Abstract
Noninvasive gene delivery to the spinal dorsal horn (SDH) remains challenging because existing methods to directly microinject vectors require laminectomy, which leads to tissue damage and inflammation. Such responses might hamper accurate readouts of cellular and behavioural effects of an introduced gene. Here we develop a new minimally-invasive SDH microinjection technique without the need of laminectomy in which a microcapillary is inserted into the SDH parenchyma through an intervertebral space. Using this method, we microinjected adeno-associated virus with an astrocytic promoter into the SDH and achieved efficient gene expression in an astrocyte-specific manner without gliosis, neuronal loss or inflammation. Furthermore, astrocytic loss- and gain-of-function of the transcription factor STAT3 by expressing a dominant-negative form and a constitutive-active form of STAT3, respectively, demonstrated the necessity and sufficiency of astrocytic STAT3 in the maintenance of neuropathic pain following peripheral nerve injury, a debilitating chronic pain state in which currently available treatments are frequently ineffective. Thus, our technique enables manipulation of gene expression in cell type- and spatial-specific manners without adverse effects, and may be useful for research in SDH physiology and pathology.
The spinal dorsal horn (SDH) receives sensory information from primary afferent sensory fibres following nociceptive stimuli1,2,3,4. Information is processed in the SDH through a complex network of interneurons and projection neurons and conveyed to several regions in the brain. Furthermore, the neuronal networks in the SDH are modulated and modified under pathological conditions such as peripheral tissue inflammation and peripheral nerve injury (PNI)1,4. Recent evidence indicates that glial cells in the SDH contribute to these pathologically altered neurotransmissions5,6. Thus, elucidating the roles of each cell type and understanding the communication between neurons and glial cells in the SDH will advance our understanding of the mechanisms involved in the activation, modulation and modification of sensory processing in the SDH under physiological and pathological conditions. Several approaches have been established to regulate gene expression, such as intrathecal injection of antisense oligonucleotides or siRNA and the conditional deletion of a gene using the Cre-loxP system. However, technical limitations of these methods include lack of segment-, region- and cell type-specificity, or the conditional deletion of genes at the developmental stage. Recently, several viral vectors have been reported to be useful tools for region- and cell type-specific gene transduction7,8,9. Several groups have tried to introduce genes into target cells in mice and rats using an intra-spinal injection method10,11,12,13,14,15,16. However, existing methods need complicated surgical procedures such as laminectomy10,11,12,13,14 or drilling of a hole in the vertebra15,16 for direct access to the spinal cord parenchyma, which results in an inflammatory response and extensive tissue damage that could hamper cellular and behavioural phenotypes of sensory processing. Indeed, recent studies have shown that inflammatory factors such as pro-inflammatory cytokines affect neuronal excitability and pain behaviours17,18,19.
To overcome the limitations described above, we set out to establish a new microinjection technique into the SDH. In this study, we developed a minimally-invasive SDH microinjection technique in mice without the need of laminectomy and any complicated surgical procedures: a microcapillary is inserted into the SDH parenchyma through an intervertebral space. This method is much easier and less invasive than previously reported methods. Furthermore, using this new technique, we successfully introduced genes using recombinant adeno-associated viral (rAAV) vectors into 4th lumbar (L4)-SDH astrocytes without any apparent detrimental effects. In addition, we provide the first evidence indicating the necessity and sufficiency of astrocytic signal transducer and activator of transcription 3 (STAT3) in the maintenance of neuropathic pain. Thus, our established microinjection technique enables the manipulation of gene expression in the SDH in a cell type- and segment-specific manner, without adverse effects, and may be useful for basic research in SDH physiology and pathology.
Results
Establishment of a minimally invasive intra-SDH injection method
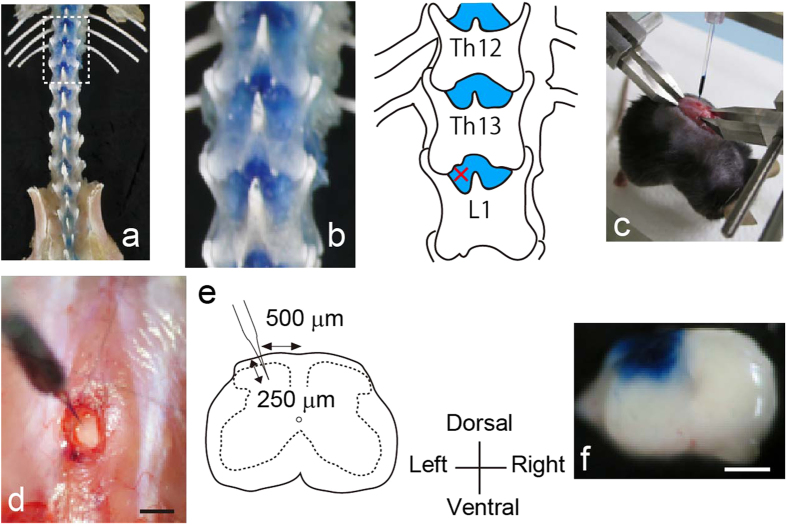
To search for a site enabling intra-SDH microinjection without laminectomy, we first visualized the skeleton of the vertebrae at the thoracic and lumbar levels of the mouse that had been fixed and injected intrathecally with Evans blue (Fig. 1a). We found that there was a dense blue area at each interspace between vertebrae that was not covered by the spine (Fig. 1b). Next, we determined if microinjection through this intervertebral space enabled delivery of substances into the SDH without laminectomy. An incision was made in the back of an anaesthetized mouse placed in a stereotaxic apparatus (Fig. 1c) and a small opening in the muscles around the left side of the interspace between Th13 and L1 vertebrae (Fig. 1d) was made. The dura mater and the arachnoid membrane were incised to make a small window to allow insertion of the microcapillary directly into the SDH. We inserted the microcapillary into the SDH (250 μm in depth from the surface of the dorsal root entry zone; Fig. 1d,e) and injected the dye. After injection, we removed the lumbar spinal cord and confirmed that the injected dye was successfully localized in the SDH (Fig. 1f). Thus, this technique enabled injection of a substance into the SDH without laminectomy in the mouse.
