Abstract
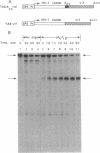
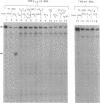
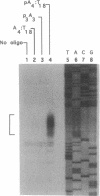
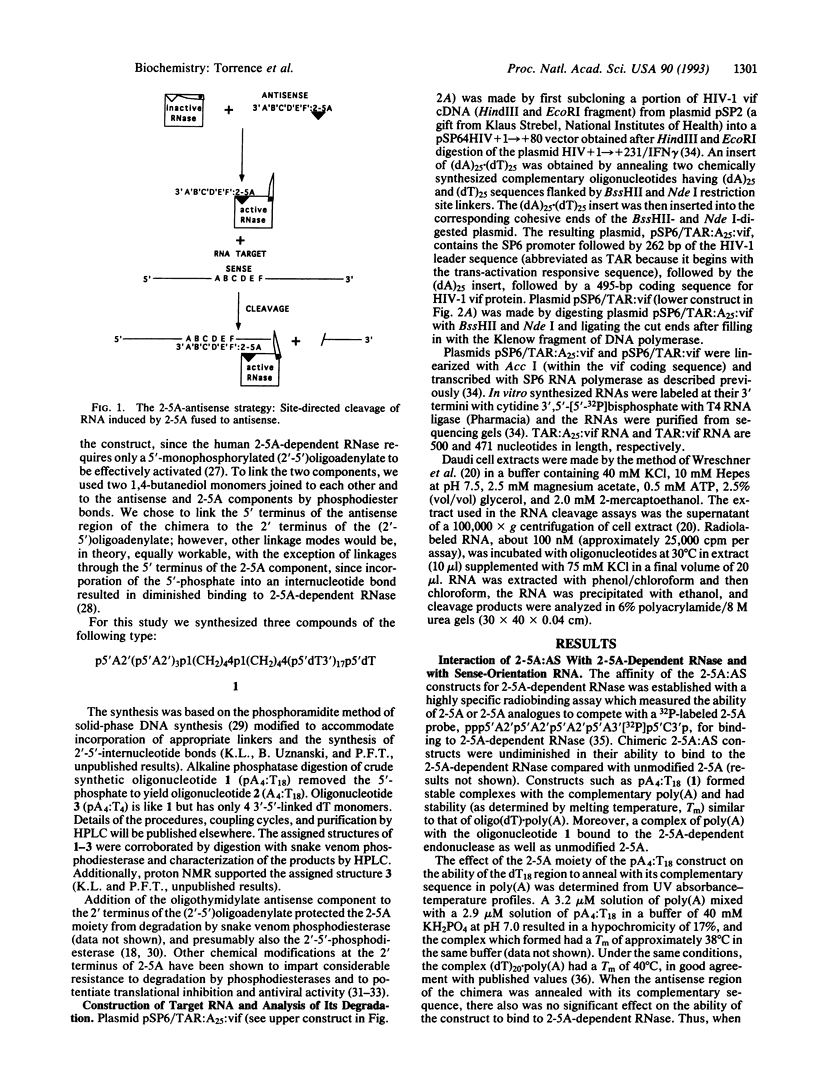
Antisense oligonucleotides hold considerable promise both as research tools for inhibiting gene expression and as agents for the treatment of a myriad of human diseases. However, targeted destruction of RNA has been difficult to achieve in a versatile, efficient, and reliable manner. We have developed an effective strategy for cleaving unique RNA sequences with 2-5A-dependent RNase, an endoribonuclease that mediates inhibitory effects of interferon on virus infection and is activated by 5'-phosphorylated 2'-5'-linked oligoadenylates known as 2-5A [pn5' A2'(p5' A2')mp5'A], resulting in the cleavage of single-stranded RNA predominantly after UpUp and UpAp sequences. To direct 2-5A-dependent RNase to cleave unique RNA sequences, p5' A2' p5' A2'p5'A was covalently linked to an antisense oligonucleotide to yield a chimeric molecule (2-5A:AS). The antisense oligonucleotide component of 2-5A:AS bound a specific RNA sequence while the accompanying 2-5A component activated 2-5A-dependent RNase, thereby causing the cleavage of the RNA in the targeted sequence. This strategy was demonstrated by inducing specific cleavage within a modified human immunodeficiency virus type 1 vif mRNA in a cell-free system from human lymphoblastoid cells. Because 2-5A-dependent RNase is present in most mammalian cells, the control of gene expression based on this technology--including therapies for cancer, viral infections, and certain genetic diseases--can be envisioned.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cayley P. J., White R. F., Antoniw J. F., Walesby N. J., Kerr I. M. Distribution of the ppp(A2'p)nA-binding protein and interferon-related enzymes in animals, plants, and lower organisms. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1243–1250. doi: 10.1016/0006-291x(82)92133-7. [DOI] [PubMed] [Google Scholar]
- Chebath J., Benech P., Revel M., Vigneron M. Constitutive expression of (2'-5') oligo A synthetase confers resistance to picornavirus infection. Nature. 1987 Dec 10;330(6148):587–588. doi: 10.1038/330587a0. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper: sequence-specific targeting. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7147–7151. doi: 10.1073/pnas.83.19.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P., Huez G., Verhaegen-Lawalle M., De Clercq E., Imai J., Torrence P., Content J. Antiviral activity of a chemically stabilized 2-5A analog upon microinjection into HeLa cells. FEBS Lett. 1986 Mar 31;198(2):326–332. doi: 10.1016/0014-5793(86)80430-6. [DOI] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Wynne J. K., Wallis S. C., Scott J. An in vitro system for the editing of apolipoprotein B mRNA. Cell. 1989 Aug 11;58(3):519–525. doi: 10.1016/0092-8674(89)90432-7. [DOI] [PubMed] [Google Scholar]
- Etienne-Smekens M., Vandenbussche P., Content J., Dumont J. E. (2'-5')Oligoadenylate in rat liver: modulation after partial hepatectomy. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4609–4613. doi: 10.1073/pnas.80.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd-Smith G., Slattery E., Lengyel P. Interferon action: RNA cleavage pattern of a (2'-5')oligoadenylate--dependent endonuclease. Science. 1981 May 29;212(4498):1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh M. C., Cayley P. J., Serafinowska H. T., Norman D. G., Reese C. B., Kerr I. M. Analogues and analogue inhibitors of ppp(A2'p)nA. Their stability and biological activity. Eur J Biochem. 1983 Apr 15;132(1):77–84. doi: 10.1111/j.1432-1033.1983.tb07327.x. [DOI] [PubMed] [Google Scholar]
- Imai J., Johnston M. I., Torrence P. F. Chemical modification potentiates the biological activities of 2-5A and its congeners. J Biol Chem. 1982 Nov 10;257(21):12739–12745. [PubMed] [Google Scholar]
- Imai J., Lesiak K., Torrence P. F. Respective role of each of the purine N-6 amino groups of 5'-O-triphosphoryladenylyl(2'----5')adenylyl(2----5')adenosine in binding to and activation of RNase L. J Biol Chem. 1985 Feb 10;260(3):1390–1393. [PubMed] [Google Scholar]
