Abstract
Climate change is thought to have major effects on groundwater resources. There is however a limited knowledge of the impacts of past climate changes such as warm or glacial periods on groundwater although marine or glacial fluids may have circulated in basements during these periods. Geochemical investigations of groundwater at shallow depth (80–400 m) in the Armorican basement (western France) revealed three major phases of evolution: (1) Mio-Pliocene transgressions led to marine water introduction in the whole rock porosity through density and then diffusion processes, (2) intensive and rapid recharge after the glacial maximum down to several hundred meters depths, (3) a present-day regime of groundwater circulation limited to shallow depth. This work identifies important constraints regarding the mechanisms responsible for both marine and glacial fluid migrations and their preservation within a basement. It defines the first clear time scales of these processes and thus provides a unique case for understanding the effects of climate changes on hydrogeology in basements. It reveals that glacial water is supplied in significant amounts to deep aquifers even in permafrosted zones. It also emphasizes the vulnerability of modern groundwater hydrosystems to climate change as groundwater active aquifers is restricted to shallow depths.
The last glacial period resulted in drastic modifications of the hydrogeological regime1,2. Sedimentary basins show evidence of glacial recharge1,3,4,5,6,7,8. The case for fractured aquifers in basements is less clear. Modeling of the hydrogeological regime in the Fennoscandian and Canadian shield during the last 10,000 yrs, has also indicated potential phases of glacial groundwater circulation in the basement9,10. However, whether recharge of glacial water is continuous or not during the last glacial maximum is not known. Furthermore, relationships of recharge with permafrosted areas remain unclear as well as recharge mechanisms11,12,13.
In recent decades, basement saline fluids have been sampled at great depths (0.5–5 km) in the context of nuclear-waste disposal investigations, and other general research programs. There is now evidence that such fluids are relatively ubiquitous in basements worldwide14,15. However their origin remains difficult to decipher due to intensive water-rock interactions and the extremely long residence-times13. In several cases, analyses with geochemical tracers have suggested that the solutes are of marine origin16,17,18,19,20,21. There is however no clear evidence of a potential marine component in hard rock aquifers. As an example, some of the brines in the Canadian shield are suggested to be of Pleistocene cryogenic origin22,23 but are also suggested to be of marine origin, which would require a Devonian age19,24,25.
The mechanisms that could explain how and when marine fluids could be introduced into the basement remain poorly understood26,27. Furthermore, no knowledge of the mechanisms involved in the preservation of marine or glacial fluids and the time length of such preservation has been available until now.
Basement rocks also constitute major groundwater resources which have been little exploited. However, there is now growing pressure for more intensive use of these resources. In recent decades, anthropogenic pressure has resulted in major drawdown and chemical evolution28,29. Such pressure accentuates the modifications induced by modern global warming as temperature increase should limit groundwater recharge in a large part of the earth surface before the end of this century, although groundwater recharge should increase in more restricted areas30,31. Deciphering the effects of climate change on paleo-hydrogeological regimes in basements is therefore of great importance. More precisely, the effects and ages of marine transgression on fluid circulation in basements need to be reliably identified, and the velocity of solute leaching quantified, together with the effects and duration of glacial fluid circulation.
Materials and Methods
Geological and hydrogeological setting
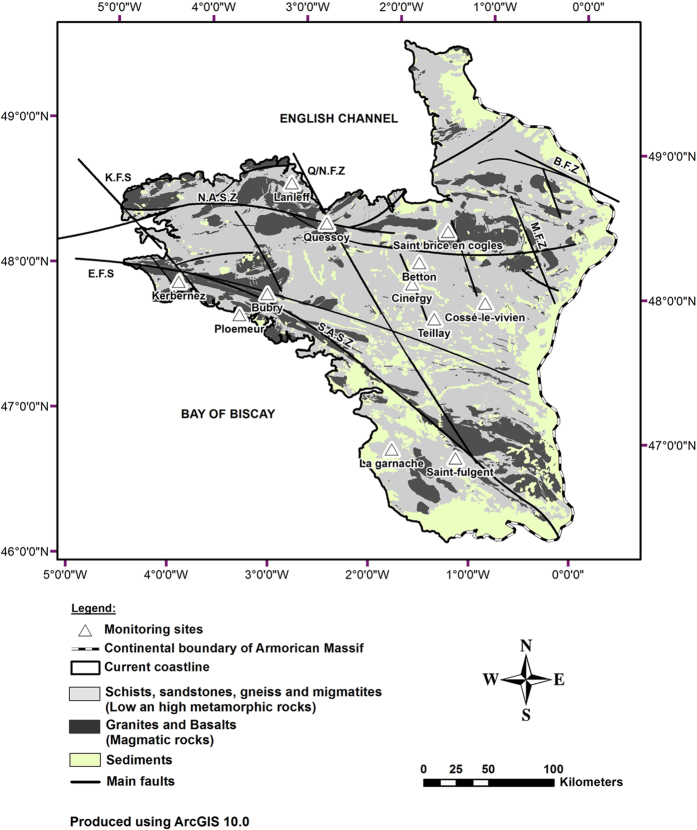
The Armorican basement covers an area of 68,500 km2 in western France (Fig. 1). The Armorican Massif is a Variscan basement including some relics of the Cadomian orogenic belt32,33 (Fig. 1). The Armorican basement is made of three major units of Upper Proterozoic to Paleozoic formations separated by major N110E shear zones: the North Armorican Shear Zone (NASZ) and the South Armorican Shear Zone (SASZ). The Armorican basement is made of low and high metamorphic rocks (schist, sandstone, micaschist and gneiss), various plutonic rocks and locally some basaltic rocks. The Armorican basement was further densely fractured (N140E faults) during the Permian–Triassic extension. Some scattered remnants of Mesozoic and Cenozoic sediments are preserved on the Armorican basement. Although the Armorican basement has been thought as mainly continental during the Jurassic and Cretaceous times, it may have been at least partially flooded during this period34. During the Early Eocene global warming, the Armorican basement recorded the development of lateritic weathering several of meters thick, which was followed by semi-arid conditions (Late Eocene to Oligocene) until the Late Miocene cooling and the Mio–Pliocene boundary where the current climatic conditions prevailed across Europe35,36. During these periods, the Armorican Massif was flooded by rising sea-levels during the Early Eocene (Ypresian), the Late Eocene (Bartonian), the early Oligocene (Rupelian) and the Lower to Middle Miocene until about 11 M yrs34,37. According to stratigraphic records studies and to the eustatic sea-level fluctuations38, three main marine transgressions can be identified during most recent periods with three different paleocoastlines: (1) the oldest and the highest, is Mio-Pliocene (Messinian ~ 5.3+/−0.8 Myr) with sea level +90 m asl, (2) the second is Pliocene (Reuverian ~ 2.7+/−0.3 Myr) with sea level +60 m asl; and (3) the most recent is Pleistocene (Gelasian/Calabrian ~ 1.8+/−0.2 Myr) with sea level +30 m asl (Fig. S1 Supporting Information). Although the sea-level fluctuation curve shows three small events including the Pleistocene Gelasian/Calabrian one, sedimentological relicts seem to only characterize one of these events. As the Miocene events occurred more than 10 M yrs in the past, we focus in this study on the more recent Mio-Pliocene to Pleistocene events that are more likely the potential mechanisms for the observed salinization of the aquifers.
