Abstract
Purpose
The vascular ischemic hypothesis attributes nerve damage in the retina to decreased blood flow in the ophthalmic artery, reduced oxygenation, and impaired axonal transport. Activation of calpain enzymes contributes to retinal cell death during hypoxia. However, we still do not know in which specific retinal layers calpains are activated. Thus, the purpose of the present study was to investigate where and when calpains are activated in an improved culture model of hypoxic monkey retina.
Methods
Monkey retinal explants were cultured on microporous membranes with the retinal ganglion cell (RGC) side facing up. Explants were incubated under hypoxic conditions, with or without additional reoxygenation. When it was used, the calpain inhibitor SNJ-1945 was maintained throughout the culture period. Immunohistochemistry and immunoblotting assays for α-spectrin, calpains 1 and 2, calpastatin, β-III tubulin, and γ-synuclein were performed with specific antibodies. Cell death was assessed by TUNEL staining.
Results
Under normoxic conditions, TUNEL-positive cells were minimal in our improved culture conditions. As early as 8 hours after hypoxia, the 150-kDa calpain-specific α-spectrin breakdown product appeared in the nerve fiber layer (NFL), where calpains 1 and 2 were localized. TUNEL-positive RGCs then increased at later time periods. The calpain inhibitor SNJ-1945 ameliorated changes induced by hypoxia or hypoxia/reoxygenation.
Conclusions
During hypoxia/reoxygenation in an improved, relevant monkey model, calpains were first activated in the NFL, followed by death of the parent RGCs. This observation suggest that calpain-induced degeneration of retinal nerve fibers may be an underlying mechanism for RGC death in hypoxic retinal neuropathies.
Keywords: calpain, nerve fiber layer, retinal ganglion cells, proteolysis, cell death, monkey retinal explant culture, SNJ-1945
Hypoxia causes activation of calpains and production of spectrin breakdown products in the nerve fiber layer of monkey retinal explants, followed by induction of RGC death.
Retinal ganglion cells (RGCs) contain branched dendrites that extend into the retinal inner plexiform layer (IPL), where they collect photoreceptor-derived input from the amacrine and bipolar neurons. Axons of the RGCs assemble to form the optic nerve and pass on the photoreceptor nerve impulses to the brain.1 Optic nerves are also important for survival of RGCs. Optic nerve diseases are thus among the most devastating disorders in ophthalmology and can lead to RGC degeneration, visual field loss, and blindness.2 Although the mechanisms are not fully understood, diseases that cause optic neuropathies are glaucoma, axial optic neuritis, Leber's hereditary optic neuropathy, and anterior ischemic optic neuropathy.
The clinical features of glaucoma, one of the leading causes of blindness, include elevated intraocular pressure (IOP), excavation of the optic nerve head (ONH), and loss of RGCs. Elevated IOP can lead to insufficient blood perfusion to the ONH, deprivation of neurotrophic factors by blockage of retrograde transport along the RGC axons,3–5 and glial cell activation by changes in the extracellular matrix of the ONH.6–8 Blood pressure fluctuations or disturbed autoregulation of blood flow also cause insufficient blood perfusion at ONH.9–12 These changes can trigger ischemia-hypoxic stress, glutamate excitotoxicity, and abnormal immune responses and lead to rapid RGC death.13–15
Calpains comprise a 15-member family of Ca2+-activated cysteine proteases, which are organized into typical and atypical subfamilies based on differences in domains.16,17 Calpains modulate cell functions, such as cell death, by cleavage of specific regulatory proteins.18,19 In retinal diseases, calpain-associated proteolysis has been shown to be an underlying mechanism in neuronal degeneration and cell death.20 For example, elevated calcium levels followed by calpain activation are observed in autoimmune optic neuritis.21 In vivo rat models such as those using ischemia-reperfusion22 or acute ocular hypertension23 show that calpains are involved in RGC death. We also showed that calpains were involved in retinal cell death in human and non-human primate retinas cultured under hypoxic conditions.24,25 However, we could not determine where calpains were activated in specific retinal layers because the fragile retinal tissues were mechanically disrupted during culture. The present study investigated where calpains were activated by hypoxia in monkey retinas and determined the mechanism of RGC death.
Materials and Methods
Experimental Animals
Eyes from rhesus monkeys (Macaca mulatta) were obtained at necropsy from the Oregon National Primate Research Center (Beaverton, OR, USA). For ethical reasons, ages varied from 1 to 11 years. The wide age range was unavoidable but acceptable for the purposes of our experiments (see below) because monkey tissues could be obtained only when they became available from experiments that were unrelated to the present studies. Excised eyes were soaked in ice-cold Hanks' balanced salt solution (Life Technologies, Carlsbad, CA, USA), and the average time between death and dissection was less than 1.5 hours. Experimental animals were handled in accordance with the Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and with the Guiding Principles in the Care and Use of Animals (Department of Health Education and Welfare publication National Institutes of Health 80–23).