Figure 1. New minimally invasive method for intra-spinal dorsal horn (SDH) microinjection.
(a) Skeletal preparation of mouse vertebral column and pelvis viewed from the dorsal aspect. (b) High magnification of dashed line in the panel (a) and its schematic illustration. Blue stained regions are uncovered by vertebra. X; intra-SDH microinjection site. (c) Photograph showing intra-SDH microinjection. The mouse was fixed by attaching custom-made clamps to the vertebral column. A microcapillary was filled with Evans blue. (d) High magnification view of intra-SDH injection site (Scale bar, 1 mm). (e) Schematic illustration of intra-SDH injection. (f) Spinal cord section of mouse after intra-SDH microinjection of Evans blue (Scale bar, 500 μm).
Specific transgene expression in SDH astrocytes by intra-SDH injection of rAAV
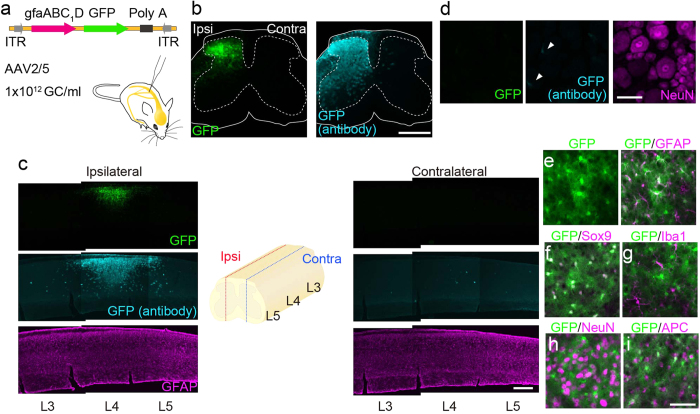
Using this new technique, we attempted to express genes in the SDH in a cell type-specific manner. In this study, we focused on astrocytes, the most abundant cell-type in the central nervous system (CNS), because they play a pivotal role in neuronal function under physiological and pathological conditions20,21,22, including chronic pain. We microinjected AAV2/5 (a serotype showing a preferential tropism for astrocytes23) containing a gene encoding green fluorescent protein (GFP) under the control of a gfap promoter (gfaABC1D; which increases astrocyte-specific expression24,25,26,27) (AAV2/5-gfaABC1D-GFP; Fig. 2a) unilaterally into the SDH through the window on the interspace between Th13 and L1 vertebrae. Twenty-eight days later, we observed strong GFP fluorescence exclusively in the ipsilateral SDH, but not contralateral to the injection site (Fig. 2b). GFP expression was predominantly localized in the L4 segment of the SDH (Fig. 2c). Consistent with these results, a similar spatial localization of GFP immunofluorescence was detected with an antibody directed against GFP (Fig. 2b,c). In contrast, we did not observe GFP fluorescence in dorsal root ganglion (DRG) neurons and only observed faint GFP immunofluorescence with the antibody (Fig. 2d, arrowheads). To determine the cell type-specificity of transgene expression, we performed immunolabelling using cell type-specific markers for identification of GFP-expressing cells. Almost all GFP-positive cells double-labelled with glial fibrillary acidic protein (GFAP) (Fig. 2e) and Sox9 (Fig. 2f), both of which are markers of astrocytes: 99.0% of total GFP-positive cells had Sox9 immunofluorescence, and 73.5% of Sox9-positive cells were positive to GFP (n = 5), indicating that the majority of total SDH astrocytes were efficiently transduced. We also observed no co-localization of GFP with ionized calcium-binding adapter molecule-1 (Iba1)- (Fig. 2g), neuronal nuclei (NeuN)- (Fig. 2h) or adenomatous polyposis coli (APC)-positive cells (Fig. 2i). Therefore, these results indicate that intra-SDH microinjection of AAV2/5-gfaABC1D-GFP induces selective gene expression in L4-SDH astrocytes.
Figure 2. Astrocyte-specific gene expression by intra-SDH microinjection of AAV2/5-gfaABC1D.
(a) Schematic illustration of a viral vector construct. (b,c) GFP expression in the spinal cord of mice 28 days after intra-SDH injection of AAV2/5-gfaABC1D-GFP (b transverse; c sagittal sections). GFAP (magenta) was used to counterstain the spinal cord. Ipsi, ipsilateral; C contralateral (Scale bar, 500 μm). (d) GFP expression in the 4th lumbar (L4) dorsal root ganglion (DRG) of intra-SDH injected mice. NeuN (magenta) was used to counterstain the DRG (Scale bar, 50 μm). (e–i) Immunohistochemical identification of GFP-expressing cells using cell-type markers (magenta; e GFAP; f Sox9; g Iba1; h NeuN; i APC) in the L4-SDH sections 28 days after intra-SDH injection of AAV2/5-gfaABC1D-GFP (Scale bar, 50 μm).
