspond to the anatomical space as defined in the MNI standard brain atlas. The initial statistical threshold was P < 0.01, and all reported clusters are FWE-corrected at the cluster level.
The main effect of “placebo” during the test sequence, defined as the neural activation during “low cue versus “control cue” trials, irrelevant of exposure type, revealed increased activation in the rostral ACC (rACC) (Montreal Neurological Institute coordinates [9, 26, −8]); a commonly reported region in placebo analgesia (Bingel et al. 2006; Kong et al. 2006). A comparison of pain responses during nonconscious versus conscious placebo trials revealed higher activation in the OFC during nonconscious trials ([36, 29, −2]). During clearly conscious placebo trials, there was a trend toward higher activation in the visual cortex; however, it did not reach significance (P = 0.07).
The main effect of “nocebo” during the test sequence, defined as the pain response during “high cue” versus “control cue” trials, irrelevant of exposure type, revealed increased activation in several regions involved in nociceptive processing, for example, ACC, bilateral insula, thalamus, and brainstem. A within-subject comparison of nocebo responses during nonconscious versus conscious trials revealed higher activation of the brainstem, thalamus, amygdala, and hippocampus (Table 1; Figure 2).
Figure 2.
Pain ratings during conscious and nonconscious placebo and nocebo trials. Identical moderate temperatures were paired with a conditioned “high pain cue,” “low pain cue,” or “control cue” to test how predictive cues changed participants' pain perception. Participants rated pain intensity on a 0–20 Numerical Response Scale (NRS). Left panel: representation of the within-subject pain ratings during the test sequence that followed the initial conditioning sequence. Bars represent the average pain rating in response to identical moderate temperatures. Error bars represent 2 intrasubject standard errors. Top asterisks (*) represent overall significance for “cue type” and lower asterisks represent significant pairwise comparisons between the cues. Right panel: illustration of the interaction of “cue type” (high, low, control) by “exposure type” (conscious, nonconscious), based on a within-subject design.
To further investigate the fMRI signal change in the amygdala during nonconscious nocebo trials, the signal in right and left amygdala was extracted and correlated with the reported pain ratings. There was a positive correlation between pain ratings during nonconscious nocebo trials and activation of the right amygdala (r = 0.52, P < 0.05) but not for the left amygdala (r = 0.32, P = 0.10) (Figures 3 and 4).
Figure 3.
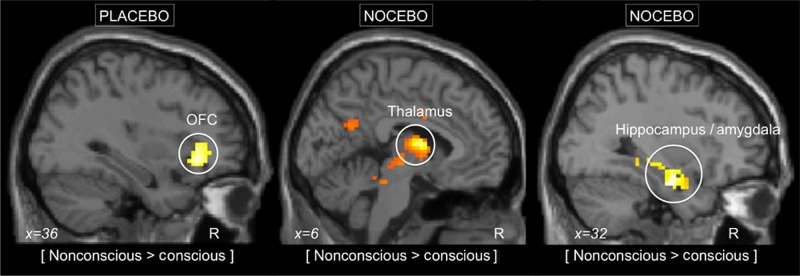
Neural correlates to nonconscious placebo and nocebo responses. Left panel: the nonconscious placebo condition, compared with conscious placebo, was associated with increased activity in the OFC, as illustrated by the circle. Middle and right panel: the nonconscious nocebo condition, compared with conscious nocebo, was associated with increased activity in the amygdala, hippocampus, and thalamus. The initial statistical threshold was P < 0.01, family-wise corrected for ROI.
Figure 4.
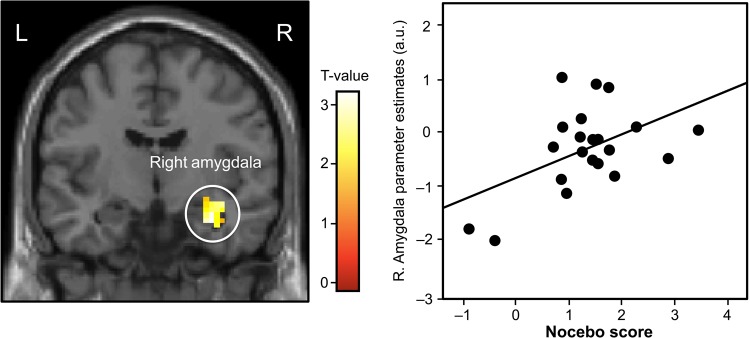
Amygdala activation during nocebo and correlation to pain ratings. Left panel: coronal representation of the increased activation in the right amygdala during nonconscious nocebo. The initial statistical threshold was P < 0.01, family-wise corrected for ROI. Right panel: a correlation analysis between extracted amygdala parameter estimates during nonconscious nocebo trials and the associated nocebo rating (r = 0.60, P < 0.005).
