Abstract
Muscarinic cholinergic neurotransmission in the amygdala is critical for memory consolidation in emotional/motivational learning tasks, but little is known about the neuronal distribution of different receptor subtypes. Immunohistochemistry was used in the present investigation to localize the m2 receptor (M2R). Differential patterns of M2R-immunoreactivity (m2R-ir) were observed in the somata and neuropil of the various amygdalar nuclei. Neuropilar m2R-ir was strongest in rostral portions of the basolateral nuclear complex (BLC). M2R+ somata were seen in low numbers in all nuclei of the amygdala. Most M2R+ neurons associated with the BLC were in the lateral nucleus and external capsule. These cells were nonpyramidal neurons that contained glutamatic acid decarboxylase (GAD), somatostatin (SOM) and neuropeptide Y (NPY), but not parvalbumin (PV), calretinin (CR), or cholecystokinin (CCK). Little or no M2R-ir was observed in GAD+, PV+, CR+, or CCK+ axons in the BLC, but was seen in some SOM+ axons and many NPY+ axons. M2R-ir was found in a small number of spiny and aspiny neurons of the central nucleus that were mainly located along the lateral and ventral borders of its lateral subdivision. Many of these cells contained SOM and NPY. M2R+ neurons were also seen in the medial nucleus, including a distinct subpopulation of neurons that surrounded its anteroventral subdivision. The latter neurons were negative for all neuronal markers analyzed. The intercalated nuclei (IN) were associated with two types of large M2R+ neurons, spiny and aspiny. The small principal neurons of the INs were M2R-negative. The somata and dendrites of the large spiny neurons, which were actually found in a zone located just outside of the rostral INs, expressed SOM and NPY, but not GAD. These findings indicate that acetylcholine can modulate a variety of discrete neuronal subpopulations in various amygdalar nuclei via M2Rs, especially neurons that express SOM and NPY.
Keywords: acetylcholine, interneurons, somatostatin, neuropeptide Y, GABA, intercalated nuclei
1. INTRODUCTION
The amygdala is one of the most important brain regions for the generation of emotional behavior and the formation of emotional memories, particularly those related to fear and anxiety (Sah et al., 2003; Pape and Paré, 2010). Several neuromodulators, including acetylcholine, are critical for mnemonic functions performed by the amygdala (McGaugh, 2004). Thus, posttraining infusions of muscarinic cholinergic antagonists into the basolateral nuclear complex of the amygdala (BLC), or lesions of the portions of the basal forebrain cholinergic system projecting to the amygdala, produce impairments in several types of emotional/motivational learning including inhibitory avoidance, contextual fear conditioning, food reward magnitude learning, conditioned place preference, and drug-stimulus learning (Power et al., 2003a). Investigations in the rat have shown that the levels of choline acetyltransferase (ChAT; the synthetic enzyme for acetylcholine) and acetylcholinesterase (the catabolic enzyme for acetylcholine) in the BLC were among the highest in the brain (Ben-Ari et al., 1977). Subsequent studies obtained similar findings in all other mammalian species investigated including human and non-human primates (Girgis, 1980; Svendsen and Bird, 1985; Hellendall et al., 1986; Amaral and Bassett, 1989). Studies combining ChAT immunohistochemistry with retrograde tract tracing demonstrated that the cholinergic basal forebrain, especially the Ch4 group in the substantia innominata, was the main source of these cholinergic inputs to the amygdala in both rodents (Mesulam et al., 1983a; Woolf et al., 1984, Carlsen et al., 1985; Zaborsky et al., 1986; Rao et al., 1987) and primates (Mesulam et al., 1983b; Koliatsos et al., 1988; Kordower et al., 1989).
Although cholinergic inputs to the amygdala are associated with both nicotinic and muscarinic receptors, most studies of memory consolidation in the amygdala utilized muscarinic antagonists (Power et al., 2003a). Pharmacological studies have found at least 4 muscarinic receptor subtypes (designated by upper case letters as M1-M4), whereas molecular biological techniques have identified 5 distinct subtypes (designated by lower case letters as m1-m5) (Ehlert et al., 1995). These muscarinic receptor subtypes exhibit a differential distribution in the brain and are characterized by specific signal transduction mechanisms (Richelson, 1995). Receptor binding autoradiographic studies in rodents and primates, including humans, have demonstrated that the amygdala contains both of the two major pharmacologically-defined receptor subtypes, M1 and M2 (Cortés and Palacios, 1986; Cortés et al., 1986; Spencer et al., 1986; Mash and Potter, 1986; Mash et al., 1988), as well as putative M3 and/or M4 receptors (Smith et al., 1991).
Memory consolidation requires activation of both M1 and M2 receptors in the amygdala (Power et al., 2003b). Although knowledge of the cellular and subcellular localization of these receptors in the amygdala is critical for understanding the actions of acetylcholine involved in consolidation of memory, previous receptor binding autoradiographic studies and film-based in situ hybridization studies lacked the resolution necessary to identify which neurons in the BLC express different muscarinic receptor subtypes. Likewise, electrophysiological investigations of neuronal responses to muscarinic drugs in the amygdala have been hampered by the lack of receptor subtype specific agonists and antagonists (Ehlert et al., 1995). However, the development of antibodies to specific muscarinic receptor subtypes has permitted immunohistochemical localization of these receptor proteins at the light and electron microscopic levels (Levey et al., 1991; Mrzljak et al., 1993). An immunohistochemical study of the rat forebrain revealed that both m1 and m2 muscarinic receptor subtypes are expressed in the in the amygdala, but no details of the nuclear or neuronal localization of these receptors was provided (Levey et al., 1991). A recent study examined the localization of the m1 receptor in the amygdala (McDonald and Mascagni, 2010). In the present investigation single and dual labeling immunohistochemistry was used to study the neuronal localization of the m2 receptor in the rat amygdala.
2. EXPERIMENTAL PROCEDURES
2.1 Tissue Preparation
A total of 16 adult male Sprague-Dawley rats (250–350g; Harlan, Indianapolis, IN) were used in this study. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of South Carolina. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Rats were anesthetized with chloral hydrate (350 mg/kg) and perfused intracardially with phosphate buffered saline (PBS; pH 7.4) containing 0.5% sodium nitrite (50 ml) followed by 3.0% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 (500 ml). Some rats received bilateral injections of colchicine (100 μg total; Sigma Chemical Co., St. Louis, MO) into the lateral cerebral ventricles 1 day before perfusion (see below). Following perfusion, brains were removed and postfixed for 3 hours in 3.0% paraformaldehyde. Brains were then sectioned on a vibratome at a thickness of 50 μm in the coronal plane. Sections were processed for immunohistochemistry in wells of tissue culture plates. All antibodies were diluted in 0.1M PBS containing 0.3% Triton X-100 1% normal goat serum, and 1% bovine serum albumin.
2.2 Immunoperoxidase experiments
Localization of the m2 muscarinic cholinergic receptor (M2R) was performed in 6 rats using the avidin-biotin immunoperoxidase (ABC) technique. Sections were incubated in a rabbit antibody to m2 (1:1000; catalog # M-9558; Sigma Chemical Co., St. Louis, MO) for 48 hrs at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a Vectastain rabbit ABC kit (Vector Laboratories, Burlingame, CA). Nickel-enhanced DAB (3, 3′-diaminobenzidine-4HCl, Sigma) was used as a chromogen to generate a black reaction product (Hancock, 1986). Although the abbreviation “M2R” will be used to refer to this receptor in this study, the antibody was actually raised against the “m2” receptor (lower case m) identified using molecular biological techniques (see Introduction).
Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in ethanols, cleared in xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). Sections from one brain were counterstained with pyronin Y (a pink Nissl stain) prior to coverslipping. Sections from another brain were counterstained with pyronin Y after coverslips were removed by incubating the slides in xylene; this brain was used to plot the locations of M2R-positive (M2R+) perikarya in the amygdala before the coverslips were removed. Plotting before counterstaining ensured that lightly labeled perikarya could be observed at the low magnification (100X) used for plotting, and not be obscured by the counterstain. Subsequent counterstaining of the sections from this brain allowed the borders of amygdalar nuclei to be visualized and drawn on the plots. Because of the low density of M2R+ neurons in the amygdala, immunoreactive perikarya were plotted from three adjacent sections at each of 5 anteroposterior levels of the amygdala to depict the spatial distribution of M2R+ neurons. Blood vessels were also plotted and used as fiducial landmarks to align the three adjacent sections. Neurons at the upper and lower surfaces of the middle section (that were cut during sectioning) might also have been plotted in the first and third sections, respectively, of the series. To ensure that this double-plotting did not occur, perikarya at the interface of adjacent sections were carefully examined, and when perikarya corresponding in size and shape were also observed at the same location in the adjacent section, these neurons were erased from the plots of the latter. Sections were analyzed using an Olympus BX51 microscope, and digital light micrographs were taken with an Olympus DP2-BSW camera system. Brightness and contrast were adjusted using Photoshop 6.0 software.
2.3 Dual-labeling immunofluorescence experiments
The results of the immunoperoxidase studies revealed that many of the M2R+ neurons in the amygdala were nonpyramidal interneurons of the basolateral nuclear complex (BLC). Previous studies have shown that most nonpyramidal neurons are spine-sparse interneurons that utilize GABA as an inhibitory neurotransmitter. Dual-labeling immunohistochemical studies suggest that the BLC contains at least four distinct subpopulations of GABAergic interneurons that can be distinguished on the basis of their content of calcium-binding proteins and peptides. These subpopulations are: (1) parvalbumin+/calbindin+ neurons, (2) somatostatin+/calbindin+ neurons, (3) large multipolar cholecystokinin+ neurons that are often calbindin+, and (4) small bipolar and bitufted interneurons that exhibit extensive colocalization of calretinin, cholecystokinin, and vasoactive intestinal peptide (Kemppainen and Pitkänen, 2000; McDonald and Betette, 2001; McDonald and Mascagni, 2001, 2002, Mascagni and McDonald, 2003; Mascagni et al., 2009). In addition, it has been demonstrated that the expression of neuropeptide Y defines a distinct subpopulation of somatostatin-positive neurons in the BLC (McDonald, 1989; McDonald et al., 1995). Dual-localization of M2R with neuronal markers for BLC GABAergic neurons (glutamic acid decarboxylase [GAD], parvalbumin [PV], somatostatin [SOM], calretinin [CR], neuropeptide Y [NPY], or cholecystokinin [CCK]) was investigated in 10 rats to determine if M2R was expressed by specific interneuronal subpopulations. Seven of these 10 rats received colchicine injections one day before perfusion to enhance perikaryal staining.
Sections were incubated for 48 hrs at 4° C in a cocktail of two primary antibodies, an anti-M2R antibody and one of several neuronal marker antibodies: mouse anti-GAD67 (1:300; Sigma), mouse anti-PV (1:5000, Swant, Bellinzona, Switzerland), mouse anti-SOM (1:3000; SOMA-8 antibody donated by Alison Buchan, University of British Columbia), mouse anti-CR (1:1500, Millipore, Billerica, MA), mouse anti-CCK (1:500, antibody 93903 donated by Dr. John H. Walsh, UCLA), or rabbit anti-NPY (1:3000; Peninsula Laboratories, San Carlos, CA). In these cocktails the same rabbit M2R antibody (1:300) used in the immunoperoxidase experiments was paired with mouse neuronal marker antibodies (GAD, PV, SOM, CR, CCK) whereas a rat anti-M2R antibody (1:300; catalog # mAB367, Millipore) was paired with the rabbit NPY antibody. All antibodies were diluted in 0.1M PBS containing 0.3% Triton X-100, 1% normal goat serum, and 1% bovine serum albumin.
After incubation in the primary antibody cocktails, sections were rinsed in 3 changes of PBS (10 min each), and then incubated in a cocktail of species-appropriate Alexa-488 antibodies (to visualize M2R immunoreactivity) and Alexa-546 antibodies (to visualize marker immunoreactivity) raised in goat (1:300; Invitrogen, Eugene, OR) for 3 hrs at room temperature. These secondary antibodies were highly cross-adsorbed by the manufacturer to ensure specificity for primary antibodies raised in each species. Sections were then rinsed in 3 changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories). Sections were examined with a Zeiss LSM 510 Meta confocal microscope. Fluorescence of Alexa-488 (green) and Alexa-546 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm and 543 nm channels. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software. In each of the immunofluorescence cases some control sections were processed with one of the two primary antibodies omitted from the antibody cocktail. In all cases only the labeling with the secondary fluorescent antibody corresponding to the non-omitted antibody was observed, and only on the appropriate channel. These results indicated that the secondary antibodies were specific for IgGs and that there was no “crosstalk” between the red and green channels (Wouterlood et al., 1998).
Cell counts of M2R-marker colocalization in neuronal perikarya of the amygdala were performed in 2–3 brains for each marker. Since the extent of colocalization of each marker in each nucleus was very similar among brains, cell counts were pooled. In each brain, a total of 9–12 sections (18–24 amygdalae) were analyzed at 200X or 400X magnifications. Because the lateral nucleus and external capsule extend for much of the length of the amygdala, cell counts in these structures were performed in most of the sections in each brain. Cell counts in smaller structures, such as the central, medial, and intercalated nuclei, used fewer sections (3–4 sections [6–8 amygdalae] per brain). Because of the low density of M2R+ neurons in the amygdala, these counting procedures yielded a total of 20–45 M2R+ neurons in each nucleus that could be examined for colocalization with each marker. For all but the M2R-NPY preparations, counts were made of single-labeled M2R+ neurons and double-labeled M2R+/marker+ neurons. In the M2R-NPY preparations, counts were made of single-labeled M2R+ neurons, single-labeled NPY+ neurons, and double-labeled M2R+/NPY+ neurons. Additional qualitative analyses of M2R expression in axons of marker-positive neurons were performed in the anterior subdivision of the basolateral nucleus (BLa) and ventromedial subdivision of the lateral nucleus (Lvm) in three non-colchicine-injected brains.
2.4 Antibody Specificity
The rabbit polyclonal M2R primary antiserum (catalog # M-9558; Sigma Chemical Co., St. Louis, MO) was raised in rabbit to a highly purified glutathione S-transferase (GST) fusion protein of a part of the third intracellular loop of the human m2 receptor corresponding to amino acids 227–356. In immunoblots this antiserum recognized the M2R fusion protein, but showed no cross-reactivity to other muscarinic receptor subtypes (Levey et al., 1990). Preadsorption of the antiserum with the M2R fusion protein, but not GST, abolished immunohistochemical staining in rat brain (Levey et al., 1991).
The rat monoclonal M2R primary antibody (catalog # mAB367, Millipore) was raised against a highly purified GST fusion protein of a part of the third intracellular loop of the human m2 receptor corresponding to amino acids 225–359. In immunoblots this antibody recognized the M2R fusion protein, but showed no cross-reactivity to other muscarinic receptor subtypes (Levey et al., 1995a). The immunohistochemical staining in rat brain appeared to be identical to that obtained with the rabbit polyclonal M2R antiserum used in this study, as previously demonstrated by Levey and co-workers (Levey et al., 1995a). This complete overlap of M2R immunoreactivity with the two M2R antibodies was verified in the amygdala in the present study by pairing the two antibodies in dual-labeling immunofluorescence preparations. No immunostaining with this M2R antibody was observed in M2R knockout mice in pulmonary smooth muscle cells, dorsal root ganglion cells, or atrial or pulmonary vein cardiomyocytes, but was seen in these cells in wild-type mice (Jositsch et al., 2009).