Titre- and time-dependent inflammation and alteration in pain responses by intra-SDH injection of AAV2/5
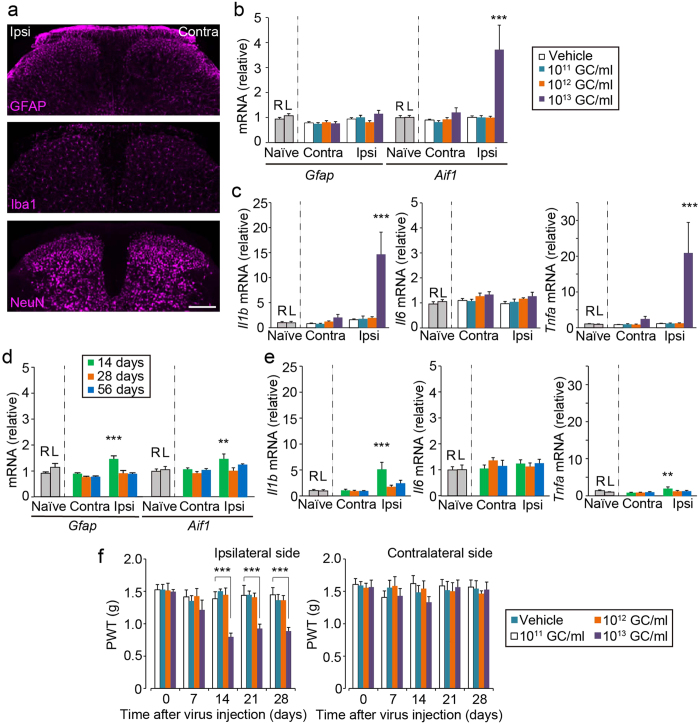
A previous study has shown that AAV2/5-transduced astrocytes become activated in a titre-dependent manner23. However, under our conditions in which AAV2/5-gfaABC1D-GFP [1012 genome copies (GC)/ml] was injected, neither gliosis (astrocytes and microglia) nor neuronal loss in the L4-SDH were observed (Fig. 3a). Consistent with this result, there was no change in the expression GFAP (Gfap) and Iba1 (Aif1) mRNA in the SDH (Fig. 3b). Furthermore, the levels of mRNA for interleukin (IL)-1β (Il1b), IL-6 (Il6) and tumor necrosis factor (TNF)-α (Tnfa) were not elevated (Fig. 3c). In contrast, a higher titre (1013 GC/ml) of AAV2/5-gfaABC1D-GFP not only increased the expression of GFP (Supplementary Fig. 1a), but also Aif1, Il1b and Tnfa, suggesting that this titre induces an inflammatory response (Fig. 3b,c). Furthermore, we investigated the time-dependent inflammatory response after SDH microinjection of 1012 GC/ml of AAV2/5-gfaABC1D-GFP. While a slight upregulation of Gfap, Aif1, Il1b and Tnfa mRNA was observed 14 days after intra-SDH injection, their expression returned to basal levels over the next 14 or 42 days (Fig. 3d,e). GFP expression was not different on either day 14 or day 28 after injection and significantly increased on day 56 (Supplementary Fig. 1b).
Figure 3. Titre-dependent adverse effects following intra-SDH AAV2/5-gfaABC1D injection.
(a) Immunofluorescence labelling for cell-type markers (GFAP, astrocytes; Iba1, microglia; NeuN, neurons) in the L4-SDH sections 28 days after intra-SDH injection of AAV2/5-gfaABC1D-GFP (Scale bar, 200 μm). (b,c) mRNA expression of genes 28 days after intra-SDH injection of vehicle or rAAV (1011–1013 GC/ml), and in naïve mice. Values represent the relative ratio of mRNA (normalized to Gapdh mRNA) to the mean expression level of mRNA in naïve mice. R, right; L, left (b,c; n = 6–8, ***P < 0.001 versus naïve spinal cord). (d,e) mRNA expression of genes 14, 28 and 56 days after intra-SDH injection of rAAV (1012 GC/ml) and in naïve mice. Values represent the relative ratio of mRNA (normalized to Gapdh mRNA) to the mean expression level of mRNA in naïve mice. (d,e; n = 6–8, **P < 0.01; ***P < 0.001 versus naïve spinal cord). (f) Paw withdrawal threshold (PWT) of mechanical stimulation to the ipsilateral and contralateral hindpaws of vehicle and virus injected mice (1011–1013 GC/ml) before (day 0) and after intra-SDH injection (n = 8; ***P < 0.001 versus vehicle injected group).
To examine the influence of these viral infection and inflammatory events on animal behaviour in vivo, we measured paw withdrawal threshold (PWT) to mechanical stimulation, which was applied to the hindpaw. Mice injected with AAV2/5-gfaABC1D-GFP (1011 or 1012 GC/ml) into the SDH showed no significant change in PWT (Fig. 3f). In contrast, the high titre of AAV2/5-gfaABC1D-GFP (1013 GC/ml) resulted in a marked decrease in PWT of the ipsilateral side from 14 days after intra-SDH injection, but not on the contralateral side (Fig. 3f). Therefore, under our experimental conditions, 28 days after SDH microinjection of 1012 GC/ml viral-titre of AAV2/5-gfaABC1D-GFP was an optimal condition to introduce a gene of interest into SDH astrocytes without an inflammatory response or sensory abnormalities.
Astrocytic STAT3 in the SDH is required for maintaining neuropathic pain
We have previously shown that PNI causes activation of STAT3 in spinal astrocytes and that intrathecal administration of an inhibitor of JAK alleviates the developed pain hypersensitivity after PNI28. These results suggest the involvement of the astrocytic JAK-STAT3 signalling pathway in the maintenance of neuropathic pain. In contrast, other studies have reported that STAT3 signalling in spinal microglia contributes to neuropathic pain development16,29. Therefore, the role of astrocytic STAT3 in neuropathic pain remains controversial. To clarify this, we utilized the established intra-SDH microinjection technique to express a dominant-negative form of STAT3 (dnSTAT3)30 in a SDH astrocyte-specific manner (Fig. 4a).