To elucidate the link between the predictive value of the aversive high pain signal, and subsequent neural responses to nonconscious nocebo cues, a correlation analysis between high pain reports during conditioning and amygdala activity during nonconscious nocebo trials was performed. Results demonstrate that high pain ratings during conditioning correlated with high activation of the amygdala during nonconscious trials, both for the right amygdala (r = 0.49, P < 0.05) and the left amygdala (r = 0.48, P < 0.05) but not for conscious nocebo trials.
Discussion
The present study demonstrates a neural mechanism for nonconscious activation of conditioned placebo/nocebo responses, including cortical as well as subcortical brain regions. During nonconsciously activated placebo analgesia, we found increased activation in the OFC, compared with consciously presented trials. The OFC is a phylogenetically old part of the prefrontal cortex (Thorpe et al. 1983) and has been widely described in studies of reward processing (Kringelbach 2005; O'Doherty and Dolan 2006) but also in placebo analgesia (Wager et al. 2004; Petrovic et al. 2010). Emerging evidence suggest that placebo analgesia share neural substrates with reward processing (Schweinhardt et al. 2009; Scott et al. 2009), which might suggest that nonconscious placebo activation of the OFC represents a reward-related signal rather than cognitive inhibition of nociception (Leknes et al. 2011). A recent study on monetary reinforcements demonstrated that we can learn to associate rewards with contextual cues, even without conscious processing (Pessiglione et al. 2008). Hence, it is possible that conscious expectation of pain relief, often reflected in the lateral prefrontal cortex, is not necessary to activate the more archaic OFC in response to conditioned placebo cues. Previous placebo studies, using consciously perceived cues, often report a mix of reward-related regions and inhibitory regions in the lateral prefrontal cortex (Kong et al. 2006, 2013), suggesting that there is considerable overlap between the brain regions recruited during conscious and nonconscious placebo. Results from the animal literature, however, support our finding by reports showing that stimulation of the OFC can activate endogenous analgesia through neural projections independently from conscious evaluation (Zhang et al. 1997). The OFC is not only activated by reward stimuli per se, and studies (Rolls 2000; O'Doherty et al. 2002) have demonstrated significant OFC activation during anticipation as well as receipt of a reward. This is similar to placebo responses since they may be seen as (conscious or nonconscious) representations of expected relief. Also, studies suggest that reward processing in the OFC represents prediction of error responses that mediate learning through updating expected/actual rewards (O'Doherty 2007). Along these lines, it is possible that the OFC in our study was more involved in coding the mismatch between the expected and perceived painful stimulus during nonconscious, compared with conscious trials. We suggest that the brain's internal representation of the painful stimulus can be adjusted and accounted for through different circuitry, with more or less flexibility, leading to the differential neural representations of placebo analgesia seen in the present study.
Nonconscious nocebo responses were associated with increased activations in fear-related subcortical structures of the brain, possibly reflecting processing of a perceived threat, since participants learn to associate the high cue with a highly aversive outcome. Previous neuroimaging studies have demonstrated how fear responses can occur without conscious perception of the fear cue (Phelps 2005), with special emphasis on the role of the amygdala and adjacent subcortical structures known to facilitate rapid recognition of threat (Morris et al. 1998; Carlsson et al. 2004). We found increased activity in the right hippocampus and amygdala during nonconscious nocebo, compared with conscious nocebo trials, furthering the role of the amygdala and hippocampus in conditioned responses of an aversive domain that is not necessarily fear-related, but related to pain (Kong et al. 2008; Bingel et al. 2011). Moreover, we found that higher activation of the amygdala during nonconscious nocebo trials correlated with the pain ratings during these trials. The link between the deliberate choice of a pain rating and the activation of the right amygdala during nonconscious nocebo trials supports previous suggestions of ways that unconscious motivation may affect our decisions and goal pursuits (Dijksterhuis et al. 2005; Custers and Aarts 2010).