The neuronal marker antibodies used in this investigation have been characterized and used extensively in previous studies of the basolateral amygdala and cerebral cortex (McDonald, 1989; McDonald and Mascagni, 2001, 2002, 2010; Mascagni and McDonald, 2003; Apergis-Schoute et al., 2007). Each antibody produced the characteristic pattern of labeling for each of the neuronal subpopulations seen in previous studies.
3. Results
3.1 Immunoperoxidase studies
All of the immunoperoxidase studies were performed using the rabbit M2R antiserum. The pattern of M2R immunoreactivity (M2-ir) in the forebrain at the level of the amygdala appeared to be identical to that obtained in previous studies using this antiserum (Levey et al., 1991, 1995b). Thus, staining in the cerebral cortex was predominately neuropilar, and exhibited a bistratified configuration. M2-ir in the striatum was mainly found in a population of large multipolar neurons. The cornu ammonis of the hippocampus exhibited many M2R+ neurons in the stratum oriens, and there were many M2R+ puncta surrounding the cell bodies of pyramidal cells in the pyramidal layer (Figs. 1, 2). Presumptive basal forebrain cholinergic neurons in the magnocellular preoptic nucleus and globus pallidus were also M2R+ (Fig. 2). M2R-ir was very similar in all 6 peroxidase-stained brains.
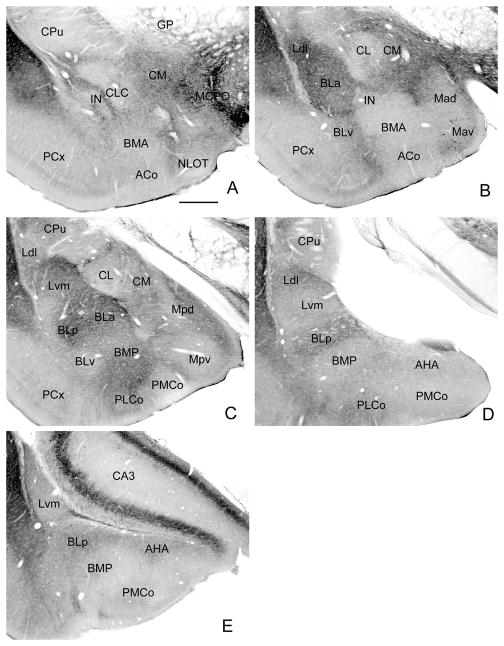
Fig. 1.
Low power photomicrographs of M2R immunoreactivity at five anteroposterior levels of the amygdala in one representative brain (see Fig. 2 for the bregma levels of these coronal sections as well as nuclear borders drawn after the sections were Nissl counterstained). Scale bar = 0.5 mm. Abbreviations: ACo, anterior cortical nucleus; AHA, amygdalohippocampal area; BLa, anterior basolateral nucleus; BLp, posterior basolateral nucleus; BLv, ventral basolateral nucleus; BMA, anterior basomedial nucleus; BMP, posterior basomedial nucleus; CA3; cornu ammonis field 3; CL, lateral central nucleus, CLC, lateral capsular central nucleus; CM, medial central nucleus; CPu, caudate-putamen; GP, globus pallidus; IC, internal capsule; IN, intercalated nucleus; Ldl, laterodorsal lateral nucleus; Lvm, ventromedial lateral nucleus; Mad, anterodorsal medial nucleus; Mav, anteroventral medial nucleus; MCPO, magnocellular preoptic nucleus; Mpd, posterodorsal medial nucleus; Mpv, posteroventral medial nucleus; NLOT, nucleus of the lateral olfactory tract; PCx, piriform cortex; PLCo, posterolateral cortical nucleus; PMCo, posteromedial cortical nucleus.
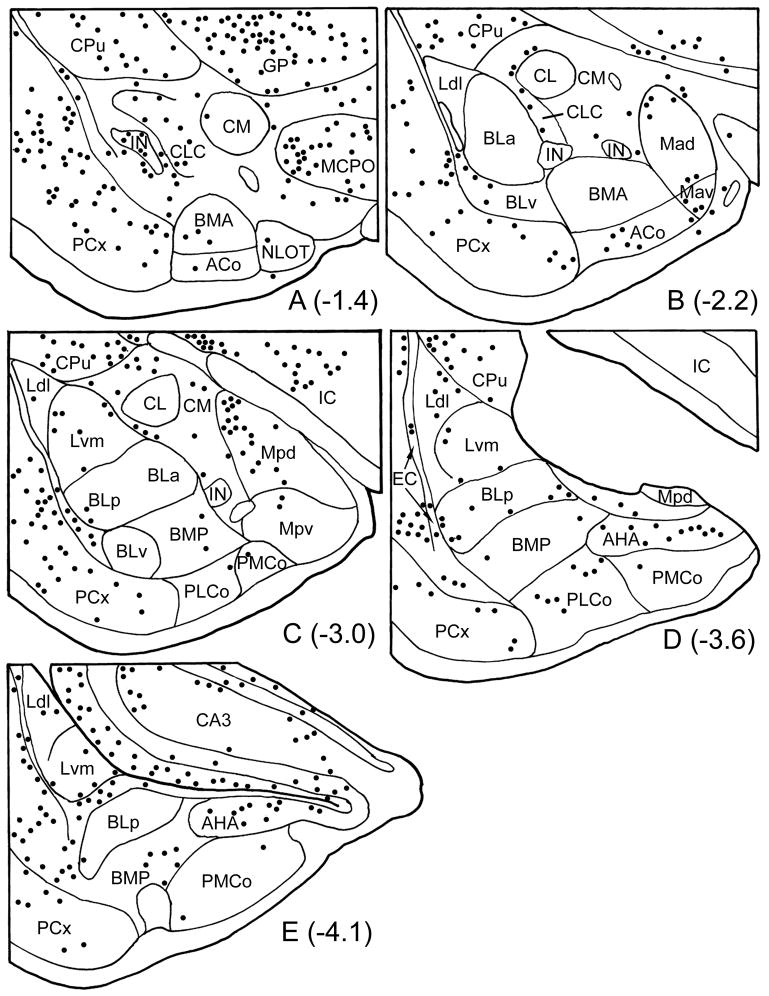
Fig. 2.
Drawings showing the locations of M2R+ neurons (dots) at the five levels of the amygdala of the brain shown in Fig. 1. Bregma levels are taken from the atlas by Paxinos and Watson (1997). Neurons were plotted from three adjacent sections at each level, including the sections shown in Fig. 1. Nuclear borders were drawn from these sections after coverslips were removed and sections were counterstained with pyronin Y (see Experimental Procedures). See Fig. 1 for scale bar and abbreviations. EC, external capsule.
