Abstract
Studies comparing gray matter (GM) volume of schizophrenic patients with or without auditory verbal hallucinations (AVHs) to that of normal controls remain controversial. This project aims to investigate changes of GM volumes of drug-naïve schizophrenic patients with and without AVHs. Eighteen first episode schizophrenic (FES) patients with AVHs, 18 FES patients without AVHs, and 18 healthy controls were scanned using structural MRI. Voxel-based morphometry (VBM) analysis was conducted to investigate changes of GM volume among the three groups. Patients with and without AVHs exhibited reduced GM volumes relative to normal controls in the left superior temporal gyrus, frontal regions, cerebellum and caudate. Further analysis of the GM of subcortical structures found that patients with AVHs had reduced thalamic volume than healthy controls. No significant difference was found between patients with and without AVHs. Significant correlation was found between the total scores of the Positive and Negative Syndrome Scale and bilateral thalamic volume. ROC analysis of thalamic volumes of the patients with AVHs and normal controls showed that the area under the curve was 0.698 (P = 0.043). The decreased thalamic volumes might serve as a biomarker for discriminating FES AVHs patients from normals.
Schizophrenia is a severe and disabling brain disorder. Nearly 60–80% of schizophrenic patients are liable to experience auditory verbal hallucinations (AVHs)1,2. It has been reported that about one fourth of patients with AVHs are chronic and resistant to medication3.
Abnormalities of brain structures have been well established in schizophrenia4,5. However, the reported nature of abnormality has been inconsistent. On the one hand, neuroimaging studies on FES patients have found reduced gray matter volume in several cortical regions compared to normal controls. For example, using the region-of-interest (ROI) approach, studies on adolescent schizophrenia found reduced volume in the ventricle system, the superior temporal gyrus (STG), and the thalamus6,7. Reduced thalamic and nucleus accumben volumes in FES patients have also been reported8,9. On the other hand, some longitudinal studies found no volumetric difference of any brain structure between patients with schizophrenia and healthy controls10. Several meta-analyses7,11 supported brain volume changes in the frontal, temporal and parietal regions. The inconsistency might be due to the complex pathogenesis and long-term chronicity of the disease and different methodological approaches of these studies6. The lack of a clear understanding of auditory hallucinations12 may also contribute to the inconsistency. In the current drug-naïve first-episode schizophrenia study, evaluating a specific subgroup of schizophrenia, i.e., those with AVHs, may allow us to reduce the underlying neural variability and resolve some of the inconsistencies13. In a schizophrenia mouse model, Chun et al. have demonstrated that specific disruptions of the thalamic-auditory cortical pathway altered auditory information processing and led to AVHs when the animals were put under stress or other confounding conditions14.
For structural brain analysis, the whole brain VBM has many advantages over the ROI approach. It is automated rather than observer-based15; it covers the whole brain and involves less laborious processing16. In this study, we conducted a whole brain VBM analysis to investigate GM volume changes in FES patients with and without AVHs. We hypothesize that GM volume in a few brain regions, including the left superior temporal gyrus, inferior frontal gyrus (IFG), and thalamus, is reduced in FES patients with AVHs compared to normal controls.
Results
Demographic and clinical-scale characteristics of the subjects
The demographic and clinical-scale characteristics of the patients and normal controls are listed in Table 1. There are obvious differences in education level and PANSS positive and total scores, but the three groups do not differ significantly in terms of age and gender.
Table 1. Demographic and clinical-scale characteristics of study participants.
| AVHs | Non-AVHs | NC | P value | |
|---|---|---|---|---|
| Demographic variables | ||||
| Cases | 18 | 18 | 18 | |
| Age, years (mean ± SD) | 22.56 ± 6.73 | 22.67 ± 3.85 | 25.06 ± 2.44 | 0.200(F = 1.63) |
| Gender (Male/Female) | 10/8 | 9/9 | 9/9 | 0.929(χ2 = 0.148) |
| Left/Right Handiness | 0/18 | 0/18 | 0/18 | |
| Education Level, years (mean ± SD) | 12.44 ± 2.30 | 12.56 ± 2.19 | 15.44 ± 2.48 | 0.000(F = 9.72)*** |
| Clinical variables | ||||
| Duration of illness, months (mean ± SD) | 5.94 ± 5.93 | 12.44 ± 18.16 | 0.16(t = −1.44) | |
| PANSS-positive symptoms | 31.11 ± 6.92 | 18.61 ± 8.68 | 0.006(t = 4.78)** | |
| PANSS-negative symptoms | 25.78 ± 3.87 | 22.06 ± 10.43 | 0.17(t = 1.42) | |
| PANSS-general psychopathology | 49.56 ± 9.01 | 47.39 ± 9.84 | 0.50(t = 0.69) | |
| PANSS-total score | 106.44 ± 13.55 | 88.06 ± 23.90 | 0.008(t = 2.84)** | |
| HAHRS | 26.22 ± 8.10 | |||
AVHs: Schizophrenic patients with auditory verbal hallucinations; Non-AVHs: Schizophrenic patients without auditory verbal hallucinations; NC: Normal controls. **P < 0.01, ***P < 0.001.