Retinal Explant Culture
Retinas were dissected into fan-shaped explants with 6 petals in Hanks' balanced salt solution. Each explant was cultured with the RGC side facing up on microporous membranes (Millipore, Billerica, MA, USA) in six-well culture plates at 37°C under 5% CO2 in Neurobasal-A medium supplemented with B27 and N2 supplement, and 2 mM l-glutamine (all from Life Technologies), and 100 μg/mL primocine (Invivogen, San Diego, CA, USA). After 3 hours, retinal explants were cultured under hypoxic conditions for 8 or 16 hours in culture medium with 0.5 mM glucose, using a gas-generating pouch system that reportedly maintains oxygen levels at less than 1% (GasPack EZ Anaerobe Pouch System; Becton Dickinson, Franklin Lakes, NJ, USA)26–28 and then reoxygenated for 8 hours in culture medium with 5.5 mM glucose. Low glucose was used in the hypoxia groups as in previous studies29 because reduced glucose is a contributing factor in many ischemia studies.25 Because of the absence of bubbling, the present monkey retinal explant model allowed preparation of retinal thin sections and flat mounts for layer-specific localization of calpain-related proteins. This could be not performed in the previous model probably because the retinas were disrupted by bubbling with 95% N2/5% CO2 gas to induce hypoxia.24 When it was used, the calpain inhibitor SNJ-1945 was maintained throughout the culture period at a final concentration of 100 μM in the medium. The 100 mM SNJ-1945 stock solution was dissolved in dimethyl sulfoxide (DMSO). SNJ-1945 was synthesized with the chemically reactive α-ketoamide masked by a cyclopropane residue. The masked α-ketoamide produces membrane permeability and calpain specificity.30 SNJ-1945 binding to the active site was confirmed in crystallized minicalpains.30
Protein Extraction From Explants and Immunoblotting
Lysates from retinal explants were obtained by sonication in buffer containing 20 mM Tris (pH 7.5), 5 mM EGTA, 5 mM EDTA, 2 mM dithioerythritol, and protease inhibitors (Complete Mini-EDTA-free; Roche, Indianapolis, IN, USA). The supernate was then collected by centrifugation at 16,100g for 10 minutes at 4°C. Protein concentrations were measured using BCA assay (Thermo Fisher Scientific, Rockford, IL, USA), using bovine serum albumin as the standard. For immunoblotting, 10 μg of each sample was loaded and run on 4% to 12% NuPAGE with 2-(N-morpholino)ethanesulfonic acid or MOPS buffer (Life Technologies). Proteins were then electrotransferred to polyvinylidene fluoride membrane at 100 V for 1.5 hours. After being blocked with 5% skim milk in Tris-buffered saline (Bio-Rad Lab, Hercules, CA, USA) containing 0.05% Tween 20, blots were probed with primary antibodies against the calpain-specific α-spectrin breakdown product at 150 kDa (SBDP150kDa) (1:1000 dilution),31 calpain 1 (1:1000 dilution; Thermo Fisher Scientific, Waltham, MA, USA), calpain 2 (1:1000 dilution; Sigma-Aldrich Corp., St. Louis, MO, USA, or Gene Tex, Irvine, CA, USA), calpastatin (1:500 dilution; Santa Cruz Biotechnology, Dallas, TX, USA), γ-synuclein (1:1000 dilution; Abcam, Cambridge, MA, USA), GAPDH (1:2000 dilution; Abcam), or α-spectrin (1:2000 dilution; Enzo Life Science, Plymouth Meeting, PA, USA). Immunoreactivity was visualized with secondary antibodies conjugated to horseradish peroxidase enzyme (1:4000 dilution; Santa Cruz Biotechnology) and ECL Plus detection reagents (GE Heath Care, Buckinghamshire, UK). Images of membranes were captured with FluorChem FC2 imager (Cell Biosciences, Inc., Santa Clara, CA, USA). Band intensities were measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA). To compensate for variability of staining between membranes, the densities of the bands were normalized to the density of endogenous GAPDH.
Incubation of Normal Retinal Soluble Proteins With Ca2+ in the Test Tube (Test-Tube Assay)
To investigate proteolysis of substrates by endogenous calpains in the test tube, the soluble proteins from homogenized, noncultured retinas were incubated with 10 mM CaCl2 for 30 minutes at 37°C, with or without the calpain inhibitor SNJ-1945 at 100 μM. Proteolysis was terminated by addition of EGTA at 10 mM final concentration. Proteolysis was visualized by immunoblotting as described above.
Immunohistochemistry of Retinal Thin Sections
Five-micrometer, formalin-fixed, paraffin-embedded sections of retinal explants were subjected to immunohistochemistry. After deparaffinization and rehydration, the sections were incubated in antigen retrieval buffer (10 mM citrate buffer, pH 6.0) for 10 minutes at 90°C. Then the sections were incubated for 15 minutes with PBS containing 0.2% Triton-X 100 for permeabilization, followed by blocking with phosphate buffered saline (PBS, pH 7.4; Life Technologies) containing 10% goat serum (Santa Cruz Biotechnology) for 30 minutes. The sections were incubated with primary antibodies against α-spectrin (1:100 dilution; Enzo Life Science), breakdown product SBDP150kDa (1:100 dilution), calpain 1 (1:100 dilution; Thermo Fisher Scientific), calpain 2 (1:100 dilution; Gene Tex), calpastatin (1:100 dilution; Santa Cruz Biotechnology), γ-synuclein (marker for retinal ganglion cell bodies and nerve fibers; 1:100 dilution; Abcam), and β-III tubulin (marker for neurons; 1:100 dilution; Sigma-Aldrich Corp.) overnight at 4°C. After being washed three times with PBS, the sections were incubated with secondary antibodies conjugated to Alexa Fluor 488 or 568 (1:200 dilution; Life Technologies), together with Hoechst 33342 dye (1:500 dilution; Life Technologies) for visualization of the nuclei.32–34 After sections were washed with PBS three times, Prolong Gold Antifade reagent (Life Technologies) was added and sealed with a coverslip. Images were observed using fluorescence microscopy (Axiovert 200; Carl Zeiss, Hallbergmoos, Germany). Both of the antibodies for γ-synuclein and SBDF150kDa were produced in rabbits. Thus, when SBDP150kDa and an NFL marker were costained in the same section, an antibody for β-III tubulin raised in mice was used instead of the antibody for γ-synuclein. Although β-III tubulin is a marker for neurons, not a specific marker for NFL, immunological localization provided specificity of β-III tubulin.35,36
Retinal Flat Mounts
Formalin-fixed retinal explants were incubated overnight at −30°C in DMSO-ethanol (1:4 dilution) to promote penetration of antibodies. Retinal explants were subjected to two cycles of freezing and thawing between −80°C and room temperature in 100% ethanol for 20 minutes. Retinal explants were then rehydrated in 70%, 50%, and 15% ethanol and PBS for 20 minutes each and incubated overnight with 0.2% Triton X-100. Samples were stained as described above, and images were observed with a model SP5 AOBS spectral confocal system (Leica, Wetzlar, Germany). Nerve fibers from RGCs were evaluated 3 to 7 mm from the tips of the fan-shaped retinal flat mounts. This was necessary because visualization of individual fibers in the optic nerve area was difficult due to their high density, and observations in far peripheral areas (tips of the fan) were not useful because of the low fiber density.