Both neuropilar and perikaryal M2R-ir was seen in the amygdala. The immunoreactivity seen at low power mainly represented neuropilar staining and varied in density in individual amygdalar nuclei (Fig. 1; see Fig. 2 for boundaries of nuclei depicted in Fig. 1). Neuropilar immunoreactivity was strongest in rostral portions of the basolateral and lateral nuclei, where it consisted of scattered puncta, occasional neuronal processes, and diffuse staining (Figs. 1B, 1C, 3). At caudal levels of the basolateral nuclear complex, M2R-ir was considerably lighter and was mainly observed in the posterior subdivision of the basolateral nucleus (BLp) and the dorsolateral subdivision of the lateral nucleus (Ldl) (Figs. 1D, E). M2R levels in the caudal part of the amygdalohippocampal nucleus (AHA) appeared to equal that in the adjacent BLp (Fig. 1E). Neuropilar M2R immunoreactivity in the cortical nuclei (Co) and nucleus of the lateral olfactory tract (NLOT) was generally very light, except in the posterolateral cortical nucleus (PLCo) which exhibited moderate levels of immunoreactivity (Figs. 1C, D).
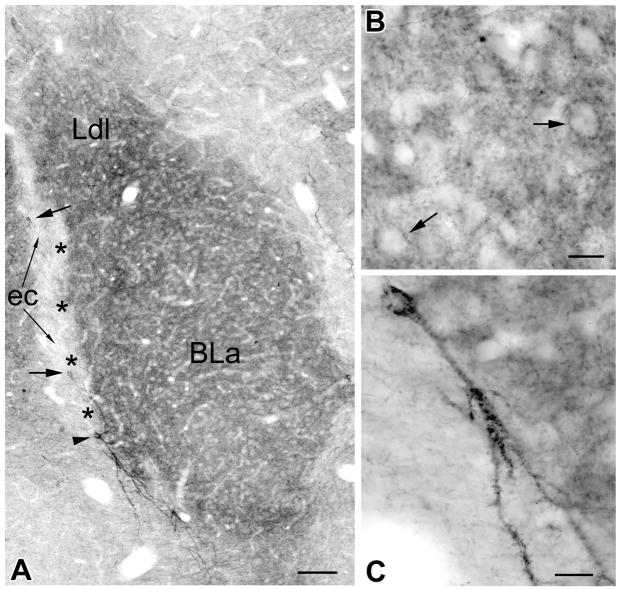
Fig. 3.
A) Higher power photomicrograph of the portion of the basolateral nuclear complex shown in Fig. 1B. Note dense neuropilar M2R-ir in the anterior subdivision of the basolateral nucleus (BLa) and the dorsolateral subdivision of the lateral nucleus (Ldl). The lateral paracapsular portion of the intercalated nucleus (asterisks), located between the BLa and the external capsule (EC), exhibits no M2R-ir; it was identified following subsequent counterstaining of the section. Large arrows indicate two medium-sized aspiny M2R+ neurons located in the external capsule. A large spiny “SPIN” neuron (see text) is located at the ventral border of the intercalated nucleus (arrowhead); its dendrites avoid entering the intercalated nucleus. B) High power photomicrograph of a field in the BLa showing neuropilar M2R-ir. Note that some perikarya of M2R-negative putative pyramidal cells appear to be surrounded by M2R+ puncta (arrows). C) High power photomicrograph of the SPIN neuron shown in A. Note spines on the dendrites in the lower portion of the field. Scale bars = 100 μm in A, 20 μm in B and C.
Individual nuclei of the centromedial amygdala also differed greatly in neuropilar M2R-ir. In the medial nuclear complex the medial portion of the posterodorsal medial nucleus (Mpd) exhibited the greatest M2R-ir (Fig. 1C). The medial subdivision of the central nucleus (CM) was densely stained, whereas M2R-ir in the lateral subdivision (CL) was very light (Fig. 1B). The intercalated nuclei (IN) exhibited almost no M2R-ir, but a thin zone adjacent to these nuclei was moderately stained (Figs. 1A, B). M2R+ axons were observed in the lateral portion of the stria terminalis and in a thin zone that extended medially, forming a dorsal rim along the presumptive commissural bundle of the stria (de Olmos and Ingram, 1972). M2R neuropilar staining, as well as small aspiny M2R+ neurons, were seen in the lateral supracapsular portion of the bed nucleus of the stria terminalis (BSTs of Alheid et al., 1995), which is associated with the lateral part of the stria terminalis along its entire extent.
M2R+ perikarya were seen in low numbers in all nuclei of the amygdala. Approximately one dozen M2R+ neurons were seen per section at all levels of the amygdala (Fig. 2; note that M2R+ neurons from three adjacent sections from the same brain were plotted at each level of the amygdala in this figure). Two distinct types of M2R+ neurons were associated with the intercalated nuclei (IN): large spiny and large aspiny. Both types of M2R+ neurons had cell bodies that were about twice the size of the principal neurons of the INs. These neurons were mainly associated with the portions of the main IN located just rostral to the BLa (bregma −1.4 level, Fig. 2A). Three to six neurons of each type were usually seen per section at these rostral levels (Figs. 1A, 2A, 4A–D). However, large spiny and aspiny neurons were occasionally seen in and around more caudal portions of the IN complex as well. Cell bodies of the large M2R+ spiny neurons were located in a zone just outside of the borders of the intercalated nuclei. These large spiny peri-intercalated nuclear neurons (“SPIN” neurons) were associated with all portions of the INs, including the lateral paracapsular INs located adjacent to the external capsule (Fig 3A, C), and the medial paracapsular INs located adjacent to the intermediate capsule (which lies just medial to the basolateral nuclear complex; Figs. 4E, 5), but most were observed adjacent to the main intercalated nucleus at rostral levels of the amygdala (Figs. 2A, 4A, 4B, 4D). SPIN neurons had cell bodies that were 15–20 μm long and 12–15 μm wide, and had 3–4 primary dendrites that branched sparingly. Secondary and more distal dendrites had a dense covering of M2R+ spines (Figs. 3C, 4D). The dendrites of SPIN neurons usually avoided entering the adjacent INs, sometimes extending along their border (Figs. 4E, 5). Some of the more ventrally situated SPIN neurons had long dendrites that extended ventrally along the border separating the piriform cortex from the anterior cortical nucleus and anterior basomedial nucleus, and terminated in the molecular layer at the amygdaloid fissure.
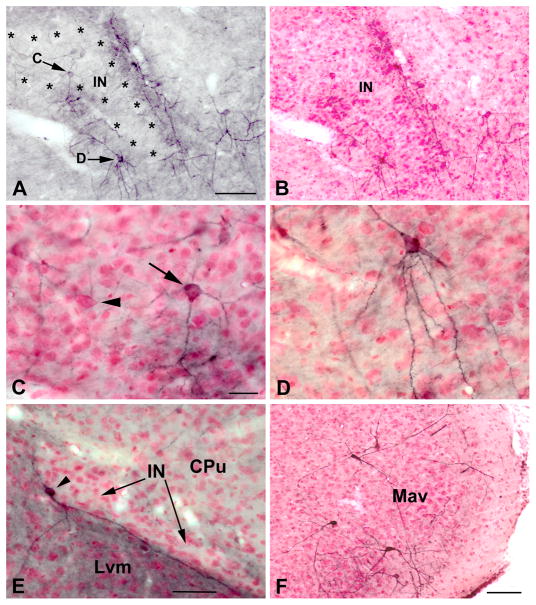
Fig. 4.
