Abstract
Ankylosing spondylitis (AS) is a chronic inflammatory disease that affects 1% of the general population. As one of the most severe types of spondyloarthropathy, AS affects the spinal vertebrae and sacroiliac joints, causing debilitating pain and loss of mobility. The goal of this review is to provide an overview of AS, from the pathophysiological changes that occur as the disease progresses, to genetic factors that are involved with its onset. Considering the high prevalence in the population, and the debilitating life changes that occur as a result of the disease, a strong emphasis is placed on the diagnostic imaging methods that are used to detect this condition, as well as several treatment methods that could improve the health of individuals diagnosed with AS.
Keywords: Ankylosing spondylitis, Magnetic resonance imaging, Ultrasound, Computed tomography, Treatment, Diagnosis
Core tip: Considering the high prevalence of ankylosing spondylitis (AS) in the population, and the debilitating life changes that occur as a result of the disease, this article places a strong emphasis on the diagnostic imaging methods that are used to detect this condition, as well as several treatment methods that could improve the health of individuals diagnosed with AS. However, we have also tried to provide a summary of current knowledge on AS in this manuscript, which can be used as a handbook for physicians handling patients with this condition.
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic and systemic seronegative inflammatory spondyloarthropathy, which causes destruction and fusion of the spinal vertebrae and sacroiliac joints. It has been proposed that the sites of attachment of the ligaments or tendons to the bone, called entheses, are the major target of the inflammatory, traumatic and degenerative pathological changes occurring in AS. Enthesitis is believed to play a primary role in the ligament calcification process, which results in pain. It can lead to reduced flexibility of the spine, and eventually complete loss of spinal mobility, destruction as well as ankylosis (fusion) of the spine and sacroiliac joints.
Accordingly, comprehension of the microanatomy and mechanical function of spinal ligaments and entheses is necessary in order to provide a better understanding of the operating mechanisms of AS, and propose treatment options.
The goal of this review is to provide an overview of AS, from the anatomy and pathophysiological changes that occur as the disease progresses, to genetic factors that are involved with its onset. Considering the high prevalence of AS in the population, and the debilitating life changes that occur as a result of the disease, a strong emphasis is placed on the diagnostic imaging methods that are used to detect this condition, as well as several treatment methods that could improve the health of individuals diagnosed with AS.
ANATOMY OF THE SPINAL LIGAMENTS
The articulation mechanisms between any two vertebrae and the connecting soft tissues form what is called the motion segment. The anterior and posterior longitudinal ligaments connect the vertebral bodies. The intervertebral disc consists of an external fibrous ring, the annulus fibrosus, and a gelatinous core, the nucleus pulposus. On each side of the vertebral arch, the laminae of the upper and lower vertebrae are joined by a ligamentum flavum. There is a spinous process at the junction of the two laminae. The interspinous ligament joins the spinous processes of the upper and lower vertebrae, and the supraspinous ligament connects their tips. The zygapophyseal joints (facet joints) form between the superior articular process of the lower vertebra and the inferior articular process of the upper vertebrae[1].
Anatomy of sacroiliac joints
The sacroiliac (SI) joint is a strong diarthrodial weight-bearing compound joint. It consists of an anterior synovial joint (between the articular surfaces of the sacrum and ilium), whose irregular interlocking surfaces are covered with articular cartilage as well as a posterior syndesmosis located between the tuberosities of these bones. The SI joint has unique characteristics not typically found in other diarthrodial joints. These characteristics include the presence of fibrocartilage in addition to hyaline cartilage, discontinuity of the posterior capsule, many ridges and depressions in the articular surfaces, and limited range of mobility compared to other synovial joints. Three main ligaments stabilize the SI joint: The thin anterior SI ligament, the thick interosseous ligament lying deep between the tuberosities of the sacrum and ilium, which is critically involved in transmitting weight from the sacrum to the iliac bones, and the posterior SI ligament, which is the external continuation of the same mass of fibrous tissue[2,3]. Histological examination reveals rich innervation of the SI joint as well as the presence of free nerve fibers within the joint capsule and the adjoining ligaments[4].
Structure of the entheses
There are two different types of entheses. Fibrous entheses, characterized by pure, dense fibrous tissue; and fibrocartilaginous entheses, which have a transition zone of fibrocartilage at the bony interface. Typically, four zones of tissue are present in the latter type: Pure, dense fibrous tissue, un-calcified fibrocartilage, calcified fibrocartilage and bone. The two-fibrocartilage zones are separated by a basophilic (calcification) line known as the tidemark. Type I-collagen is the dominant collagen in the tendons/ligaments and bone, whereas type II-collagen is a special feature of fibrocartilage tissue, both calcified and un-calcified, as it accounts for its compression-tolerance properties[5]. Most tendons and ligaments have fibrocartilaginous entheses. Typically in AS, enthesitis is the primary trigger of the disease, while all other joint manifestations are secondary.
Diffusion of the pathological changes in the tissues surrounding entheses, e.g., fibrocartilages, bursa, fat pads and the entheses collectively constitute the concept of entheses organ, which helps to explain synovitis and osteitis in AS[6,7]. Entheses are metabolically active and are nourished by blood supplies from the perichondrium and periosteum. However, normal entheses organs are avascular in their fibrocartilaginous regions, and are completely devoid of immune cells. Microdamage of the entheses appears to be associated with tissue repair responses and vessel ingrowth[6,8].
Pathophysiology
Enthesopathy occurs in the subchondral bone area, with an erosive inflammatory infiltrate composed of lymphoplasmocytes and sometimes polymorphonuclear cells, followed by fibrous tissue proliferation leading to cartilage and then bone formation. Spinal and SI enthesitis limit the ability of the spine to move and the ability of the thoracolumbar fascia to influence the alignment of the lumbar vertebrae, thereby it increases their risk of destructive injury[9]. This phenomenon decreases the length of the visco-elastic portion of the spinal ligaments, and affects the myxoid subchondral bone marrow. As the disease progresses, it destroys the nearby articular tissues or joint tissues. The calcification of the entheses organ is the final step before the appearance of the “bamboo spine” where original and new cartilages are replaced by bone causing fusion of the joint bones, leading to stiffness and immobility. Enthesitis is also associated with underlying osteitis. Whether mechanically induced or inflammatory-related, the extent of osteitis is determined by human leukocyte antigen-B27 gene[10].
BONE DENSITY LOSS IN AS
Bone mineral density (BMD) loss occurs in the course of AS with high prevalence. The severity of BMD loss depends on the disease duration and the presence of syndesmophytes in the spine. A decrease in BMD can be found both in the hip as well as in the spine in both early and late stages of the disease. Dual-energy X-ray Absorption (DEXA) is the most reliable method for the measurement of BMD. Normal bone density is defined as T score ≥ -1.0, osteopenia as -2.5 < T score < -1.0, and osteoporosis as T score ≤ -2.5[11]. The T score corresponds to the number of standard deviations (SD) from any result of the peak bone mass. Osteoporosis of the spine (L1-L4) is much more common than that of the hip in AS, and BMD of the spine still remains the most important site to define osteoporosis in patients with AS[12]. Low BMD becomes clinically pertinent as it increases the risk of fracture, since these fractures are a considerable cause of morbidity and reduced quality of life[13].
Significant local and systemic inflammatory responses may play an important role in the development of osteoporosis (defined as T scores less than -2.5 in one region in the lumbar spine or proximal femur) in clinically established AS patients. Genetic susceptibility, immobility and impaired calcium and vitamin D absorption are other possible mechanisms that facilitate the bone loss process in AS. DEXA measurements of the hip can detect continuing bone loss represented by a low BMD with better sensitivity than in the spine. Although the deleterious effects of AS are considered to be more distinguished in the spine, the Bath Ankylosing Spondylitis Disease Activity Index, an accepted indicator of disease activity, demonstrates pronounced activity in the hips rather than the spine[14].
Increased bony sclerosis that is seen in the expected disease evolution of AS can artificially cause an augmentation of BMD in routine DEXA of the spine, despite the ongoing bone loss that is depicted in hip measurements of DEXA. Enthesitis of the vertebral margins, sclerosis of vertebral end-plates, syndesmophyte formation, interapophyseal joint and interpedicular joint ankylosis can all justify this paradoxical increased BMD of spinal involvement in AS. Studies where BMD has not increased may reflect the heterogeneity of the selected sample, since they have included AS patients in all stages of the disease, probably some of them without syndesmophytes. It has been shown that BMD measured by lateral DEXA or on Quantitative Computerized Tomography is less affected by syndesmophytes than anteroposterior lumbar DEXA in late stage AS patients[12].
Genetics in AS
AS is a systemic disease with a strong genetic predisposition. Previous studies have indicated that several genetic factors implicate the susceptibility to AS[15-17]. Brown et al[15] in 1997 reported a disease concordance of about 12.5% and 75% in di- and monozygotic twins, respectively (18). In addition to the role of genetics in susceptibility to AS, some studies have focused on the impact of genetic predisposition on important clinical parameters, including the age of disease onset and disease activity in AS patients. Brophy et al[13] found a correlation between disease severity among siblings and a parent-child concordance for ophthalmic involvement at the onset of disease in early adulthood[18].
The major histocompatibility complex (MHC) locus on chromosome 6p and other non-MHC loci have been shown to be associated with the genetic basis of AS[19]. In 1973, Brewerton et al[20] revealed the amazingly strong association between HLA–B27 and AS. Human leukocyte antigen (HLA) B27 is a surface antigen class-I that presents antigenic peptides to T-cells. It is encoded in the MHC[20,21]. HLA-B27 consists of a family of more than 40 subtypes named HLA-B*2701 to HLA-*B2728. HLA-B*2702, B*2704, and B*2705 have the strongest association with AS[22]. The overall prevalence of HLA-B27 in the general population is 8%, however, there are regional differences in prevalence. For instance, the prevalence of HLA-B27 among the general population in the United States is 6.1%, however, in New Zealand the prevalence is 9.2%[23-25]. HLA-B27 seems to be rare in the African population, which is consistent with a low disease incidence[26].