Figure 1. Location map of the sampling sites and geology of the Armorican Massif.
Twelve sites showing high Cl concentration were chosen from the ADES national groundwater monitoring database. The well characteristics are presented in the Table S1. Two scientific monitoring sites (Kerbernez—Agrhys observatory and St Brice en Coglès) presenting shallow modern groundwater were also included to get a representative modern end-member. Three main geological domains are separated by large shear zones (South and North Armorican Shear Zones) and divided by more recent N140° fault zones (Elorn, Kerforne, Quessoy/Nort). The geological shapes have been manually created from detailed geological maps available on-line from BRGM (http://infoterre.brgm.fr). The coast line has been manually pointed as well as the sampling sites which have been pointed from their GPS coordinates.
The altitudes of the Armorican basement reach 400 m in places but commonly range from 100 to 200 m. The climate is temperate. From a hydrogeological point of view, the weathered layer (0–30 m) constitutes an aquifer which is highly sensitive to diffuse agricultural pollution39. Additional aquifers can be found in the fractured rocks below this aquifer where water circulation is related to fault zones40.
More than 1,800 wells designed for groundwater exploitation are recorded in the ADES database for Brittany (http://www.ades.eaufrance.fr/). This database was used to analyze the spatial distribution of chloride concentrations in wells located further than 1 km from the coast line41.
Twelve sites in the area with very high chloride concentrations (>80 mg/L) were selected for geochemical investigation. Groundwater was collected after pumping in the deeper part of the aquifers below the weathered layer at depths of 50 to 150 m. One sample was collected from a deep research well (“Cinergy sample”) which intersected the basement from 405 m down to 675 m below a local sedimentary basin. This was the deepest and most saline sample (Cl = 1,208 mg/L). The samples collected, with Cl concentrations ranging from 74 to 144 mg/L, show evidences of mixing with modern and shallow groundwater (Cl < 50 mg/L). A sub-group of three sites (Bubry, Betton and Cinergy sites) with the most saline samples (Cl > 150 mg/L) is defined from PCA analysis (supporting information, section Sample origin and water-rock interaction). Below we compare these most saline fluids and other mixed samples to groundwater in the shallow weathered zone which contains modern and non saline groundwater. Although the number of saline samples is limited, it appeared clear from the chloride data base that very high chloride concentrations could be observed in various sites. The 12 sites were chosen in order to represent various geological formations as well as various places in the Armorican Massif (see sample location and characteristics Fig. 1 and Table S1, supporting Information). Although limited, this database is thought to be representative of the scale of the whole Armorican basement.
Analytical methods section
Wells were pumped trying to install the pump in front of the most permeable fractures. Physico-chemical parameters (pH, Eh, T°C, Cond.) were monitored until stabilization was reached i.e. about 20 to 30 mn, usually 30 to 50% of the borehole volume. Samples were collected in polyethylene bottles rinsed with ultrapure water and acidified for cation analysis. Anions, cations and trace-elements were analysed in the Geosciences Rennes Laboratory using chromatography (anions) and ICP-MS (cations and trace).
Dissolved gases were collected without air contamination in steel bottles using a specific Grundfoss pump. CFC and SF6 were measured in the Rennes Condate-Eau analytical laboratory through gas-chromatographic analysis with ECD detector. Ne and Ar gases were measured though gas-chromatographic analysis with a catharometric detector. Uncertainty is about 3% for CFC and SF6 and about 5% for other gases.
All the isotopic analysis were carried out in the BRGM Laboratory. Water stable isotopes were determined through equilibration with δ18O and δD uncertainties of +/−0.1 and 0.5‰. Sulphate isotopic compositions were determined using a Delta S mass spectrometer (Thermo Finnigan). The δ34S of sulphates was measured from SO2 obtained from CdS precipitated after sulphate reduction and δ18O-SO4 was determined from the CO2 produced by the reaction of BaSO4 with C at 1050 °C. The Canon Diablo Troilite and Vienna-SMOW standards were used for S and O isotopes respectively with uncertainty about +/−0.3‰. Boron isotopes were determined by positive thermal ionization mass spectrometry on a Finnigan MAT 261 single collector solid source mass spectrometer. Values are reported on the δ scale relative to NBS951 boric acid standard. The long-term external reproducibility based on replicate analysis is ±0.4‰. The internal error is often better than 0.2‰
36Cl/35Cl ratio was measured by AMS at the French national ASTER facility. Chemical separation of Cl was conducted based on the protocol of Conard et al. (1986)42. To prevent S isobaric interferences, BaSO4 was precipitated by adding BaNO3 and was removed by filtration. Next, AgCl was precipitated by adding AgNO3 and recovered by centrifugation. To improve SO4 removal, the AgCl precipitate was dissolved in NH4 before repeating the procedure. AgCl is then precipitated again by adding NH4OH. A blank solution was prepared with Merck Standard NaCl 99.91%. The measured blanks provided 36Cl/35Cl ratios that were less than 10−15 at/at.
The carbon species (i.e. CO2, TDIC and carbonates) were converted into CO2 by direct acidification, and the 13C contents were measured by mass spectrometry (SIRA) at the IDES Laboratory (University of Paris Sud). The 13C content is reported using δ (‰) notation, as a deviation from the V-PDB (Vienna-Belemnite from the Pee Dee formation, North Carolina, USA). Graphite sources for 14C analyses were prepared from TDIC in the IDES Laboratory, and measured using accelerator mass spectrometry (UMS LMC14, Gif-sur-Yvette, France). The 14C contents are expressed as a percentage of modern carbon (pmC). Analytical errors, including laboratory errors, are of ±0.2‰ vs V-PDB for the δ13C, and between 0.1 and 0.3 pmC for the A14C.
Results
Solute origin
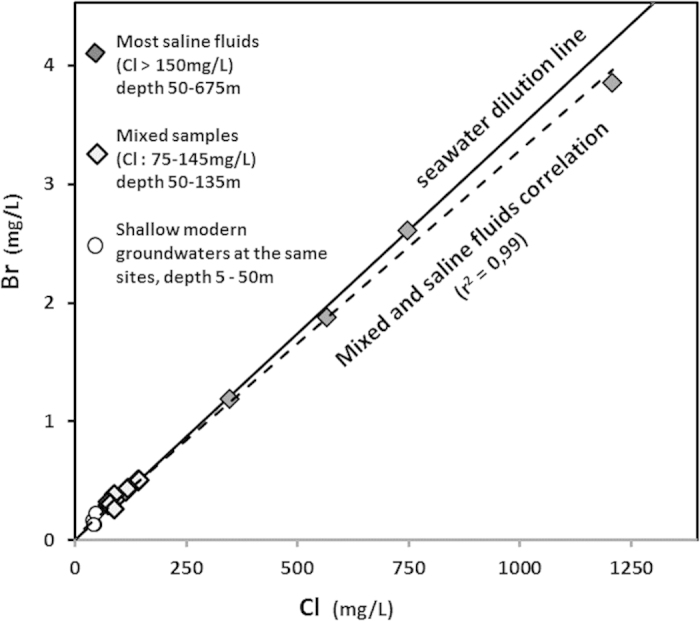
Whereas the Cl/Br mass-ratio range in the shallow and modern groundwater (<30 m depth) was very large (100 to 600) the Cl/Br mass-ratio of the saline fluids (Cl > 80 mg/L) collected in the Armorican basement was 283 +/−16 (Fig. 2). This ratio is very close to that of seawater (288). The linear Br to Cl relationship (R2 = 0.957) closely matches a dilution line ranging from the deepest, most saline fluid (Cinergy sample) to the most dilute shallow ones. This relationship is equal to the dilution line between the most dilute shallow samples and a modern seawater end-member. The boron isotope value for the more saline fluid (Cinergy) was +40.7‰, which is very similar to the isotopic composition of boron in seawater (+39‰). The boron isotope ratios in all the other saline fluids were close to this signature except in one sample where the ratios were lower, possibly as a result of interaction with clay minerals.