Our findings of differential brain activation with nonconscious cues, compared with conscious cues, add to previous evidence of 2 processes by which placebo and nocebo responses are conveyed. Amanzio and Benedetti (1999) reported that conscious expectations of pain relief were associated with placebo effects that were naloxone reversible but that placebo effects induced by conditioning with a non-steroidal anti-inflammatory drug, without conscious expectations of an analgesic response, were not naloxone reversible. In another study from the same laboratory (Benedetti et al. 2003), verbally induced expectations completely reversed the effects of conditioning on conscious placebo responses (pain and motor movements), but not on nonconscious responses (hormonal secretion). Our study is clearly related to these previous studies, and in addition to adding neuroimaging, we also adopted a modified theoretical approach. The previous 2 studies compared conscious versus nonconscious bodily functions (i.e., pain ratings versus hormonal secretion), always presenting the treatment cues in plain sight (e.g., intravenous injection with a syringe, which the subjects are clearly seeing and feeling). The present study used conscious versus nonconscious conditioned cues, which means that the subject was either aware or not aware of the treatment cue. In its most simple form, our experiment would be as if Pavlov would ring his bell (or more correctly he might have used tones or buzzers) at a consciously undetectable frequency (conditioned stimulus) to test the effects of conditioned pain responses. Nonetheless, all 3 studies, taken together, support the conclusion that “conditioning procedures and other sources of information sometimes shape conscious expectancies and that these expectancies mediate some placebo effects; however, in other cases conditioning procedures appear to shape placebo effects that are not mediated by conscious cognition” (Stewart-Williams and Podd 2004).
The behavioral data revealed significant placebo and nocebo effects in response to both conscious and nonconscious cues; however, an interaction analysis revealed a trend toward larger conscious than nonconscious placebo responses. This difference is most likely due to enhanced analgesia expectancies during conscious trials. This interpretation is consistent with previous studies, in which it has been shown that placebo analgesia induced by classical conditioning and verbal suggestions together is of greater magnitude than placebo analgesia induced by verbal suggestions alone (Amanzio and Benedetti 1999; Benedetti et al. 2003; Colloca et al. 2008; Colloca and Benedetti 2009) and that the effect of a conditioned stimulus is also mediated by expectancy (Montgomery and Kirsch 1997; Watson et al. 2006, 2007; Kirsch et al. 2014).
The present study has several limitations. First of all, the placebo and nocebo effects were obtained in an experimental setting and cannot be directly translated to clinical situations. Also, the differences in pain ratings were statistically significant but might not represent large enough effects from a clinical perspective. Instead, our experiment was optimized for probing differences in neural processing of nonconscious versus conscious treatment cues, using well-defined a priori hypotheses based on previous neuroimaging studies. The statistical effects were modest but similar to the effects observed in other placebo neuroimaging studies with similar sample size. Furthermore, the correlation analyses of extracted amygdala values and pain ratings are to be considered as exploratory since the measures are not completely independent. Finally, the use of faces as conditional stimuli (CS) may have introduced a bias toward finding results involving the amygdala, as the amygdala is involved in face perception and evaluation (Todorov 2011). In line with previous neuroimaging experiments that used faces in backward-masking paradigms (Breiter et al. 1996; Whalen et al. 1998), we found that the right amygdala was more activated during nonconscious versus conscious trials, indicating that there is something about nonconscious processing of salient stimuli that activates the right amygdala (and not differences in face perception per se). To prevent a possible bias from differences in face perception between different face stimuli, faces were counterbalanced across pain levels, such that each face used as a CS for high pain for half of the participants and as a CS for low pain for the others, and the same faces were used in conscious and nonconscious conditions.
In conclusion, we propose a neural mechanism that conveys nonconscious perception of treatment cues into placebo and nocebo responses. Our results suggest that health-related behaviors can be triggered by cues that are not consciously perceived, not only for pain, but perhaps also for other medical problems with demonstrated placebo effects, like anxiety (Petrovic et al. 2005), asthma (Wechsler et al. 2011), and depression (Kirsch et al. 2008). It is possible that the increased understanding of how implicit treatment cues are conveyed into change in clinical outcomes can stimulate changes of clinical practice and improve therapeutic decisions.
Funding
This work was supported by the COFAS Marie Curie Postdoc Program and Osher Center for Integrative Medicine at Karolinska Institutet to K.J.; R01AT006364 (NCCAM), R21AT004497 (NCCAM), R03AT218317 (NIDA) to J.K.; K24 AT004095 (NCCAM) to T.K.; R01AT005280 (NCCAM) to R.G.; UL1 RR025758-01 (NCRR) P41RR14075 (NCRR) to Bruce Rosen. Funding to pay the Open Access publication charges for this article was provided by the Karolinska Institute.
Notes
Conflict of Interest: None declared.
