Abstract
Purpose.
To investigate the effect of pressure/flow on the barrier function and protein expression of normal and senescent porcine aqueous humor plexus (AAP) cells, which are the porcine equivalent of human Schlemm's canal endothelial cells.
Methods.
AAP cells were grown for 2 weeks in physiological (5% O2) or hyperoxic conditions (40% O2) to model cell senescence. Control and senescent AAP cells were subjected to control and elevated hydrostatic pressure gradient of 10 mm Hg for 72 hours. Hydraulic conductivity (HC) and transendothelial electric resistance (TEER) were measured. The expressions of senescence-associated β-galactosidase and DNA damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) were monitored, and the protein expression profile was analyzed by Western blot.
Results.
After 14 days of hyperoxia, AAP cells stained positive for 8-OHdG and β-galactosidase. Pressure elevation/flow resulted in significant increase of HC in control cells (from 1.37 ± 0.12 to 1.64 ± 0.18 μL/mm Hg/min/cm2, P < 0.05), but not in senescent cells (1.15 ± 0.17 and 1.08 ± 0.10 μL μL/mm Hg/min/cm2). TEER changes were consistent with the HC results. Western blot analysis showed that the expression level of myosin light chain, claudin-5, and VE-cadherin significantly reduced under pressure elevation in control cells but not in senescent cells.
Conclusions.
AAP cells are mechano-sensitive; however, cell senescence rendered the cells less responsive to mechanical stimulus, which may have pathological consequences.
Keywords: glaucoma, aging, Schlemm's canal
This study investigated the effect of aging on the mechanotransduction sensitivity of the porcine Schlemm's canal cells.
Introduction
Glaucoma is a group of complex and heterogeneous blinding diseases, affecting almost 75 million people worldwide. Clinically, glaucoma is characterized by cupping of the optic nerve head, resulting from apoptosis of damaged retinal ganglion cells. Although the molecular basis of glaucoma pathology is poorly understood, the risk of developing primary open angle glaucoma clearly increases with elevated IOP and age.1–7 Evidence suggests that death of retinal ganglion cells is the consequence of both ischemic and mechanical injury,8 likely due to elevated IOP.
In older human eyes, up to 90% of the aqueous humor exits the eye through trabecular meshwork (TM)/Schlemm's canal (SC). The inner wall of SC is formed by a continuous layer of endothelial cells, which is located at the major site of resistance to aqueous humor outflow and is thought to be important in controlling IOP.9,10 SC cells are joined together by tight junctions and desmosomes. Early studies showed that agents can act to alter cytoskeleton integrity of the SC cells to change outflow facility.11–13
SC cells are exposed to unusually large mechanical forces. Despite the relatively slow flow rate of aqueous humor in the eye, the shear stress in the SC was calculated to be in the range of 2 to 8 dynes/cm2, which could reach those in large arteries (2–25 dynes/cm2).14,15 The pressure gradient in normotensive eyes is estimated to be between 2 and 6 mm Hg (IOP minus episcleral venous pressure). SC cells are able to sense them and respond to them by modifying their physiological and biochemical properties.14,15 This functional link is likely important in cell tensional homeostasis and maintaining normal tissue structure and function. Similar to the blood vessel endothelial cells, SC endothelial cells experience shear stress and hydraulic pressure gradients, both of which can alter cell physiology.
Although pressure elevation and aging are important risk factors for glaucoma, unknown are effects of aging on SC cells in response to pressure gradients. In glaucoma patients, oxidative damage to TM/SC16–18 positively correlated with visual field damage and IOP.16 Using an in vitro model for human SC cells (porcine aqueous humor plexus [AAP] cells), we observed that cell senescence (due to hyperoxic damage) altered cytoskeletal and cell-cell adhesion protein expression, which impacted on its cell barrier functions.19 The purpose of this study was to investigate the barrier function and protein expression of AAP cells (normal versus senescent) subjected to a pressure gradient and associated flow across the cell monolayer.