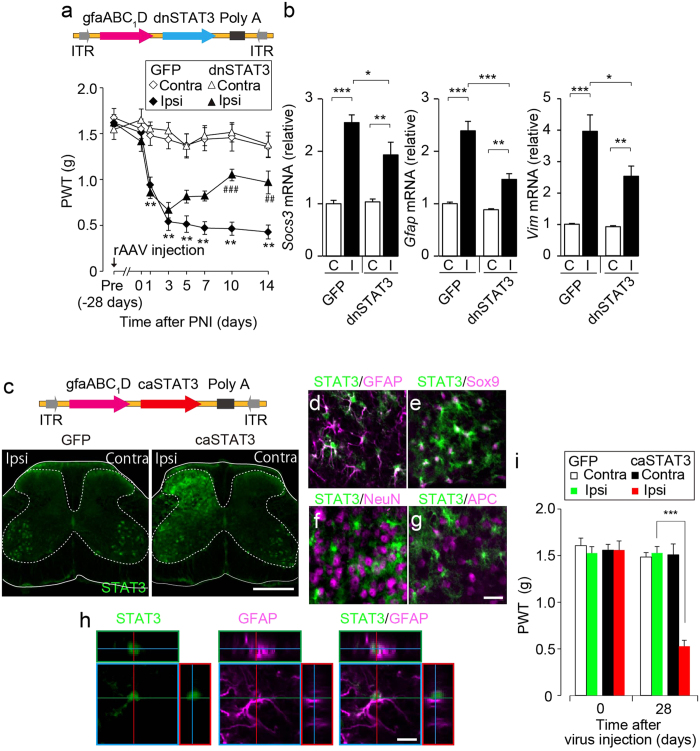
Figure 4. The L4-SDH astrocytic STAT3 signalling is necessary and sufficient for the maintenance of neuropathic pain.
(a) PWT of C57BL/6J mice injected with AAV2/5-gfaABC1D-GFP or -dnSTAT3 pre (−28 days), before (day 0) and after peripheral nerve injury (PNI) (n = 8, **P < 0.01 versus pre, ##P < 0.01; ###P < 0.001 versus ipsilateral side of AAV2/5-gfaABC1D-GFP-injected group). (b) Real-time PCR analysis of mRNA in mice injected with AAV2/5-gfaABC1D-GFP or -dnSTAT3 14 days after PNI. Values represent the relative ratio of mRNA (normalized to actb mRNA) to the contralateral side of AAV2/5-gfaABC1D-GFP injected group. C, contralateral; I, ipsilateral. (n = 7–8, *P < 0.05; **P < 0.01; ***P < 0.001). (c) Immunofluorescence of STAT3 in L4 spinal cord sections 28 days after intra-SDH injection of AAV2/5-gfaABC1D-GFP or -caSTAT3 (Scale bar, 500 μm). (d-g) Double immunofluorescence labelling for STAT3 (green) and cell-type markers (magenta; d GFAP; e Sox9; f NeuN; g APC) in L4-SDH sections 28 days after intra-SDH injection of AAV2/5-gfaABC1D-GFP or -caSTAT3 (Scale bar, 20 μm). (h) Representative confocal z-stack digital images of a single cell double-immunolabelled with STAT3 (green) and GFAP (magenta). Scale bar, 10 μm. (i) PWT of AAV2/5-gfaABC1D-GFP- or -caSTAT3-injected mice before (day 0) and after virus injection (n = 8, ***P < 0.001 versus ipsilateral side of AAV2/5-gfaABC1D-GFP injected group).
AAV2/5-gfaABC1D-dnSTAT3-treated mice showed no effect of basal mechanical sensitivity before PNI (Fig. 4a). Following PNI, PWT decreased, which was comparable between mice injected with AAV2/5-gfaABC1D-GFP and -dnSTAT3 from day 1 to day 3 (Fig. 4a). However, AAV2/5-gfaABC1D-dnSTAT3-treated mice produced a progressive recovery in decreased PWT from day 5 to day 14 (Fig. 4a). Furthermore, PNI-induced upregulation of suppressor of cytokine signalling 3 (Socs3), Gfap and vimentin (Vim) on day 14 was significantly suppressed by introducing dnSTAT3 in L4-SDH astrocytes (Fig. 4b). These results indicate that interfering with astrocytic STAT3 activation suppresses the maintenance of PNI-induced tactile allodynia. To validate the effect of astrocytic introduction of dnSTAT3, we used a conditional knockout approach in which we crossed Stat3fl/fl mice with transgenic mice bearing Cre recombinase under the control of the GFAP promoter (Gfap-cre mice) and generated Gfap-cre;Stat3fl/fl mice in which the loss of STAT3 function occurs preferentially in astrocytes31,32. Consistent with the result of AAV2/5-gfaABC1D-dnSTAT3-injected mice, Gfap-cre;Stat3fl/fl mice displayed significant recovery of PNI-induced tactile allodynia (Supplementary Fig. 2a) and a marked suppression of PNI-induced upregulation of Socs3, Gfap and Vim (Supplementary Fig. 2b). Together, these results strongly suggest that L4-SDH astrocytic STAT3 signalling is necessary for the transition of astrocytes to a reactive state and for the maintenance of neuropathic pain after PNI.
We then examined whether STAT3 activation in SDH astrocytes produces tactile allodynia in mice without nerve injury. To induce gain of function of STAT3 in SDH astrocytes, we microinjected AAV2/5 with the constitutive active form of STAT3 (caSTAT3)33 into the SDH (Fig. 4c). Strong STAT3 immunofluorescence was observed in the L4-SDH 28 days after intra-SDH injection of AAV2/5-gfaABC1D-caSTAT3 compared with AAV2/5-gfaABC1D-GFP (Fig. 4c). Double-immunolabelling experiments using cell markers revealed that caSTAT3-positive cells were double-labelled with GFAP (Fig. 4d,h) and Sox9 (Fig. 4e), but not with NeuN (Fig. 4f) and APC (Fig. 4g). Under such conditions, mice injected with AAV2/5-gfaABC1D-caSTAT3 showed a significant decrease in PWT on the ipsilateral side (Fig. 4i). These results imply that L4-SDH astrocytic STAT3 activation is sufficient to produce tactile allodynia.