Figure 2. Bromide to chloride mass ratios.
Three groups are distinguished on the basis of a PCA analysis (supporting information): (1) Shallow modern groundwater from the investigated sites and two monitoring sites, (2) samples showing mixing between deep saline samples and modern shallow groundwater, (3) three saline sites.
Modern and shallow groundwater present a wide range of SO4/Cl mass-ratios (0.3 to 1.8 Fig. 3), generally far above the marine mass-ratio (0.14) and the SO4/Cl mass-ratio in local precipitation (0.44 +/−0.3). Oxidation of sulphide minerals such as pyrite, ubiquitous in igneous or metamorphic rocks increases the sulphate concentrations in the shallow groundwater. Furthermore, nitrate constitutes a major electron acceptor in modern agricultural catchments, which enhances sulphide oxidation whereas the oxidation mechanisms in groundwater that are not anthropogenically influenced are much more limited43,44. The sulphate concentrations in all the collected saline samples were high (mean concentration = 105 mg/L) with SO4/Cl mass-ratios ranging from 0.04 to 0.4 with a mean of 0.2 +/−0.14 when Cl concentrations is greater than 150 mg/L, close to the ratio found in seawater (0.22).
Figure 3. Sulphate to chloride mass ratios.
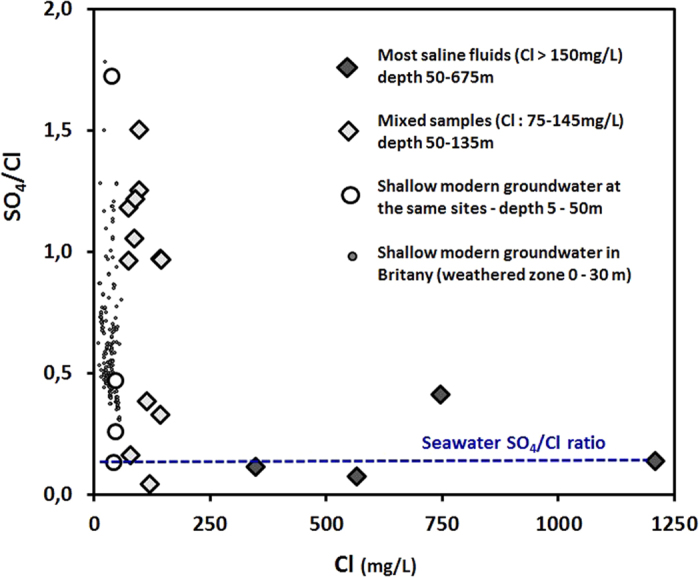
A large modern groundwater data set (depth ranging from 5 to 25 m) from the Agrhys observatory (Kernernez site in Fig. 1) is presented.
Different domains within the δ18O-SO4 vs δ34S-SO4 plot can be observed (Fig. 3). The δ34S-SO4 values for shallow groundwater, particularly nitrate-contaminated groundwater (i.e. NO3 > 5 mg/L), are within the range of +8 to +16‰. Groundwater influenced by sulphide dissolution present lower isotopic ratios (down to −10‰) as the isotopic ratios for sulphide δ34S-SO4 are usually negative45. δ34S-SO4 of the most saline fluids (Cl > 150 mg/l), ranges from +23 to +25‰. The other saline fluids can be plotted along a mixing line (arrow in Fig. 4) between two end-members: (1) a 34S depleted end-member containing sulphate originating from sulphide oxidations (denitrified fluids in Fig. 4) and (2) the fluids with the highest salinity. Both δ34S-SO4 (+22 to +25‰) and a δ18O-SO4 (+13 to +16‰) of the most saline fluid exceed that of present day seawater (around +21‰ and 9.5‰, respectively). Subsequent 0.8‰ and 6‰ decline of both δ34S-SO4 and δ18O-SO4 marine sulphate have taken place over the last 3 M yrs46,47, Therefore, the dataset indicates values for saline saline fluids slightly above those reported for 2 to 5 Myr seawater. However the slight difference can be explained either by a diagenetic reduction of sulphates within the first few centimeters of the sediment column, inducing a sulphate isotope fractionation or the occurrence of transitional environments such as lagoons and marshes.
Figure 4. Sulphate isotopic ratios.
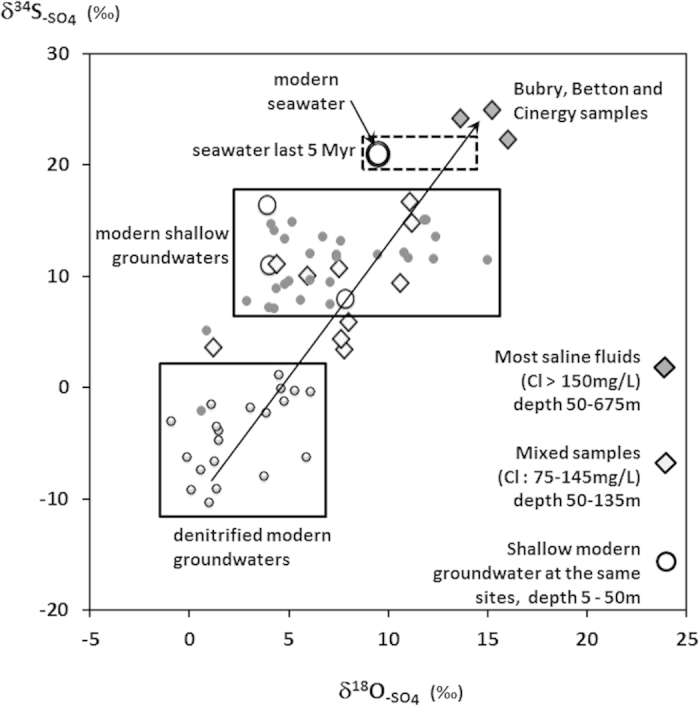
A framework of interpretation is provided from previous data (Pauwels et al., 2010). Two domains are identified: modern shallow groundwater and sites where autotrophic denitrification is assumed, i.e. groundwater showing low nitrate and high sulphate concentrations. Mio-Pliocene (Paytan et al., 1998; Turchyn et al., 2006) and modern seawater signature is presented. Arrow represents a potential mixing line between the denitrified groundwater, mixed groundwater and saline groundwater.
Chloride vertical distribution
The chloride concentrations of all the Brittany wells in the ADES database have been plotted against altitude (asl) in Fig. 5. A 10 m depth-average is also presented (red curve). An increase of the chloride range and clear concentration increase with depth is observed, especially below 100 m asl. The mean chloride concentrations in the highest 250 m (i.e. above 100 m asl), vary between 15 and 30 mg/L. Such concentrations are indicative of meteoric and anthropogenic sources41. The sharp chloride increase observed below 100 m requires a third source. A more detailed investigation revealed that the chloride concentrations precisely reflect the last three transgression events41. This result strongly supports a marine origin for the water solutes below 100 m depth, whatever the site.