TUNEL Staining and Cell Counting
Cleavage of DNA in sections and flat mounts of retinal explants was determined using TUNEL technique (Cell Death Detection kit; Roche). TUNEL-positive cells were assumed to be undergoing apoptosis. For quantitative measurement, γ-synuclein- and TUNEL-positive cells were counted in two square areas (775 × 775 μm) at 3 to 5 and at 5 to 7 mm away from the tips of the fan-shaped retinal flat mounts. Measurements were averaged, and the percentage of TUNEL-positive RGCs was calculated as [γ-synuclein and TUNEL-double positive cells/all γ-synuclein-positive cells] × 100.
Statistical Analysis
The Wilcoxon rank sum test (JMP 10 statistical software; SAS Institute Inc., Cary, NC, USA) was used to evaluate the differences between groups.
Results
Hypoxia Activates Calpains in Explanted Monkey Retinas
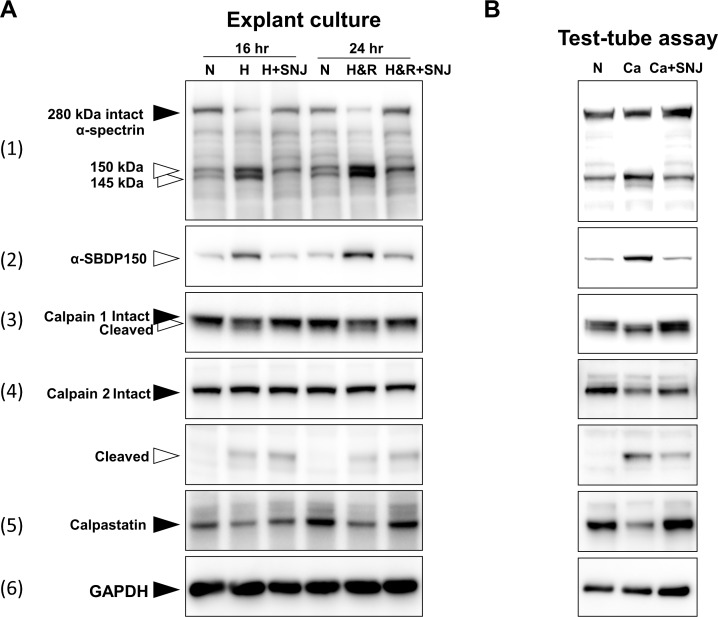
To detect calpain activation, monkey retina explants were cultured for 16 or 24 hours, and then the entire explant containing all retinal layers was homogenized and immunoblotted (Fig. 1A). After hypoxia, the band at 280 kDa for endogenous intact α-spectrin, a well-known substrate for calpain, decreased, and fragments of α-spectrin at 150 and 145 kDa were observed (Fig. 1A-1). An antibody specific for the calpain-specific spectrin breakdown product at 150 kDa (SBDP150kDa) detected such a band after hypoxia (Fig. 1A-2). Autolysis of calpains indicated activation,37 and the active fragments for calpain 1 (Fig. 1A-3) and for calpain 2 at 43 kDa (Fig. 1A-4) were detected after hypoxia. Calpastatin, the endogenous inhibitor of calpains, appeared to decrease slightly after 24 hours (Fig. 1A-5). Using semiquantitative densitometry to examine the immunoblotted calpastatin bands, we found the decreases in calpastatin to be statistically significant (data not shown). Incubation with the synthetic calpain inhibitor SNJ-1945 ameliorated these changes. Loading controls for GAPDH (Fig. 1A-6) indicated that equal amounts of proteins were added to the immunoblot wells. These data indicated that, despite abundant calpastatin, hypoxia activated calpain proteolysis in whole explanted monkey retinas.
Figure 1.
(A, left panels) Immunoblots from retinal explants cultured under hypoxia/reoxygenation for 16 or 24 hours. Black arrowheads show intact protein bands, and white arrowheads show their breakdown products. N, normoxia; H, hypoxia; H+SNJ, hypoxia plus the calpain inhibitor SNJ-1945; H&R, hypoxia for 16 hours followed by reoxygenation for 8 hours (hypoxia/reoxygenation); H&R+SNJ, hypoxia/reoxygenation plus SNJ-1945. (B, right panels) Immunoblots of normal, noncultured retinal soluble proteins incubated in the test tube for 30 minutes without Ca2+ (N), with Ca2+ (Ca), or with Ca2+ plus SNJ-1945 (Ca+SNJ). Endogenous GAPDH was used as the normalizing loading control.
Figure 2.