Voxel-based morphometry analysis of gray matter volume
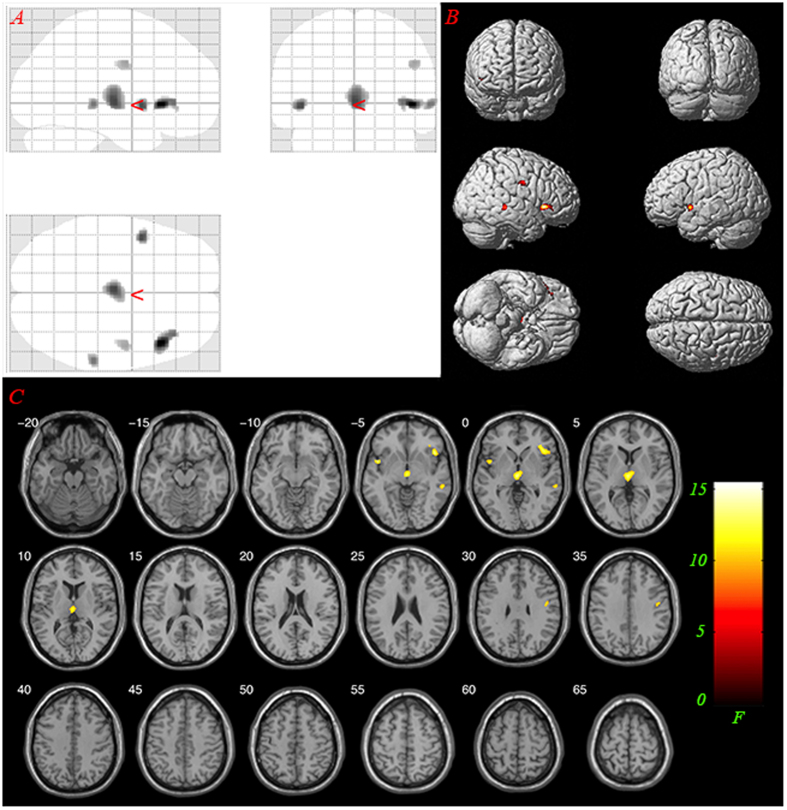
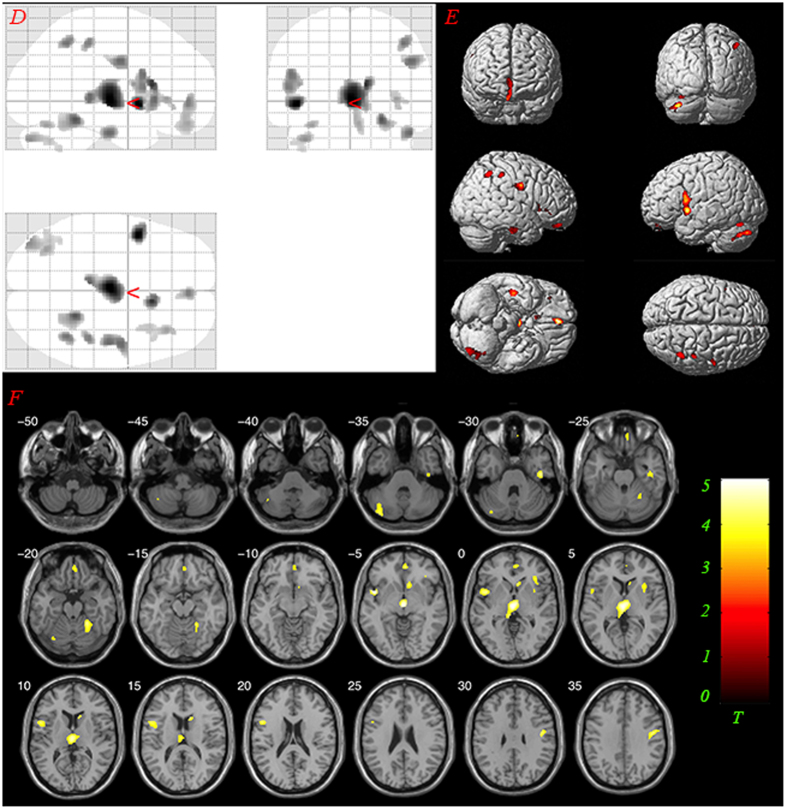
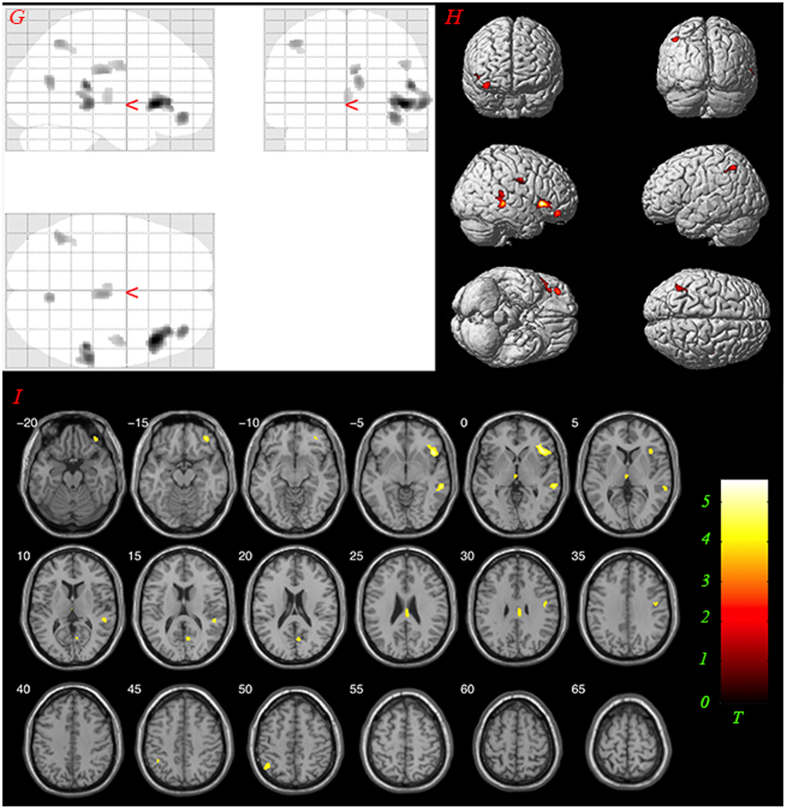
Voxel-based morphometry (VBM) analysis and one-way ANOVA were conducted. Difference in GM volume was found in the bilateral thalamus, left STG, right IFG and precentral gyrus among the three groups (F(2, 48) = 8.004, P < 0.001 uncorrected, cluster size >65 (expected voxels per cluster). See details in Fig. 1). Post-hoc analysis showed that the gray matter volumes of the patients with AVHs was significantly reduced in the bilateral thalamus, left STG, left IFG and inferior parietal lobule, inferior temporal gyrus, cerebellum posterior lobe, cerebellum anterior lobe, caudate, medial orbital frontal gyrus, and precentral gyrus compared to those of normal controls (T = 3.268, P < 0.001 uncorrected, cluster size >95 (expected voxels per cluster). See details in Fig. 2). Post-hoc analysis also showed that the gray matter volumes of the patients without AVHs was significantly reduced in the left thalamus, IFG, inferior parietal lobule, middle orbital frontal gyrus and middle temporal gyrus compared to those of normal controls (T = 3.268, P < 0.001 uncorrected, cluster size >95 (expected voxels per cluster). See details in Fig. 3). Detailed results are provided in Figs 1,2,3 and Table 2.
Figure 1. Main effects: significant GM differences in the bilateral thalamus, left Superior Temporal Gyrus, right Inferior Frontal Gyrus, Inferior Parietal Lobule and Precentral Gyrus (F(2, 48) = 8.004, P < 0.001 uncorrected, cluster size >65).