Figure 5. Vertical distribution of chloride concentrations.
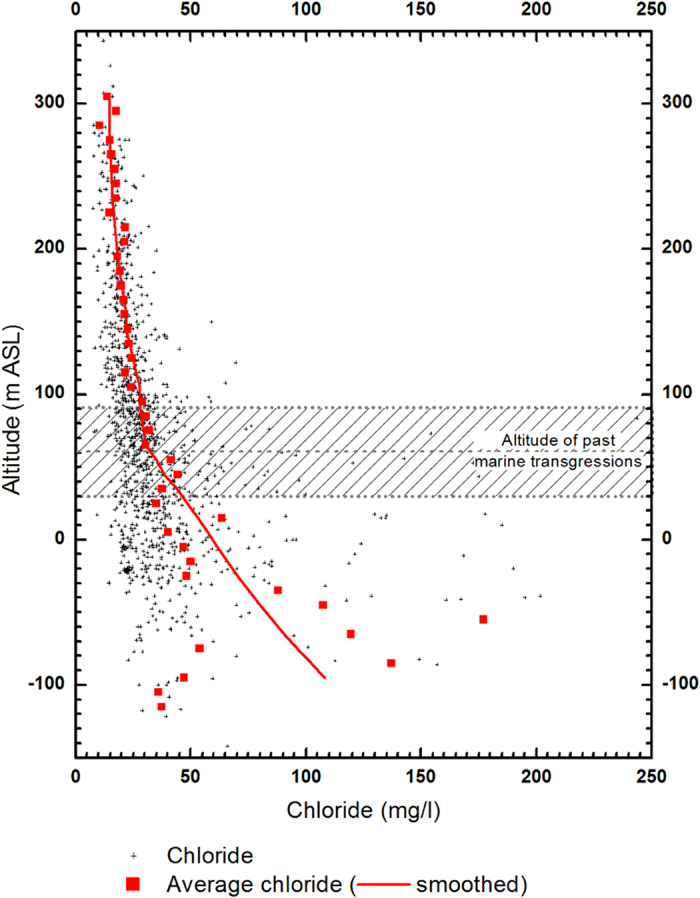
Chloride concentrations from 1,800 wells in the Armorican Massif recorded in the ADES national database are presented versus the well mean altitude above sea level. The paleo-coastlines of the three last transgressions are presented. A mean chloride concentration for each 5m layer is computed.
Recharge temperature
The stable isotopes in the most saline fluids can all be plotted along the local meteoric water line (LMWL) and represent meteoric continental waters (Supporting information, stable isotopes section). Only the composition of the deepest and most saline Cinergy-sample differs from the LMWL. When possible, the most saline fluids were compared with shallow groundwater at the same location. A systematic shift towards more negative values was observed along the LMWL. Such a shift indicates that the recharge conditions were colder than modern ones. This shift is correlated to both the chloride concentration and the recharge temperature (Supporting information, stable isotopes and noble gas section). These correlations favor the temperature interpretation to other potential explanations (altitude, continental effect, cloud internal fractionation) although the extent of the shift is lower than expected from recharge temperatures. As atmospheric conditions may have evolved along with temperature from the last glacial maximum to modern conditions, the stable isotope shift is only used as a temperature indication.
Recharge temperatures were deduced, using an inverse model, from the Ar and Ne concentrations48 (supplementary information, stable isotopes and noble gas section). The recharge temperatures for the collected fluids ranged from 5 to 10 °C, except for the deepest and most saline Cinergy sample which presented a recharge temperature close to 0 °C and also extremely high excess air. The present-day recharge temperature in Brittany is about 12 °C and the noble gas measurements in shallow groundwater agree with such temperatures40. It is thus clearly apparent from the recharge temperatures that the recharge conditions were colder than today. This is particularly true for the deepest and most saline Cinergy sample, which would have required glacial or immediately post-glacial recharge conditions.
A clear correlation was observed between recharge temperature and chloride concentrations (Fig. 6). The three sites with most saline samples display a linear correlation between surface shallow groundwater and the deepest and most saline Cinergy sample.
Figure 6. Recharge temperature deduced from noble gases vs chloride concentration.
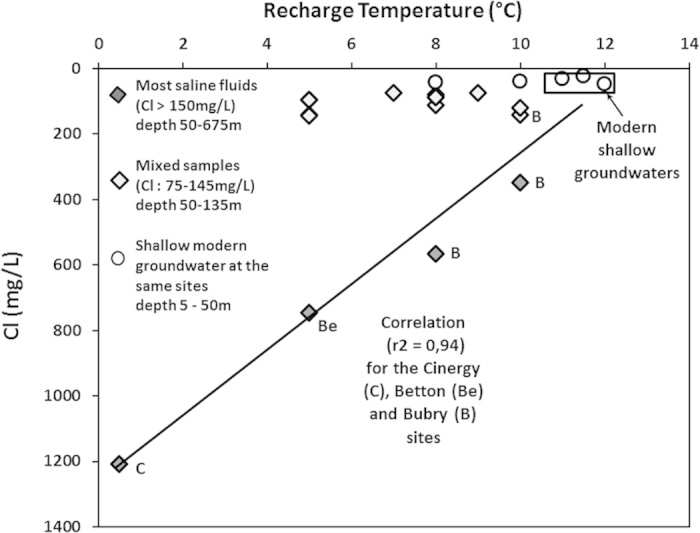
Paleo recharge temperature is deduced from the Ar and Ne content and is presented against the chloride concentration in the 12 sites investigated.
Groundwater dating
CFC, tritium and SF6 groundwater dating shows that modern groundwater presents residence times close to 20 yrs in the weathered part of the aquifers (0–30 m) and 20 to 50 yrs and more in the deeper part33,40 (with uncertainties +/−less than 2 yrs). All the saline fluids present CFC concentrations close to the detection limits which is interpreted as “old” fluids (>50 yrs) mixed with a limited amount of modern surface waters. On the basis of the nitrate and CFC content, the amount of modern surface fluids contained in the samples ranges from less than 1% to 15% (see supporting information for end-member used, mixing section and Table S2). 36Cl and 14C analysis were carried out within 3 samples including the two most saline ones (Cinergy sample Cl = 1,208 mg/L; Betton sample Cl = 700 mg/L). A detailed interpretation of these data is provided in supporting information (Section Groundwater dating, figure S2 and Tables S4 and S5). 36Cl in these samples is close to the equilibrium with the neutron production of the Proterozoic formations where the waters have been collected. This equilibrium process requires residence times of at least 106 yr. Conversely, 14C indicates residence times of 17,100 to 17,600 yrs for the two most saline fluids. The 36Cl and 14C data are interpreted as representing two different events as presented below. The chemical basis for the scenario is also given in supplementary information (mixings section).