A) Higher power photomicrograph of the portion of the rostral intercalated nucleus shown in Fig. 1A. The same field is shown after pyronin Y counterstaining in B. Asterisks indicate the borders of the intercalated nucleus. Arrows indicate M2R+ neurons shown at higher power in C and D after counterstaining. The upper arrow indicates a large aspiny M2R+ neuron that is located in the intercalated nucleus; several more lightly-stained large aspiny M2R+ neurons are seen above and to left of this cell. The M2R+ zone surrounding the intercalated nucleus is mainly filled with dendrites and cell bodies of large spiny M2R+ neurons (“SPIN” neurons), one of which is indicated by the lower arrow. C) Higher power photomicrograph of the large aspiny M2R+ neuron shown in A after counterstaining (arrow). Note that it is larger than the small principal neurons of the intercalated nucleus which surround it. The perikaryon of a large M2R-negative neuron of the intercalated nucleus is also seen in this field (arrowhead). D) Higher power photomicrograph of the M2R+ SPIN neuron shown in A after counterstaining; its spiny dendrites avoid entering the intercalated nucleus above. E) An M2R+ SPIN neuron (arrowhead) associated with one of the medial paracapsular nuclei (IN) located between the ventromedial subdivision of the lateral nucleus (Lvm) and the caudate-putamen (CPu) at the bregma −3.3 level. Note that its dendrites extend along the border of the IN and into the Lvm, but avoid entering the IN. Also note the lack of neuropil staining in the IN. See Fig. 5 for a drawing of this neuron. F) Higher power photomicrograph of the M2R+ neurons surrounding the anteroventral subdivision of the medial nucleus at the level of Figs. 1B and 2B after counterstaining. Scale bars = 100 μm in A (B is the same magnification), 25 μm in C (D is the same magnification), 50 μm in E, and 100 μm in F.
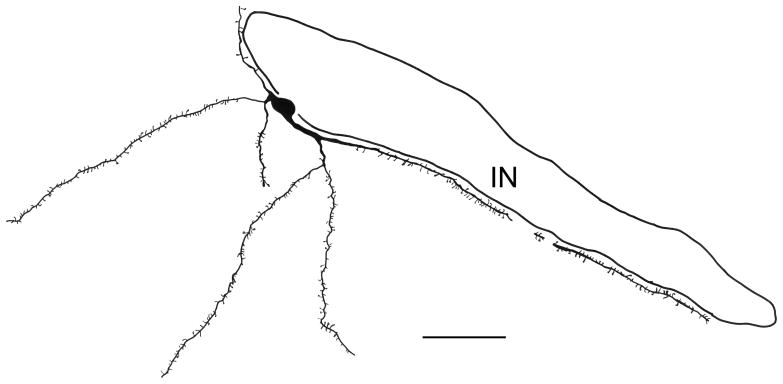
Fig. 5.
Camera lucida drawing of the SPIN neuron shown in Fig. 4E. It was located adjacent to one of the medial paracapsular intercalated nuclei (IN) and was reconstructed from two adjacent sections. Scale bar = 50 μm.
The large aspiny M2R+ neurons associated with the INs had cell bodies that were usually located within the boundaries of the INs (Figs. 4A–C). Their cell bodies and dendritic arborizations resembled those of SPIN neurons, but their dendrites were aspiny. Although they were seen in all portions of the INs, there was a concentration of these aspiny M2R+ neurons in the portion of the IN located just rostral to the anterior subdivision of the basolateral nucleus (Figs. 2A, 4A–C). Cell bodies of large M2R-negative neurons were also seen within the INs (Fig. 4C, arrowhead).
The central nucleus contained a small number of medium-sized M2R+ neurons (3–4 per section). The perikarya of many of these were in a narrow zone along the lateral and ventral borders of the lateral subdivision of the central nucleus (CL) (Figs. 2B, 2C, 6A, 6B). The great majority of these neurons had spiny dendrites but a small number of aspiny neurons of similar morphology were also seen. The cell bodies of these neurons were 12–15 μm long and 9–10 μm wide. Their dendrites tended to run tangential to the lateral and ventral borders of the CL.
Fig. 6.
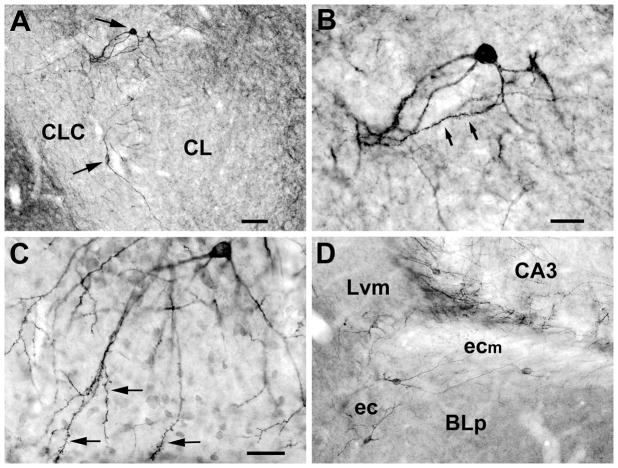
A) Photomicrographs of two spiny M2R+ neurons (arrows) located along the lateral and dorsal edges of the lateral subdivision of the central nucleus (CL). The ventral neuron is bitufted and located at the border between CL and the lateral capsular subdivision of the central nucleus (CLC). The dorsal neuron is multipolar and located between the central nucleus and the caudate-putamen. B) Higher power view of the dorsal neuron in A, showing a spiny dendritic segment (arrows). C) M2R+ neuron in the anteroventral subdivision of the medial nucleus (Mav) in a counterstained section. Its cell body is located in the ventral part of the cellular layer, and its dendrites, and those of other M2R+ Mav neurons, extend ventrally into the molecular layer. Note complex dendritic appendages on the portions of the dendrites in the molecular layer (arrows). D) Several M2R+ neurons in the region of the external capsule (ec) and its medial extension (ecm) between the ventromedial lateral nucleus (Lvm) and BLp. See Fig. 9 for a drawing of these neurons. Scale bars = 65 μm in A, 25 μm in B, 30 μm in C, and 50 μm in D.
The medial nucleus contained a subpopulation of medium-sized M2R+ aspiny neurons that were mainly found surrounding its anteroventral subdivision (Mav; Fig. 2B) and in the medial part of its posterodorsal subdivision (Mpd; Fig. 2C). M2R+ neurons surrounding the Mav (1–5 per section) had cell bodies that were 15–20 μm long and 10–15 μm wide (Figs. 4F, 6C). The entire dendritic arborization of each neuron was stained in a Golgi-impregnation-like fashion. These cells had 4–5 primary dendrites that branched sparingly. Most portions of the dendritic arborization were spine-sparse or moderately spiny, but distal dendrites extending into the molecular layer at the junction of the Mav and ACo exhibited a profusion of complex dendritic appendages (Fig. 6C). M2R+ neurons in the Mpd were very lightly stained and had cell bodies that were 14–17 μm long and 10–12 μm wide. These neurons had 3–4 aspiny primary dendrites that branched sparingly. However, usually just the cell bodies and proximal dendrites were stained.
The cortex-like nuclei, including the basolateral nuclear complex (BLC; comprised of the basolateral, lateral, and basomedial nuclei) and cortical nuclei, contained a numerically small subpopulation of M2R+ nonpyramidal neurons (see Figs. 7 and 8 for examples from the BLC). These neurons had ovoid or fusiform cell bodies that were 13–16 μm long and 8–12 μm wide, and had 3–4 primary dendrites that branched sparingly. Dendritic branches were either aspiny or spine-sparse. Most of these neurons were found in the lateral nucleus (Fig. 2C–E, 7C–E, 8), and were often seen near the boundary separating the ventromedial subdivision of the lateral nucleus (Lvm) from the dorsomedial subdivision of the lateral nucleus (Ldl) or posterior subdivision of the basolateral nucleus (BLp). Similar cells were seen in the amygdalohippocampal area (AHA) in moderate numbers (approximately 3–5 neurons per section; Fig. 2D, E) but the staining there was light and usually confined to the cell bodies and proximal dendrites. Nonpyramidal neurons resembling those in the BLC were also seen in the external capsule (Figs. 2, 7A, 8C, 9), and in a medial extension of the external capsule that separates the caudal part of the lateral nucleus from the BLp (Figs. 2E, 6D, 9). Neurons in these fiber bundles had dendrites that were oriented in the plane of the fiber bundles.