Materials and Methods
AAP Isolation and Culture
AAP endothelial cells were isolated according to an established method developed by our group.20 For each cell line, the TM and AAP tissue from six porcine eyes (4- to 6-month-old pigs) was pooled to generate a primary mixed culture. After collagenase I (1 mg/mL; Sigma-Aldrich, Shanghai, China) digestion, cells were allowed to proliferate for 8 days and then were treated with puromycin (4 μg/mL, Invivo Gen, San Diego, CA, USA) for 2 days. The cultures were allowed to recover and selected cells populated the culture. Cells were characterized by unique expression of vascular endothelial (VE)-cadherin and von Willebrand factor. For the experiments, normal and senescent cells were exposed to elevated hydrostatic pressure, and a total of 10 cell lines were used.
Chronic Oxidative Stress Model
Chronic oxidative stress was induced by subjecting cells to normobaric hyperoxia condition (40% O2, 5% CO2) for 14 days in a triple-gas incubator (Smart cell, Shanghai, China) as described previously.19,21 Control cultures were grown under physiological oxygen conditions (5% O2, 5% CO2).
Elevated Hydrostatic Pressure Model
AAP cells were seeded at confluence onto the bottom side of a Transwell permeable filter membrane insert (Corning 3460; Corning, Shanghai, China; 0.4-μm pore diameter, 12-mm membrane diameter; 4 ×106 pores/cm2) and cultured. Before putting the AAP cells under a pressure gradient, they were typically cultured for 10 to 14 days and transendothelial electric resistance (TEER) was stable for 5 consecutive days. Cells were cultured under 10 mm Hg hydrostatic pressure (perfused in basal to apical direction) for 72 hours using a peristaltic pump system described by our group22 with slight modifications. This setup allowed testing of the effect of pressure without altering the gas tension on the cells.22 Briefly, the Transwell insert was closed by a silicon stopper and connected to a peristaltic pump (Kamoer, Shanghai, China), which was used to circulate medium between a reservoir exposed to incubator gases and the culture chamber. The medium reservoir was placed at a height of 5 and 136 mm above the cells to produce a control pressure and an elevated pressure of 10 mm Hg. This arrangement supplied fresh medium in equilibrium with the incubator gases to the cells inside the culture chamber. The flow rate of the pump was determined by perfusing the cell monolayer at the target pressure. After 72 hours of pressure elevation, the Transwell was disconnected from the reservoir and tested for hydraulic conductivity (HC) and TEER.
Hydraulic Conductivity
HC was measured using an established method by our group.19 After AAP cells were exposed to control and high pressure in the Transwell, they were perfused in the basal to apical direction at 4 mm Hg using a perfusion system described previously23 that delivered a variable flow rate (Q) across the cell layer to maintain a pressure drop (ΔP). Hydraulic conductivity (Lp) was calculated from Lp = Q/(ΔP × A), where A is the membrane area.
Transendothelial Electrical Resistance
TEER was measured after cells were exposed to control and high pressure. TEER measurements across confluent AAP cell monolayers grown on Transwells were performed at room temperature with STX-2 Ag/AgCl electrodes and an EVOM2 Voltohmeter (both from World Precision Instruments, Sarasota, FL, USA), according to manufacturer's instructions. Ten measurements were taken per each well, and three 12-well plates were measured for each strain of cells. All measurements of TEER were corrected to account for the resistance of the filter membrane.
Western Blot
Cell lysates were prepared using RIPA solution and protein concentration was estimated by the Bradford method. Equal amounts of protein (50 μg protein/lane) were separated by SDS-PAGE (10.0% or 12.5% acrylamide gel slabs), followed by electrophoretic transfer of resolved proteins to nitrocellulose filters. The membrane was blocked by 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween-20 for 2 hours. Filters were then probed using primary antibodies that specifically recognize myosin light chain (1:1000, Abcam, Shanghai, China), VE-cadherin (1:500, Abcam), and claudin-5 (1:500, Abcam), followed by incubation with peroxidase-linked secondary antibodies. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Signals in the linear range of the X-ray film were captured digitally and densitometry was performed using Kodak Molecular Imaging Software (Kodak, Shinkawa, Japan).
Statistics
For TEER and HC measurements, data distribution normality was first tested. Data were analyzed by the Mann-Whitney U test (SPSS 17 for Windows; IBM-SPSS, Chicago, IL, USA). In all cases, differences were considered significant at P less than 0.05.