Discussion
A two-phase evolution
As already suggested by several studies of old, saline fluids, the solute and water may have different origins and represent different phases of fluid circulation corresponding to geologic or global climatic changes15,16,19. They will therefore be discussed separately. The agreement between chloride vertical distribution and the altitudes of the last transgressions, from 5.3 to 2 M yrs old, supports a marine origin of the solutes in groundwater below 100 m asl. High Cl concentrations, requiring a marine contribution, are observed throughout the Armorican basement except in the zones preserved from past transgressions at altitudes higher than 90 m asl where the Cl concentrations remain below 50 mg/L. This correlation is also supported by the geochemical relationships, Br/Cl and SO4/Cl mass-ratios, as well as the B and S isotopic ratios. The marine signature of the solutes from saline fluids in the basement thus represents the generalized influence of transgressions prior to the glacial period at the scale of the Armorican basement. 36Cl data that suggest secular equilibrium are in good agreement with the transgression timing.
It was concluded in several earlier studies of saline fluids worldwide that the solutes were of marine origin16,17,18,19,20,21. However it was difficult to confirm this due to intensive water-rock interactions. In contrast, this signature is clearly apparent in the Armorican aquifers for various solutes (Cl, SO4, Br, Cl/Br and Cl/SO4 ratios, B and S isotopic ratios). This may be related to the very short time since the last transgression (1.8 +/−0.2 M yrs).
On the contrary, the noble gases and water stable isotopic ratios clearly demonstrate that the water in these saline fluids is of meteoric origin and that recharge occurred during glacial or immediately post-glacial conditions. Glacial water strongly diluted the older seawater already contained in the basement rocks. We interpret this contradiction as reflecting a two-stage evolution namely: (1) Introduction of seawater-derived fluids into the basement 5.3 to 2 M yrs ago, which constitute the source of the solutes collected in most of the wells below 100 m asl in the Armorican basement. (2) Recharge of cold meteoric water. Glacial melt water introduction follows the end of the last glacial maximum 19,000 yrs ago at the onset of deglaciation of the permafrost in northwestern Europe 18 to 17,000 yrs ago49. Although it might be possible that glacial recharge has occurred several times during the past, following the various glaciation cycles, 14C ages mainly agree with a limited period at the onset of the last deglaciation.
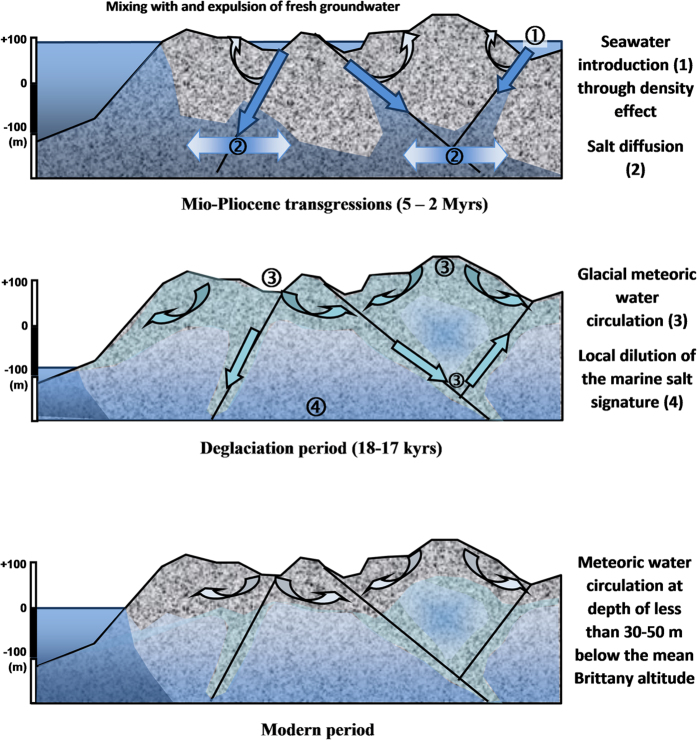
A hydrogeological model based on these observations is depicted in Fig. 7 and presents this two-phased circulation and the very different modern hydrogeological regime. These 3 situations are discussed below.
Figure 7. Hydrogeological model derived from the geochemical data.
The hydrogeological model presents the three steps that have been established through the geochemical data. The first stage represents the introduction of seawater in the basement through density-driven process within major faults. Seawater is introduced in the lower parts of the basement where it is mixed with freshwater. Following introduction which is spatially focused, seawater is introduced in the rock matrix (micro-porosity) through a slow diffusion process. During the second stage, permafrost breakthrough induces the introduction of cold freshwater within the same major faults. This introduction is induced by large hydrogeological loops that occur probably during a relatively short period (less than a few thousand years). The third stage represents the modern period where the hydrogeological loops are less extended towards depth which allows for the preservation of the previous signatures below 100 m depth. Figure is original and has been drawned by L.A.
Seawater introduction into the basement
The attribution of a marine signature to several deep saline samples worldwide may indicate that the mechanisms responsible for seawater introduction into basements occur in various places through geological time. The introduction of saline fluid into basements requires drivers. These may be upward fluxes related to basin brine expulsion. A typical example of such mechanisms is the fluid migration responsible for MVT-type metal deposits. However, such fluid circulation seems to be mainly restricted to the basin/basement interface16,50. As transgression occurs, the simplest mechanism of seawater introduction into the basement is by gravity- and density-driven displacement of former fresh groundwater by seawater. Such a process may account for saline fluid circulation at relatively great depth in the most permeable fractures. However, this mechanism cannot explain the relatively ubiquitous occurrence of saline fluids throughout the Armorican basement. Furthermore, the most saline fluids were collected in the Cinergy well at a depth of 405–750 m below a small Mesozoic sedimentary basin, away from the border faults of this grabben. These elements require that the process was thorough and efficient, allowing the saline fluid to penetrate all the scales of porosity and not only the major structures.
Diffusion may account for the penetration of marine fluid within the whole basement rocks. Molecular diffusion can be estimated from the diffusion of water molecules in water Dm, which is about 10−9 m2.s−1 for typical groundwater temperatures. The diffusion length scale51 – i.e. the length of the mixed zone – is about 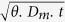 , where t is the time since recharge, and θ is the porosity ranging from 0.01 to 0.1, leading to a mixing length of about 20 to 60m for 1 Myr, and from 2 to 6 m for 10 kyr. It would seem from these estimates that the marine chlorides are probably well mixed at the scale of the investigation, i.e. a few hundred meters. As seawater has been introduced into the basement at higher time-scales than the million years diffusion scale, it should have diffused throughout the hard rock porosity. Even the immobile water in the microporosity could contain marine solutes. This does not mean that the concentration is constant throughout the aquifer since (1) the flow is likely rapidly renewed in the upper part of the geological section, and (2) an increase in concentration, due to the density effect, should still be observed as the time required for complete homogeneous diffusion of the entire domain exceeds several M yrs. In particular, a depth gradient should be observed as the density-driven flow should result in a greater influence at depth.
, where t is the time since recharge, and θ is the porosity ranging from 0.01 to 0.1, leading to a mixing length of about 20 to 60m for 1 Myr, and from 2 to 6 m for 10 kyr. It would seem from these estimates that the marine chlorides are probably well mixed at the scale of the investigation, i.e. a few hundred meters. As seawater has been introduced into the basement at higher time-scales than the million years diffusion scale, it should have diffused throughout the hard rock porosity. Even the immobile water in the microporosity could contain marine solutes. This does not mean that the concentration is constant throughout the aquifer since (1) the flow is likely rapidly renewed in the upper part of the geological section, and (2) an increase in concentration, due to the density effect, should still be observed as the time required for complete homogeneous diffusion of the entire domain exceeds several M yrs. In particular, a depth gradient should be observed as the density-driven flow should result in a greater influence at depth.