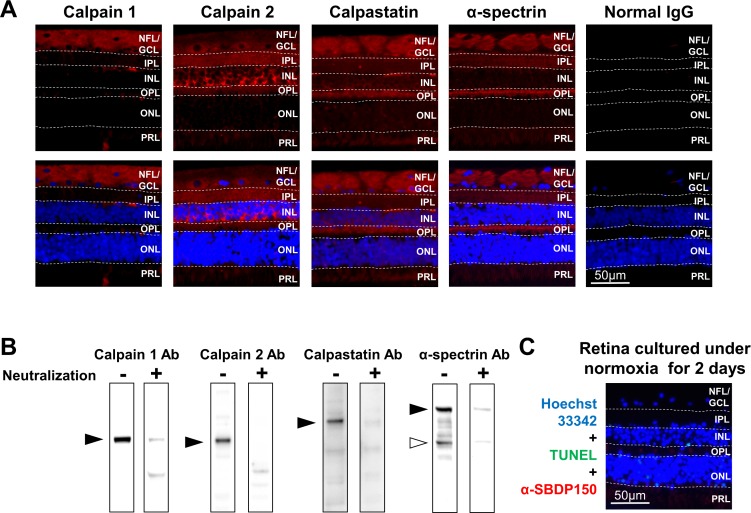
(A, top panels) Immunohistochemistry results of normoxic, noncultured retinas showing localization of calpain 1, calpain 2, calpastatin, and α-spectrin. (A, bottom panels) Images after merging with sections stained by Hoechst 33342 nuclear stain (blue). (A, far right panel) Negative-staining controls using nonimmunized IgG for calpain 1, calpain 2, calpastatin, and α-spectrin. Dashed lines show the margins of each retinal layer. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; NFL, nerve fiber layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor layer. (B) Immunoblots show the specificity of antibodies used for immunohistochemistry assay of calpains 1 and 2, calpastatin, and α-spectrin before (−) and after (+) neutralization with excess immunizing peptide. (C) Immunohistochemistry results of normoxic retina cultured for 2 days. Cells were triple stained for TUNEL-positive cells (green), calpain-specific breakdown product SBDP150kDa (red), and Hoechst 33342 nuclear stain (blue).
Figure 3.
(Upper panels) NFL from retinal explants cultured under hypoxia or hypoxia/reoxygenation show staining for calpain-specific breakdown product SBDP150kDa (red under white arrows) at 16 and 24 hours. Images were merged with Hoechst 33342-stained sections (blue). (Middle panels) Immunohistochemical staining of retinal explants show neuronal marker β-III tubulin (green) localized mostly in the NFL. (Lower panels) Double-stained retinal explants cultured under hypoxia/reoxygenation conditions show immunohistochemical colocalization of SBDP150kDa (red) and neuron-specific β-III tubulin (green) merged to form a yellow band of proteolyzed α-spectrin by calpains in the NFL. Abbreviations as in Figure 2 legend.
Figure 5.
(A) Flat mounts of GCL stained for TUNEL (green, white arrows). Compared to controls that were cultured for 24 hours (A, left panels), hypoxia/reoxygenation increased TUNEL-positive RGCs (A, middle panels). SNJ-1945 inhibited the increase in TUNEL-positive RGCs (A, right panels). Mounts countered stained for γ-synuclein (A, bottom panels) also demonstrate decreases in γ-synuclein in the RGCs during hypoxia/reoxygenation (middle panel), which was ameliorated by SNJ-1945 (right panel). Abbreviations as in Figure 2 legend. (B) Cross-sections show NFL/GCL stained for γ-synuclein. (C) Percentage of TUNEL-positive RGCs was calculated. Both hypoxia (H) and hypoxia plus reoxygenation (H&R) caused statistically significant increases in the percentage of TUNEL-positive RGCs and amelioration by SNJ-1945 (SNJ). Data are means ± SEM (n = 5). *P < 0.05 (Wilcoxon rank sum test). (D) Immunoblots for γ-synuclein from retinal explants cultured under hypoxia/reoxygenation conditions. (E) Band intensities from γ-synuclein in (D) normalized to GAPDH, confirming statistically significant decreases in γ-synuclein caused by hypoxia/reoxygenation and inhibition by SNJ-1945. Data are mean ± SEM (n = 9). *P < 0.05 relative to H&R. (F) Immunoblots for γ-synuclein from noncultured retinal soluble proteins incubated in the test tube without (−Ca) or with (+Ca) 10 mM Ca.
Figure 6.
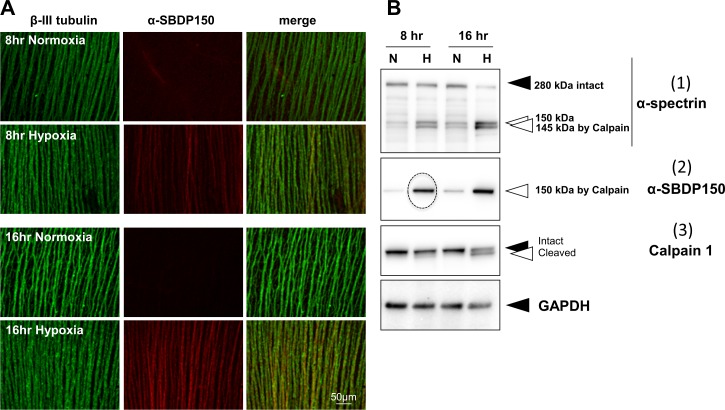
(A) Flat mounts with NFL immunostained for β-III tubulin (green) and for the breakdown product SBDP150kDa (red). SBDP150kDa appeared in the NFL as early as 8 hours after hypoxia (second row middle panel). (B) Immunoblots of retinal explants show that (1) hypoxia caused decreases in intact α-spectrin, (2) appearance of SBDP150kDa fragments (dotted circle), and (3) autolysis of calpain 1 as early as 8 hours. Black arrowheads show intact protein bands, and white arrowheads show proteolyzed fragments from the parent protein.
Most calpain is usually cytosolic and activated by calcium.38 The soluble proteins from noncultured normal monkey retinas were incubated with 10 mM calcium in the test tube. After 30 minutes, positive indicators of calpain activation were observed (Fig. 1B), and these changes were similar to those caused by hypoxia (Fig. 1A). This indicated that hypoxia probably activates calpains by causing an influx of calcium into at least some layers of the retina.
Calpain System Is Prominent in the NFL and GCL
Of the seven layers in normal monkey retinas, calpain 1 generally immunolocalized in the nerve fiber layer (NFL) and ganglion cell layer (GCL) (Fig. 2A). Calpain 2 was observed in the NFL, GCL, IPL, and outer plexiform layer (OPL) and at high levels in the inner nuclear layer (INL). Calpastatin was observed throughout the retina but was particularly prominent in the NFL and GCL. α-Spectrin was also located throughout the retina but was intense in the NFL, IPL, and OPL.