(A) glass view; (B) 3-D render; (C) slice view. Only clusters thresholded with the number of expected voxels are shown.
Figure 2. Results of post hoc analysis comparing GM volumes between groups AVHs and NC.
The yellow-colored areas indicate brain regions, including bilateral thalamus, left Superior Temporal Gyrus, left Inferior Frontal Gyrus, right Inferior Parietal Lobule, Inferior Temporal Gyrus, Cerebellum Posterior Lobe, Cerebellum Anterior Lobe, Caudate, Medial Orbital Frontal Gyrus, and Precentral Gyrus, with significantly reduced GM volumes relative to normal controls. The statistical threshold was set at P < 0.001 uncorrected. (AVHs: auditory verbal hallucinations; NC: normal controls). (D) glass view; (E) 3-D render; (F) slice view. Only clusters thresholded with the number of expected voxels are shown.
Figure 3. Results of post hoc analysis comparing GM volumes between groups Non-AVHs and NC.
The yellow-colored areas indicate brain regions, including left thalamus, right Inferior Frontal Gyrus, left Inferior Parietal Lobule, Middle Temporal Gyrus, Middle Orbital Frontal Gyrus, with significantly reduced GM volumes relative to normal controls. The statistical threshold was set at P < 0.001 uncorrected. (AVHs: auditory verbal hallucinations; NC: normal controls). (G) glass view; (H) 3-D render; (I) slice view. Only clusters thresholded with the number of expected voxels are shown.