The prevalence of polymorphisms of the HLA-B27 gene is different around the world. B*2705 is the most prevalent variant among HLA-B27 carriers in the white British population[27]. However, a combination of B*2704 and B*2705 is the prevalent variant in Chinese populations[28].
To explain the association of HLA-B27 with the pathogenesis of AS two essential theories have been proposed, namely the canonical and non-canonical theories. The “arthritogenic peptide” theory is a canonical theory that suggests HLA-B27 mediated antigen presentation as the center of pathogenesis. The theory suggests that the T-cell mediated cytotoxic response to self-antigens can result in autoimmunity and inflammation[29-33]. “Misfolded protein” and “HLA-B27 surface homodimer” hypotheses are two non-canonical theories explaining the pathogenesis through the accumulation of inappropriate HLA-B27 proteins and abnormal intracellular signaling, respectively[34-40].
Although the association of HLA-B27 with AS is very significant, some non-B-27 MHC genes and non-MHC genes may be associated with this disease. The HLA-B60 gene, an MHC class I gene, is associated with AS in British and Chinese populations[41,42]. In addition, Brown et al[43] (1998) and Sims et al[19] (2007) showed that the HLA-DRB1 gene, MHC class II, can be associated with AS. In the case of non-MHC genes, the IL-1 gene complex is associated with AS[44-47]. The CYP2D6 gene encoding cytochrome P450 debrisoquine 4-hydroxylase may be implicated in AS pathogenesis[48,49]. Other identified candidate non-MHC genes associating with AS are ERAP1, IL23R, ANTXR2, RUNX3, and LTBR-TNFRSF1 A[50-52].
In AS, the association of radiographic severity with HLA-B27 seems to be different to those with spondyloarthritis (SpA). Non-B27 genes such as SNP rs8092336 and SNP rs1236913 were found to be associated with HLA-B27 radiographic severity rather than HLA-B2[53].
Diagnosis of AS
AS is the most severe subtype of spondyloarthropathy, affecting up to 1% of the general population[54,55]. Its main clinical manifestations are inflammatory back pain (IBP), inflammation in other parts of the body or the axial skeleton, anterior uveitis, enthesitis, as well as peripheral arthritis[54] (Figures 1 and 2).
Figure 1.
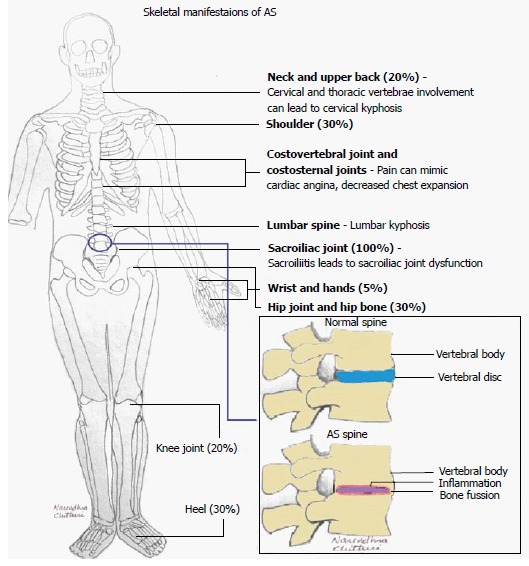
Schematic of skeletal manifestations of ankylosing spondylitis. AS: Ankylosing spondylitis.
Figure 2.

Schematic of extra-skeletal manifestations of ankylosing spondylitis. AS: Ankylosing spondylitis.
Genetic factors play a large role in the diagnosis of AS. Although the effect of the gene is unclear, more than 90% of patients with AS carry HLA-B27[54,55]. However, only 1 in 15 people who carry this gene are likely to develop AS; this fact brings speculations of environmental factors and stress having the ability to influence the progress of the disease[55]. The average age of onset for patients who were HLA-B27 positive (in a study involving 1080 patients) was 24.8 years, whereas those who were HLA-B27 negative had an average onset age of 27.7 years[56,57].
One of the main problems in diagnosing AS is the fact that there are no diagnostic criteria, making early diagnosis difficult. Due to this issue, there is a delay in diagnosis between five to ten years, leading to unnecessary diagnostic and therapeutic procedures as well as increased morbidity[54,55]. Early diagnosis of the disease is important in order to allow for effective therapy and to improve patient outcome[58-60].
The first standardized classification criteria were brought forth by the European Spondyloarthropathy Study Group in 1991[61]. The classification criteria for SpA were seen as the presence of inflammatory back pain (IBP) or asymmetric synovitis, along with one of the following: Positive family history of SpA or related disease, inflammatory bowel disease (IBD), psoriasis, enthesopathy, alternating pain in the buttocks, preceding infection in the urogenital or enteral tract[54,61]. More recently, this work has been continued by the Assessment of Spondyloarthritis International Society (ASAS), who have published recommendations for clinical trials and management of AS. In 2009, the ASAS also published a statement on the classification of axial SpA, which was written with the objective of validating and refining the classification/diagnostic criteria of axial SpA[55,56,62]. The brief criteria from this publication, which can be seen in Table 1, have a sensitivity of 82.9% and a specificity of 84.4%[55,62].
Table 1.
Classification criteria for axial spondyloarthritis defined by the Assessment of SpondyloArthritis International Society, to be used for patients with back pain for more than 3 mo and an onset of less than 45 years
| Sacroiliitis on imaging plus one or more axial spondyloarthritis feature |
| or |
| Positive test for HLA-B27 gene plus 2 or more other axial spondyloarthritis features |
| Sacroiliitis on imaging |
| Active (acute) inflammation on magnetic resonance imaging highly suggestive of sacroiliitis associated with axial spondyloarthritis |
| Definite radiographic sacroiliitis according to the modified New York criteria |
| Axial spondyloarthritis features |
| Inflammatory back pain Arthritis Enthesitis (heel) Uveitis Dactylitis Psoriasis Crohn’s disease or diagnosis of colitis Good response to non-steroidal anti-inflammatory drugs Family history of axial spondyloarthritis Positive test for HLA-B27 gene Elevated C-reactive protein levels |
Earlier criteria include the Rome criteria (published in 1963 and revised in 1968)[63], and modifications made to this in New York (1984)[64], which have been scrutinized for some shortcomings, including inability to establish early diagnosis of AS[55,65]. The newer ASAS criteria allow for a broader spectrum of diagnostic features[54,61].
There is some confusion between diagnostic and classification criteria for SpA. As of now, all criteria that have been developed are classification criteria, although they are sometimes used as diagnostic criteria[54]. This can be problematic as using classification criteria to diagnose patients with possible AS leads to overshooting the probability of the diagnosis, as the pretest probability of the disease is not considered[54]. The classification criteria are also more suited to detect later stages of the disease, rather than helping make an early diagnosis[56].
As a solution to the lack of diagnostic criteria, a “diagnostic algorithm” has been developed by Rudwaleit et al[66] (2004). With this algorithm, patients experiencing chronic back pain can be diagnosed with axial SpA if they experience IBP along with three or more of the outlined SpA features[65,66]. Additionally, HLA-B27 genotyping may be recommended for patients experiencing IBP and one or two SpA features. If HLA-B27 typing is positive, patients can then be diagnosed with axial SpA. Finally, patients who have IBP, but no other SpA features, should undergo HLA-B27 testing as well as MRI imaging, from which they will be diagnosed as having axial Spa if HLA-B27 is positive and MRI demonstrates inflammation of the sacroiliac joints[65,66].
To come closer to diagnosing AS in its earlier stages, the distinction must be made between a patient’s back pain that is caused by AS (characterized as IBP) vs pain caused by mechanical lower back pain (MLBP). Four parameters have been proposed to distinguish AS from MLBP. The parameters defining IBP include: Improvement of back pain with exercise, but not with rest, waking up during the second half of the night or early morning due to back pain, experiencing morning stiffness of at least 30 min, and alternating buttock pain[54,65,67]. The criteria can be used on adults aged less than 50 years with chronic back pain, and are considered positive if two or more of the four parameters are experienced (sensitivity = 70.3% and specificity = 81.2%)[54].
To attain this information, clinical symptoms, history, examination, laboratory parameters and imaging must be obtained from the patient[54]. Clinical symptoms that may indicate the presence of SpA include IBP, arthritis (seen by swelling, joint effusion or by imaging), enthesitis (seen as swelling where tendons and ligaments attach to the bone - commonly near the heel if there is pain during walking, specifically in the morning[55]) and any accompanying features such as psoriasis, Crohn-like colitis and anterior uveitis[54]. Other symptoms can include stiffness that can take anywhere from a few minutes to two hours to relieve, particularly in the morning, and can be seen to reappear after long periods of sitting or rest[55]. Symptoms can also include fatigue that does not subside with sleep, pain in the spine that is worse during rest and that improves with exercise[68,69]; shortness of breath once AS is in the later stages, as fusion of thoracic vertebrae can restrict expansion of the chest[55]; feverishness or night sweats, however, these can also be associated with other types of inflammatory and autoimmune diseases[70]; as well as flare-ups of AS, where it has been seen that 70% of patients with AS will have flares in any one week[55,71].
It is important to determine if the patient has a family history of SpA or diseases like psoriasis or IBD. A history of rheumatic symptoms or other suspicious skin or gut findings must also be noted[54].