The relevant controls for these immunohistochemical studies indicated that (1) immunostaining with normal IgG in place of specific antibodies produced minimal background staining (Fig. 2A far right); (2) calpains, calpastatin, and α-spectrin antibodies were monospecific (note, SBDP stained together with intact α-spectrin, [Fig. 2A, white arrow head]) as the appropriate bands (Fig. 2B, dashes) were attenuated by first incubating the antibodies with their immunizing peptides (Fig. 2B, +); and (3) monkey retinal explants cultured under normoxia for up to 2 days produced visibly normal retina layering (Fig. 2C, blue). Furthermore, under normoxic conditions, TUNEL-positive cells (Fig. 2C, green) were negligible at 1 day (data not shown) and slightly elevated in INL and outer nuclear layer (ONL) after 2 days (Fig. 2C, green spots). SBDP150kDa was slightly observable at 2 days in PRL and OPL (Fig. 2C, red) under normoxic conditions.
These controls indicated that the normal explant culture conditions for 24 hours were relatively nontoxic and did not appreciably activate calpains and that the immunohistologic stains were monospecific.
Hypoxia Activates Calpain in NFL/GCL
Hypoxia (16 hours) or hypoxia/reoxygenation (24 hours) caused staining of SBDP150kDa specifically in the NFL (Fig. 3, white arrows, upper panels). To confirm localization of SBDP150kDa, costaining with the neuronal marker β-III tubulin, which localized principally in the NFL (Fig. 3, green, middle panels), was performed. SBDP150kDa localized mainly in the NFL after hypoxia and reoxygenation (Fig. 3, red and yellow, lower panels). Thus, hypoxia activated calpains in the NFL and GCL as early as 16 hours. The staining for calpastatin decreased slightly in retina treated with hypoxia (16 hours) or hypoxia/reoxygenation (24 hours), but distribution of calpastatin was similar to that observed in normoxic retina (data not shown). This was also supported by immunoblotting assay (Fig. 1A).
Calpain Activity in NFL/GCL Is Inhibited by SNJ-1945
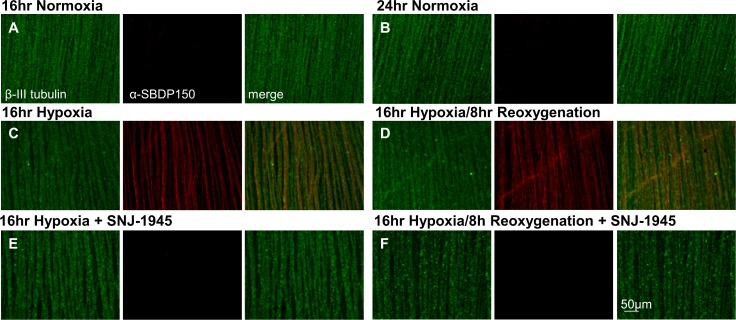
Flat mount sections counter-stained with β-III tubulin showed the NFL fibers as parallel rods (Fig. 4A, B, green). As described above with cross-sections, calpain activation during hypoxia (Figs. 4C, D, middle panels, red α-SBDP150) was confirmed in the NFL. Importantly, hypoxia-induced calpain activation was ameliorated by SNJ-1945 (Figs. 4E, F, lack of orange staining, lower right panels). Because calpain is an intracellular protease, these data indicated that this synthetic inhibitor, SNJ-1945, was able to penetrate the NFL/GCL and prevent calpain-induced proteolysis. As with calpain activation, obvious differences were not observed between young and adult monkeys (data not shown).
Figure 4.
(A, B) Normoxic controls. Flat mounts of retinal explants cultured under normoxia at 16 and 24 hours, show the NFL was stained for β-III tubulin (green), but not for the breakdown product SBDP150kDa (red). (C, D, middle panels) After hypoxia, abundant SBDP150kDa was found in the NFL. (E, F, lower panels) Formation of SBDP150kDa was almost completely inhibited by calpain inhibitor SNJ-1945.
Apoptosis Follows Hypoxia-Induced Calpain Proteolysis in the NFL
A causal relationship between hypoxia-induced calpain proteolysis and cell death in the NFL/GCL was tested next. Two stains were used and showed (a) TUNEL staining marking DNA fragmentation associated with apoptotic cell death and inhibition by SNJ-1945 were observed after 24 hours of hypoxia/reoxygenation (Fig. 5A, green dots with white arrows, upper panels); and (b) red counter-staining for γ-synuclein tagged the NFL and GCL layers (Fig. 5A, bottom panels and Fig. 5B).
Because of the low abundance of apoptotic cells (<10%), microscopic cell counting data for TUNEL-stained cells more clearly demonstrated increasing apoptosis at the later times, after hypoxia (Fig. 5C). After hypoxia, the percentage of TUNEL-positive-cells was significantly increased at 16 and 24 hours to 3% and 8%, respectively but was not elevated at 8 hours. SNJ-1945 significantly reduced the percentage of TUNEL-positive cells (Fig. 5C, 16 and 24 hours, “H&R+SNJ”).
We also noted that the staining for γ-synuclein appeared to decrease in the NFL/GCL in retinas treated with hypoxia/reoxygenation (Fig. 5A, bottom left “N” vs. bottom middle panel “H&R”), and this was ameliorated by SNJ-1945 (Fig. 5A, bottom right panel). To quantify changes in γ-synuclein, immunoblotting was performed. Immunoblot analysis confirmed the decrease in γ-synuclein and amelioration by SNJ-1945 (Fig. 5D). Band density analysis of the γ-synuclein bands demonstrated a statistically significant decrease due to hypoxia/reoxygenation (Fig. 5E) that was significantly ameliorated by SNJ-1945.