Results
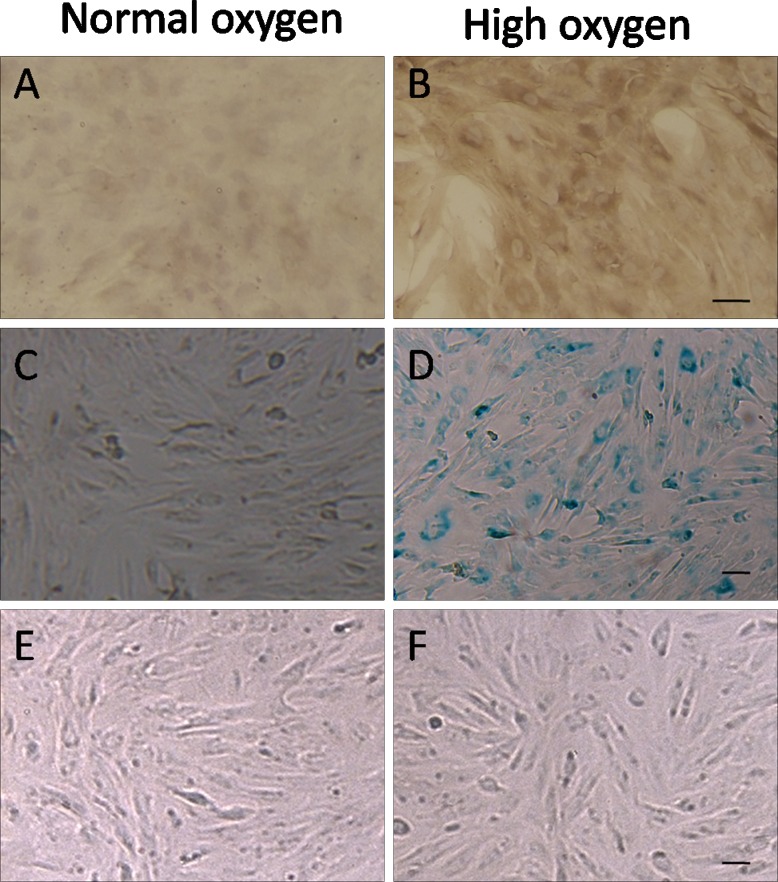
After 14 days of hyperoxia, AAP cells stained positive for the DNA damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) (Figs. 1A, 1B) and cell senescence marker β-galactosidase (Figs. 1C, 1D). Figures 1E and 1F show normal and senescent AAP cells subjected to 10 mm Hg pressure for 72 hours; cell morphology was not markedly different except that aged cells seemed slightly larger, which was likely due to oxidative stress, as reported previously.19
Figure 1.
AAP cells cultured in control and in high oxygen conditions. Cells cultured under normal conditions have negligible staining for DNA damage marker 8-OHdG and (A) senescence marker β-galactosidase (C), whereas cells exposed to high oxygen levels stained positive for these markers (B, D). Shown are AAP cells before (E) and after (F) being cultured under 10 mm Hg pressure for 72 hours.