Clinical examination for early AS is limited, as major deformities of the spine have probably not yet occurred, and sacroiliitis and spondylitis cannot be diagnosed through clinical means alone. The earliest signs to look for include reduced lateral spinal flexion of the lumbar spine (10 cm), decreased chest expansion (4 cm) and limited cervical rotation (70o)[54]. If only pain is present, further imaging is recommended. The two main laboratory parameters useful for diagnosis include HLA-B27 genotype and C-reactive protein levels[54]. HLA-B27 remains of high importance, especially early on in the disease, whereas only one half of patients with AS also have high serum levels of C-reactive protein[54,72].
X-RAY
Imaging in AS has been synonymous for decades with conventional radiography (CR). However, developments in computed tomography (CT), ultrasonography (US) and magnetic resonance imaging (MRI) have increased the amount of information that can be obtained by imaging. Imaging is necessary in AS to establish diagnoses, determine the extent of disease in axial or peripheral joints and/or entheses, and monitor the change in disease[73,74].
Radiographs are the most important imaging technique for the detection, diagnosis, and follow-up monitoring of patients with AS[75]. Typical findings of AS are sacroiliitis and bridging syndesmophyte of the spine, which usually take many years to develop. Sacroillitis is the hallmark of AS and is found in the early stages[73]. Subchondral bone erosion on the iliac side of the SI joint is seen first, followed by subchondral sclerosis and bony proliferation[76], as seen in Figure 3.
Figure 3.
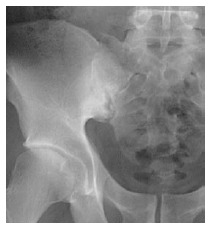
Anteroposterior pelvic X-ray shows pseudodilatation of the sacroiliac joint due to subchondral erosion and subchondral bone sclerosis.
In the spine, first visualized are small erosions at the corner of the vertebral bodies that are caused by periosteal bone formation[77,78]. After these erosions, ossification of outer fibers results in syndesmophytes, which leads to bridging between the vertebrae. Thickening of the syndesmophytes results in the appearance of the “bamboo sign” in radiographs[78], as in Figure 4.
Figure 4.
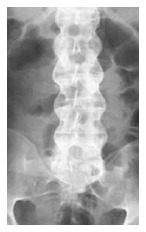
Anteroposterior vertebral X-ray shows bilateral thickening of the syndesmophytes resulting in the appearance of the “bamboo sign” in radiographs.
The “dagger sign” is another finding that results from ossification of the posterior interspinous ligament[78]. Pseudoarthrosis manifested by disco-vertebral destruction and sclerosis presents as a hypodense and linear shape in the sclerotic border. Pseudoarterosis appears in radiographs similar to vertebral disks infected by diseases such as tuberculosis, although it is caused by undetected vertebral fractures or unfused segments[74]. Ill-defined erosions with sclerosis at the side of ligaments and tendonitis are seen as enthesopathy. These lesions are typically bilateral and symmetrical in distribution. Enthesopathic changes are particularly prominent at certain sites around the pelvis, such as the ischial tuberosity[79] (Figure 5).
Figure 5.
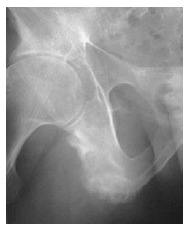
Anteroposterior pelvic X-ray shows ill-defined erosions with sclerosis at the side of ligaments, and tendonitis is seen as enthesopathy at certain sites around the pelvis, at the ischial tuberosity.
Knee changes consist of uniform joint space narrowing and surrounding bony proliferation. The glenohumeral joint space may be narrow and a large erosion may be present in the upper greater tuberosity. The hips are typically involved symmetrically demonstrated as joint space narrowing, femoral head axial migration, and osteophyte formation at the femoral head-neck junction. In the hands, the joints are usually involved asymmetrically. Erosions are smaller and shallower. Marginal periostitis is seen, and bone density is usually preserved. Lung manifestations of AS are seen as progressive fibrosis and bullous changes in the upper lobes. Lung changes are usually seen several years after the joint disease develops[74]. Conventional radiography is relatively inexpensive and widely available, however, it is not sensitive in the early stages of AS. The amount of data documenting a prognostic value of CR findings is limited in low-grade (grade 1) sacroiliitis, and it has a predictive value for the progression of AS[80].
CT
CT scanning may be useful in patients with suspected AS where radiography results are normal or equivocal. CT is rapid, reliable and produces high-resolution images.
CT demonstrates pathological findings similar to CR such as erosion, osteoporosis, scleroses as well as new bone formation, with better visualization and localization[81] (Figure 6).
Figure 6.
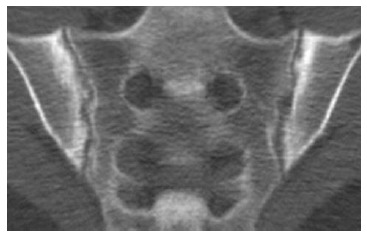
Computed tomography of sacroiliac joint shows bilateral subchondral sclerosis and dentate joint contour due to bone erosion.
CT provides better visualization for the measurement of syndesmophyte growth[82]. Other useful applications of CT scanning are the detection of atlantoaxial instability, manubriosternal disease, paraspinal muscle atrophy and costovertebral disease. In patients with advanced AS, CT is the imaging of choice for the assessment of fractures of the cervical spine and soft-tissue injuries[81]. CT is also effective as a navigation tool in screw fixation of spinal and lumbar fractures[83,84].
APPLICATION OF US IN THE MANAGEMENT OF PATIENTS WITH AS
Although MRI has become a reference modality in the lack of any gold standard method for definite diagnosis of AS, US still maintains a major role in the diagnosis of AS because it is simple, inexpensive and pervasively available. Also, recent studies and the efforts of the ultrasound taskforce (OMERACT-EULAR) have validated US as a diagnostic tool, proving it to be a highly sensitive, non-invasive and a practical tool in the assessment of joint pathology[85].
US of the sacroiliac joint
The presence or absence of sacroiliitis continues to be the mainstay of early diagnosis of AS[86]. Several recent studies have provided data on the usefulness of color Doppler ultrasound (CDUS) in the assessment of the sacroiliac joints and spine[87-89] (Figure 7).
Figure 7.
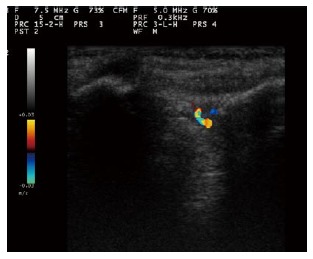
Color Doppler sonogram of sacroiliac joint in a patient with active sacroiliitis reveals vascularization within the posterior portion of the right sacroiliac joint.
Klauser et al[90] showed sensitivity, specificity, positive and negative predictive values of 100% for contrast-enhanced US in the detection of clinically active sacroiliitis (Figure 8). Although contrast-enhanced CDUS has been reported to have a high negative predictive value for the detection of sacroiliitis[63], the role of US in the assessment of sacroiliitis and spine involvements in AS is still minimal.
Figure 8.
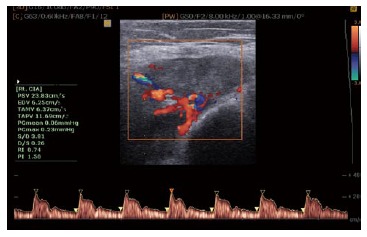
Color and spectral waveform Doppler ultrasonogram of the ischial tuberosity in a patient with severe enthesopathy with a mean resistive index of 0.74 before treatment.
US in the assessment of enthesitis
US is superior in the evaluation of rheumatologic diseases with peripheral involvement. Active enthesitis is an ultrasonographic finding highly suggestive of AS[91-95]. Enthesitis is the inflammation of the insertion of tendons, ligaments and capsules into the bone. Calcaneal entheses (plantar fascia and Achilles tendon), knee entheses (quadricipital tendon and proximal and distal attachments of patellar tendon), hip entheses (gluteus medius tendon) and elbow entheses (medial and lateral epicondyle tendon) are common sites of enthesitis in patients with AS. A national consortium of Rheumatology experts in 2006 recommended the use of Doppler US (or MRI) to evaluate the entheseal involvement in patients with AS (level of evidence 2b/3; grade of recommendation D)[96].
Gandjbakhch et al[97] have systematically reviewed the studies regarding the use of US in the evaluation of entheses; they found a heterogeneity in the US technique and definitions in different studies, and thus suggested the determination of specific US definitions for enthesitis to be used universally in both research and clinical settings[97]. The ultrasound taskforce (OMERACT-EULAR) has made an enormous effort to provide definitions and to validate US techniques in the diagnosis of rheumatologic diseases.
US in the evaluation of atherosclerosis and cardiovascular complications
Accelerated atherosclerosis is a major problem in rheumatic inflammatory disorders[98,99], therefore US has a substantial role in the evaluation of patients with AS. Two US techniques have been widely accepted as non-invasive methods for evaluating atherosclerosis. One is brachial artery flow-mediated dilation, which was demonstrated to be lower in AS patients compared to healthy controls[98], and is an indicator of endothelial dysfunction, which itself is an initiator of atherosclerotic processes. The second method is the measurement of intima-media thickness (IMT) for the early detection of atherosclerotic lesions. In the study by Sari et al[98], there was no significant difference between the carotid IMT of AS patients and controls, but it was positively correlated with age and the severity of AS disease, suggesting that patients with severe AS suffer from more advanced intimal thickening or atherosclerotic changes[98] (Figure 9).
Figure 9.
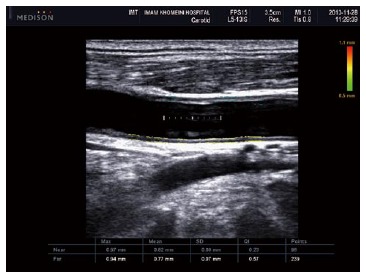
Ultrasonogram shows increased intima-media thickness of both near (0.82 mm) and far wall (0.77 mm) of common carotid artery in patients with ankylosing spondylitis.