Glacial recharge
In contrast, glacial meteoric recharge presents the characteristics of a relatively intensive and short process. First, the deepest and most saline Cinergy sample contains extremely high excess air. Such excesses are thought to be characteristic of rapid recharge of glacial melt3,52, which would agree with the low recharge temperatures. This trapping of excess air implies a rapid recharge event. Second, the preservation of these glacial signatures in all the fluids investigated indicates a generalized process which has not been erased by further mixing with modern groundwater. Third, the Cinergy sample indicates that glacial fluids have penetrated at great depth which may occur as i) glacial groundwater has a high density and ii) seawater level increase is slower than permafrost melt. It is interpreted as a succession of rapid recharge events which occurred during a short period between 18 and 17,000 yrs ago after the glacial maximum. This period corresponds to the first retreat of northwestern glaciers and could also correspond to the breakthrough of the permafrost in Brittany49. Resumption of recharge processes have also been observed in sedimentary aquifers in northwestern Europe at a similar period53. During this period, the sea level was about 80m below its present level, which has allowed deeper penetration than the modern groundwater regime.
Hydrodynamic dispersion, which is the second process that might have diluted the original glacial or marine signal, is classically modeled by a diffusion equation. The diffusion coefficient in this equation is the product of the average flow velocity v by the hydrodynamic dispersivity length A. The dispersion length is thus equal to 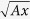 , where x is the distance of flow since recharge. The distance from recharge zones is reasonably between 100 m and 1000 m for most groundwater samples. Hydrodynamic dispersivity values of between 10−2 and 103 m have been reported in field scale studies but the reliability of values greater than 1 is poor54. The resulting estimated dispersion length is between 1 and 30 m. In contrast with molecular diffusion, the mixing length scale is only dependent on the flow distance and not on the time since the recharge event. This back-of-the-envelope calculation explains why we can have in the same place relatively high chloride concentrations, signatures of marine transgressions during warmer climates, as well as a glaciation recharge signature.
, where x is the distance of flow since recharge. The distance from recharge zones is reasonably between 100 m and 1000 m for most groundwater samples. Hydrodynamic dispersivity values of between 10−2 and 103 m have been reported in field scale studies but the reliability of values greater than 1 is poor54. The resulting estimated dispersion length is between 1 and 30 m. In contrast with molecular diffusion, the mixing length scale is only dependent on the flow distance and not on the time since the recharge event. This back-of-the-envelope calculation explains why we can have in the same place relatively high chloride concentrations, signatures of marine transgressions during warmer climates, as well as a glaciation recharge signature.
In contrast to the marine signature, the signature of the last glaciations has not been diluted at a distance of more than two meters from fracture zones allowing recharge fluid circulation. Although glacial water may exchange solutes with the microporosity at a local scale, it did not have time to equilibrate through diffusion with the surrounding rocks. Major fractures allow water circulation and microporosity acts as a reservoir for solutes. If water circulation is slow, the water in the fracture may equilibrate through diffusive processes with solutes from the microporosity immediately around the fracture without modifying the water signature16,55. However, the glacial signature will remain much more heterogeneously distributed than the much older marine signature.
The correlation between recharge temperature and chloride concentrations also supports this interpretation even though these parameters are related to two distinct processes (marine transgression and glacial melting). It shows that the last major circulation events were triggered by conditions extremely different to present conditions. Density-gravity-driven circulation is required to inject marine solutes at depth and the glacial recharge may be related to a much lower sea level and much deeper piezometric levels in the continent. Such lowering of the water table could have allowed efficient recharge as it limits the river network extension and increases the distance with the watershed surface. As the groundwater could not be discharged into the river network deeper flow i.e. regional loops could have been promoted. The most saline and deepest samples present the highest salinity and the coldest recharge temperature, as the original zones of marine signature, and also reflect the most rapid and active circulation of post-glacial water with high excess air and low recharge temperatures.
Recent modeling of groundwater infiltration during the glacial cycle in Canada indicated that major infiltration occurred below the glacier during the ice sheet progression whilst it remained limited in the permafrost areas56. A transient groundwater discharge regime also seemed to dominate during the deglaciation period. Geochemical observations may however indicate more complex processes in the periglacial areas27. The Armorican basement is considered to have been a discontinuous periglacial permafrosted area during the last glacial cycle49. The evidence of ubiquitous glacial water about 17,000 yrs ago rather indicates a continuous permafrost and a massive breakthrough at the onset of the glaciers retreat before the generalized deglaciation.
Modern recharge
Although the salinities are not high, in comparison to the original seawater, the homogeneous preservation of salinity throughout the Armorican basement and the clear increase with depth indicates the limited downward circulation of modern groundwater. Circulation loops are mainly constrained by potential discharge zones. The depth of groundwater penetration is limited both by the surface hydrological network and the high sea level (as compared to during the glacial period). The non-existence of high elevation recharge zones within the Armorican basement, which could have flushed the deep saline fluids, has allowed the signatures of both the marine transgression and the glacial circulation events to be preserved.
Conclusion
Investigation of a large database of chloride concentrations in the groundwater of hard rock aquifers in the Armorican basement in western France, revealed higher chloride concentrations below 100 m asl than could be expected from meteoric and anthropogenic sources. Twelve sites presenting chloride concentrations higher than 80 mg/L were investigated for isotopic, geochemical and dissolved gases analyses.
These data are interpreted as reflecting: (1) Introduction of marine seawater during the last transgressions 5.3 to 2 M yrs ago through gravity- and density-driven infiltration. This introduction was followed by diffusion of the marine solutes throughout the entire hard rock domain at the scale of several hundred meters, and exchanges with the rock microporosity. (2) Rapid recharge of meteoric water immediately following the last glacial maximum. Glacial water circulated in the main active fluid circulation zones. This water may have equilibrated with the surrounding rocks during the last 17,000 yrs thus allowing a single sample to contain both marine solutes and meteoric glacial solutes. (3) Following these two phases, modern groundwater circulation seems to be depth-limited as no dilution of these signatures has occurred.
This study indicates that marine transgression leads to a generalized introduction of seawater into hard rock aquifers. Diffusion allows the marine signature to be present throughout the area. It should thus be quite a common component of groundwater in hard rock aquifers. Conversely glacial recharge seems a rapid event, related to a restricted period related to the breakthrough of the permafrost. It indicates that glacial water may circulate even in permafrosted areas, at least during short time periods. The presence of a glacial signature throughout the Armorican basement indicates that glacial water, probably permafrost melt water, has a huge impact on groundwater even if it represents a time-limited event.
As compared to saline fluids in basement elsewhere, the Armorican fluids agree with the overall depth-salinity relationship41. The most striking difference is the relatively shallow depth of these fluids, probably induced by the lack of relief, and the relatively recent age of the transgressions. These two points have allowed both the marine and glacial signatures to be preserved, however the scenario described should have a large applicability, at least as test hypothesis, in crystalline rocks worldwide. The Armorican saline fluids thus confirm that fluid signatures in basements may record the various signatures of geologic and climatic events. They can be considered as archives of “crisis” events at the geological scale, which are recorded in the basement aquifers. It also emphasizes the role of fault-zones in the fluid transfer at depth. Our results also indicate that the extent of hydrogeological circulation of fresh water has been limited to less than 50 to 100 m during the last 17,000 yrs. Thus our findings emphasize the high sensitivity of groundwater resources to global climate changes as well as to anthropogenic pressure. They provide time-constraints for groundwater modeling under various climatic scenarios.