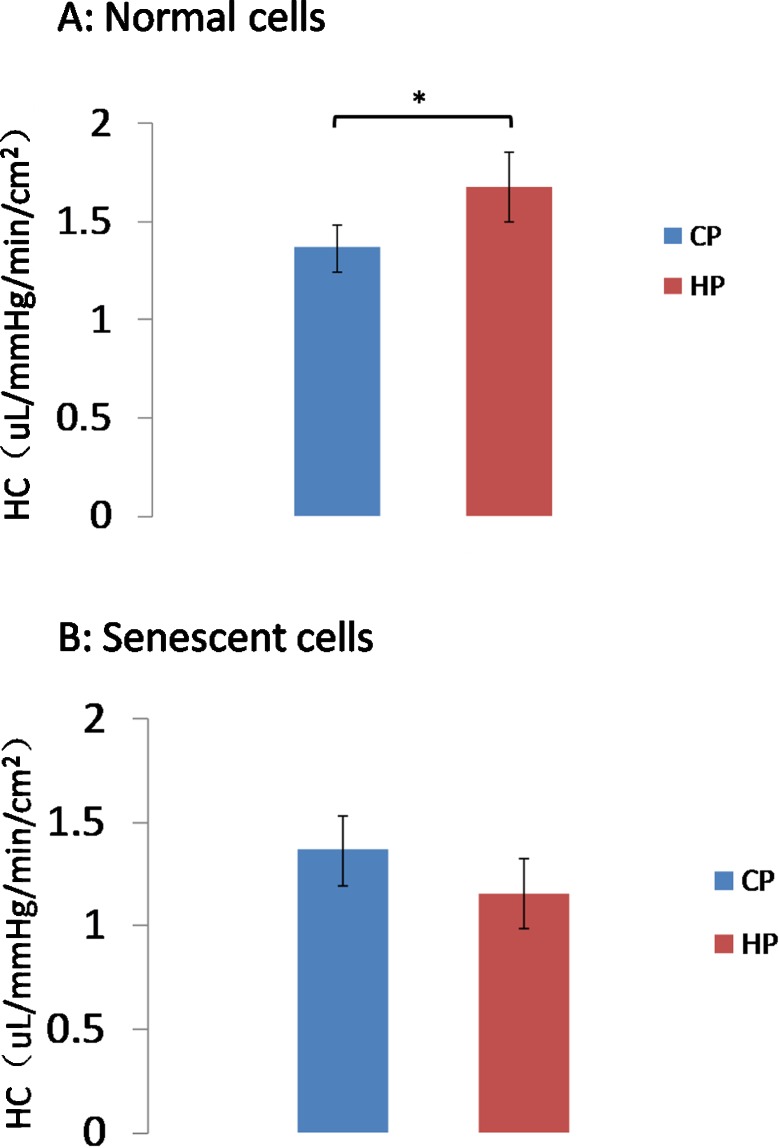
We measured HC of AAP monolayers of normal and senescent cells. In normal cells, HC was 1.37 ± 0.12 and 1.68 ± 0.18 μL/mm Hg/min/cm2 in control and high-pressure groups, respectively; HC significantly increased in the high-pressure condition compared with control (n = 10, P < 0.05; Fig. 2A). In senescent cells, HC was 1.15 ± 0.17 μL/mm Hg/min/cm2 and 1.08 ± 0.10 μL/mm Hg/min/cm2 in control and high-pressure groups, respectively, which did not differ significantly (n = 10, P > 0.05; Fig. 2B).
Figure 2.
HC of AAP cell monolayers. HC of control cells exposed to high pressure gradient was significantly greater than control pressure (A), *P < 0.05, n = 10. However, high pressure did not change HC significantly in senescent (hyperoxic) cells (B). CP, control pressure; HP, high pressure.
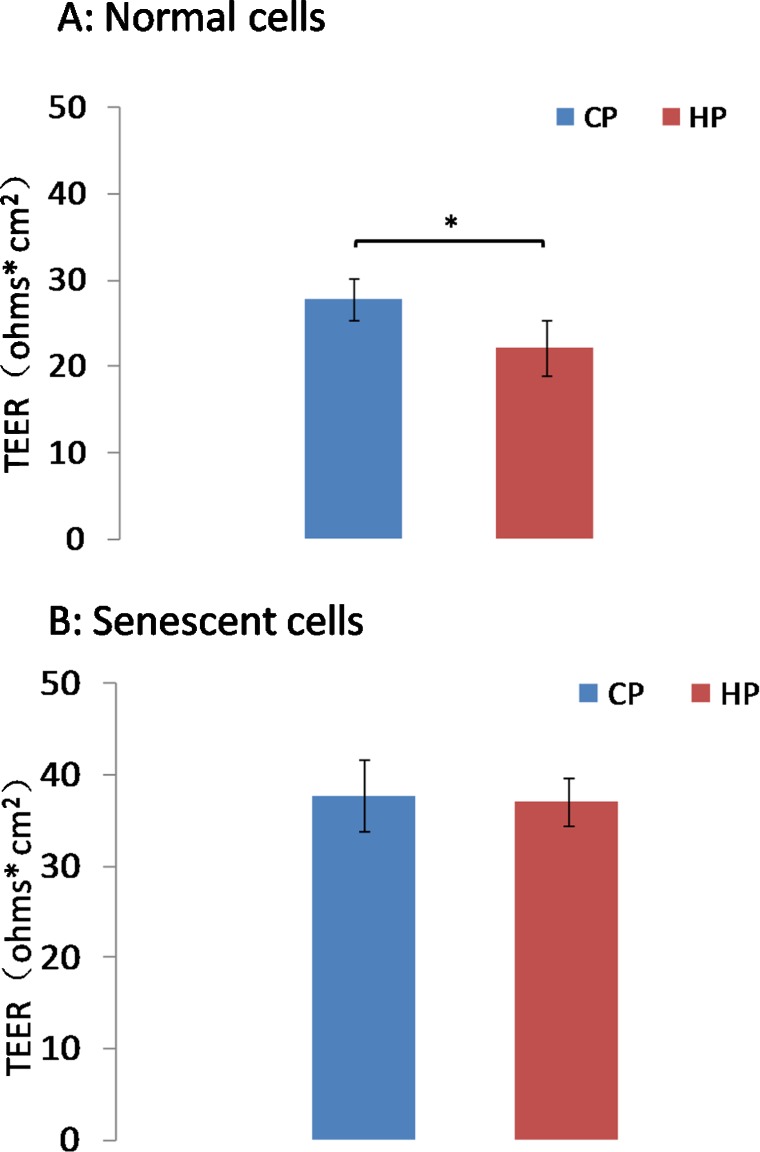