A recent meta-analysis demonstrated a higher weighted IMT in AS patients (n = 1214) compared to controls (n = 1000) using 15 case-control studies and 9 abstracts[100].
Cardiac abnormalities are well recognized in AS. It has been shown that in many cases, cardiac change could even be prior to the onset of clinical AS disease[101,102]. Trans-thoracic echocardiography and Doppler US are useful techniques in the assessment of cardiac disease in AS, and may detect aortic valve disease at an early pre-clinical stage[103].
Aortic root and valve disease is a frequent complication in AS patients. It includes aortic root thickening and dilation, aortic cusp thickening and retraction, subaortic bump as well as aortic and mitral regurgitation[103-106]. The prevalence ranges between 24% and 100% according to autopsy reports, and 8%-31% according to trans-thoracic echocardiography[103,107-110]. The prevalence rate was higher when using trans-esophageal echocardiography as the diagnostic modality (82% in AS compared to 24% in healthy controls)[106]. Except for the duration of AS, these anatomical changes are reported to be unrelated to disease features (activity, severity or therapy), but they are associated with clinically important cardiovascular morbidity[106]. Conduction disturbances, yet another cardiovascular complication in AS, result from the progression of fibrosis through the interventricular septum to the atrioventricular node, thus they occur after aortic and valvular changes, and could be predicted in advance by early diagnosis of valvular changes[111,112].
Early detection of AS-related valvular abnormalities may provide the potential to prevent their progression to irreversible phases. Townend et al[113] reported that immunosuppressive therapy could prevent or delay valvular replacement in rheumatic patients with aortic regurgitation.
Cardiomyopathy and pericarditis, two other comorbidities in patients with AS, can be assessed by echocardiography[103,114-116]. The myocardial dysfunction seen in AS patients, which was demonstrated to be a diastolic variant (lower E wave velocity, higher A wave velocity and overall low E/A ratio) rather than a systolic variant, could be evaluated by echocardiography as well[102,117]. Caliskan et al[101] used trans-thoracic Doppler echocardiography to measure the coronary flow reserve (CFR), which is an indicator of coronary microvascular circulation, and they reported a decline in CFR in AS patients.
US in treatment monitoring
Doppler US was demonstrated to be useful in monitoring the patients’ response to enthesitis treatment[89,94,118-120]. Some studies have suggested the use of the resistive index (RI) of CDUS for following up the patients’ response to treatment[88].
Limitations of the ultrasound study
Since the bone cortex blocks ultrasound beams, US cannot visualize the foci of the bone marrow inflammation in the process of AS disease[121]. Operator dependency and its inter-observer variability is always a challenge for the clinical application of US.
Considerations regarding the physician performing the Ultrasound study
Another issue that should be addressed here is whether US for diagnosing AS should be performed by rheumatologists during the patient’s clinic visit or by radiologists.
There are no data to support the superiority of one over the other in the setting of AS. A study related to the assessment of rotator cuff tears, compared arthrography performed by radiologists to sonography performed by rheumatologists, and showed equivalency between their sensitivity and specificity rates[122]. So far, no one has studied the competency of the ultrasonographer, whether a radiologist or a rheumatologist, in the assessment of musculoskeletal disease[123].
Regardless of who performs the US assessment, it is important for the ultrasonographer to be competent in order to minimize the risk of misdiagnosis or unnecessary examination[123,124].
Nearly one-quarter of rheumatologists in US are using this technique, but it is still far from being incorporated into routine clinical practice[125]. In many European countries such as Germany, Italy and Spain, rheumatologists routinely perform US; and in some countries, musculoskeletal US training is a compulsory part of rheumatology training[126,127].
There is no doubt that it is unreasonable to expect every rheumatologist to be competent in all US procedures indicated for rheumatology[123], and that would remain within the remit of radiologists[128], however, training rheumatologists for a more selective list of procedures such as identifying synovitis in a joint, etc., which could improve clinical practice in rheumatology and enhance the care provided for patients, would be beneficial.
The best case scenario is a close cooperation between rheumatologists and radiologists. The cooperation and task division between these two specialties would facilitate a cost-effective, more convenient and the least risky care for patients, whilst providing the required information for diagnosis and treatment of patients for the physician[128].
MRI
Spinal inflammation can be demonstrated by MRI using the fat-saturating short tau inversion recovery (STIR) technique, especially in early and active disease, by showing inflammation, bone marrow edema, and pre-radiographic erosions at the sacroiliac joint Figure 10.
Figure 10.
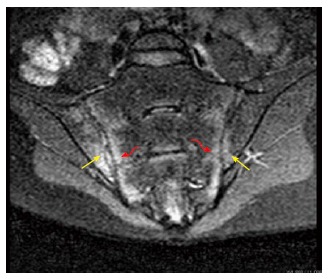
Oblique coronal magnetic resonance imaging (short tau inversion recovery sequence) of a patient with active ankylosing spondylitis revealed bilateral high signal intensity in both the sacral and iliac components of the sacroiliac joint.
The ability to diagnose AS before any bony deformities occur can significantly improve patient outcome and allow for effective therapies to be used before the later and more detrimental phases of the disease occur[58-60]. MRI is the most sensitive tool for imaging early AS and it can show both active inflammation as well as chronic structural changes near the sacroiliac joints and near the spine, both typical places for AS[58]. More recently, diffusion-weighted MRI (DWI) has been shown to be helpful in the early diagnosis of AS[58] In comparison, CT is limited by its lower sensitivity to characterize lesions in soft tissue and bone marrow, and is associated with considerable radiation exposure[58]. For these reasons, MRI has been recommended in recent classification criteria of AS[60,129].
In a study by Ai et al[58], 34 patients aged 15-38 years experiencing lower back pain for 3 mo to 2 years were studied using MRI examination. By comparing the apparent diffusion coefficient (ADC) values in subchondral bone marrow and sacroiliac joints, patients with typical uncomplicated musculoskeletal low back pain were able to be differentiated from those patients with AS[58]. In the study, the mean ADC values in early AS patients were significantly higher than the ADC values in LBP patients and healthy patient controls[58]. Early AS patients had mean ADC values of subchondral bone marrow along the bilateral sacroiliac joints that increased because of ongoing pathologically inflammatory penetration[58].
In addition, as shown by Ai et al[58], WB-MRI can detect many abnormalities in the sacroiliac joints, peripheral joints, attachment points of ligaments and tendons as well as in the spine, with one single scan. This is ideal because AS can manifest in parts of the body other than the sacroiliac joints[130], which it is well known for. MRI combined with a number of novel techniques, such as spectral pre-saturation inversion recovery echo planar imaging (SPIR-EPI) or short TI inversion recovery echo planar imaging (STIR-EPI), can create high-quality images, allowing for a better early diagnosis of AS[58]. For example, an efficient evaluation of AS patients can be done with WB-DWI combined with the background signal suppression technique (DWIBS) to create better lesion contrast and higher spatial resolution[58]. These novel techniques can create better imaging of lesion sites, with decreased interfering signal from other tissues, which leads to earlier diagnosis of patients with AS[58].
TREATMENT OF AS
Treatment of AS consists of a broad range of methods, which target different pathways involved with the progression of the disease. The most common treatment methods involve reduction of the inflammatory response, which is the primary complication resulting from AS, physical therapy, as well as surgical treatment to address deformities of the spine.
Anti-inflammatory treatment
The focus of the anti-inflammatory therapy in AS patients with high disease activity is the inhibition of the tumor necrosis factor α (TNF-α). Several studies have shown TNF-α inhibitors to be successful in reducing the inflammatory response caused by AS[131,132]. The use of TNF-α inhibitors as effective treatment methods for AS is considered a relatively new method, with its effects being recognized only in the last two decades. This treatment method is mostly effective in the early stages of the disease, where reduction of inflammation can prevent deformities of the skeleton. The two categories of TNF-α inhibitor drugs are anti-TNF antibodies and TNF receptors. Infliximab and adalimumab are monoclonal antibodies, while etanercept is a TNF receptor drug; both of these drugs are approved for use in AS treatment[133]. A study that analyzed the results of the effect of 5 inhibitor drugs through 13 controlled trials (adalimumab, etanercept, golimumab, infliximab and infliximab-biosimilar), showed that all of these drugs were significantly better than placebo in treating the symptoms of AS[134]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are another line of therapy used in AS. NSAIDs are more commonly used to alleviate back pain and increase mobility in patients with AS, however, several studies have shown that NSAIDs are also effective in reducing progression of the disease[135,136]. A recent study showed that patients not exposed to NSAIDs had a higher risk of developing morbidities and cardiovascular diseases, when compared to NSAIDs such as etoricoxib, celecoxib, as well as non-selective NSAIDs[137].
Physical therapy
Physical therapy is employed to increase muscle strength and mobility and reduce pain in patients with AS. Different exercise therapy methods have been used in the rehabilitation of AS patients, including weight training, cardiovascular training, and aquatic exercises. In general, these exercise methods provide similar treatment outcomes, which result in an increase in mobility, decrease in back stiffness, as well as a decrease in pain and fatigue[138]. However, compared to the more common exercise therapies, cardiovascular training has been shown to increase fitness in AS patients[139], while aquatic exercise has been more effective in reducing pain[140]. Spa therapy is another method recently introduced, which provides a more passive treatment[141]. Despite some significant indication of the effectiveness of these therapies, several studies have shown that improvement in patients is much higher when physical therapies are combined with anti-inflammatory drug treatments[142,143]. Considering the importance of exercise in the quality of life and mobility in AS patients, home exercise programs have been developed for patients to perform on their own after hospitalization. However, several studies have discussed the possibility that many patients do not perform these exercises at home on a regular basis, likely due to pain. Pain associated with AS not only induces fatigue in patients[144], but can also be a factor in the lack of exercise. A combination of anti-inflammatory drugs with physical therapy can be beneficial in this aspect, as pain reduction due to anti-inflammatory drugs can ultimately increase motivation for exercise[145].