Table 2. Brain clusters with significant gray matter volume changes.
| Brain region | L/R | No. of voxels | MNI coordinates (X Y Z) | P value | ||
|---|---|---|---|---|---|---|
| Main-effect | P < 0.001 uncorrected | |||||
| Superior Temporal Gyrus | L | 67 | −45 | 6 | −3 | |
| Thalamus | R | 224 | 4 | −16 | 2 | |
| Thalamus | L | 268 | −4 | −16 | 6 | |
| Inferior Frontal Gyrus | R | 300 | 45 | 22.5 | −3 | |
| Precentral Gyrus | R | 99 | 46.5 | −7.5 | 31.5 | |
| Post-hoc (volume reduction in AVHs vs. NC) | P < 0.001 uncorrected | |||||
| Superior Temporal Gyrus | L | 241 | −46.5 | 6 | −4.5 | |
| Thalamus | R | 448 | 4.5 | −10.5 | 1.5 | |
| Thalamus | L | 651 | −1.5 | −19.5 | 1.5 | |
| Inferior Frontal Gyrus | L | 143 | −49.5 | 9 | 19.5 | |
| Inferior Parietal Lobule | R | 158 | 42 | −52.5 | 49.5 | |
| Inferior Temporal Gyrus | R | 121 | 46.5 | −16.5 | −30 | |
| Precentral Gyrus | R | 272 | 48 | −9 | 32 | |
| Cerebellum Posterior Lobe | L | 332 | −39 | −82.5 | −34.5 | |
| Cerebellum Anterior Lobe | R | 228 | 24 | −45 | −18 | |
| Caudate | R | 255 | 10.5 | 18 | 12 | |
| Medial Orbital Frontal Gyrus | R | 183 | 3 | 48 | −3 | |
| Post-hoc (volume reduction in Non-AVHs vs. NC) | P < 0.001 uncorrected | |||||
| Thalamus | L | 97 | −1.5 | −18 | 1.5 | |
| Inferior Frontal Gyrus | R | 578 | 45 | 21 | −3 | |
| Inferior Parietal Lobule | L | 144 | −43.5 | −57 | 49.5 | |
| Middle Orbital Frontal Gyrus | R | 150 | 37.5 | 43.5 | −13.5 | |
| Middle Temporal Gyrus | R | 292 | 51 | −36 | 13.5 | |
No. Number; MNI: Montreal Neurological Institute; L/R: Left or Right hemisphere; Cluster size > expected voxels per cluster; AVHs: Schizophrenic patients with auditory verbal hallucinations; Non-AVHs: Schizophrenic patients without auditory verbal hallucinations; NC: Normal controls.
Comparison of thalamic volumes between patients and normal controls
Each subjects’ bilateral thalamic volumes were corrected for differences in whole brain size to eliminate potential head size bias. Age, gender, and education years were considered as covariates as well. Thalamic volumes decreased from normal controls (group NC, 1.330 ± 0.086%) to patients without AVHs (group Non-AVHs, 1.295 ± 0.112%) and patients with AVHs (group AVHs, 1.269 ± 0.086%). We found that the group factor (F(2, 47) = 3.284 , P = 0.046) had significant impact on thalamus volume. Further post-hoc analysis (Least Significant Difference, LSD) showed that, significance difference was found between groups NC and AVHs (P = 0.014). The results are shown in Fig. 4. No significant difference was found between groups AVHs and Non-AVHs, nor between groups Non-AVHs and NC.
Figure 4. Comparison of corrected thalamic volumes between the two patient and normal control groups.
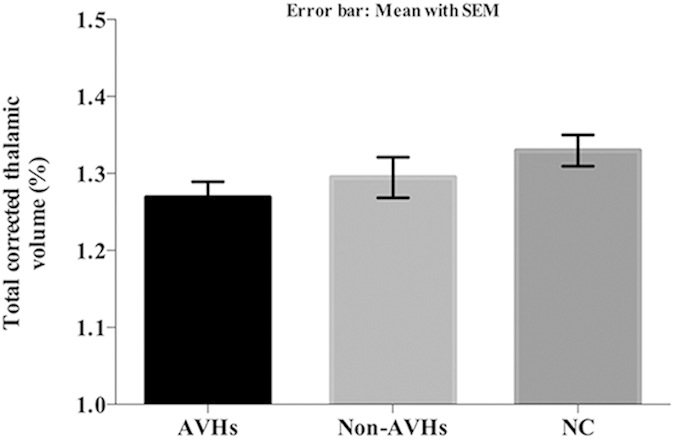
Significant difference was found between groups AVHs and group NC. (AVHs: Schizophrenic patients with auditory verbal hallucinations; Non-AVHs: Schizophrenic patients without auditory verbal hallucinations; NC: Normal controls).
