Abstract
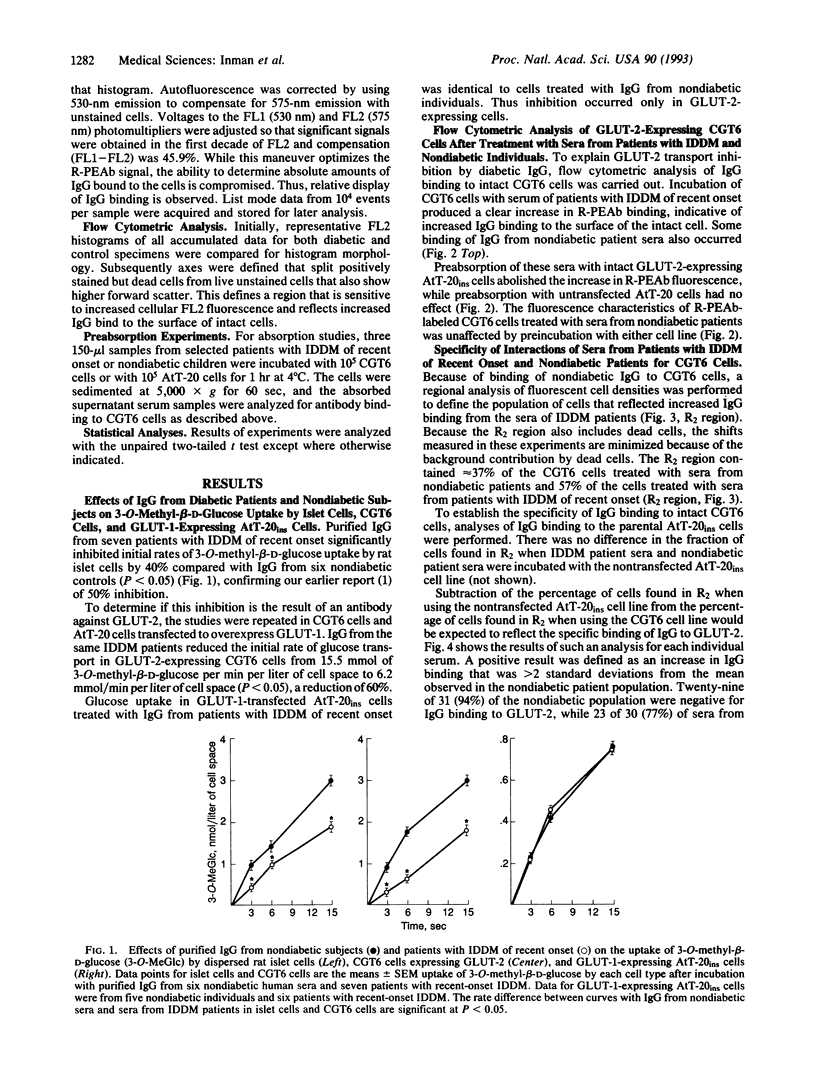
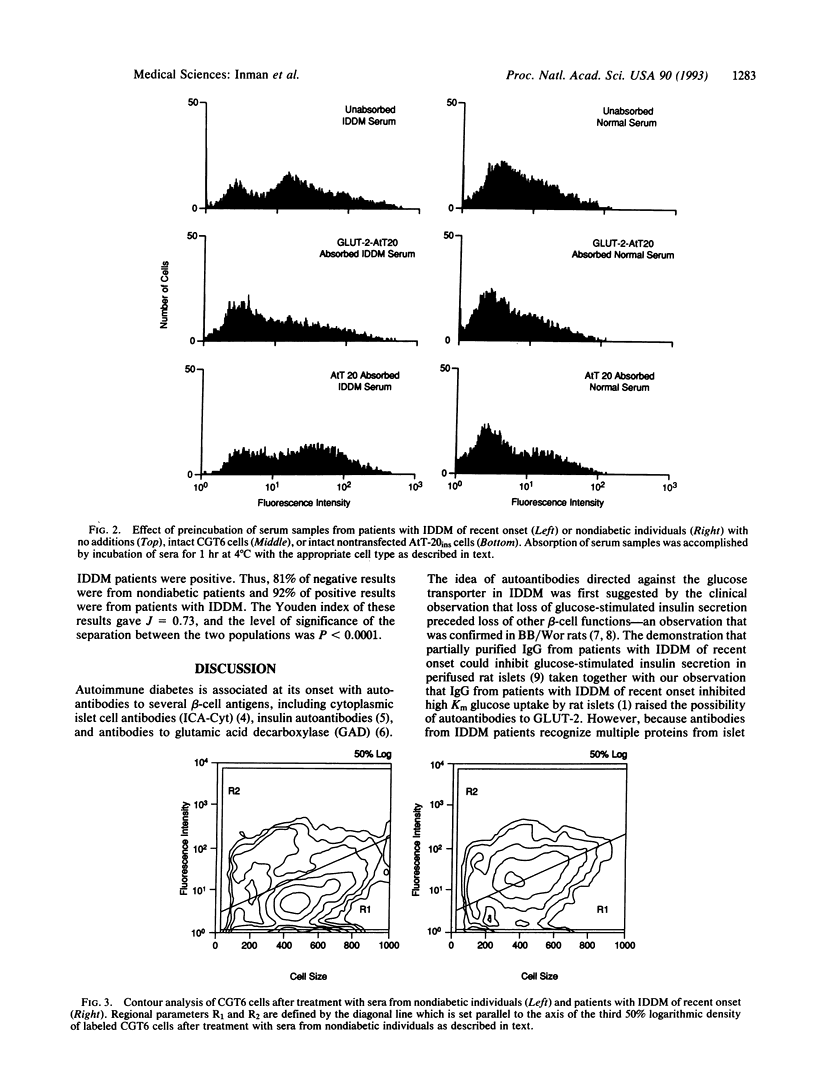
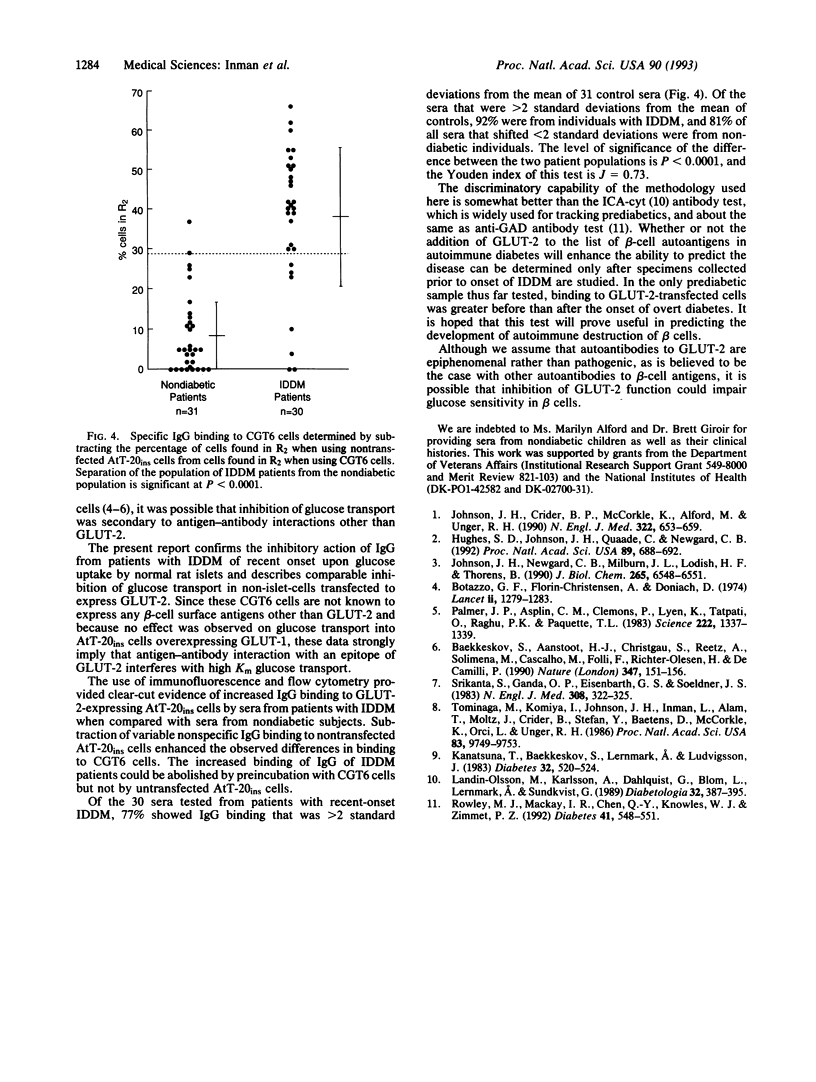
Purified immunoglobulin G (IgG) from the serum of patients with insulin-dependent diabetes mellitus (IDDM) of recent onset inhibits high-Km uptake of 3-O-methyl-beta-D-glucose by rat pancreatic islets. To determine if the inhibition is the result of antibodies against GLUT-2, the high-Km glucose transporter of beta cells, we incubated IDDM sera with rat islet cells and with AtT-20ins cells transfected to express GLUT-2. IDDM sera inhibited glucose uptake in islet cells and in GLUT-2-expressing AtT-20ins cells but not in AtT-20ins cells transfected to express the low-Km isoform, GLUT-1. In 24 of 30 (77%) patients with newly diagnosed IDDM, IgG binding as measured by immunofluorescence and flow cytometry of the cells transfected to express GLUT-2 was > 2 standard deviations from the mean of the nondiabetic population; 29 of 31 (96%) of nondiabetic children were negative (P < 0.0001). Increased IgG binding could be removed by absorption with GLUT-2-expressing cells but not with GLUT-1-expressing cells. We conclude that most patients with IDDM of recent onset have autoantibodies to GLUT-2.