Surgical intervention
Progression of AS may lead to deformities in the axial skeleton. One of the most common deformities in AS patients is the fixed thoracolumbar kyphotic deformity (TLKD) of the spine. The sole treatment method of this deformity is surgical. There are currently three surgical techniques used in spine surgery to fix TLKD, namely the opening wedge osteotomy (OWO), closing wedge osteotomy (CWO) and polysegmental wedge osteotomy (PWO). The OWO technique involves performing osteotomies of vertebrae L1, L2 and L3, followed by a manual extension of the lumbar spine to open a wedge of the anterior column, by closing the posterior osteotomies[146]. Unfortunately, disruption of the anterior longitudinal ligament from the manual extension of the spine is entailed in the procedure and major risks from this method include vascular and neurological complications[147,148]. The CWO technique involves resecting the posterior elements of a vertebra, followed by an extension of the spine, which closes the posterior osteotomy by creating an opening on the anterior part of the column[149]. The PWO technique involves performing several closing wedges of posterior lumbar osteotomies, which results in smaller closing angles of the osteotomies, and leads to a more gradual extension of the spine[147]. In general, there is no preference in choosing between the three techniques. However, studies have shown that the CWO and PWO have resulted in better outcomes in patients, compared to the OWO[150].
Spinal fractures in the thoracolumbar region are another complication that can result from AS. Surgical intervention is the ideal treatment method as it prevents further fractures of the spine, and improves the neurological status of patients[151]. Surgery has also been used for the treatment of other complications that arise from AS, such as deformities in the cervical region of the spine and in the sacroiliac joint.
CONCLUSION
This review provides a detailed summary of AS, one of the most common spondyloarthropathies. AS affects quality of life by causing debilitating pain and a significant decrease in mobility, but also occurs in high prevalence with a loss of bone mineral density and atherosclerosis. The discovery of several genetic factors that increase susceptibility to AS, as well as the use of CT, MRI and US in the diagnosis of the disease, could result in the early diagnosis of AS, which is very important in improving treatment outcome. Physical therapy, NSAIDs and inhibitor drugs have proven successful in alleviating disease symptoms, and in preventing further disease progression. Surgical intervention is necessary when the disease causes deformities to the spine in the later stages.
ACKNOWLEDGMENTS
Many thanks to Narendra Chetori for drawing the first two figures.
Footnotes
Conflict-of-interest statement: All authors declare that there is no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 22, 2014
First decision: December 26, 2014
Article in press: June 16, 2015
P- Reviewer: Essex MN, Sakkas L, Storto G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Hukins DWL, Meakin JR. Relationship between structure and mechanical function of the tissues of the intervertebral joint. American Zoology. 2000;40:42–52. [Google Scholar]
- 2.Vleeming A, Schuenke MD, Masi AT, Carreiro JE, Danneels L, Willard FH. The sacroiliac joint: an overview of its anatomy, function and potential clinical implications. J Anat. 2012;221:537–567. doi: 10.1111/j.1469-7580.2012.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KL, Dalley AF, Agur AMR. Clinically Oriented Anatomy, 7th ed. PA: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 4.Forst SL, Wheeler MT, Fortin JD, Vilensky JA. The sacroiliac joint: anatomy, physiology and clinical significance. Pain Physician. 2006;9:61–67. [PubMed] [Google Scholar]
- 5.Benjamin M, Ralphs JR. Entheses--the bony attachments of tendons and ligaments. Ital J Anat Embryol. 2001;106:151–157. [PubMed] [Google Scholar]
- 6.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D, Aydin SZ, Tan AL. The synovio-entheseal complex and its role in tendon and capsular associated inflammation. J Rheumatol Suppl. 2012;89:11–14. doi: 10.3899/jrheum.120233. [DOI] [PubMed] [Google Scholar]
- 9.Willard FH. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. Movement, stability and lumbopelvic pain Intergration of Research and Therapy. PA: Lippincott Williams & Wilkins; 2007. pp. 5–45. [Google Scholar]
- 10.McGonagle D, Benjamin M, Marzo-Ortega H, Emery P. Advances in the understanding of entheseal inflammation. Curr Rheumatol Rep. 2002;4:500–506. doi: 10.1007/s11926-002-0057-2. [DOI] [PubMed] [Google Scholar]
- 11.Singh HJ, Nimarpreet K, Ashima S, Kumar A, Prakash S. Study of bone mineral density in patients with ankylosing spondylitis. J Clin Diagn Res. 2013;7:2832–2835. doi: 10.7860/JCDR/2013/6779.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Weijden MA, Claushuis TA, Nazari T, Lems WF, Dijkmans BA, van der Horst-Bruinsma IE. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol. 2012;31:1529–1535. doi: 10.1007/s10067-012-2018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brophy S, Hickey S, Menon A, Taylor G, Bradbury L, Hamersma J, Calin A. Concordance of disease severity among family members with ankylosing spondylitis? J Rheumatol. 2004;31:1775–1778. [PubMed] [Google Scholar]
- 14.Kaya A, Ozgocmen S, Kamanli A, Ardicoglu O. Bone loss in ankylosing spondylitis: does syndesmophyte formation have an influence on bone density changes? Med Princ Pract. 2009;18:470–476. doi: 10.1159/000235897. [DOI] [PubMed] [Google Scholar]
- 15.Brown MA, Kennedy LG, MacGregor AJ, Darke C, Duncan E, Shatford JL, Taylor A, Calin A, Wordsworth P. Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 1997;40:1823–1828. doi: 10.1002/art.1780401015. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JH, Rose FC. The familial occurrence of ankylosing spondylitis. Br Med J. 1954;2:1139–1140. doi: 10.1136/bmj.2.4897.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Järvinen P. Occurrence of ankylosing spondylitis in a nationwide series of twins. Arthritis Rheum. 1995;38:381–383. doi: 10.1002/art.1780380313. [DOI] [PubMed] [Google Scholar]
- 18.Brown MA, Brophy S, Bradbury L, Hamersma J, Timms A, Laval S, Cardon L, Calin A, Wordsworth BP. Identification of major loci controlling clinical manifestations of ankylosing spondylitis. Arthritis Rheum. 2003;48:2234–2239. doi: 10.1002/art.11106. [DOI] [PubMed] [Google Scholar]
- 19.Sims AM, Barnardo M, Herzberg I, Bradbury L, Calin A, Wordsworth BP, Darke C, Brown MA. Non-B27 MHC associations of ankylosing spondylitis. Genes Immun. 2007;8:115–123. doi: 10.1038/sj.gene.6364362. [DOI] [PubMed] [Google Scholar]
- 20.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 21.Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16:675–690. [PubMed] [Google Scholar]
- 22.Khan MA, Mathieu A, Sorrentino R, Akkoc N. The pathogenetic role of HLA-B27 and its subtypes. Autoimmun Rev. 2007;6:183–189. doi: 10.1016/j.autrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 2012;64:1407–1411. doi: 10.1002/art.33503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts RL, Wallace MC, Jones GT, van Rij AM, Merriman TR, Harrison A, White D, Stamp LK, Ching D, Highton J, et al. Prevalence of HLA-B27 in the New Zealand population: effect of age and ethnicity. Arthritis Res Ther. 2013;15:R158. doi: 10.1186/ar4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Firestein GS, Kelley WN. Kelley’s Textbook of Rheumatology. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013. [Google Scholar]
- 26.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 27.Brown MA. Human leucocyte antigen-B27 and ankylosing spondylitis. Intern Med J. 2007;37:739–740. doi: 10.1111/j.1445-5994.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Jiang L, Cai Q, Danoy P, Barnardo MC, Brown MA, Xu H. Predominant association of HLA-B*2704 with ankylosing spondylitis in Chinese Han patients. Tissue Antigens. 2010;75:61–64. doi: 10.1111/j.1399-0039.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin R, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- 30.Hermann E, Yu DT, Meyer zum Büschenfelde KH, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 31.Scofield RH, Kurien B, Gross T, Warren WL, Harley JB. HLA-B27 binding of peptide from its own sequence and similar peptides from bacteria: implications for spondyloarthropathies. Lancet. 1995;345:1542–1544. doi: 10.1016/s0140-6736(95)91089-1. [DOI] [PubMed] [Google Scholar]
- 32.Frauendorf E, von Goessel H, May E, Märker-Hermann E. HLA-B27-restricted T cells from patients with ankylosing spondylitis recognize peptides from B*2705 that are similar to bacteria-derived peptides. Clin Exp Immunol. 2003;134:351–359. doi: 10.1046/j.1365-2249.2003.02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos M, Alvarez I, Sesma L, Logean A, Rognan D, López de Castro JA. Molecular mimicry of an HLA-B27-derived ligand of arthritis-linked subtypes with chlamydial proteins. J Biol Chem. 2002;277:37573–37581. doi: 10.1074/jbc.M205470200. [DOI] [PubMed] [Google Scholar]