Discussion
In the current study, we established a new technique for intra-SDH microinjection in mice. This method does not need any complicated surgical procedures such as laminectomy or drilling of a hole in the vertebra, which are commonly necessary for intra-spinal injection. In our method, both surgical operation and microinjection into the SDH was completed on average within 15 min per mouse. Thus, our established method is much easier and faster than that of existing methods and enables microinjection of substances into the mouse SDH without any substantial tissue and SDH damage. This minimally invasive method enables microinjection of a variety of substances into the SDH. Here, we showed cell type- and segment-specific expression of genes of interest in the SDH. In this study, by using the astrocyte tropic AAV serotype AAV2/5 with the gfap promoter gfaABC1D, we achieved efficient and selective gene transduction in L4-SDH astrocytes. In addition, intra-SDH microinjection did not lead to gene expression in the DRG. Using a viral titre of 1012 GC/ml, almost all transduced cells expressed the astrocytic marker Sox9, and over 70% of total astrocytes in the L4-SDH were transduced. The transduction efficiency (and also gene expression per cell) could be increased by intra-SDH injection of AAV2/5 at higher viral titres. Indeed, a high titre (1013 GC/ml: approximately 5–6 × 109 GC per injection) of AAV2/5-gfaABC1D resulted in increased GFP expression. However, it should be noted that microinjection of this viral titre led to a marked increase in the expression of the microglial marker Iba1 and proinflammatory cytokines in the SDH, implying that inflammatory responses might be induced. The detrimental responses also affected animal behaviour, because mice displayed long-lasting pain hypersensitivity. Therefore, the viral titre is a critical factor when determining the effect of an introduced gene on behavioural phenotypes. However, it appears that titre-dependent adverse effects of intraparenchymal injection of rAAV may vary depending on the method, site of injection, or vector construction. Intra-hippocampal injection of AAV2/5-gfa104-eGFP (3 × 1010 GC per injection) induces reactive astrocytosis without microgliosis23. Injection of AAV2/5-gfaABC1D-cyto-GCaMP3 (3 × 1010 GC per injection) and Lck-GCaMP3 (2.4 × 1010 GC per injection) into the hippocampus did not cause astrogliosis24. However, these studies have not measured the expression of proinflammatory factors after viral infection. In contrast, 1012 GC/ml of AAV2/5-gfaABC1D-GFP, which resulted in efficient and selective gene expression in L4-SDH astrocytes, did not affect behavioural sensitivity to mechanical stimulation over the 1 month of testing after the injection, however, a slight increase in the expression of proinflammatory cytokines, Aif1 and Gfap were observed on day 14. Thus, we conclude that intra-SDH microinjection of AAV2/5-gfaABC1D at 1012 GC/ml of viral titre would be optimal to enable efficient and selective gene transduction in L4-SDH astrocytes without detrimental inflammatory responses or sensory abnormalities.
Spinal astrocytes have been proposed to play an important role in the maintenance of neuropathic pain based on studies where manipulation of the function or expression of molecules activated in spinal astrocytes (such as the transcription factor STAT3) by intrathecal administration of either pharmacological inhibitors, antisense oligodeoxynucleotides or siRNAs28,34,35,36,37,38,39 affects established neuropathic pain after PNI. However, there is no direct evidence showing that SDH astrocyte-specific gene manipulation modulates neuropathic pain maintenance. In addition, the involvement of STAT3 in spinal astrocytes has remained controversial because it has been reported that PNI activates STAT3 in astrocytes and microglia and that intrathecal administration of an inhibitor of JAK, which is an upstream activator of STAT3, suppresses the induction or established tactile allodynia after PNI in rats16,28,29. In this study, by using our established astrocyte-specific gene expression method, we demonstrated for the first time the pivotal role of astrocytic STAT3 in the SDH for the maintenance of neuropathic pain. We showed that expression of dnSTAT3 in L4-SDH astrocytes produced a progressive reversal of established tactile allodynia after PNI. This reversal is supported by our previous study showing that pharmacological inhibition of JAK reduces established allodynia after PNI in rats28. Interestingly, despite the expression of dnSTAT3 in L4-SDH astrocytes prior to PNI, the induction of tactile allodynia was not affected. This finding strengthens the role of astrocytes in the maintenance phase. Furthermore, the reversal of PNI-induced tactile allodynia and astrocytic gene expression in mice with L4-SDH astrocyte-specific expression of dnSTAT3 was also observed in Gfap-cre;Stat3fl/fl mice. Cre recombinase activity in Gfap-cre;Stat3fl/fl mice occurs not only in SDH astrocytes but also other regions including ventral spinal cord astrocytes and DRG satellite glia (Supplementary Fig. 3). Therefore, this similar phenotype obtained from two independent approaches provides compelling evidence indicating that astrocytic STAT3 in the L4-SDH is necessary for maintaining tactile allodynia. In addition, it is conceivable that the observed phenotype in Gfap-cre;Stat3fl/fl mice may be associated with STAT3 inactivation in L4-SDH astrocytes. This view is supported by our gain-of-function approach in which L4-SDH-specific introduction of caSTAT3 alone produced tactile allodynia in normal mice. Collectively, our findings from astrocyte-specific loss- and gain-of-function approaches and conditional knockout mice strongly indicate that STAT3 in L4-SDH astrocytes is not only necessary for maintaining tactile allodynia but is also sufficient to induce allodynia.
Our established intra-SDH microinjection technique is useful to investigate the role of a gene of as-yet-unknown function or of disease-related mutation reported in humans in neuropathic pain and other sensory processing. This technique also yields a versatile method adaptable for multiple applications. Applying this method for optogenetics40,41 or chemogenetics (designer receptors exclusively activated by designer drugs; DREADD42) approaches by expressing genes such as for channelrhodopsin-2 or synthetic G-protein-coupled receptors, respectively, can specifically control astrocytic activity in the L4-SDH in vitro and in vivo. This would be a powerful tool for revealing new insights into the role of astrocytes in the SDH, including their contribution to pain and other sensory processing. Furthermore, by utilizing a promoter for neurons or other glial cells, it will allow the introduction of genes of interest into other cell types in the L4-SDH. Thus, combination of our new intra-SDH microinjection technique with recent genetic technologies would be a useful method to advance our understanding of spinal cord physiology and pathology.