Correlation between thalamic volumes and clinical scale scores
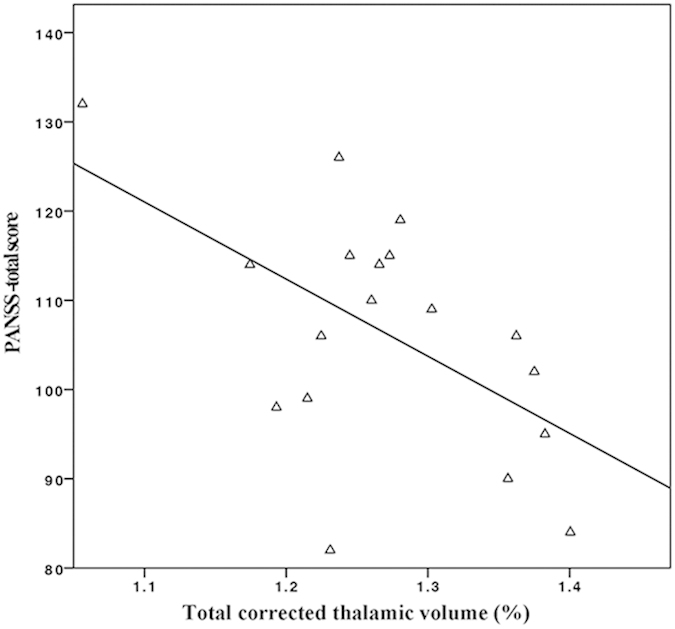
We found a significant correlation between the PANSS total scores and corrected bilateral thalamic volumes in the AVHs group (r = −0.551, P = 0.018, see Fig. 5). HAHRS scores were obtained for patients with AVHs. We did not find a significant correlation between the HAHRS scores and corrected bilateral thalamic volumes in the group (r = −0.149, P = 0.555).
Figure 5. The PANSS total scores were significantly correlated with the corrected bilateral thalamic volumes in the group AVHs (PANSS: Positive and Negative Syndrome Scale, AVHs: Schizophrenic patients with auditory verbal hallucinations, r = −0.551, P = 0.018).
ROC analysis of thalamic volumes of patients with AVHs and normal controls
A receiver operating characteristics (ROC) graph is a technique for visualizing, organizing and selecting classifiers based on their performance17. ROC analysis, which has been widely used in the evaluation of diagnostic tests18, was performed on the corrected thalamic volume to classify patients with AVHs and normal controls into their respective categories. We found that all the corrected thalamic volumes were located within Mean ± 3SD in the NC and AVHs groups. Grubbs test19 indicates that there is no outlier in the dataset (α = 0.05). The area under the ROC was 0.698 (P = 0.043). According to Youden’s index20, when the corrected thalamic volume is greater than 1.267%, the corresponding sensitivity and specificity of the ROC analysis was 77.8% and 55.6% respectively (see Fig. 6).
Figure 6. ROC curve for discriminating schizophrenic patients with AVHs from normal controls.

The area under the ROC curve was 0.698 (P = 0.043). ROC: Receiver Operating Characteristic; AVHs: Schizophrenic patients with auditory verbal hallucinations.
Discussion
In the current study, we found that the GM volumes of a number of brain structures, including the bilateral thalamus, left superior temporal gyrus (STG), frontal regions, precentral gyrus, cerebellum and caudate, were significantly reduced in drug-naïve FES patients with AVHs, compared to those of normal controls. There were significant GM volume differences of GM in left thalamus, IFG, inferior parietal lobule, middle orbital frontal gyrus and middle temporal gyrus, between the FES patients without AVHs and normal controls. No significant difference was found between FES patients with and without AVHs.
The STG is involved in speech perception12. Our finding that GM volume of the left STG is reduced in schizophrenic patients with AVHs is consistent with that of Modinos and colleagues21 who found that the level of AVHs was significantly correlated with GM volume reduction in the left STG in a meta-analysis. The left STG includes the auditory cortex and has been shown to be the aetiology of AVHs22. All subjects were right-handed in our study. It is conceivable that the left STG may play a critical role in AVHs. Kubera et al. has found that the GM volume of the frontotemporal region was reduced in persistent AVHs patients23. We have found reduced GM volume in the left inferior frontal gyrus (Brodmann’s areas 44 and 45), medial frontal gyrus (Brodmann’s areas 8, 11). The left inferior frontal gyrus is one of the classical left-hemisphere language-processing areas24; the medial frontal gyrus is associated with language and memory processing25,26. A number of studies have suggested that the cerebellum is involved in schizophrenia27,28. For instance, a significant reduction of the volume of the cerebellum vermis in the schizophrenic group has been reported29, and Neckelmann et al.30 found that the severity of hallucinations associated with reduced grey matter volume in left and right cerebellum. In the current study, we found the reduced cerebellum volume in the AVHs group compared to normal controls. The cerebellum is thought to be involved in the monitoring process22. However, conventional VBM method lacks some precision with respect to cerebellar morphology, so we would treat this conclusion with care. The structural studies, e.g., gray matter volume changes in caudate31 and precentral gyrus32, have shown that the caudate and precentral gyrus were associated with hallucination or inner speech.
In this study, we found no significant correlation between scores of the auditory hallucination rating scale and thalamic volume in the AVHs group. But there was a significant correlation between PANSS total scores and thalamic volume in the AVHs group. Our ROC analysis suggests that it was possible to use thalamic volume to discriminate schizophrenic patients with AVHs from normal controls with modest success.