- 34.Mear JP, Schreiber KL, Münz C, Zhu X, Stevanović S, Rammensee HG, Rowland-Jones SL, Colbert RA. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–6670. [PubMed] [Google Scholar]
- 35.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 36.Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 37.Bowness P. HLA B27 in health and disease: a double-edged sword? Rheumatology (Oxford) 2002;41:857–868. doi: 10.1093/rheumatology/41.8.857. [DOI] [PubMed] [Google Scholar]
- 38.Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–5048. [PubMed] [Google Scholar]
- 39.Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–2982. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 40.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–759. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 41.Brown M, Bunce M, Calin A, Darke C, Wordsworth P. HLA-B associations of HLA-B27 negative ankylosing spondylitis: comment on the article by Yamaguchi et al. Arthritis Rheum. 1996;39:1768–1769. doi: 10.1002/art.1780391028. [DOI] [PubMed] [Google Scholar]
- 42.Wei JC, Tsai WC, Lin HS, Tsai CY, Chou CT. HLA-B60 and B61 are strongly associated with ankylosing spondylitis in HLA-B27-negative Taiwan Chinese patients. Rheumatology (Oxford) 2004;43:839–842. doi: 10.1093/rheumatology/keh193. [DOI] [PubMed] [Google Scholar]
- 43.Brown MA, Kennedy LG, Darke C, Gibson K, Pile KD, Shatford JL, Taylor A, Calin A, Wordsworth BP. The effect of HLA-DR genes on susceptibility to and severity of ankylosing spondylitis. Arthritis Rheum. 1998;41:460–465. doi: 10.1002/1529-0131(199803)41:3<460::AID-ART12>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 44.Timms AE, Crane AM, Sims AM, Cordell HJ, Bradbury LA, Abbott A, Coyne MR, Beynon O, Herzberg I, Duff GW, et al. The interleukin 1 gene cluster contains a major susceptibility locus for ankylosing spondylitis. Am J Hum Genet. 2004;75:587–595. doi: 10.1086/424695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Paardt M, Crusius JB, García-González MA, Baudoin P, Kostense PJ, Alizadeh BZ, Dijkmans BA, Peña AS, van der Horst-Bruinsma IE. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms in ankylosing spondylitis. Rheumatology (Oxford) 2002;41:1419–1423. doi: 10.1093/rheumatology/41.12.1419. [DOI] [PubMed] [Google Scholar]
- 46.McGarry F, Neilly J, Anderson N, Sturrock R, Field M. A polymorphism within the interleukin 1 receptor antagonist (IL-1Ra) gene is associated with ankylosing spondylitis. Rheumatology (Oxford) 2001;40:1359–1364. doi: 10.1093/rheumatology/40.12.1359. [DOI] [PubMed] [Google Scholar]
- 47.Chou CT, Timms AE, Wei JC, Tsai WC, Wordsworth BP, Brown MA. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann Rheum Dis. 2006;65:1106–1109. doi: 10.1136/ard.2005.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown MA, Laval SH, Brophy S, Calin A. Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2000;59:883–886. doi: 10.1136/ard.59.11.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beyeler C, Armstrong M, Bird HA, Idle JR, Daly AK. Relationship between genotype for the cytochrome P450 CYP2D6 and susceptibility to ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis. 1996;55:66–68. doi: 10.1136/ard.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, Jin R, Zhou X, Bradbury LA, Appleton LH, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, Oppermann U, Dilthey A, Pirinen M, Stone MA, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, Stone MA, Corr M, Gensler LS, Gladman D, et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 2015;74:1387–1393. doi: 10.1136/annrheumdis-2013-204835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braun J, Sieper J. Early diagnosis of spondyloarthritis. Nat Clin Pract Rheumatol. 2006;2:536–545. doi: 10.1038/ncprheum0296. [DOI] [PubMed] [Google Scholar]
- 55.Bond D. Ankylosing spondylitis: diagnosis and management. Nurs Stand. 2013;28:52–9; quiz 60. doi: 10.7748/ns2013.12.28.16.52.e7807. [DOI] [PubMed] [Google Scholar]
- 56.Shaikh SA. Ankylosing spondylitis: recent breakthroughs in diagnosis and treatment. J Can Chiropr Assoc. 2007;51:249–260. [PMC free article] [PubMed] [Google Scholar]
- 57.Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–66. doi: 10.1007/s00296-002-0237-4. [DOI] [PubMed] [Google Scholar]
- 58.Ai F, Ai T, Li X, Hu D, Zhang W, Morelli JN. Value of diffusion-weighted magnetic resonance imaging in early diagnosis of ankylosing spondylitis. Rheumatol Int. 2012;32:4005–4013. doi: 10.1007/s00296-011-2333-9. [DOI] [PubMed] [Google Scholar]
- 59.Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11. [PubMed] [Google Scholar]
- 60.Weber U, Maksymowych WP. Sensitivity and specificity of magnetic resonance imaging for axial spondyloarthritis. Am J Med Sci. 2011;341:272–277. doi: 10.1097/MAJ.0b013e31820f8c59. [DOI] [PubMed] [Google Scholar]
- 61.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 62.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 63.Moll JM, Wright V. New York clinical criteria for ankylosing spondylitis. A statistical evaluation. Ann Rheum Dis. 1973;32:354–363. doi: 10.1136/ard.32.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goie The HS, Steven MM, van der Linden SM, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a comparison of the Rome, New York and modified New York criteria in patients with a positive clinical history screening test for ankylosing spondylitis. Br J Rheumatol. 1985;24:242–249. doi: 10.1093/rheumatology/24.3.242. [DOI] [PubMed] [Google Scholar]
- 65.Song IH, Sieper J, Rudwaleit M. Diagnosing early ankylosing spondylitis. Curr Rheumatol Rep. 2007;9:367–374. doi: 10.1007/s11926-007-0059-1. [DOI] [PubMed] [Google Scholar]
- 66.Rudwaleit M, van der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–543. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rudwaleit M, Metter A, Listing J, Sieper J, Braun J. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum. 2006;54:569–578. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 68.Mengshoel AM. Life strain-related tiredness and illness-related fatigue in individuals with ankylosing spondylitis. Arthritis Care Res (Hoboken) 2010;62:1272–1277. doi: 10.1002/acr.20216. [DOI] [PubMed] [Google Scholar]
- 69.Sieper J, van der Heijde D, Landewé R, Brandt J, Burgos-Vagas R, Collantes-Estevez E, Dijkmans B, Dougados M, Khan MA, Leirisalo-Repo M, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS) Ann Rheum Dis. 2009;68:784–788. doi: 10.1136/ard.2008.101501. [DOI] [PubMed] [Google Scholar]
- 70.Mold JW, Holtzclaw BJ, McCarthy L. Night sweats: a systematic review of the literature. J Am Board Fam Med. 2012;25:878–893. doi: 10.3122/jabfm.2012.06.120033. [DOI] [PubMed] [Google Scholar]
- 71.Cooksey R, Brophy S, Gravenor MB, Brooks CJ, Burrows CL, Siebert S. Frequency and characteristics of disease flares in ankylosing spondylitis. Rheumatology (Oxford) 2010;49:929–932. doi: 10.1093/rheumatology/kep435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spoorenberg A, van der Heijde D, de Klerk E, Dougados M, de Vlam K, Mielants H, van der Tempel H, van der Linden S. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol. 1999;26:980–984. [PubMed] [Google Scholar]
- 73.Braun J, Sieper J, Bollow M. Imaging of sacroiliitis. Clin Rheumatol. 2000;19:51–57. doi: 10.1007/s100670050011. [DOI] [PubMed] [Google Scholar]
- 74.Karasick D, Schweitzer ME, Abidi NA, Cotler JM. Fractures of the vertebrae with spinal cord injuries in patients with ankylosing spondylitis: imaging findings. AJR Am J Roentgenol. 1995;165:1205–1208. doi: 10.2214/ajr.165.5.7572504. [DOI] [PubMed] [Google Scholar]
- 75.Resnick D. Diagnosis of Bone and Joint Disorders. Philadelphia, PA: WB Saunders; 2002. [Google Scholar]
- 76.Resnick D, Niwayama G, Goergen TG. Comparison of radiographic abnormalities of the sacroiliac joint in degenerative disease and ankylosing spondylitis. AJR Am J Roentgenol. 1977;128:189–196. doi: 10.2214/ajr.128.2.189. [DOI] [PubMed] [Google Scholar]
- 77.Berens DL. Roentgen features of ankylosing spondylitis. Clin Orthop Relat Res. 1971;74:20–33. [PubMed] [Google Scholar]
- 78.Jacobson JA, Girish G, Jiang Y, Resnick D. Radiographic evaluation of arthritis: inflammatory conditions. Radiology. 2008;248:378–389. doi: 10.1148/radiol.2482062110. [DOI] [PubMed] [Google Scholar]
- 79.Vinson EN, Major NM. MR imaging of ankylosing spondylitis. Semin Musculoskelet Radiol. 2003;7:103–113. doi: 10.1055/s-2003-41344. [DOI] [PubMed] [Google Scholar]