Surprisingly, direct activation of calpains by addition of calcium to normal retinal soluble proteins in the test tube did not cause proteolysis of γ-synuclein (Fig. 5F). Thus, γ-synuclein was not a substrate for calpains. These data suggested that loss of γ-synuclein was a consequence of hypoxia, but the loss was not due to direct hydrolysis by calpain.
The mechanism of γ-synuclein loss was in marked contrast to that of α-spectrin. α-Spectrin is a sensitive substrate for activated calpains in calcium incubation studies (Fig. 1B) and occurred as early as 8 hours after hypoxia in cultured explants (Fig. 6A, red, middle panels). The early hydrolysis of α-spectrin was supported by immunoblot analysis of the hypoxic explants at 8 hours that showed fragmentation of the 280-kDa α-spectrin band (Fig. 6B-1), the appearance of the strong band of the calpain-specific α-SBDP150 (Fig. 6B-2, dotted circle), and the appearance of the active cleavage product of calpain 1 (Fig. 6B-3). This suggested that, under hypoxic conditions, the early hydrolysis of α-spectrin by calpain might contribute to later apoptotic cell death and loss of γ-synuclein.
Discussion
The data above provide two new contributions, as follows. (1) Activation of calpain, hydrolysis of its substrates such as α-spectrin, and apoptosis occur in the NFLs and GCLs of the primate retina during hypoxia. (2) Inhibition of calpain activation by the cell-permeable inhibitor SNJ-1945 was demonstrated in an improved monkey model that may be relevant to treatment of hypoxic retinal neuropathies in man.
Mechanism of Cell Death
Numerous studies in rodents support the idea that hypoxia leads to influx of calcium into retina and activation of calpains. For example, hypoxia caused elevation of intracellular Ca2+ concentrations in retinal ganglion cells.39 Lack of oxygen would inhibit mitochondrial energy production, reduce active transport of calcium out of the RGC, and elevate free intracellular calcium levels. The active calpain heterodimer can sequester up to 10 calcium molecules that cause reconfiguration of relatively distant polypeptide regions into a functional active site.38,40 This active site is narrower and deeper than in similar proteases, and calpain thus prefers to hydrolyze at the unstructured regions of proteins.41
One such substrate is α-spectrin, which was rapidly hydrolyzed by calpain both in vitro and in vivo.42 In the present study, calpain-specific SBDP150kDa was observed in the NFL in retinal explants under hypoxic conditions (Figs. 3, 4), where calpains 1 and 2 were localized (Fig. 2A). α-Spectrin is also localized in plasma membranes and concentrated in axons and presynaptic terminals (Fig. 2A).43 In mammalian axons, α-spectrin is required to stabilize transmembrane proteins at the nodes of Ranvier,44 indicating a critical role of α-spectrin in axonal function. Because SBDP150kDa was not observed in the GCL (Fig. 3), calpains may also proteolyze other critical proteins in the soma. β-Tubulin, enolase, and heat shock protein 70 were hydrolyzed by calpains in an earlier model of primate retinas incubated under hypoxic conditions.24
Axonal damage due to calpain-induced hydrolysis of critical proteins may trigger retrograde signaling back to the soma of individual neurons through endoplasmic reticulum (ER) structures. ER structures are distributed along the entire lengths of axons and are connected with those in the neuronal soma.45 This could lead to the activation of proapoptotic signaling pathways in RGCs. This scenario is consistent with the timing of events in the present study, that is, TUNEL-positive RGCs were found after calpain-specific proteolysis of α-spectrin was observed in the NFL of hypoxic retinas. The number of TUNEL-positive RGCs was relatively small, presumably due to the initial stage of hypoxia. Loss due to necrosis may be an alternative mechanism. These mechanisms will be elucidated by future studies.
Paradoxically, the endogenous inhibitor calpastatin colocalized with calpains in the GCL (Fig. 2A). The presence of large amounts of calpastatin in other tissues exhibiting calpain-induced proteolysis is a common, although puzzling, finding.46 Some reports indicate that under the influence of elevated Ca2+, calpains translocate to membranes where they escape cytosolic calpastatin and where phospholipids facilitate calpain activation.47 Calpastatin is, in fact, a known substrate for calpains.48 This would allow further activation of calpains. In the present study, degradation of calpastatin was also observed, but it occurred after α-spectrin and suggested initial partial inhibition of calpains by calpastatin.
The sequence of events described above is consistent with the following hypothesis for the role of calpains in hypoxic primate retina: hypoxia inhibited mitochondrial energy production; active transport of calcium out of the NFL was inhibited; increased intracellular Ca2+ activated calpains; proteolysis of α-spectrin occurred in axons; calpain degraded calpastatin; and proapoptotic signaling pathways were activated in the axons, resulting in apoptosis in the soma and accompanied by loss of nonsubstrate proteins such as γ-synuclein.
Efficacy SNJ-1945 in a Relevant Monkey Model
Hypoxia is widely implicated in loss of RGCs, which occurs in many ophthalmic conditions.49–51 Importantly, the present study showed that the calpain inhibitor SNJ-1945 inhibited calpain activation and apoptosis of the RGCs in hypoxic retinal explants. SNJ-1945 also inhibited proteolysis of all calpain substrates when normal retinal soluble proteins were incubated with Ca2+ (Fig. 1B). In hypoxia-induced retinal explants, however, autolysis of calpain 2 was not inhibited by SNJ-1945 (Fig. 1A). This discrepancy may be due to localization of calpain 2 in the middle layers. Even though SNJ-1945 was present on both NFL and PRL sides, our explant model has no blood circulation. Even though SNJ-1945 was specifically designed for improved membrane permeability,52 SNJ-1945 may not have penetrated into the middle layers, such the INL. This may need to be addressed before SNJ-1945 is tested in clinical trials.