Similar to HC results, TEER of control cells was significantly lower in cells exposed to high pressure (22 ± 3.2 Ω* cm2) compared with those exposed in control pressure (28 ± 2.4 Ω* cm2, n = 10, P < 0.05; Fig. 3A). However, TEER of senescent cells subjected to high pressure gradient was 33 ± 2.3 Ω* cm2, which did not differ significantly from control pressure (32 ± 2.1 Ω* cm2, P > 0.05; Fig. 3B).
Figure 3.
TEER of AAP endothelial cell monolayers. In control cells (A), pressure elevation (10 mm Hg) resulted in significantly lower TEER than control pressure (*P < 0.05, n = 10). However, the TEER of senescent cells exposed to the same pressure elevation is similar to control cells (B).
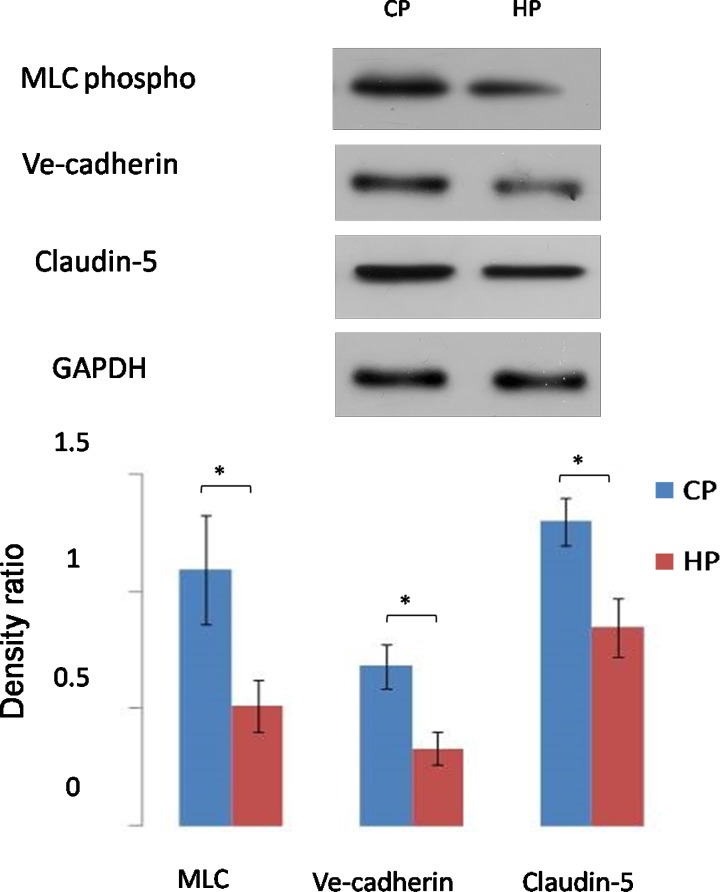
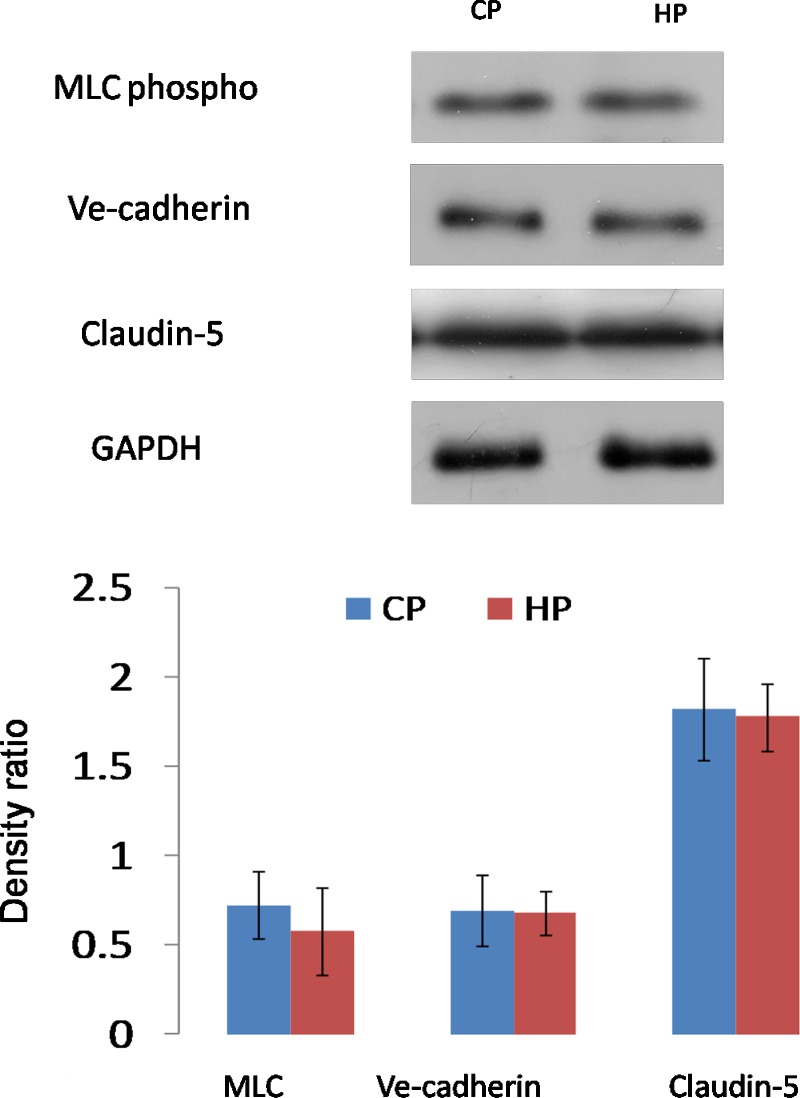
We then studied the barrier protein expressions using Western blot analysis (Figs. 4, 5). Data showed that in normal cells, MLC, VE-cadherin, and claudin-5 were significantly downregulated by pressure elevation. Densitometry analysis showed that the mean percentage reduction of the myosin light chain (MLC), VE-cadherin, and claudin-5 was 53%, 48%, and 26%, respectively (n = 10, P < 0.05; Fig. 4). Interestingly, senescent cells failed to respond to the pressure elevation, and the protein expression level of these three proteins was similar to control cells (n = 10, P > 0.05; Fig. 5).
Figure 4.
Western blot analysis of barrier protein expression by AAP cells subjected to control or high pressure gradient in normal cells. Pressure elevation resulted in significant reduction in MLC phospho, claudin-5, and VE-cadherin expression (*P < 0.05). Representative Western blots (above) and the corresponding densitometric analysis of blots (below) are for MLC phospho, claudin-5, and VE-cadherin. The y-axis is the blot density ratio of protein and GADPH. Shown are representative images of 1 experiment of 10 in total. Error bar is the mean ± SD of the mean.