Additional Information
How to cite this article: Aquilina, L. et al. Impact of climate changes during the last 5 million years on groundwater in basement aquifers. Sci. Rep. 5, 14132; doi: 10.1038/srep14132 (2015).
Supplementary Material
Acknowledgments
Financial support was provided by the CNRS through the EC2CO—AQUADIV project. Financial support was also provided by the EU-RDF INTERREG IVA France (Channel)—England program, (Climawat project). The investigations also benefited from the support of INRA and CNRS (Environmental Research Observatories H+ and AgrHys) and of INSU-CNRS and CEA (LMC14) Artemis accelerator facilities for 14C analyses as well as the water agencies Cap Lorient and Collectivité eau du basin rennais. Kevin Hiscock and Lee Gum (East Anglia Univ.) are thanked for the Betton Pz6 sample noble gas analysis within the Climawat project. The ASTER Team (M. Arnold, G. Aumaître, D. Bourlès, K. Keddadouche, University of Aix-Marseille, CEREGE, CNRS-IRD UM 34) is thanked for the 36Cl analysis. Diana Warwick is warmly acknowledged for detailed English editing. The first author (L.A.) is indebted to W.A. Mozart piano concertos for healing musical support.
Footnotes
Author Contributions L.A., V.V., T.L., A.A. and H.P. established the original research project. A.A., V.V., T.L., C.R., S.B.M., M.K., C.L.G.L.S., E.P.G. and F.B. made the geochemical analysis. L.A., H.P., V.V., T.L. and F.B. interpreted the geochemical analysis. A.A., P.D. and L.A. interpreted the physical mechanisms described in the paper. L.A., P.D., A.A., V.V. and H.P. wrote the initial version of the paper. E.P.G., T.L., C.R., O.B., C.L.G.L.S., A.D., E.C. and F.B. reviewed the manuscript.
References
- McIntosh J. C., Garven G. & Hanor J. S. Impacts of Pleistocene glaciation on large-scale groundwater flow and salinity in the Michigan Basin. Geofluids 11, 18–33 (2011). [Google Scholar]
- Person M., McIntosh J., Bense V. & Remenda V. H. Pleistocene hydrology of North America: The role of ice sheets in reorganizing groundwater flow systems. Rev. Geophys. 45, 1–28 (2007). [Google Scholar]
- Klump S., Grundl T., Purtschert R. & Kipfer R. Groundwater and climate dynamics derived from noble gas, C-14, and stable isotope data. Geology 36, 395–398 (2008). [Google Scholar]
- McMahon P. B., Bohlke J. K. & Christenson S. C. Geochemistry, radiocarbon ages, and paleorecharge conditions along a transect in the central High Plains aquifer, southwestern Kansas, USA. Appl. Geochemistry 19, 1655–1686 (2004). [Google Scholar]
- McIntosh J. C. C. & Walter L. M. M. Paleowaters in Silurian-Devonian carbonate aquifers: Geochemical evolution of groundwater in the Great Lakes region since the Late Pleistocene. Geochim. Cosmochim. Acta 70, 2454–2479 (2006). [Google Scholar]
- Edmunds W. M. & Smedley P. L. Residence time indicators in groundwater: the East Midlands Triassic sandstone aquifer. Appl. Geochemistry 15, 737–752 (2000). [Google Scholar]
- Stute M. et al. Cooling of Tropical Brazil (5 ºC) During the Last Glacial Maximum. Science 269, 379–83 (1995). [DOI] [PubMed] [Google Scholar]
- Ntosh J. C. M. C. I. et al. Pleistocene recharge to midcontinent basins: Effects on salinity structure and microbial gas generation. Geochim. Cosmochim. Acta 66, 1681–1700 (2002). [Google Scholar]
- Lemieux J.-M., Sudicky E. A., Peltier W. R. & Tarasov L. Simulating the impact of glaciations on continental groundwater flow systems: 2. Model application to the Wisconsinian glaciation over the Canadian landscape. J. Geophys. Res. 113, F03018 (2008). [Google Scholar]
- Lemieux J.-M., Sudicky E. A., Peltier W. R. & Tarasov L. Simulating the impact of glaciations on continental groundwater flow systems: 1. Relevant processes and model formulation. J. Geophys. Res. 113, F03017 (2008). [Google Scholar]
- Chen Z., Qi J., Xu J., Ye H. & Nan Y. Paleoclimatic interpretation of the past 30 ka from isotopic studies of the deep confined aquifer of the North China plain. Appl. Geochemistry 18, 997–1009 (2003). [Google Scholar]
- Castro M. C., Hall C. M., Patriarche D., Goblet P. & Ellis B. R. A new noble gas paleoclimate record in Texas - Basic assumptions revisited. Earth Planet. Sci. Lett. 257, 170–187 (2007). [Google Scholar]
- Stotler R. L., Frape S. K. & Shouakar-Stash O. An isotopic survey of delta Br-81 and delta Cl-37 of dissolved halides in the Canadian and Fennoscandian Shields. Chem. Geol. 274, 38–55 (2010). [Google Scholar]
- Bucher K. & Stober I. Fluids in the upper continental crust. Geofluids 10, 241–253 (2010). [Google Scholar]
- Frape S. K., Blyth A., Blomqvist R., McNutt R. H. & Gascoyne. in Surf. Gr. Water, Weather. Soils, Treatise Geochemistry. (Eds. Drever J. I., Holland H. D., Turekian K. K.) 541–580 (Elsevier, 2003). [Google Scholar]
- Aquilina L. & Dreuzy J.-R. De. Relationship of present saline fluid with paleomigration of basinal brines at the basement/sediment interface (Southeast basin – France). Appl. Geochemistry 26, 1933–1945 (2011). [Google Scholar]
- Aquilina L., Genter A., Elsass P. & Pribnow D. in Hydrogeol. Cryst. rocks (eds. Stober. I. & Bucher. K.) (Kluwer, 2000). [Google Scholar]
- Beaucaire C., Gassama N., Tresonne N. & Louvat D. Saline groundwaters in the hercynian granites (Chardon Mine, France): geochemical evidence for the salinity origin. Appl. Geochemistry 14, 67–84 (1999). [Google Scholar]
- Bottomley D. J., Gregoire D. C. & Raven K. G. Saline groundwaters and brines in the Canadian shield: geochemical and isotopic evidence for a residual evaporite brine component. Geochim. Cosmochim. Acta 5, 1483–1498 (1994). [Google Scholar]
- Bottomley D. J. et al. The origin and evolution of Canadian Shield brines: evaporation or freezing of seawater? New lithium isotope and geochemical evidence from the Slave craton. Chem. Geol. 155, 295–320 (1999). [Google Scholar]
- Louvat D., Michelot J. L. & Aranyossy J. F. Origin and residence time of salinity in the Aspö groundwater system. Appl. Geochemistry 14, 917–925 (1999). [Google Scholar]
- Starinsky A. & Katz A. The formation of natural cryogenic brines. Geochim. Cosmochim. Acta 67, 1475–1484 (2003). [Google Scholar]
- Herut B., Starinsky A., Katz A. & Bein A. The role of sea-water freezing in the formation of subsurface brines. Geochim. Cosmochim. Acta 54, 13–21 (1990). [Google Scholar]
- Bottomley D. J. et al. Iodine-129 constraints on residence times of deep marine brines in the Canadian Shield: Comment and Reply - Reply. Geology 31, 94–94 (2003). [Google Scholar]