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974 Nov 30;2(7892):1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. D., Johnson J. H., Quaade C., Newgard C. B. Engineering of glucose-stimulated insulin secretion and biosynthesis in non-islet cells. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):688–692. doi: 10.1073/pnas.89.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Crider B. P., McCorkle K., Alford M., Unger R. H. Inhibition of glucose transport into rat islet cells by immunoglobulins from patients with new-onset insulin-dependent diabetes mellitus. N Engl J Med. 1990 Mar 8;322(10):653–659. doi: 10.1056/NEJM199003083221003. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Kanatsuna T., Baekkeskov S., Lernmark A., Ludvigsson J. Immunoglobulin from insulin-dependent diabetic children inhibits glucose-induced insulin release. Diabetes. 1983 Jun;32(6):520–524. doi: 10.2337/diab.32.6.520. [DOI] [PubMed] [Google Scholar]
- Landin-Olsson M., Karlsson A., Dahlquist G., Blom L., Lernmark A., Sundkvist G. Islet cell and other organ-specific autoantibodies in all children developing type 1 (insulin-dependent) diabetes mellitus in Sweden during one year and in matched control children. Diabetologia. 1989 Jun;32(6):387–395. doi: 10.1007/BF00277264. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R., Chen Q. Y., Knowles W. J., Zimmet P. Z. Antibodies to glutamic acid decarboxylase discriminate major types of diabetes mellitus. Diabetes. 1992 Apr;41(4):548–551. doi: 10.2337/diab.41.4.548. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Eisenbarth G. S., Soeldner J. S. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med. 1983 Feb 10;308(6):322–325. doi: 10.1056/NEJM198302103080607. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Komiya I., Johnson J. H., Inman L., Alam T., Moltz J., Crider B., Stefan Y., Baetens D., McCorkle K. Loss of insulin response to glucose but not arginine during the development of autoimmune diabetes in BB/W rats: relationships to islet volume and glucose transport rate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9749–9753. doi: 10.1073/pnas.83.24.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]