- 80.Huerta-Sil G, Casasola-Vargas JC, Londoño JD, Rivas-Ruíz R, Chávez J, Pacheco-Tena C, Cardiel MH, Vargas-Alarcón G, Burgos-Vargas R. Low grade radiographic sacroiliitis as prognostic factor in patients with undifferentiated spondyloarthritis fulfilling diagnostic criteria for ankylosing spondylitis throughout follow up. Ann Rheum Dis. 2006;65:642–646. doi: 10.1136/ard.2005.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geijer M, Sihlbom H, Göthlin JH, Nordborg E. The role of CT in the diagnosis of sacro-iliitis. Acta Radiol. 1998;39:265–268. doi: 10.1080/02841859809172191. [DOI] [PubMed] [Google Scholar]
- 82.Tan S, Yao J, Flynn JA, Yao L, Ward MM. Quantitative syndesmophyte measurement in ankylosing spondylitis using CT: longitudinal validity and sensitivity to change over 2 years. Ann Rheum Dis. 2015;74:437–443. doi: 10.1136/annrheumdis-2013-203946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huwart L, Amoretti N. CT- and fluoroscopy-guided percutaneous screw fixation of a “carrot-stick” spinal fracture in an elderly man with ankylosing spondylitis. Skeletal Radiol. 2013;42:1767–1773. doi: 10.1007/s00256-013-1684-6. [DOI] [PubMed] [Google Scholar]
- 84.Fan Chiang CY, Tsai TT, Chen LH, Lai PL, Fu TS, Niu CC, Chen WJ. Computed tomography-based navigation-assisted pedicle screw insertion for thoracic and lumbar spine fractures. Chang Gung Med J. 2012;35:332–338. doi: 10.4103/2319-4170.106137. [DOI] [PubMed] [Google Scholar]
- 85.Roemer FW, van Holsbeeck M, Genant HK. Musculoskeletal ultrasound in rheumatology: a radiologic perspective. Arthritis Rheum. 2005;53:491–493. doi: 10.1002/art.21318. [DOI] [PubMed] [Google Scholar]
- 86.O’Shea F, Salonen D, Inman R. The challenge of early diagnosis in ankylosing spondylitis. J Rheumatol. 2007;34:5–7. [PubMed] [Google Scholar]
- 87.Klauser A, Halpern EJ, Frauscher F, Gvozdic D, Duftner C, Springer P, Schirmer M. Inflammatory low back pain: high negative predictive value of contrast-enhanced color Doppler ultrasound in the detection of inflamed sacroiliac joints. Arthritis Rheum. 2005;53:440–444. doi: 10.1002/art.21161. [DOI] [PubMed] [Google Scholar]
- 88.Mohammadi A, Ghasemi-rad M, Aghdashi M, Mladkova N, Baradaransafa P. Evaluation of disease activity in ankylosing spondylitis; diagnostic value of color Doppler ultrasonography. Skeletal Radiol. 2013;42:219–224. doi: 10.1007/s00256-012-1412-7. [DOI] [PubMed] [Google Scholar]
- 89.Unlü E, Pamuk ON, Cakir N. Color and duplex Doppler sonography to detect sacroiliitis and spinal inflammation in ankylosing spondylitis. Can this method reveal response to anti-tumor necrosis factor therapy? J Rheumatol. 2007;34:110–116. [PubMed] [Google Scholar]
- 90.Klauser AS, De Zordo T, Bellmann-Weiler R, Feuchtner GM, Sailer-Höck M, Sögner P, Gruber J. Feasibility of second-generation ultrasound contrast media in the detection of active sacroiliitis. Arthritis Rheum. 2009;61:909–916. doi: 10.1002/art.24648. [DOI] [PubMed] [Google Scholar]
- 91.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–533. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 93.Fournié B, Margarit-Coll N, Champetier de Ribes TL, Zabraniecki L, Jouan A, Vincent V, Chiavassa H, Sans N, Railhac JJ. Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power-doppler study versus rheumatoid arthritis. Joint Bone Spine. 2006;73:527–531. doi: 10.1016/j.jbspin.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 94.Kiris A, Kaya A, Ozgocmen S, Kocakoc E. Assessment of enthesitis in ankylosing spondylitis by power Doppler ultrasonography. Skeletal Radiol. 2006;35:522–528. doi: 10.1007/s00256-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 95.Spadaro A, Iagnocco A, Perrotta FM, Modesti M, Scarno A, Valesini G. Clinical and ultrasonography assessment of peripheral enthesitis in ankylosing spondylitis. Rheumatology (Oxford) 2011;50:2080–2086. doi: 10.1093/rheumatology/ker284. [DOI] [PubMed] [Google Scholar]
- 96.Pavy S, Dernis E, Lavie F, Maillefert JF, Mariette X, Schaeverbeke T, Cantagrel A, Claudepierre P, Flipo RM, Goupille P, et al. Imaging for the diagnosis and follow-up of ankylosing spondylitis: development of recommendations for clinical practice based on published evidence and expert opinion. Joint Bone Spine. 2007;74:338–345. doi: 10.1016/j.jbspin.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Gandjbakhch F, Terslev L, Joshua F, Wakefield RJ, Naredo E, D’Agostino MA. Ultrasound in the evaluation of enthesitis: status and perspectives. Arthritis Res Ther. 2011;13:R188. doi: 10.1186/ar3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sari I, Okan T, Akar S, Cece H, Altay C, Secil M, Birlik M, Onen F, Akkoc N. Impaired endothelial function in patients with ankylosing spondylitis. Rheumatology (Oxford) 2006;45:283–286. doi: 10.1093/rheumatology/kei145. [DOI] [PubMed] [Google Scholar]
- 99.Skare TL, Verceze GC, Oliveira AA, Perreto S. Carotid intima-media thickness in spondyloarthritis patients. Sao Paulo Med J. 2013;131:100–105. doi: 10.1590/S1516-31802013000100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mathieu S, Gossec L, Dougados M, Soubrier M. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:557–563. doi: 10.1002/acr.20364. [DOI] [PubMed] [Google Scholar]
- 101.Caliskan M, Erdogan D, Gullu H, Yilmaz S, Gursoy Y, Yildirir A, Yucel E, Muderrisoglu H. Impaired coronary microvascular and left ventricular diastolic functions in patients with ankylosing spondylitis. Atherosclerosis. 2008;196:306–312. doi: 10.1016/j.atherosclerosis.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Yildirir A, Aksoyek S, Calguneri M, Oto A, Kes S. Echocardiographic evidence of cardiac involvement in ankylosing spondylitis. Clin Rheumatol. 2002;21:129–134. doi: 10.1007/s10067-002-8271-x. [DOI] [PubMed] [Google Scholar]
- 103.O’Neill TW, King G, Graham IM, Molony J, Bresnihan B. Echocardiographic abnormalities in ankylosing spondylitis. Ann Rheum Dis. 1992;51:652–654. doi: 10.1136/ard.51.5.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bulkley BH, Roberts WC. Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation. 1973;48:1014–1027. doi: 10.1161/01.cir.48.5.1014. [DOI] [PubMed] [Google Scholar]
- 105.Davidson P, Baggenstoss AH, Slocumb CH, Daugherty GW. Cardiac and aortic lesions in rheumatoid spondylitis. Proc Staff Meet Mayo Clin. 1963;38:427–435. [PubMed] [Google Scholar]
- 106.Roldan CA, Chavez J, Wiest PW, Qualls CR, Crawford MH. Aortic root disease and valve disease associated with ankylosing spondylitis. J Am Coll Cardiol. 1998;32:1397–1404. doi: 10.1016/s0735-1097(98)00393-3. [DOI] [PubMed] [Google Scholar]
- 107.Alves MG, Espirito-Santo J, Queiroz MV, Madeira H, Macieira-Coelho E. Cardiac alterations in ankylosing spondylitis. Angiology. 1988;39:567–571. doi: 10.1177/000331978803900702. [DOI] [PubMed] [Google Scholar]
- 108.Brunner F, Kunz A, Weber U, Kissling R. Ankylosing spondylitis and heart abnormalities: do cardiac conduction disorders, valve regurgitation and diastolic dysfunction occur more often in male patients with diagnosed ankylosing spondylitis for over 15 years than in the normal population? Clin Rheumatol. 2006;25:24–29. doi: 10.1007/s10067-005-1117-6. [DOI] [PubMed] [Google Scholar]
- 109.Thomas D, Hill W, Geddes R, Sheppard M, Arnold J, Fritzsche J, Brooks PM. Early detection of aortic dilatation in ankylosing spondylitis using echocardiography. Aust N Z J Med. 1982;12:10–13. doi: 10.1111/j.1445-5994.1982.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 110.Tucker CR, Fowles RE, Calin A, Popp RL. Aortitis in ankylosing spondylitis: early detection of aortic root abnormalities with two dimensional echocardiography. Am J Cardiol. 1982;49:680–686. doi: 10.1016/0002-9149(82)91946-4. [DOI] [PubMed] [Google Scholar]
- 111.Dik VK, Peters MJ, Dijkmans PA, Van der Weijden MA, De Vries MK, Dijkmans BA, Van der Horst-Bruinsma IE, Nurmohamed MT. The relationship between disease-related characteristics and conduction disturbances in ankylosing spondylitis. Scand J Rheumatol. 2010;39:38–41. doi: 10.3109/03009740903096101. [DOI] [PubMed] [Google Scholar]
- 112.Yildirir A, Aksoyek S, Calguneri M, Aytemir K, Kabakci G, Ovunc K, Nazli N, Ozmen F, Oto A, Kes S. QT dispersion as a predictor of arrhythmic events in patients with ankylosing spondylitis. Rheumatology (Oxford) 2000;39:875–879. doi: 10.1093/rheumatology/39.8.875. [DOI] [PubMed] [Google Scholar]
- 113.Townend JN, Emery P, Davies MK, Littler WA. Acute aortitis and aortic incompetence due to systemic rheumatological disorders. Int J Cardiol. 1991;33:253–258. doi: 10.1016/0167-5273(91)90355-s. [DOI] [PubMed] [Google Scholar]
- 114.Momeni M, Taylor N, Tehrani M. Cardiopulmonary manifestations of ankylosing spondylitis. Int J Rheumatol. 2011;2011:728471. doi: 10.1155/2011/728471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moyssakis I, Gialafos E, Vassiliou VA, Boki K, Votteas V, Sfikakis PP, Tzelepis GE. Myocardial performance and aortic elasticity are impaired in patients with ankylosing spondylitis. Scand J Rheumatol. 2009;38:216–221. doi: 10.1080/03009740802474672. [DOI] [PubMed] [Google Scholar]
- 116.Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34:585–592. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Han C, Robinson DW, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. [PubMed] [Google Scholar]