Methods
Animals
C57BL/6J Jcl mice (Clea Japan, Tokyo, Japan), Gfap-cre mice (line 73.12, The Jackson Laboratory, ME, USA) and Stat3fl/fl mice43 were used. Stat3fl/fl mice were crossed with Gfap-cre mice to generate Gfap-cre;Stat3fl/fl mice and Stat3fl/fl littermates were used as a control. To explore the specificity of Cre recombinase activity in Gfap-cre mice, Gfap-cre mice were crossed with CAG-CATfl/fl-EGFP (CCE) mice, a reporter mouse line having a CAG [cytomegalovirus (CMV) early enhancer element and chicken β-actin] promotor, followed by a loxP-flanked chloramphenicol acetyltransferase (CAT) sequence upstream of EGFP. We used male C57BL/6J mice and male or female Stat3fl/fl, Gfap-cre; Stat3fl/fl mice and Gfap-cre; CCE mice. All mice used were 8–12 weeks of age at the start of each experiment and were housed at 22 ± 1 °C with a 12-h light-dark cycle. All animals were fed food and water ad libitum. All animal experiments were conducted according to relevant national and international guidelines contained in the ‘Act on Welfare and Management of Animals’ (Ministry of Environment of Japan) and ‘Regulation of Laboratory Animals’ (Kyushu University) and under the protocols approved by the Institutional Animal Care and Use committee review panels at Kyushu University.
Recombinant adeno-associated virus (rAAV) vector production
To produce rAAV vector for astrocyte specific gene transduction, a vector containing the astrocytic promoter gfaABC1D44 was generated from pZac2.1 by substituting the CMV promoter with gfaABC1D (amplified from addgene #19974). We then cloned AcGFP (referred to as GFP), the dominant negative form of STAT3 (dnSTAT3, amplified from addgene #8709) and the constitutive active form of STAT3 (caSTAT3, amplified from addgene #13373) into the above modified pZac2.1 to generate pZac2.1-gfaABC1D-GFP, pZac2.1-gfaABC1D-dnSTAT3 and pZac2.1-gfaABC1D-caSTAT3, respectively. The rAAV vector was produced from human embryonic kidney 293 (HEK293) cells with triple transfection [pZac, cis plasmid; pAAV2/5, trans plasmid; pAd DeltaF6, adenoviral helper plasmid (All plasmids were purchased from the University of Pennsylvania Gene Therapy Program Vector Core)]45,46 and purified by two cesium chloride density gradient purification steps47. The vector was dialyzed against phosphate-buffered saline (PBS) containing 0.001% (v/v) Pluronic-F68 using Amicon Ultra 100K filter units (Millipore, Darmstadt, Germany). The Genome titre of rAAV was determined by Pico Green fluorometric reagent (Molecular Probes, OR, USA) following denaturation of the AAV particle. Vectors were stored in aliquots at −80 °C until use.
Skeletal preparations of mouse vertebral column
Under isoflurane [2% (v/v)] anaesthesia, 1% (w/v) Evans blue (10 μL) was intrathecally injected through the L5–L6 intervertebral space48 to visualize the skeleton of the vertebrae. After several minutes, mice were deeply anaesthetized by intraperitoneal (i.p.) injection of pentobarbital and perfused transcardially with PBS, followed by ice-cold 4% (w/v) paraformaldehyde/PBS. The vertebral column was removed and further dissection was performed to clearly visualize the vertebral, sacral and medial iliac bones by removing the muscles or other connective tissue. The dissected preparation was briefly bleached and photographed (Fig. 1a,b).
Intra-SDH injection of dye or rAAV vector
Mice were deeply anaesthetized by subcutaneous (s.c.) injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1). The skin was incised at Th12–L3, and custom-made clamps were attached to the rostral and caudal sites of the vertebral column (Fig. 1c). Paraspinal muscles around the left side of the interspace between Th13 and L1 vertebrae were removed, and the dura mater and the arachnoid membrane were carefully incised using the tip of a 30G needle to make a small window to allow the microcapillary Femtotip (Eppendorf, NY, USA) insert directly into the SDH (Fig. 1d). The microcapillary was inserted with a pre-flow of 1% (w/v) Evans blue or rAAV solution through the small window (approximately 500 μm lateral from the midline) and inserted into the SDH (250 μm in depth from the surface of the dorsal root entry zone) (Fig. 1e). Evans blue or rAAV solution was pressure-ejected (600 hPa) for 50–60 seconds (approximately 500–600 nL) using the FemtoJet Express (Eppendorf, NY, USA). After microinjection, the inserted microcapillary was removed from the SDH, the skin was sutured with 3-0 silk, and mice were kept on a heating pad until recovery.
Peripheral nerve injury
We used the spinal nerve injury model49 with some modifications50. Under isoflurane (2%) anaesthesia, a small left-side incision at L3-S1 was made. Paraspinal muscles and fats were removed from L5 traverse process, and the part of this traverse process was removed to expose the parallel-lying L3 and L4 spinal nerves. The L4 nerve was carefully isolated and cut. The wound was sutured with 3-0 silk. The surrounding skin was pulled together and sutured with 3-0 silk.