References
- Amanzio M, Benedetti F. 1999. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 19:484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G. 2006. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 26:12014–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. 2003. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 23:4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. 2006. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 120:8–15. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. 2011. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 3:70ra14. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. 1996. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 17:875–887. [DOI] [PubMed] [Google Scholar]
- Buchel C, Geuter S, Sprenger C, Eippert F. 2014. Placebo analgesia: a predictive coding perspective. Neuron. 81:1223–1239. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Öhman A. 2004. Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 4:340–353. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. 2009. Placebo analgesia induced by social observational learning. Pain. 144:28–34. [DOI] [PubMed] [Google Scholar]
- Colloca L, Sigaudo M, Benedetti F. 2008. The role of learning in nocebo and placebo effects. Pain. 136:211–218. [DOI] [PubMed] [Google Scholar]
- Custers R, Aarts H. 2010. The unconscious will: how the pursuit of goals operates outside of conscious awareness. Science. 329:47–50. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Aarts H, Smith PK. 2005. The power of the subliminal: on subliminal persuasion and other potential applications. In: The New Unconscious—Social Cognition and Social Neuroscience. New York: Oxford University Press. [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. 2009. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 63:533–543. [DOI] [PubMed] [Google Scholar]
- Esteves F, Parra C, Dimberg U, Ohman A. 1994. Nonconscious associative learning: pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 31:375–385. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. 2012. Nonconscious activation of placebo and nocebo pain responses. PNAS. 109:15959–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. 2008. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 5:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Kong J, Sadler P, Spaeth R, Cook A, Kaptchuk TJ, Gollub R. 2014. Expectancy and conditioning in placebo analgesia: separate or connected processes? Psychol Conscious: Theory Res Pract. 1:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Lynn SJ, Vigorito M, Miller RR. 2004. The role of cognition in classical and operant conditioning. J Clin Psychol. 60:369–392. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, LaViolette P, Vangel M, Rosen B, Kaptchuk TJ. 2008. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 28:13354–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. 2006. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 26:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. 2013. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 154:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Dehaene S. 2007. Levels of processing during non-conscious perception: a critical review of visual masking. Philos Trans R Soc Lond B Biol Sci. 362:857–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M. 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 6:691–702. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Ann Rev Neurosci. 23:155–184. [DOI] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. 2011. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 6:e17870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. 1998. From sensation to cognition. Brain. 121:1013–1052. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. 2009. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 29:12236–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery GH, Kirsch I. 1997. Classical conditioning and the placebo effect. Pain. 72:107–113. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. 1998. Conscious and unconscious emotional learning in the human amygdala. Nature. 393:467–470. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. 2007. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 1121:254–272. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. 2002. Neural responses during anticipation of a primary taste reward. Neuron. 33:815–826. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dolan RJ. 2006. The role of human orbitofrontal cortex in reward prediction and behavioural choice: insights from neuroimaging. In: Zald D, Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press. [Google Scholar]
- Öhman A, Carlsson K, Lundqvist D, Ingvar M. 2007. On the unconscious subcortical origin of human fear. Physiol Behav. 92:180–185. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Petrovic P, Daunizeau J, Palminteri S, Dolan R, Frith C. 2008. Subliminal instrumental conditioning demonstrated in the human brain. Neuron. 59:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. 2007. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 316:904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. 2005. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 46:957–969. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. 2010. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 150:59–65. [DOI] [PubMed] [Google Scholar]
- Phelps E. 2005. The interaction of emotion and cognition: the relation between the human amygdala and cognitive awareness. In: Hassin RR, Uleman JS, Bargh JA, editors. The New Unconscious—Social Cognition and Social Neuroscience. New York: Oxford University Press. [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JNP, Tracey I. 2001. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 21:9896–9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. 2000. The orbitofrontal cortex and reward. Cereb Cortex. 10:284–294. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. 2009. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 29:4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. 2009. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 55:325–336. [DOI] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. 2004. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull. 130:324–340. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. 1983. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 49:93–115. [DOI] [PubMed] [Google Scholar]
- Todorov A. 2011. Evaluating faces on social dimensions. In: Todorov A, Fiske S, Prentice D, editors. Social Neuroscience: Toward Understanding the Underpinnings of the Social Mind. Oxford: Oxford University Press. [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. 2004. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 303:1162–1167. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AKP. 2006. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 126:115–122. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Vogt BA, Jones AKP. 2007. Placebo analgesia is not due to compliance or habituation: EEG and behavioural evidence. Neuro Report. 18:771–775. [DOI] [PubMed] [Google Scholar]
- Wechsler ME, Kelley JM, Boyd IO, Dutile S, Marigowda G, Kirsch I, Israel E, Kaptchuk TJ. 2011. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 365:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. 1998. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 18:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Tang JS, Yuan B, Jia H. 1997. Inhibitory effects of electrically evoked activation of ventrolateral orbital cortex on the tail-flick reflex are mediated by periaqueductal gray in rats. Pain. 72:127–135. [DOI] [PubMed] [Google Scholar]