Fig. 7.
Photomicrographs of M2R+ nonpyramidal cells in the basolateral nuclear complex and external capsule. A) M2R+ neuron in the external capsule. Asterisks indicate the border separating BLp from the external capsule, which is located dorsolateral to the nucleus. B) M2R+ neuron in the posterior subdivision of the basolateral nucleus (BLp). Asterisks indicate the border separating BLp from the medial extension of the external capsule, which is located dorsal to the nucleus. C–E) M2R+ neurons in the lateral nucleus. Scale bar = 25 μm for all photomicrographs.
Fig. 8.
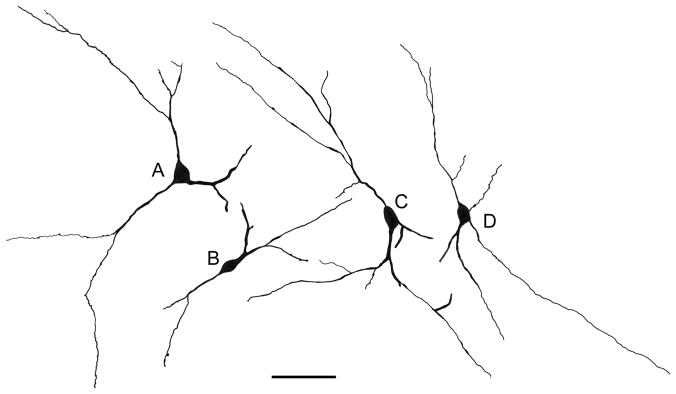
Camera lucida drawings of representative M2R+ nonpyramidal neurons cells in the lateral nucleus and external capsule. A) The M2R+ neuron in the lateral nucleus shown in Fig. 7C. B) The M2R+ neuron in the lateral nucleus shown in Fig. 7E. C) The M2R+ neuron in the external capsule shown in Fig. 7A. D) M2R+ neuron in the lateral nucleus. Scale bar = 50 μm.
Fig. 9.
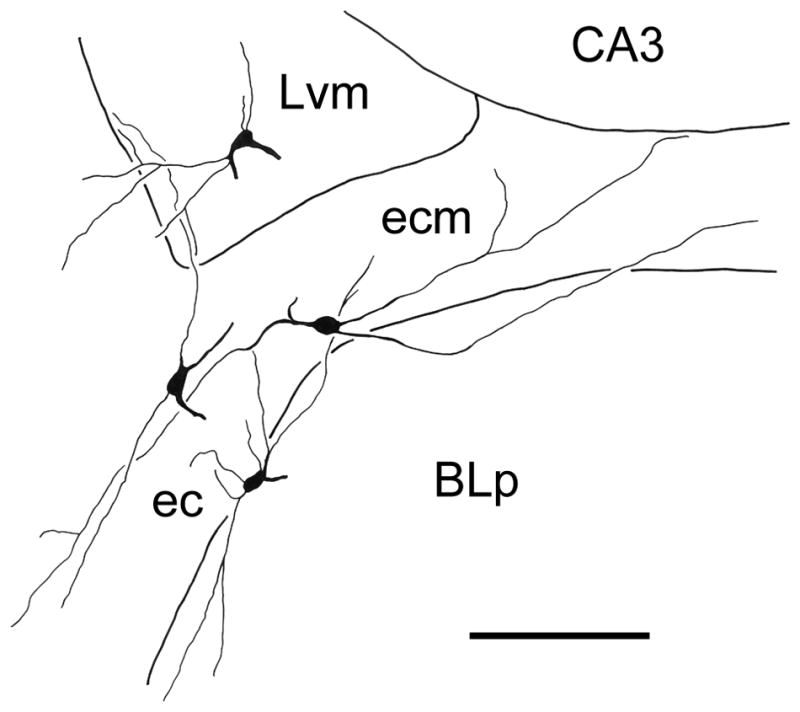
Camera lucida drawing of M2R+ nonpyramidal neurons cells in the lateral nucleus (Lvm) and external capsule (ec), including its medial extension between the lateral nucleus and the BLp (ecm), that was reconstructed from 3 adjacent sections. One of the sections is depicted in Fig. 6D. Scale bar = 100 μm.
3.2 Dual-labeling Immunofluorescence studies
Many of the M2R+ neurons in the amygdala were nonpyramidal neurons of the basolateral nuclear complex (BLC), most of which are known to be GABAergic interneurons. Recent studies have demonstrated that there are at least four separate subpopulations of GABAergic interneurons in the BLC which can be selectively labeled using antibodies to PV, SOM, CCK, or CR/VIP (see Experimental Procedures). In addition, expression of neuropeptide Y (NPY) defines a distinct subpopulation of somatostatin-positive neurons (McDonald, 1989; McDonald et al., 1995). Dual-labeling immunofluorescence studies using antibodies to GAD, PV, SOM, CCK, CR, or NPY were performed to determine whether these cell type specific markers are expressed by M2R+ neurons in the BLC. Since perikaryal M2R-ir was light in immunofluorescence preparations, all rats used for cell counts in dual-labeling experiments were injected with colchicine one day before perfusion to enhance perikaryal labeling. Although this enhanced perikaryal M2R-ir in individual neurons, the distribution and density of M2R+ perikarya appeared to be identical to that seen in non-colchicine-injected rats. In addition to examining the BLC, expression of these markers was also studied in other amygdalar neuronal populations that exhibited robust M2R-ir, including neurons of the intercalated nuclei, the M2R+ neurons that surrounded the anteroventral subdivision of the medial nucleus (Mav), and the M2R+ neurons in the central nucleus. Table 1 summarizes the percentages of M2R+ neurons that co-expressed different neuronal markers.
Table 1.
Percentages of M2R-positive neurons that express neuropeptide Y (NPY), somatostatin (SOM), or GAD in different amygdalar regions (see text for detailed cell count data)
| Amygdalar Region | NPY | SOM | GAD |
|---|---|---|---|
| Lateral nucleus | 100% | 100% | 92% |
| External capsule | 100% | 100% | 68% |
| Peri-intercalated nucleus* | 100% | 100% | 0% |
| Lateral central nucleus | 22% | 57% | 0% |
The term “peri-intercalated nucleus” refers to the “SPIN” neurons that surround the intercalated nuclei (see text).