- 118.Aydin SZ, Karadag O, Filippucci E, Atagunduz P, Akdogan A, Kalyoncu U, Grassi W, Direskeneli H. Monitoring Achilles enthesitis in ankylosing spondylitis during TNF-alpha antagonist therapy: an ultrasound study. Rheumatology (Oxford) 2010;49:578–582. doi: 10.1093/rheumatology/kep410. [DOI] [PubMed] [Google Scholar]
- 119.Ozgocmen S, Kiris A, Ardicoglu O, Kocakoc E, Kaya A. Glucocorticoid iontophoresis for Achilles tendon enthesitis in ankylosing spondylitis: significant response documented by power Doppler ultrasound. Rheumatol Int. 2005;25:158–160. doi: 10.1007/s00296-004-0488-3. [DOI] [PubMed] [Google Scholar]
- 120.Stone M, Salonen D, Lax M, Payne U, Lapp V, Inman R. Clinical and imaging correlates of response to treatment with infliximab in patients with ankylosing spondylitis. J Rheumatol. 2001;28:1605–1614. [PubMed] [Google Scholar]
- 121.Chary-Valckenaere I, d’Agostino MA, Loeuille D. Role for imaging studies in ankylosing spondylitis. Joint Bone Spine. 2011;78:138–143. doi: 10.1016/j.jbspin.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 122.Swen WA, Jacobs JW, Neve WC, Bal D, Bijlsma JW. Is sonography performed by the rheumatologist as useful as arthrography executed by the radiologist for the assessment of full thickness rotator cuff tears? J Rheumatol. 1998;25:1800–1806. [PubMed] [Google Scholar]
- 123.Wakefield RJ, Brown AK, O’Connor PJ, Karim Z, Grainger A, Emery P. Musculoskeletal ultrasonography: what is it and should training be compulsory for rheumatologists? Rheumatology (Oxford) 2004;43:821–822. doi: 10.1093/rheumatology/keh227. [DOI] [PubMed] [Google Scholar]
- 124.Larché MJ, McDonald-Blumer H, Bruns A, Roth J, Khy V, de Brum-Fernandes AJ, Wakefield RJ, Brown AK, Bykerk V. Utility and feasibility of musculoskeletal ultrasonography (MSK US) in rheumatology practice in Canada: needs assessment. Clin Rheumatol. 2011;30:1277–1283. doi: 10.1007/s10067-011-1743-0. [DOI] [PubMed] [Google Scholar]
- 125.Samuels J, Abramson SB, Kaeley GS. The use of musculoskeletal ultrasound by rheumatologists in the United States. Bull NYU Hosp Jt Dis. 2010;68:292–298. [PubMed] [Google Scholar]
- 126.Kane D, Grassi W, Sturrock R, Balint PV. Musculoskeletal ultrasound--a state of the art review in rheumatology. Part 2: Clinical indications for musculoskeletal ultrasound in rheumatology. Rheumatology (Oxford) 2004;43:829–838. doi: 10.1093/rheumatology/keh215. [DOI] [PubMed] [Google Scholar]
- 127.Wakefield RJ, Goh E, Conaghan PG, Karim Z, Emery P. Musculoskeletal ultrasonography in Europe: results of a rheumatologist-based survey at a EULAR meeting. Rheumatology (Oxford) 2003;42:1251–1253. doi: 10.1093/rheumatology/keg367. [DOI] [PubMed] [Google Scholar]
- 128.Tins BJ, Butler R. Imaging in rheumatology: reconciling radiology and rheumatology. Insights Imaging. 2013;4:799–810. doi: 10.1007/s13244-013-0293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ostergaard M, Poggenborg RP, Axelsen MB, Pedersen SJ. Magnetic resonance imaging in spondyloarthritis--how to quantify findings and measure response. Best Pract Res Clin Rheumatol. 2010;24:637–657. doi: 10.1016/j.berh.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Konca S, Keskin D, Cılız D, Bodur H, Sakman B. Spinal inflammation by magnetic resonance imaging in patients with ankylosing spondylitis: association with disease activity and outcome parameters. Rheumatol Int. 2012;32:3765–3770. doi: 10.1007/s00296-011-2248-5. [DOI] [PubMed] [Google Scholar]
- 131.De Keyser F, Van den Bosch F, Mielants H. Anti-TNF-alpha therapy in ankylosing spondylitis. Cytokine. 2006;33:294–298. doi: 10.1016/j.cyto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Sengupta R, Stone MA. The assessment of ankylosing spondylitis in clinical practice. Nat Clin Pract Rheumatol. 2007;3:496–503. doi: 10.1038/ncprheum0591. [DOI] [PubMed] [Google Scholar]
- 133.Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210. doi: 10.1159/000289205. [DOI] [PubMed] [Google Scholar]
- 134.Baji P, Péntek M, Szántó S, Géher P, Gulácsi L, Balogh O, Brodszky V. Comparative efficacy and safety of biosimilar infliximab and other biological treatments in ankylosing spondylitis: systematic literature review and meta-analysis. Eur J Health Econ. 2014;15 Suppl 1:S45–S52. doi: 10.1007/s10198-014-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wanders A, Heijde Dv, Landewé R, Béhier JM, Calin A, Olivieri I, Zeidler H, Dougados M. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756–1765. doi: 10.1002/art.21054. [DOI] [PubMed] [Google Scholar]
- 136.Dougados M, Béhier JM, Jolchine I, Calin A, van der Heijde D, Olivieri I, Zeidler H, Herman H. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44:180–185. doi: 10.1002/1529-0131(200101)44:1<180::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 137.Kristensen LE, Jakobsen AK, Askling J, Nilsson F, Jacobsson LT. Safety of Etoricoxib, Celecoxib, and Nonselective Nonsteroidal Antiinflammatory Drugs in Ankylosing Spondylitis and Other Spondyloarthritis Patients: A Swedish National Population-Based Cohort Study. Arthritis Care Res (Hoboken) 2015;67:1137–1149. doi: 10.1002/acr.22555. [DOI] [PubMed] [Google Scholar]
- 138.Giannotti E, Trainito S, Arioli G, Rucco V, Masiero S. Effects of physical therapy for the management of patients with ankylosing spondylitis in the biological era. Clin Rheumatol. 2014;33:1217–1230. doi: 10.1007/s10067-014-2647-6. [DOI] [PubMed] [Google Scholar]
- 139.Niedermann K, Sidelnikov E, Muggli C, Dagfinrud H, Hermann M, Tamborrini G, Ciurea A, Bischoff-Ferrari H. Effect of cardiovascular training on fitness and perceived disease activity in people with ankylosing spondylitis. Arthritis Care Res (Hoboken) 2013;65:1844–1852. doi: 10.1002/acr.22062. [DOI] [PubMed] [Google Scholar]
- 140.Dundar U, Solak O, Toktas H, Demirdal US, Subasi V, Kavuncu V, Evcik D. Effect of aquatic exercise on ankylosing spondylitis: a randomized controlled trial. Rheumatol Int. 2014;34:1505–1511. doi: 10.1007/s00296-014-2980-8. [DOI] [PubMed] [Google Scholar]
- 141.Aydemir K, Tok F, Peker F, Safaz I, Taskaynatan MA, Ozgul A. The effects of balneotherapy on disease activity, functional status, pulmonary function and quality of life in patients with ankylosing spondylitis. Acta Reumatol Port. 2010;35:441–446. [PubMed] [Google Scholar]
- 142.Masiero S, Bonaldo L, Pigatto M, Lo Nigro A, Ramonda R, Punzi L. Rehabilitation treatment in patients with ankylosing spondylitis stabilized with tumor necrosis factor inhibitor therapy: a randomized controlled trial. J Rheumatol. 2011;38:1335–1342. doi: 10.3899/jrheum.100987. [DOI] [PubMed] [Google Scholar]
- 143.Gyurcsik Z, Bodnár N, Szekanecz Z, Szántó S. Treatment of ankylosing spondylitis with biologics and targeted physical therapy: positive effect on chest pain, diminished chest mobility, and respiratory function. Z Rheumatol. 2013;72:997–1004. doi: 10.1007/s00393-013-1240-8. [DOI] [PubMed] [Google Scholar]
- 144.Brophy S, Davies H, Dennis MS, Cooksey R, Husain MJ, Irvine E, Siebert S. Fatigue in ankylosing spondylitis: treatment should focus on pain management. Semin Arthritis Rheum. 2013;42:361–367. doi: 10.1016/j.semarthrit.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Stockdale J, Selfe J, Roddam H. An exploration of the impact of anti-TNFα medication on exercise behaviour in patients with ankylosing spondylitis. Musculoskeletal Care. 2014;12:150–159. doi: 10.1002/msc.1068. [DOI] [PubMed] [Google Scholar]
- 146.Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6–9. [PubMed] [Google Scholar]
- 147.Van Royen BJ, De Gast A. Lumbar osteotomy for correction of thoracolumbar kyphotic deformity in ankylosing spondylitis. A structured review of three methods of treatment. Ann Rheum Dis. 1999;58:399–406. doi: 10.1136/ard.58.7.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Simmons E. Relation of vascular complication to the level of lumbar extension osteotomy in ankylosing spondylitis. 61st Annual Meeting of the American Academy of Orthopedic Surgeons. New Orleans. Philadelphia, PA: WB Saunders; 1994. [Google Scholar]
- 149.Scudese VA, Calabro JJ. Vertebral wedge osteotomy. correction of rheumatoid (ankylosing) spondylitis. JAMA. 1963;186:627–631. doi: 10.1001/jama.1963.03710070029006. [DOI] [PubMed] [Google Scholar]
- 150.Arun R, Dabke HV, Mehdian H. Comparison of three types of lumbar osteotomy for ankylosing spondylitis: a case series and evolution of a safe technique for instrumented reduction. Eur Spine J. 2011;20:2252–2260. doi: 10.1007/s00586-011-1894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu ML, Tsai TT, Lai PL, Fu TS, Niu CC, Chen LH, Chen WJ. A retrospective study of treating thoracolumbar spine fractures in ankylosing spondylitis. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S117–S123. doi: 10.1007/s00590-013-1375-y. [DOI] [PubMed] [Google Scholar]