Immunohistochemistry
Mice were deeply anaesthetized by i.p. injection of pentobarbital and perfused transcardially with PBS, followed by ice-cold 4% paraformaldehyde/PBS. The L4 (transverse) or L3–L5 (sagittal) segments of the spinal cord, or the L4 DRG were removed, postfixed in the same fixative for 3 h at 4 °C, and placed in 30% sucrose solution for 24 h at 4 °C. Transverse L4 spinal cord sections (30 μm), sagittal L3-L5 spinal cord sections (30 μm) and L4 DRG sections (15 μm) were incubated in blocking solution (3% normal goat serum or normal donkey serum) for 2 h at room temperature and then incubated for 48 h at 4 °C with primary antibodies: polyclonal rabbit anti-GFP (1:2000, MBL, Aichi, Japan), polyclonal rabbit anti-STAT3 (1:1000, Cell signalling, MA, USA), polyclonal goat anti-Sox9 (1:1000, R&D Systems, MN, USA), monoclonal rat anti-GFAP (1:1000, Invitrogen, CA, USA), monoclonal mouse anti-NeuN (1:200, Millipore, Darmstadt, Germany), polyclonal rabbit anti-Iba1 (1:5000, Wako, Osaka, Japan), and monoclonal mouse anti-APC (CC1 clone, 1:500, Calbiochem, CA, USA). Following incubation, tissue sections were washed and incubated for 3 h at room temperature in secondary antibody solution (Alexa Fluor 488 and/or 546, 1:1000, Molecular Probes, OR, USA and/or CF 405, 1:1000, Biotium, CA, USA). The tissue sections were washed, slide-mounted and subsequently coverslipped with Vectashield hardmount (Vector Laboratories, PA, USA). Three to five sections from the L4 spinal cord segments of each mouse were randomly selected and analysed using an LSM510 and 700 Imaging System (Carl Zeiss, Oberkochen, Germany).
Behavioural studies
To assess tactile allodynia, mice were placed individually in an opaque plastic cylinder, which was placed on a wire mesh and habituated for 1 h to allow acclimatization to the new environment. After that, calibrated von Frey filaments (0.02–2.0 g, North Coast Medical, CA, USA) were applied to the plantar surfaces of the hindpaws of mice with or without intra-SDH injection, or PNI from below the mesh floor and the 50% PWT was determined using the up-down method51.
Quantitative real-time PCR
Mice were deeply anaesthetized with pentobarbital, perfused transcardially with PBS, and L3-L4 spinal cord was removed and separated half at middle of the spinal cord by a sagittal cut and then into dorsal and ventral horn by a horizontal cut, so that a sample contained only dorsal horn of the ipsilateral or contralateral side to the virus injection or PNI. Total RNA from the L3–L4 SDH was extracted using Trisure (Bioline, London, UK) according to the manufacturer’s protocol and purified with RNeasy mini plus kit (Qiagen, CA, USA). The amount of total RNA was quantified by measuring the OD260 using a Nanodrop spectrophotometer (Nanodrop, DE, USA). For reverse transcription, 200 ng of total RNA was transferred to the reaction with Prime Script reverse transcriptase (Takara, Kyoto, Japan). Quantitative polymerase chain reaction (PCR) was carried out with FastStart Essential DNA Probe Master (Roche, BSL, Switz) using a LightCycler 96 or a LightCycler 480 system (Roche, BSL, Switz). Expression levels were normalized with to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or β-actin. The sequence of TaqMan probe and forward and reverse primers used in this study were described below.
GFP: 5′-FAM-ACCCCAACGAGAAGCGCGATCA-TAMRA-3′ (probe), 5′-TGTGCTGCTGCCCGATAA-3′ (forward primer),
5′-GTCACGAAGCCGAAGTAGATCA-3′ (reverse primer).
Iba1 (Aif1): 5′-FAM-CAGGAAGAGAGGCTGGAGGGGATCAA-TAMRA-3′ (probe), 5′-GATTTGCAGGGAGGAAAAGCT-3′ (forward primer),
5′-AACCCCAAGTTTCTCCAGCAT-3′ (reverse primer).
GFAP: 5′-FAM-CGTCCCGCAACGCAGAGCTG-TAMRA-3′ (probe), 5′-GAGTGGTATCGGTCTAAGTTTGCA-3′ (forward primer),
5′-GCGGCGATAGTCGTTAGCTT-3′ (reverse primer).
Vimentin (Vim): 5′-FAM-TTGACACCCACTCAAAAAGAACACTCCTGA-TAMRA-3′ (probe), 5′-CCCTGAACCTGAGAGAAACTAACC-3′ (forward primer),
5′-GTCTCATTGATCACCTGTCCATCT-3′ (reverse primer).
IL-6: 5′-FAM-TCACAGAGGATACCACTCCCAACAGACCTG-TAMRA-3′ (probe), 5′-GGGACTGATGCTGGTGACAA-3′ (forward primer),
5′-TGCCATTGCACAACTCTTTTCT-3′ (reverse primer).
TNF-α: 5′-FAM-TACGTGCTCCTCACCCACACCGTCA-TAMRA-3′ (probe), 5′-GTTCTCTTCAAGGGACAAGGCTG-3′ (forward primer),
5′-TCCTGGTATGAGATAGCAAATCGG-3′ (reverse primer).
IL-1β: 5′-FAM-TGCAGCTGGAGAGTGTGGATCCCAA-TAMRA-3′ (probe), 5′-GAAAGACGGCACACCCACC-3′ (forward primer),
5′-AGACAAACCGCTTTTCCATCTTC-3′ (reverse primer).
SOCS3: 5′-FAM-CAGCTCGGACCAGCGCCACTTCT-TAMRA-3′ (probe), 5′-CGCGGGCACCTTTCTTATC-3′ (forward primer),
5′-CTCACACTGGATGCGTAGGTTCT-3′ (reverse primer).
GAPDH: 5′-FAM-ACCACCAACTGCTTAGCCCCCCTG-TAMRA-3′ (probe), 5′-TGCCCCCATGTTTGTGATG-3′ (forward primer),
5′-GGCATGGACTGTGGTCATGA-3′ (reverse primer).
β-actin (Actb): 5′-FAM- CCTGGCCTCACTGTCCACCTTCCA-TAMRA-3′ (probe), 5′-CCTGAGCGCAAGTACTCTGTGT-3′ (forward primer),
5′-CTGCTTGCTGATCCACATCTG-3′ (reverse primer).
Statistical analysis
All data are shown as the mean ± SEM. Statistical significance of differences was determined using two-way ANOVA with post hoc Bonferroni test (Figs 3f and 4a,i), one-way ANOVA with post hoc Dunnett’s multiple comparison test (Fig. 4a) or Tukey’s multiple comparison test (Figs 3b–e and 4b) using GraphPad Prism 4 software. Differences were considered significant at P < 0.05.