- Johnston M. I., Hearl W. G. Purification and characterization of a 2'-phosphodiesterase from bovine spleen. J Biol Chem. 1987 Jun 15;262(17):8377–8382. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D., Silverman R. H., Jacobsen H., Leisy S. A., Dieffenbach C. W., Friedman R. M. Regulation of ppp(A2'p)nA-dependent RNase levels during interferon treatment and cell differentiation. Eur J Biochem. 1985 Feb 1;146(3):611–618. doi: 10.1111/j.1432-1033.1985.tb08695.x. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Helene C., Chassignol M., Thuong N. T. Targeted cleavage of polynucleotides by complementary oligonucleotides covalently linked to iron-porphyrins. Biochemistry. 1986 Nov 4;25(22):6736–6739. doi: 10.1021/bi00370a002. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Praseuth D., Perrouault L., Chassignol M., Thuong N. T., Hélène C. Sequence-targeted photochemical modifications of nucleic acids by complementary oligonucleotides covalently linked to porphyrins. Bioconjug Chem. 1990 Mar-Apr;1(2):108–113. doi: 10.1021/bc00002a004. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Blake K. R., Miller P. S. Interaction of psoralen-derivatized oligodeoxyribonucleoside methylphosphonates with synthetic DNA containing a promoter for T7 RNA polymerase. Nucleic Acids Res. 1988 Nov 25;16(22):10681–10697. doi: 10.1093/nar/16.22.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guerrier-Takada C., Altman S. Targeted cleavage of mRNA in vitro by RNase P from Escherichia coli. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3185–3189. doi: 10.1073/pnas.89.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J. J., Cantin E. M., Sarver N., Chang P. F. The potential use of catalytic RNAs in therapy of HIV infection and other diseases. Pharmacol Ther. 1991;50(2):245–254. doi: 10.1016/0163-7258(91)90016-f. [DOI] [PubMed] [Google Scholar]
- SenGupta D. N., Berkhout B., Gatignol A., Zhou A. M., Silverman R. H. Direct evidence for translational regulation by leader RNA and Tat protein of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7492–7496. doi: 10.1073/pnas.87.19.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T. Y. Nucleosides and nucleotides as potential therapeutic agents. Angew Chem Int Ed Engl. 1970 Sep;9(9):678–688. doi: 10.1002/anie.197006781. [DOI] [PubMed] [Google Scholar]
- Silverman R. H. Functional analysis of 2-5A-dependent RNase and 2-5a using 2',5'-oligoadenylate-cellulose. Anal Biochem. 1985 Feb 1;144(2):450–460. doi: 10.1016/0003-2697(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Silverman R. H., Wreschner D. H., Gilbert C. S., Kerr I. M. Synthesis, characterization and properties of ppp(A2'p)nApCp and related high-specific-activity 32P-labelled derivatives of ppp(A2'p)nA. Eur J Biochem. 1981 Mar 16;115(1):79–85. doi: 10.1111/j.1432-1033.1981.tb06200.x. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Imai J., Lesiak K., Jamoulle J. C., Sawai H. Oligonucleotide structural parameters that influence binding of 5'-O-triphosphoadenylyl-(2'----5')-adenylyl-(2'----5')-adenosine to the 5'-O-triphosphoadenylyl-(2'----5')-adenylyl-(2'----5')-adenosine dependent endoribonuclease: chain length, phosphorylation state, and heterocyclic base. J Med Chem. 1984 Jun;27(6):726–733. doi: 10.1021/jm00372a004. [DOI] [PubMed] [Google Scholar]
- Toulmé J. J., Hélène C. Antimessenger oligodeoxyribonucleotides: an alternative to antisense RNA for artificial regulation of gene expression--a review. Gene. 1988 Dec 10;72(1-2):51–58. doi: 10.1016/0378-1119(88)90127-8. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M. Inhibition of protein synthesis by 2'-5' linked adenine oligonucleotides in intact cells. Nature. 1978 Nov 2;276(5683):88–90. doi: 10.1038/276088a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., McCauley J. W., Skehel J. J., Kerr I. M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981 Jan 29;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]