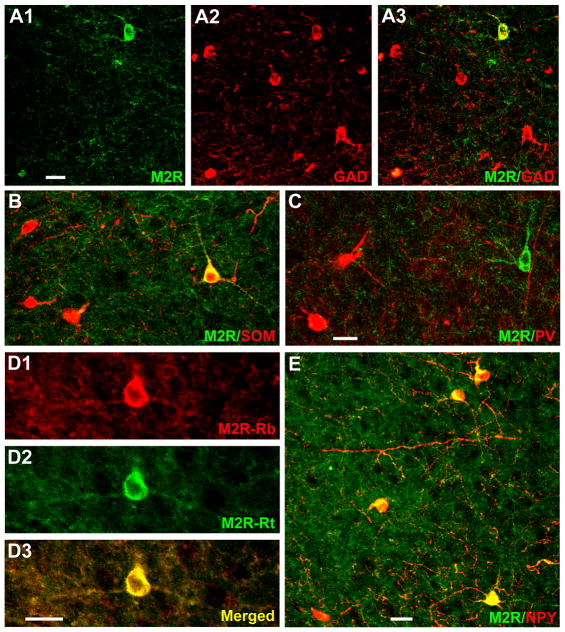
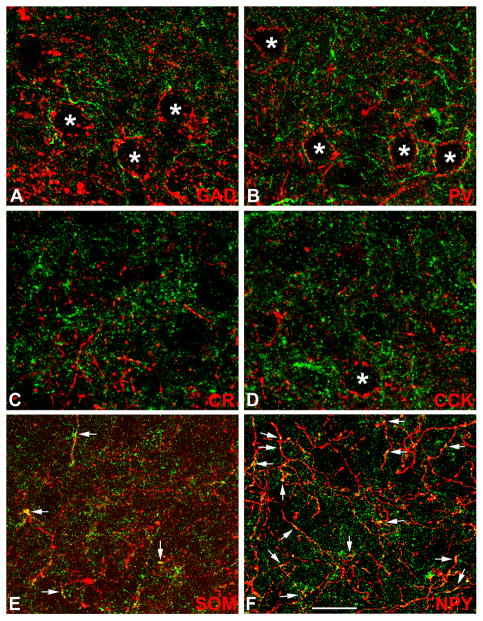
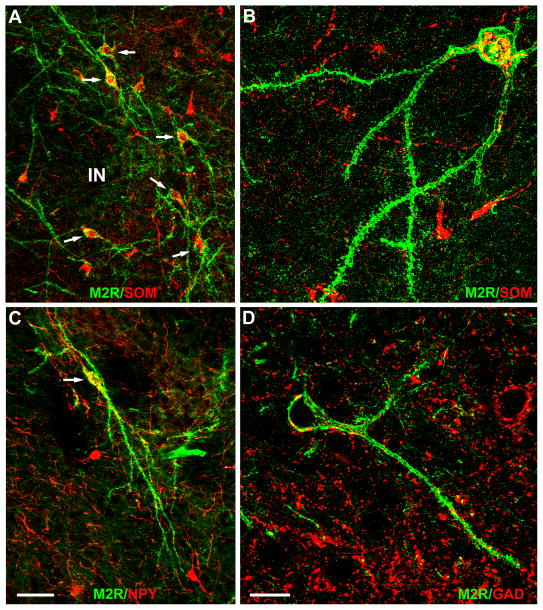
Since most M2R+ neurons of the BLC were located in the lateral nucleus, and were more easily seen in this nucleus versus other portions of the BLC due to the lighter neuropil labeling in the former, all BLC cell counts were performed in the lateral nucleus. As expected from their nonpyramidal morphology, virtually all M2R+ neurons in the lateral nucleus were GAD+ (24/26 [92%]; Fig. 10A). Moreover, cell counts revealed that all M2R+ neurons in the lateral nucleus were SOM+ (25/25; Fig. 10B), but none were PV+ (Fig. 10C), CR+ or CCK +. In fact, no M2R+ neurons anywhere in the amygdala expressed PV, CR, or CCK. In both M2R/GAD and M2R/SOM preparations double-labeled neurons formed a small percentage of the marker-labeled neurons in the lateral nucleus. In addition, cell counts revealed that all M2R+ neurons in the external capsule were SOM+ (21/21), and most were GAD+ (15/22 [68%]).
Fig. 10.
Immunofluorescence photomicrographs of M2R+ nonpyramidal cells in the lateral amygdalar nucleus. A1) An M2R+ neuron (green) is seen in the upper portion of this field, and the edge of another M2R+ neuron is seen in the lower left corner. A2) GAD+ neurons in the same field as A1 (red). A3) Merged image of A1 and A2. Coexpression of M2R and GAD is indicated in yellow. Note yellow labeling of both M2R+ neurons seen in A1. B) M2R+ structures (green) and SOM+ neurons (red) in a merged image. The single M2R+ neuron in this field co-expresses SOM (yellow). C) M2R+ neuron (green) and PV+ neurons (red) in a merged image. The single M2R+ neuron in this field does not express PV. D) The rabbit M2R antibody (D1) and the rat M2R antibody (D2) produce virtually identical patterns of labeling in both perikarya and the neuropil of the lateral nucleus (D3) and all other forebrain regions investigated. E) M2R+ structures (green) and NPY+ neurons (red) in a merged image. All four M2R+ neurons in this field co-express NPY (yellow). Scale bars: 25μm in A and E, 20μm in C and D (B is at the same magnification as C).
Since the NPY antibody was raised in rabbit, we utilized an M2R antibody raised in rat for M2R/NPY dual localization experiments. In experiments using cocktails of the two M2R antibodies there was 100% overlap in M2R labeling in the amygdala and other forebrain regions (Fig. 10D). All M2R+ neurons in the lateral nucleus were NPY+ (45/45), and these neurons constituted 64% (45/70) of all NPY+ neurons in this nucleus (Fig. 10E). Cell counts revealed that all M2R+ neurons in the external capsule were also NPY+ (30/30), and these neurons constituted 88% (30/34) of all NPY+ neurons in this fiber bundle.
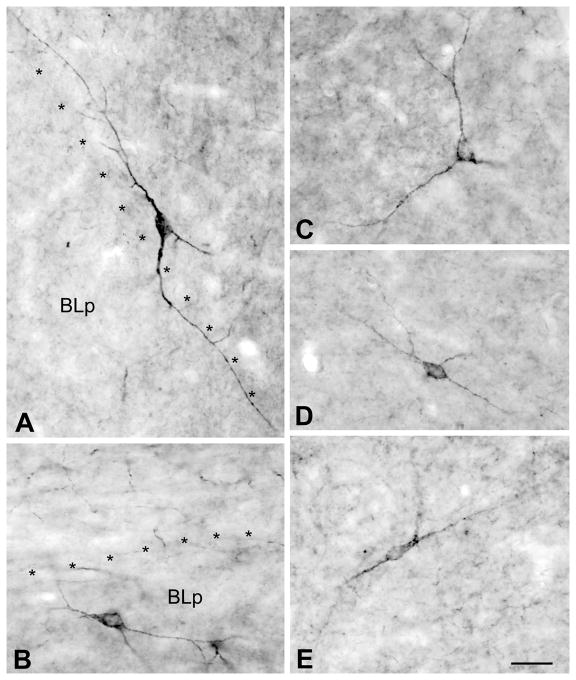
The neuropil of the basolateral and lateral nuclei was analyzed in non-colchicine-injected rats to determine if some of the axons that contain interneuronal markers exhibited M2R-ir. The results in the two nuclei were identical. Little or no M2R-ir was observed in GAD+, PV+, CR+, or CCK+ axons (Figs. 11A–D). However, M2R-ir was seen in some SOM+ axons and in many NPY+ axons in both nuclei (Figs. 11E, F). Nevertheless, the overwhelming majority of M2R+ puncta in the neuropil were marker-negative.
Fig. 11.
Immunofluorescence photomicrographs of M2R neuropilar imunoreactivity (green) and axons expressing markers for interneurons (red) in the basolateral amygdala. Yellow indicates colocalization. Asterisks indicate unlabeled somata of putative pyramidal cells. A) M2R/GAD in the BLa. B) M2R/PV in the BLa. C) M2R/CR in the BLa. D) M2R/CCK in the lateral nucleus. E) M2R/SOM in the BLa. Arrows indicate several M2R+/SOM+ axon terminals. F) M2R/NPY in the BLa. Arrows indicate some of the many M2R+/NPY+ axon terminals in this field. Note little or no M2R-ir in GAD+, PV+, CR+, or CCK+ axons. Scale bar = 25 μm.