Figure 5.
Western blot analysis of barrier protein expression by AAP cells subjected to control or high pressure gradient in senescent cells. Pressure elevation did not result in significant change in MLC phospho, claudin-5, and VE-cadherin expression. Error bar is the mean ± SD of the mean.
Discussion
In this study, we investigated the effects of senescence on the responses of AAP cells to a pressure gradient. We found for the first time that mechanotransduction of AAP cells, measured in three different ways, was reduced after cell senescence. We observed that pressure-induced changes in HC and TEER were negated in senescent AAP cells. We further observed that the barrier protein downregulation in normal AAP cells was abolished by aging. Taken together, results indicate that oxidative stress compromises physiological responses of AAP cells to a pressure gradient and associated transcellular and paracellular flow.
Previously our group found that oxidative stress rendered AAP cells more resistant to aqueous flow,19 with a significant increase in TEER and reduction in HC. Senescent cells expressed a significantly greater abundance of cytoskeleton, adhesion, and barrier proteins. In contrast, here we show that exposure of cells to a pressure gradient reduced the resistance of the AAP monolayer and the barrier proteins were downregulated, suggesting that junction disassembly is protective for further IOP elevation. However, this protective mechanism seemed to diminish in senescent cells in that they were less sensitive to the mechanical stimulus.
Although not all the primary open angle glaucoma patients have high IOP, reducing IOP is usually effective at slowing disease progression. SC and AAP cells are important in regulating IOP and therefore are the key cells to consider for pathology associated with ocular hypertension in glaucoma. In normal conditions, as other endothelial cells, the permeability of AAP cells increased with pressure elevation, which is a negative feedback mechanism to maintain a stable pressure. However, this ability of mechano-sensing seemed to be affected by cell aging, which coincides with increased risk of developing glaucoma in the elderly. Previous work also showed that the permeability of AAP cells reduced under oxidative stress and aging, which itself may result in increased IOP.19 Data collected here suggest that the aging cells are also less able to respond to pressure elevation, suggesting the development of a vicious cycle.
In our hydrostatic model, cells were exposed to a pressure gradient and paracellular and transcellular flow in the proper basal-to-apical direction. One shortcoming of the elevated hydrostatic pressure model used here is that the permeability of the cell monolayer may vary during the 72 hours of culture under elevated pressure. A stable flow of medium was supplied by the peristaltic pump to the reservoir. This may in turn result in variation in the target pressure. Several preliminary experiments were done to eliminate the error. A medium reservoir with a relatively big diameter (50-mL syringe) was used to compensate for this variation. We estimated that there was 2.2% ± 1.7% (mean ± SD) deviation from the 10 mm Hg target pressure during 72 hours. To culture the cells in basal-to-apical direction under elevated pressure was challenging, and initially we experienced cell sheets detaching from the Transwell membrane in the experiments. Cells from passage 2 were used in these studies, which were plated to Transwell membranes when it was approximately 80% confluent. In the preliminary experiments, when perfused cells under 2 to 10 mm Hg, we found that the higher the pressure the easier the cell sheets would detach from the membrane. It was important that a second needle should be threaded through the silicon stopper when the culture chamber was closed to shunt any pressure spikes that would otherwise damage the cell layer. Typically, the cells were cultured for 10 to 14 days before the experiments, too long or too short a culture time would increase the likelihood of cell sheets detaching.
In the vascular system, cell cytoskeleton also remodels and shows cell–matrix or cell–cell adhesions mediate mechanotransduction24 in response to shear stress. In eNOS overexpressed animal models, elevation of IOP induced increased pressure-dependent outflow.25 Inhibition of actin microtubules or intermediate filaments block many endothelial cell responses to flow.26 Importantly, advanced aging leads to impaired endothelial NO synthesis and enhanced endothelial cell apoptosis.27–29 In human umbilical vein endothelial cells, the application of shear stress, which exerted a profound apoptosis inhibitory effect via up regulation of NO synthesis in young cells, failed to inhibit apoptosis in aged cells.