There are several limitations in this study. First, we only conducted a VBM analysis on the data. Future research could apply source-based morphometry (SBM)33 on the dataset. Second, our sample size is relatively small due to the difficulty in recruiting drug-naïve first-episode schizophrenic patients with auditory verbal hallucinations.
Similar to many studies that have found reduced thalamic volume in patients with schizophrenia34,35, we found significant GM volume differences in brain structures between first-episode schizophrenic patients with or without hallucinations and normal controls. To our knowledge, the findings that there’s no volumetric difference in drug-naïve first-episode schizophrenia with AVHs versus Non-AVHs is a novel result. In fact, there’s just one study focusing on volumetric differences in drug- naïve AVHs versus Non-AVHs. In that study36, the authors found that the gray matter volumes in the frontal and temporal lobes were significantly larger in patients with AVHs than in patients without AVHs. The different results from the two studies are probably due to (1) smaller sample size (17 AVHs, 8 Non-AVHs), (2) no adjustment on sex, and (3) unbalanced number of patients with and without AVHs. The negative findings between AVHs group and Non-AVHs group patients may be because two of our Non-AVHs patients have reported that they’ve experienced AVHs 2 years prior to recruitment. We have excluded these two patients in the Non-AVHs group, and did the VBM again, the results were similar to the current study and the difference between two patients group is still negative.
As a debilitating and often lifelong disease37, schizophrenia is considered to be a nosology that likely reflects a group of diseases rather than a single disease entity38,39. The substantial clinical heterogeneity in this disorder is a major obstacle to the identification of the neurobiological correlates of this disorder40. In this study, the FES patients were young and didn’t have long duration of the disease. Guo et al. found that patients with longer psychosis histories had significantly smaller gray matter volumes in the right superior temporal gyrus, left fusiform gyrus, and left middle temporal gyrus41. But no such significant effect was found in this study. Although a consensual definition is still lacking, AVHs is characterized by perceiving sounds without auditory stimulus, there’s one of the most influential cognitive models proposes the impairment of self-monitoring on one’s own inner speech, then misleading to identify verbal thoughts as alien source42,43,44. There is evidence that AVHs could act as a potential taxonomy standard45. That’s probably why we found significant difference between patients with AVHs and normal controls, especially in the left STG. In order to reduce or eliminate clinical heterogeneity in a study sample, an endophenotype (intermediate phenotype) research strategy should be adopted45. An important requirement, however, is that an endophenotype should be a quantitatively measurable trait.
Conclusion
In conclusion, the current study demonstrates that volumetric reductions in language processing areas and nonsensory regions in FES patients with AVHs. Significant reduction in thalamic volume, especially the right thalamic volume, may provide an objective imaging measure for discriminating schizophrenic patients with auditory verbal hallucinations from normal controls.
Methods
Participants
The included 36 FES patients were assigned to two groups according to the presence of AVHs symptom. Those who reported AVHs at least once a day for the past four weeks were assigned to AVHs group. Patients who have never experienced AVHs or have not experienced them within two years before recruitment were allocated to Non-AVHs group. An additional 18 normal control subjects (NC group), matched for age and gender with the patients, were recruited via advertisement in the local community. All participants gave their written informed consent prior to the study, which was in accordance with the Declaration of Helsinki and all experiments protocol were approved by ethics committee in Fourth Military Medical University, Xi’an, China.
Clinical measures
Patients meeting diagnostic criteria for schizophrenia according to DSM-IV were assessed by two senior clinical psychiatrists using the Positive and Negative Symptom Scale46 (PANSS total score ≥60) at most two days before the MRI session. All patients were first-episode and drug-naïve at the time of scanning. AVHs patients were further evaluated using Hoffman Auditory Hallucination Rating Scale (HAHRS)47 before the MRI scan. This scale assesses AVHs on seven characteristics: frequency, reality, loudness, number of voices, length, attention dedicated to the hallucinations, and hallucination-induced arousal. A general score is obtained as well, by summing the items, to give a measure of general AVHs severity.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed on a 3 Tesla Allegra system (Siemens, Erlangen, Germany). A standard birdcage head coil along with foam pads was used to reduce head motion and scanner noise. High-resolution whole brain volume T1-weighted 3D anatomical data were acquired using the 3D magnetization-prepared rapid gradient echo (3D MPRAGE) sequence. The scanning parameters were: repetition time = 2530 ms; echo time = 3.5 ms; flip angle = 7°; field of view = 256 mm × 256 mm; data matrix = 256 × 256; slice thickness = 1 mm; gap = 0 mm; number of slices = 192 slices. The image resolution was 1 mm × 1 mm × 1 mm.