Note that calpain activation, location of proteolysis, and inhibition by synthetic inhibitor were demonstrated in an improved culture model. This model was less traumatic, retained apparently normal retinal layering, and, most importantly, used monkey retina. This model is obviously more relevant to the human situation than previous rodent studies and is further evidence that SNJ-1945 may be a promising drug in hypoxic optic nerve diseases.
Acknowledgments
The authors thank Anda Cornea at the Imaging and Morphology Support Core, Oregon Health and Science University, for technical assistance.
Supported in part by the National Center for Research Resources (NCRR)/National Institutes of Health Grant P51 OD011092 to the Oregon National Primate Research Center, and by NCRR Grant S10 RR024585 to the Imaging and Morphology Support Core, Oregon Health and Science University. The potential conflict of interest for Thomas R. Shearer, Mitsuyoshi Azuma, and Masayuki Hirata was reviewed, and a management plan approved by the OHSU Conflict of Interest in Research Committee was implemented.
Disclosure: M. Hirata, Senju Pharmaceutical Co. Ltd. (E); T.R. Shearer, Senju Pharmaceutical Co. Ltd. (C); M. Azuma, Senju Pharmaceutical Co. Ltd. (E)
References
- 1. Cohen-Cory S,, Lom B. Neurotrophic regulation of retinal ganglion cell synaptic connectivity: from axons and dendrites to synapses. Int J Dev Biol. 2004; 48: 947–956. [DOI] [PubMed] [Google Scholar]
- 2. Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye. 2004; 18: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 3. Anderson DR,, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974; 13: 771–783. [PubMed] [Google Scholar]
- 4. Gaasterland D,, Tanishima T,, Kuwabara T. Axoplasmic flow during chronic experimental glaucoma. 1. Light and electron microscopic studies of the monkey optic nervehead during development of glaucomatous cupping. Invest Ophthalmol Vis Sci. 1978; 17: 838–846. [PubMed] [Google Scholar]
- 5. Hayreh SS,, March W,, Anderson DR. Pathogenesis of block of rapid orthograde axonal transport by elevated intraocular pressure. Exp Eye Res. 1979; 28: 515–523. [DOI] [PubMed] [Google Scholar]
- 6. Hernandez MR,, Pena JD. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997; 115: 389–395. [DOI] [PubMed] [Google Scholar]
- 7. Pena JD,, Varela HJ,, Ricard CS,, Hernandez MR. Enhanced tenascin expression associated with reactive astrocytes in human optic nerve heads with primary open angle glaucoma. Exp Eye Res. 1999; 68: 29–40. [DOI] [PubMed] [Google Scholar]
- 8. Ricard CS,, Kobayashi S,, Pena JD,, Salvador-Silva M,, Agapova O,, Hernandez MR. Selective expression of neural cell adhesion molecule (NCAM)-180 in optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. Brain Res Mol Brain Res. 2000; 81: 62–79. [DOI] [PubMed] [Google Scholar]
- 9. Leske MC,, Heijl A,, Hyman L,, Bengtsson B,, Dong L,, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007; 114: 1965–1972. [DOI] [PubMed] [Google Scholar]
- 10. Flammer J,, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007; 52: 162–173. [DOI] [PubMed] [Google Scholar]
- 11. Leske MC,, Wu S-Y,, Hennis A,, Honkanen R,, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008; 115: 85–93. [DOI] [PubMed] [Google Scholar]
- 12. Memarzadeh F,, Ying-Lai M,, Chung J,, Azen SP,, Varma R. Blood pressure perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2010; 51: 2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehn MH,, Fingert JH,, Kwon YH. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am. 2005; 18: 383–395. [DOI] [PubMed] [Google Scholar]
- 14. Kaur C,, Foulds WS,, Ling E-A, Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008; 2: 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caprara C,, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012; 31: 89–119. [DOI] [PubMed] [Google Scholar]
- 16. Croall DE,, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991; 71: 813–847. [DOI] [PubMed] [Google Scholar]
- 17. Smith MA,, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012; 96: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goll DE,, Thompson VF,, Li H,, Wei W,, Cong J. The calpain system. Physiol Rev. 2003; 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 19. Momeni HR. Role of calpain in apoptosis. Cell J. 2011; 13 (2); 65–72. [PMC free article] [PubMed] [Google Scholar]
- 20. Paquet-Durand F,, Johnson L,, Ekström P. Calpain activity in retinal degeneration. J Neurosci Res. 2007; 85 (4); 693–702. [DOI] [PubMed] [Google Scholar]
- 21. Hoffmann DB,, Williams SK,, Bojcevski J,, Müller A,, Stadelmann C,, Naidoo V,, et al. Calcium influx and calpain activation mediate preclinical retinal neurodegeneration in autoimmune optic neuritis. J Neuropathol Exp Neurol. 2013; 72 (8); 745–757. [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto YR,, Nakajima TR,, Fukiage CR,, Sakai OR,, Yoshida YR,, Azuma MR,, et al. Involvement of calpain isoforms in ischemia-reperfusion injury in rat retina. Curr Eye Res. 2000; 21: 571–580. [PubMed] [Google Scholar]
- 23. Oka T,, Tamada Y,, Nakajima E,, Shearer TR,, Azuma M. Presence of calpain-induced proteolysis in retinal degeneration and dysfunction in a rat model of acute ocular hypertension. J Neurosci Res. 2006; 83: 1342–1351. [DOI] [PubMed] [Google Scholar]
- 24. Nakajima E,, David LL,, Bystrom C,, Shearer TR,, Azuma M. Calpain-specific proteolysis in primate retina: Contribution of calpains in cell death. Invest Ophthalmol Vis Sci. 2006; 47: 5469–5475. [DOI] [PubMed] [Google Scholar]