- Katz A. & Starinsky A. Iodine-129 constraints on residence times of deep marine brines in the Canadian Shield: Comment and Reply - Comment. Geology 31, 93–94 (2003). [Google Scholar]
- Greene S., Battye N., Clark I., Kotzer T. & Bottomley D. Canadian Shield brine from the Con Mine, Yellowknife, NT, Canada: Noble gas evidence for an evaporated Palaeozoic seawater origin mixed with glacial meltwater and Holocene recharge. Geochim. Cosmochim. Acta 72, 4008–4019 (2008). [Google Scholar]
- Stotler R. L. L. et al. The interglacial-glacial cycle and geochemical evolution of Canadian and Fennoscandian Shield groundwaters. Geochim. Cosmochim. Acta 76, 45–67 (2012). [Google Scholar]
- Galloway J. N. et al. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 (2004). [Google Scholar]
- Custodio E. Aquifer overexploitation: what does it mean? Hydrogeol. J. 10, 254–277 (2002). [Google Scholar]
- Green T. R. et al. Beneath the surface of global change: Impacts of climate change on groundwater. J. Hydrol. 405, 532–560 (2011). [Google Scholar]
- Scibek J., Allen D. M., Cannon A. J. & Whitfield P. H. Groundwater-surface water interaction under scenarios of climate change using a high-resolution transient groundwater model. J. Hydrol. 333, 165–181 (2007). [Google Scholar]
- Ballevre M., Bosse V., Ducassou C. & Pitra P. Palaeozoic history of the Armorican Massif: Models for the tectonic evolution of the suture zones. Comptes Rendus Geosci. 341, 174–201 (2009). [Google Scholar]
- Chantraine J. et al. The Cadomian active margin (North Armorican Massif, France): a segment of the North Atlantic Panafrican belt. Tectonophysics 331, 1–18 (2001). [Google Scholar]
- Bessin P., Guillocheau F., Robin C., Schroetter J.-M. & Bauer H. Planation surfaces of the Armorican Massif (western France): Denudation chronology of a Mesozoic land surface twice exhumed in response to relative crustal movements between Iberia and Eurasia. Geomorphology 233, 75–91 (2015). [Google Scholar]
- Zachos J. C., Dickens G. R. & Zeebe R. E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283 (2008). [DOI] [PubMed] [Google Scholar]
- Mosbrugger V., Utescher T. & Dilcher D. L. Cenozoic continental climatic evolution of Central Europe. Proc. Natl. Acad. Sci. USA 102, 14964–14969 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault N. et al. Mio-Pliocene to Pleistocene paleotopographic evolution of Brittany (France) from a sequence stratigraphic analysis: relative influence of tectonics and climate. Sediment. Geol. 163, 175–210 (2004). [Google Scholar]
- Hardenbol J. et al. Mesozoic and Cenozoic sequence chronostratigraphy framework of european basins. Society of Economic Paleontologists and Mineralogists, Special Publication 60, 3–11 (1998). [Google Scholar]
- Aquilina L. et al. Nitrate dynamics in agricultural catchments deduced from groundwater dating and long-term nitrate monitoring in surface and ground waters. Sci. Total Environ. 435, 167–178 (2012). [DOI] [PubMed] [Google Scholar]
- Ayraud V. et al. Compartmentalization of physical and chemical properties in hard-rock aquifers deduced from chemical and groundwater age analyses. Appl. Geochemistry 23, 2686–2707 (2008). [Google Scholar]
- Armandine Les Landes A., Aquilina L., Davy P., Vergnaud V. & Le Carlier C. Time scales of regional circulation of saline fluids in continental aquifers (Armorican massif, Western France). Hydrol. Earth Syst. Sci. 19, 1413–1426 (2015). [Google Scholar]
- Conard N. et al. The chemical preparation of AgCl for measuring Cl-36 in polar ice with accelerator mass-spectrometry. Radiocarbon 28, 556–560 (1986). [Google Scholar]
- Tarits C. et al. Oxido-reduction sequence related to flux variations of groundwater from a fractured basement aquifer (Ploemeur area, France). Appl. Geochemistry 21, 29–47 (2006). [Google Scholar]
- Böhlke J. K., Denver J. M. & Bohlke J. K. Combined use of groundwater dating, chemical, and isotopic analyses to resolve the history and fate of nitrate contamination in two agricultural watersheds, Atlantic coastal plain, Maryland. Water Resour. Res. 31, 2319–2339 (1995). [Google Scholar]
- Pauwels H., Ayraud-Vergnaud V., Aquilina L. & Molénat J. The fate of nitrogen and sulfur in hard-rock aquifers as shown by sulfate-isotope tracing. Appl. Geochemistry 25, 105–115 (2010). [Google Scholar]
- Paytan A., Kastner M., Campbell D. & Thiemens M. H. Sulfur isotopic composition of Cenozoic seawater sulfate. Science 282, 1459–1462 (1998). [DOI] [PubMed] [Google Scholar]
- Turchyn A. V. & Schrag D. P. Cenozoic evolution of the sulfur cycle: Insight from oxygen isotopes in marine sulfate. Earth Planet. Sci. Lett. 241, 763–779 (2006). [Google Scholar]
- Aeschbach-Hertig W., Peeters F., Beyerle U. & Kipfer R. Interpretation of dissolved atmospheric noble gases in natural waters. Water Resour. Res. 35, 2779–2792 (1999). [Google Scholar]
- Jiráková H., Huneau F., Celle-Jeanton H., Hrkal Z. & Le Coustumer P. Insights into palaeorecharge conditions for European deep aquifers. Hydrogeol. J. 19, 1545–1562 (2011). [Google Scholar]
- Boiron M. C. et al. Fluid transfers at a basement/cover interface Part II. Large-scale introduction of chlorine into the basement by Mesozoic basinal brines. Chem. Geol. 192, 121–140 (2002). [Google Scholar]
- Grathwohl P. Diffusion in natural porous media: Contaminant transport, sorption/desorption and dissolution kinetics. (Springer, 1998).
- Alvarado J. A. C., Leuenberger M., Kipfer R., Paces T. & Purtschert R. Reconstruction of past climate conditions over central Europe from groundwater data. Quat. Sci. Rev. 30, 3423–3429 (2011). [Google Scholar]
- Corcho Alvarado J. A., Leuenberger M., Kipfer R., Paces T. & Purtschert R. Reconstruction of past climate conditions over central Europe from groundwater data. Quat. Sci. Rev. 30, 3423–3429 (2011). [Google Scholar]
- Gelhar L. W., Welty C. & Rehfeldt K. R. A critical review of data on field-scale dispersion in aquifers. Water Resour. Res. 28, 1955–1974 (1992). [Google Scholar]
- Waber H. N. N. & Smellie J. A. T. Characterisation of pore water in crystalline rocks. Appl. Geochemistry 23, 1834–1861 (2008). [Google Scholar]
- Lemieux J.-M. M., Sudicky E. a., Peltier W. R. & Tarasov L. Dynamics of groundwater recharge and seepage over the Canadian landscape during the Wisconsinian glaciation. J. Geophys. Res. Surf. 113, F01011 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.