Image analysis
Data were preprocessed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) in the Statistical Parametric Mapping software package version 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). The structural T1-weighted anatomical images were automatically segmented into gray matter and white matter. The resulting images were spatially normalized and scaled with Jacobian matrices into the Montreal Neurological Institute (MNI) space. The absolute masking, with a threshold of 0.2 was used. Modulated images were saved by correcting for non-linear warping only. The Gaussian kernel for smoothing had an 8-mm full-width at half maximum (FWHM). Analysis of variance was conducted based on the general linear model. Age, gender, years of education were included as additional covariates in this model. The Xjview toolbox (http://www.alivelearn.net/xjview) was used to perform multi-comparison correction with P < 0.001 (uncorrected). Only clusters thresholded with the number of expected voxels (spatial extent threshold) calculated according to the theory of Gaussian random fields are reported.
Individual bilateral thalamic volumes were estimated using FIRST (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/first), which is part of FSL Version 5.0.6 (FMRIB Software Library) and a model-based segmentation/registration tool for deep gray matter, e.g., left and right thalamus. Total brain volume was estimated with SIENAX (http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/siena/index.html#sienax), which is a part of FSL48. The total volume of brain tissue, normalised for subject head size, was estimated with SIENAX49, used to correct thalamic volume for differences in head size.
Statistical analysis
Chi-square tests, independent-sample t tests, and one-way ANOVAs were conducted in this study. ANOVA and post hoc tests were applied to compare the corrected GM volumes of the three groups. Pearson correlation analysis was applied to evaluate the relationship between thalamic volume and clinical test scores in AVHs patients. A Receiver Operating Characteristic (ROC) analysis was performed to distinguish AVHs patients from normal controls based on thalamic volumes. SPSS 16.0 (IBM SPSS Statistics) was used for additional statistical analysis. P < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Huang, P. et al. Decreased bilateral thalamic gray matter volume in first-episode schizophrenia with prominent hallucinatory symptoms: A volumetric MRI study. Sci. Rep. 5, 14505; doi: 10.1038/srep14505 (2015).
Acknowledgments
We are very grateful to all the participants. Peng Huang would like to thank the China Scholarship Council for providing him the opportunity to study at the Ohio State University. This research was supported by the National Basic Research Program of China (973 Program), No. 2011CB707805.
Footnotes
Author Contributions H.Y., D.M., Y.C., P.H. and Y.X. designed and conducted the experiment, H.Y., Y.X., L.C., C.L. and W.M.L. collected the data, P.H., X.L., Z.-L.L. and Y.X. analyzed the data, P.H., Y.X. and X.Z. wrote the manuscript. H.Y., D.M., W.G.L., X.L., Q.T. and Z.-L.L. edited the manuscript and gave conceptual advice.
References
- Andreasen N. C. & Flaum M. Schizophrenia: the characteristic symptoms. Schizophr. Bull. 17, 27 (1991). [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., Welham J. & McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2, e141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill S. S., Murray R. M. & McGuire P. K. Auditory hallucinations: a review of psychological treatments. Schizophr. Res. 32, 137–150 (1998). [DOI] [PubMed] [Google Scholar]
- Ho B.-C. et al. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry 60, 585–594 (2003). [DOI] [PubMed] [Google Scholar]
- Kempton M. J., Stahl D., Williams S. C. & DeLisi L. E. Progressive lateral ventricular enlargement in schizophrenia: a meta-analysis of longitudinal MRI studies. Schizophr. Res. 120, 54–62 (2010). [DOI] [PubMed] [Google Scholar]
- Henze R. et al. Gray matter alterations in first-admission adolescents with schizophrenia. J. Neuroimaging 21, 241–246 (2011). [DOI] [PubMed] [Google Scholar]
- Glahn D. C. et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol. Psychiatry 64, 774–781 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A. R. et al. Thalamic volumes in patients with first-episode schizophrenia. Am. J. Psychiatry 158, 618–624 (2001). [DOI] [PubMed] [Google Scholar]
- Ebdrup B. H. et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J. Psychiatry Neurosci. 35, 95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz-Santianez R. et al. One year longitudinal study of the straight gyrus morphometry in first-episode schizophrenia-spectrum patients. Psychiatry Res. 202, 80–83 (2012). [DOI] [PubMed] [Google Scholar]
- Honea R., Crow T. J., Passingham D. & Mackay C. E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 162, 2233–2245 (2005). [DOI] [PubMed] [Google Scholar]
- Allen P. et al. Neuroimaging Auditory Hallucinations in Schizophrenia: From Neuroanatomy to Neurochemistry and Beyond. Schizophr. Bull. 38, 695–703 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G. et al. Differential brain glucose metabolic patterns in antipsychotic-naive first-episode schizophrenia with and without auditory verbal hallucinations. J. Psychiatry Neurosci. 36, 312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S. et al. Specific disruption of thalamic inputs to the auditory cortex in schizophrenia models. Science 344, 1178–1182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E. A., Valentine E. R., Wilding J. M. & Kapur N. Routes to remembering: the brains behind superior memory. Nat. Neurosci. 6, 90–95 (2002). [DOI] [PubMed] [Google Scholar]