- 25. Azuma M,, Hammond KB,, Nakajima E,, Shearer TR. Calpain protease causes hypoxia-induced proteolysis in cultured human retina. Curr Eye Res. 2014; 39: 421–424. [DOI] [PubMed] [Google Scholar]
- 26. Potapova IA,, Gaudette GR,, Brink PR,, Robinson RB,, Rosen MR,, Cohen IS,, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007; 25 (7); 1761–1768. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y,, László C,, Liu Y,, Liu W,, Chen X,, Evans SC,, et al. Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation. Neoplasia. 2010; 12 (1); 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sansone CL,, Blumenthal EM. Neurodegeneration in drop-dead mutant drosophila melanogaster is associated with the respiratory system but not with Hypoxia. PLoS One. 2013; 8(7);e68032. [DOI] [PMC free article] [PubMed]
- 29. Nakajima E,, Hammond KB,, Rosales JL,, Shearer TR,, Azuma M. Calpain not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells. Invest Ophthalmol Vis Sci. 2011; 52 (10); 7059–7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Azuma M,, Shearer TR. The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol. 2008; 53 (2); 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saido TC,, Yokota M,, Nagao S,, Yamaura I,, Tani E,, Tsuchiya T,, et al. Spatial resolution of fodrin proteolysis in postischemic brain. J Biol Chem. 1993; 268: 25239–25243. [PubMed] [Google Scholar]
- 32. Wu M-M,, Zhu T-T,, Wang P,, Kuang F,, Hao D-J,, You S-W,, et al. Dose-dependent protective effect of lithium chloride on retinal ganglion cells is interrelated with an upregulated intraretinal BDNF after optic nerve transection in adult rats. Int J Mol Sci. 2014; 15 (8); 13550–13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan W,, Liu Z,, Wang X,, Luo X. Dark rearing maintains tyrosine hydroxylase expression in retinal amacrine cells following optic nerve transection. Neural Regen Res. 2012; 7 (1); 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Y,, Hong D-H,, Pawlyk B,, Yue G,, Adamian M,, Grynberg M,, et al. The retinitis pigmentosa GTPase regulator (RPGR)- interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003; 100 (7); 3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mi X-S,, Feng Q,, Lo ACY,, Chang RC-C,, Lin B,, Chung SK,, et al. Protection of retinal ganglion cells and retinal vasculature by Lycium barbarum polysaccharides in a mouse model of acute ocular hypertension. PLoS One. 2012; 7(10);e45469. [DOI] [PMC free article] [PubMed]
- 36. Haenold R,, Weih F,, Herrmann K-H,, Schmidt K-F,, Krempler K,, Engelmann C,, et al. NF-κB controls axonal regeneration and degeneration through cell-specific balance of RelA and p50 in the adult CNS. J Cell Sci. 2014; 127(Pt14);3052–3065. [DOI] [PubMed]
- 37. Cong J,, Goll DE,, Peterson AM,, Kapprell HP. The role of autolysis in activity of the Ca2+ -dependent proteinases (mu-calpain and m-calpain). J Biol Chem. 1989; 264: 10096–10103. [PubMed] [Google Scholar]
- 38. Suzuki K,, Hata S,, Kawabata Y,, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004; 53: 12–18. [DOI] [PubMed] [Google Scholar]
- 39. Sasaki T,, Kaneko A. Elevation of intracellular ca(2+) concentration induced by hypoxia in retinal ganglion cells. Jpn J Ophthalmol. 2007; 51 (3); 175–180. [DOI] [PubMed] [Google Scholar]
- 40. Moldoveanu T,, Gehring K,, Green DR. Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains. Nature. 2008; 456: 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sorimachi H,, Mamitsuka H,, Ono Y. Understanding the substrate specificity of conventional calpains. Biol Chem. 2012; 393: 853–871. [DOI] [PubMed] [Google Scholar]
- 42. Czogalla A,, Sikorski AF. Spectrin and calpain: a “target” and a “sniper” in the pathology of neuronal cells. Cell Mol Life Sci. 2005; 62: 1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riederer BM,, Zagon IS,, Goodman SR. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol. 1986; 102: 2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lacas-Gervais S,, Guo J,, Strenzke N,, Scarfone E,, Kolpe M,, Jahkel M,, et al. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004; 166: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu Y,, Park KK,, Yang L,, Wei X,, Yang Q,, Cho K-S,, et al. Differential effects of unfolded protein response pathways on axon injury-induced death of retinal ganglion cells. Neuron. 2012; 73: 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vaisid T,, Kosower NS,, Katzav A,, Chapman J,, Barnoy S. Calpastatin levels affect calpain activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer's disease. Neurochem Int. 2007; 51: 391–3977. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki K,, Sorimachi H. A novel aspect of calpain activation. FEBS Lett. 1998; 433: 1–4. [DOI] [PubMed] [Google Scholar]
- 48. Blomgren K. Calpastatin is upregulated and acts as a suicide substrate to calpains in neonatal rat hypoxia-ischemia. Ann N Y Acad Sci. 1999; 890: 270–271. [DOI] [PubMed] [Google Scholar]
- 49. Wax MB,, Tezel G. Neurobiology of glaucomatous optic neuropathy: diverse cellular events in neurodegeneration and neuroprotection. Mol Neurobiol. 2002; 26: 45–55. [DOI] [PubMed] [Google Scholar]
- 50. Tezel G,, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004; 15: 80–84. [DOI] [PubMed] [Google Scholar]
- 51. Chen H-L,, Pistollato F,, Hoeppner DJ,, Ni H-T,, McKay RDG,, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007; 25: 2291–2301. [DOI] [PubMed] [Google Scholar]
- 52. Shirasaki Y,, Miyashita H,, Yamaguchi M,, Inoue J,, Nakamura M. Exploration of orally available calpain inhibitors: peptidyl alpha-ketoamides containing an amphiphile at P3 site. Bioorg Med Chem. 2005; 13: 4473–4484. [DOI] [PubMed] [Google Scholar]