In conclusion, our study showed that AAP cells were mechano-sensitive. However, senescence rendered the AAP cells less responsive to a pressure gradient, suggesting pathological consequences over time, such as the development of glaucoma.
Acknowledgments
Supported by National Science Foundation China (81100662, 81371015), Shanghai Municipal Health Bureau Young Outstanding Scientist Program (XYQ2013083), 211 Project of Fudan University (EHF158351), Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry), Allergan unlimited research Grant EY022359 (WDS), and Research to Prevent Blindness Foundation (WDS).
Disclosure: Y. Lei, Allergan (F); W.D. Stamer, None; J. Wu, None; X. Sun, None
References
- 1. Friedman DS. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004; 122: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989; 96: 26–32. [DOI] [PubMed] [Google Scholar]
- 3. Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001; 131: 699–708. [DOI] [PubMed] [Google Scholar]
- 4. Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology. 1989; 96: 1363–1368. [DOI] [PubMed] [Google Scholar]
- 5. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994; 112: 821–829. [DOI] [PubMed] [Google Scholar]
- 6. Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998; 105: 733–739. [DOI] [PubMed] [Google Scholar]
- 7. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore eye survey. JAMA. 1991; 266: 369–374. [PubMed] [Google Scholar]
- 8. Kuehn MH, Fingert JH, Kwon YH. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am. 2005; 18: 383–395, vi. [DOI] [PubMed] [Google Scholar]
- 9. Stamer WD, Roberts BC, Epstein DL. Hydraulic pressure stimulates adenosine 3′,5′-cyclic monophosphate accumulation in endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1999; 40: 1983–1988. [PubMed] [Google Scholar]
- 10. Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res. 1989; 8: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 11. Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 2246–2254. [DOI] [PubMed] [Google Scholar]
- 12. Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm's canal endothelial cell monolayers. Invest Ophthalmol Vis Sci. 2010; 51: 6633–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53: 3092–3103. [DOI] [PubMed] [Google Scholar]
- 14. Ethier CR, Read AT, Chan D. Biomechanics of Schlemm's canal endothelial cells: influence on F-actin architecture. Biophys J. 2004; 87: 2828–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allingham RR, de Kater AW, Ethier RC. Schlemm's canal and primary open angle glaucoma: correlation between Schlemm's canal dimensions and outflow facility. Exp Eye Res. 1996; 62: 101–110. [DOI] [PubMed] [Google Scholar]
- 16. Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005; 123: 458–463. [DOI] [PubMed] [Google Scholar]
- 17. Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 5251–5258. [DOI] [PubMed] [Google Scholar]
- 18. Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003; 114: 638–646. [DOI] [PubMed] [Google Scholar]
- 19. Lei Y, Stamer WD, Wu J, Sun X. Oxidative stress impact on barrier function of porcine angular aqueous plexus cell monolayers. Invest Ophthalmol Vis Sci. 2013; 54: 4827–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei Y, Overby DR, Read AT, Stamer WD, Ethier CR. A new method for selection of angular aqueous plexus cells from porcine eyes: a model for Schlemm's canal endothelium. Invest Ophthalmol Vis Sci. 2010; 51: 5744–5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci. 2008; 49: 3961–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lei Y, Rajabi S, Pedrigi RM, Overby DR, Read AT, Ethier CR. In vitro models for glaucoma research: effects of hydrostatic pressure. Invest Ophthalmol Vis Sci. 2011; 52: 6329–6339. [DOI] [PubMed] [Google Scholar]
- 23. Lei Y, Overby DR, Boussommier-Calleja A, Stamer WD, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011; 52: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stanley NR, Lazazzera BA. Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol. 2004; 52: 917–924. [DOI] [PubMed] [Google Scholar]
- 25. Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br J Pharmacol. 1996; 118: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luscher TF, Noll G. The pathogenesis of cardiovascular disease: role of the endothelium as a target and mediator. Atherosclerosis. 1995; 118: S81–S90. [PubMed] [Google Scholar]
- 28. Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans. Effects of age, atherosclerosis, hypercholesterolemia, and hypertension. J Clin Invest. 1993; 92: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001; 98: 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]