- Asami T. et al. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. NeuroImage 59, 986–996 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Pattern Recog. Lett. 27, 861–874 (2006). [Google Scholar]
- Zweig M. H. & Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39, 561–577 (1993). [PubMed] [Google Scholar]
- Grubbs F. E. Procedures for detecting outlying observations in samples. Technometrics 11, 1–21 (1969). [Google Scholar]
- Youden W. J. Index for rating diagnostic tests. Cancer 3, 32–35 (1950). [DOI] [PubMed] [Google Scholar]
- Modinos G. et al. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex 49, 1046–1055 (2013). [DOI] [PubMed] [Google Scholar]
- Allen P., Larøi F., McGuire P. K. & Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 32, 175–191 (2008). [DOI] [PubMed] [Google Scholar]
- Kubera K. M. et al. Source-based morphometry of gray matter volume in patients with schizophrenia who have persistent auditory verbal hallucinations. Prog. Neuropsychopharmacol. Biol. Psychiatry 50, 102–109 (2014). [DOI] [PubMed] [Google Scholar]
- Just M. A., Carpenter P. A., Keller T. A., Eddy W. F. & Thulborn K. R. Brain activation modulated by sentence comprehension. Science 274, 114–116 (1996). [DOI] [PubMed] [Google Scholar]
- Gabrieli J. D., Poldrack R. A. & Desmond J. E. The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. USA 95, 906–913 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn. Sci. 9, 512–518 (2005). [DOI] [PubMed] [Google Scholar]
- Andreasen N. C. & Pierson R. The role of the cerebellum in schizophrenia. Biol. Psychiatry 64, 81–88 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard H., Amado I., Mouchet-Mages S., Olié J.-P. & Krebs M.-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr. Bull. 34, 155–172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T., Okubo Y., Suhara T. & Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol. Psychiatry 49, 20–27 (2001). [DOI] [PubMed] [Google Scholar]
- Neckelmann G. et al. MR morphometry analysis of grey matter volume reduction in schizophrenia: association with hallucinations. Int. J. Neurosci. 116, 9–23 (2006). [DOI] [PubMed] [Google Scholar]
- O’Daly O. G., Frangou S., Chitnis X. & Shergill S. S. Brain structural changes in schizophrenia patients with persistent hallucinations. PSYCHIAT RES: NEUROIM 156, 15–21 (2007). [DOI] [PubMed] [Google Scholar]
- Marti-Bonmati L. et al. Chronic auditory hallucinations in schizophrenic patients: MR analysis of the coincidence between functional and morphologic abnormalities. Radiology 244, 549–556 (2007). [DOI] [PubMed] [Google Scholar]
- Xu L., Groth K. M., Pearlson G., Schretlen D. J. & Calhoun V. D. Source‐based morphometry: The use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 30, 711–724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C. et al. Gray matter volume decreases in elderly patients with schizophrenia: a voxel-based morphometry study. Schizophr. Bull. 38, 796–802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A. M. et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol. Psychiatry 56, 544–552 (2004). [DOI] [PubMed] [Google Scholar]
- Shin S.-E. et al. Segmented volumes of cerebrum and cerebellum in first episode schizophrenia with auditory hallucinations. Psychiat. Res.: Neuroimag. 138, 33–42 (2005). [DOI] [PubMed] [Google Scholar]
- Leucht S. et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. The Lancet 379, 2063–2071 (2012). [DOI] [PubMed] [Google Scholar]
- Insel T. R. & Wang P. S. Rethinking mental illness. JAMA 303, 1970–1971 (2010). [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B., Buchanan R. W., Ross D. E. & Carpenter W. T. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 58, 165–171 (2001). [DOI] [PubMed] [Google Scholar]
- Voineskos A. N. et al. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA psychiatry 70, 472–480 (2013). [DOI] [PubMed] [Google Scholar]
- Guo X. et al. Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naive schizophrenia. PLoS One. 8, e83679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P., Aleman A. & Mcguire P. K. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int. Rev. Psychiatr. 19, 407–415 (2007). [DOI] [PubMed] [Google Scholar]
- Waters F., Woodward T., Allen P., Aleman A. & Sommer I. Self-recognition deficits in schizophrenia patients with auditory hallucinations: a meta-analysis of the literature. Schizophr. Bull. 38, 741–750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley P., Fernyhough C. & Ellison A. Auditory verbal hallucinations as atypical inner speech monitoring, and the potential of neurostimulation as a treatment option. Neurosci. Biobehav. Rev. 37, 2794–2805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman I. I. & Gould T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645 (2003). [DOI] [PubMed] [Google Scholar]
- Kay S. R., Flszbein A. & Opfer L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261 (1987). [DOI] [PubMed] [Google Scholar]
- Hoffman R. E. et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. Gen. Psychiatry 60, 49–56 (2003). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004). [DOI] [PubMed] [Google Scholar]
- Smith S. M. et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage 17, 479–489 (2002). [DOI] [PubMed] [Google Scholar]