Additional Information
How to cite this article: Kohro, Y. et al. A new minimally-invasive method for microinjection into the mouse spinal dorsal horn. Sci. Rep. 5, 14306; doi: 10.1038/srep14306 (2015).
Supplementary Material
Acknowledgments
We thank Dr. Takahiro Masuda for assisting and advice with experiments, Prof. Hideyuki Okano for kindly providing STAT3fl/fl and CCE mice and the University of Pennsylvania vector core for providing pZac2.1, pAAV2/5 and pAd DeltaF6 plasmid. This work was supported by Grant-in Aid for Scientific Research (KAKEN S-23229008) from Japan Society of the Promotion of Science (JSPS; Chiyoda-ku, Tokyo, Japan; to KI) and by grants from the JSPS through the “Funding Program for Next Generation World-Leading Researchers (NEXT Program)” (to MT).
Footnotes
Author Contributions Y.K. designed and performed most of the experiments, analysed the date, and wrote the manuscript. E.S. performed the initial experiments for this project, R.T. assisted with the experiments. H.T.-S. optimized construct of plasmids. H.O. kindly provided STAT3fl/fl and CCE mice and provided critical comments. K.I. supervised the overall project and wrote the manuscript. M.T. conceived the intra-SDH microinjection method and this project, designed the experiments, supervised the overall project and wrote the manuscript.
References
- Braz J., Solorzano C., Wang X. & Basbaum A. I. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A. J. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11, 823–836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira V. E. & Ginty D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S. A., Ma Q. & De Koninck Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat Neurosci 17, 183–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J. & Woolf C. J. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10, 1361–1368 (2007). [DOI] [PubMed] [Google Scholar]
- Ji R. R., Berta T. & Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 154 Suppl 1, S10–28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdenx M., Dutheil N., Bezard E. & Dehay B. Systemic gene delivery to the central nervous system using Adeno-associated virus. Front Mol Neurosci 7, 50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merienne N., Le Douce J., Faivre E., Deglon N. & Bonvento G. Efficient gene delivery and selective transduction of astrocytes in the mammalian brain using viral vectors. Front Cell Neurosci 7, 106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S. & Kobayashi K. Dissecting circuit mechanisms by genetic manipulation of specific neural pathways. Rev Neurosci 24, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- Lu Y. et al. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-alpha and IL-1beta signaling. Pain 155, 2618–2629 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. J., Cao D. L., Zhang X., Ji R. R. & Gao Y. J. Chemokine contribution to neuropathic pain: respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 154, 2185–2197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe A. et al. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat Med 12, 677–681 (2006). [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A. et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci 27, 4165–4177 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti M. et al. Nuclear calcium signaling in spinal neurons drives a genomic program required for persistent inflammatory pain. Neuron 77, 43–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier A., Mauborgne A., Masson J., Mallet J. & Pohl M. Lentiviral-mediated targeted transgene expression in dorsal spinal cord glia: tool for the study of glial cell implication in mechanisms underlying chronic pain development. J Neurosci Methods 167, 148–159 (2008). [DOI] [PubMed] [Google Scholar]
- Dominguez E., Mauborgne A., Mallet J., Desclaux M. & Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci 30, 5754–5766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Zhang L., Cheng J. K. & Ji R. R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 28, 5189–5194 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Schoffnegger D. et al. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci 33, 6540–6551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. et al. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain 152, 419–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V. et al. Glial cells in (patho)physiology. J Neurochem 121, 4–27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M. V. & Vinters H. V. Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4; doi: 10.1042/an20120010 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski P. I. et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13, 584–591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E. et al. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141, 633–647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X. et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci 17, 694–703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustein M. D. et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wang T., Sun G. Y. & Ding S. Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170, 992–1003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M. et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain 134, 1127–1139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez E., Rivat C., Pommier B., Mauborgne A. & Pohl M. JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 107, 50–60 (2008). [DOI] [PubMed] [Google Scholar]
- Bonni A. et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278, 477–483 (1997). [DOI] [PubMed] [Google Scholar]
- Herrmann J. E. et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28, 7231–7243 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner I. B. et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33, 12870–12886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J. F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999). [DOI] [PubMed] [Google Scholar]
- Chen G. et al. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 137, 2193–2209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. J. et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 29, 4096–4108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y. et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14, 331–336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z. Y., Gerner P., Woolf C. J. & Ji R. R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 114, 149–159 (2005). [DOI] [PubMed] [Google Scholar]
- Katsura H. et al. Transforming growth factor-activated kinase 1 induced in spinal astrocytes contributes to mechanical hypersensitivity after nerve injury. Glia 56, 723–733 (2008). [DOI] [PubMed] [Google Scholar]
- Imai S. et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain 136, 828–843 (2013). [DOI] [PubMed] [Google Scholar]
- Gradinaru V., Mogri M., Thompson K. R., Henderson J. M. & Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine A. V. et al. Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C. et al. Rat nucleus accumbens core astrocytes modulate reward and the motivation to self-administer ethanol after abstinence. Neuropsychopharmacology 39, 2835–2845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161, 4652–4660 (1998). [PubMed] [Google Scholar]
- Lee Y., Messing A., Su M. & Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493 (2008). [DOI] [PubMed] [Google Scholar]
- Vandenberghe L. H. et al. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther 21, 1251–1257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M. et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 21, 1259–1271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E. et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther 17, 503–510 (2010). [DOI] [PubMed] [Google Scholar]
- Hylden J. L. & Wilcox G. L. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67, 313–316 (1980). [DOI] [PubMed] [Google Scholar]
- Kim S. H. & Chung J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 (1992). [DOI] [PubMed] [Google Scholar]
- Masuda T. et al. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 5, 3771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M. & Yaksh T. L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.