All SPIN neurons surrounding the INs were SOM+ (22/22) and NPY+ (23/23) (Fig. 12A–C). SPIN neurons did not express GAD-ir but were innervated by GAD+ axons (Fig. 12D). The large aspiny M2R+ neurons located in the INs, and the large M2R+ neurons surrounding the anteroventral subdivision of the medial nucleus also did not express any of the neuronal markers used in this study. Many of the spiny M2R+ neurons in the lateral portions of the central nucleus expressed SOM (16/28 [57%]), and some expressed NPY (6/27 [22%]). Although it appeared that all of the neurons in the lateral subdivision of the central nucleus (CL) were GAD+, none of the spiny M2R+ neurons encapsulating CL were GAD+.
Fig. 12.
Immunofluorescence photomicrographs of M2R+ SPIN neurons. A). Six SPIN neurons (arrows) exhibit robust M2R-ir in their somata and dendrites (green). All 6 express SOM-ir that is mainly confined to their somata (red). Areas of co-expression within the cytoplasm of these neurons are indicated by yellow. Note that these 6 M2R+/SOM+ neurons surround a portion of the intercalated nucleus (IN) located just rostral to the BLa. B). Higher power photomicrograph of a M2R+ SPIN neuron co-expressing SOM. C) An M2R+ SPIN neuron (green) co-expresses NPY in its soma. D) M2R-ir (green) and GAD-ir (red) in a field that contains an M2R+ SPIN neuron. The SPIN neuron does not contain GAD, but GAD+ puncta (putative axon terminals) contact the cell body and dendrites of the SPIN neuron. In some cases these red GAD+ puncta appear yellow due to their partial overlap with the green M2R-ir in the contacted SPIN neuron. Scale bars = 50 μm in C (A is at the same magnification), 20μm in D (B is at the same magnification).
4. Discussion
This is the first detailed immunohistochemical investigation of M2R localization in the amygdala. Earlier immunohistochemical and in situ hydridization studies of muscarinic receptor protein and mRNA localization in the rat brain reported that M2R+ neurons were seen in low numbers in the amygdala, but provided no details regarding their location within the nuclear complex (Levey et al., 1991). The present investigation found low numbers of M2R+ neurons in all nuclei of the amygdala, including subpopulations of neurons containing somatostatin (SOM) and NPY in the BLC, central nucleus, external capsule, and surrounding the rostral INs. There were also high levels of neuropilar M2R-ir in certain amygdalar nuclei, including the rostral portions of the BLC, medial subdivision of the central nucleus, and posterodorsal subdivision of the medial nucleus. This pattern of neuropilar staining closely resembled the pattern of M2R localization in the amygdala using receptor binding autoradiography (Spencer et al., 1986).
4.1 M2R in the basolateral amygdala and external capsule
Previous studies have shown that there are two major cell classes in the BLC, pyramidal neurons and nonpyramidal neurons. Although these cells do not exhibit a laminar organization, their morphology, synaptology, and electrophysiology are remarkably similar to their counterparts in the cerebral cortex (Carlsen and Heimer, 1988; McDonald, 1992; Washburn and Moises, 1992; Rainnie et al., 1993; Paré et al., 2003). Thus, pyramidal neurons in the BLC are projection neurons with spiny dendrites that utilize glutamate as an excitatory neurotransmitter, whereas most nonpyramidal neurons are spine-sparse or aspiny interneurons that utilize GABA as an inhibitory neurotransmitter. All of the M2R+ neurons in the BLC and adjacent external capsule appeared to be aspiny nonpyramidal neurons. Not surprisingly, the great majority of these neurons were GAD+, indicating that they are GABAergic.
Immunohistochemical studies have shown that the BLC contains several distinct subpopulations of GABAergic nonpyramidal neurons that can be distinguished on the basis of their content of calcium-binding proteins and peptides (see above). The present study indicates that the M2R+ neurons in the BLC and adjacent external capsule are a subpopulation of SOM/NPY+ neurons. The distribution of these M2R/SOM/NPY+ neurons is similar to the distribution of SOM/NPY+ neurons seen in previous studies of the rat amygdala (McDonald, 1989). Previous studies of the lateral nucleus indicate that NPY+ neurons constitute about 80% of SOM+ neurons (McDonald, 1989), and that the latter neuronal subpopulation constitutes about 16% of GABA+ interneurons (McDonald and Mascagni, 2002). Since the present study found that 64% of NPY+ neurons in the lateral nucleus were M2R+, this suggests that M2R+ neurons make up about 8% of GABAergic interneurons in this nucleus. The strong M2R labeling of the somata and dendrites of these neurons implies that the activity of these neurons is modulated by acetylcholine via M2Rs. Although physiological studies of the BLC indicate that some interneurons are activated via muscarinic receptors, the type of muscarinic receptor mediating these effects was not determined (Washburn and Moises, 1992). In the neocortex, however, there is evidence for excitation of interneurons via M2Rs (McCormick and Prince, 1985). Since the main postsynaptic targets of GABAergic SOM+ neurons in the BLC are spines and distal dendrites of pyramidal cells (Muller et al., 2007), the activation of M2R+ SOM/NPY interneurons in the BLC may contribute to the indirect hyperpolarization of pyramidal cells by muscarinic agonists (Washburn and Moises, 1992).
M2R is also expressed in nonpyramidal neurons in the neocortex, but the subpopulations exhibiting immunoreactivity vary depending on the cortical area and species investigated. In the monkey visual cortex approximately one-quarter of all PV+, CB+, and CR+ interneurons are M2R+ (Disney and Aoki, 2008). In the human and monkey neocortex the most strongly labeled M2R+ nonpyramidal neurons were observed in layer 6 and the adjacent subcortical white matter (Smiley et al, 1998). These cortical neurons were rich in acetylcholinesterase and also expressed nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) enzyme activity, which represents the activity of nitric oxide synthase (NOS), the synthetic enzyme for nitric oxide. In both primate and rat neocortex, NOS+ nonpyramidal neurons also express GABA, SOM, and NPY (Judas et al., 1999; Kubota et al., 2011). Likewise, in the present study many of the M2R+ neurons were in or adjacent to the white matter of the external capsule, and virtually all expressed GABA, SOM, and NPY. Moreover, the distribution and morphology of these neurons closely resembles the distribution of NADPH-d+ neurons in the BLC (McDonald et al., 1993), suggesting that these neurons also express NOS.
The results of colocalization of M2R and interneuronal markers in the hippocampus and entorhinal cortex are much different from the findings in the BLC in the present investigation. In the entorhinal cortex 88% of PV+ neurons and 26% of CR+ neurons were M2R+, but other markers were not investigated (Chaudhuri et al., 2005). In the hippocampus virtually all M2R+ neurons are GABAergic, but only about 50% exhibit immunoreactivity for the interneuronal markers PV, CB, SOM, VIP, CR, or CCK (Hajos et al., 1998). The main marker-positive hippocampal interneurons that were M2R+ were the CR+ and SOM+ subpopulations, which represented about 20% and 15% of all M2R+ neurons, respectively. Since the local connections of marker-specific interneuronal subpopulations in the hippocampus (Freund and Buzsaki, 1996) and the BLC (Muller et al., 2003, 2005, 2006, 2007) are similar, the differences in the expression of M2R among interneuron subpopulations suggests that M2R-mediated cholinergic mechanisms associated with interneurons may be dissimilar in these two major limbic structures.
The present investigation revealed very dense neuropilar M2R-ir in the BLC, especially at rostral amygdalar levels. Some of this M2R-ir was associated with NPY+ axon terminals in both the lateral and basolateral nuclei. If these NPY+ terminals represented axons of the NPY/SOM/GABA+ nonpyramidal cells in the BLC that exhibited M2R-ir, it would also be expected that there would also be extensive colocalization of M2R and GAD in these terminals, and that far more of these terminals would be seen in the lateral nucleus versus the basolateral nucleus, since most M2R+ perikarya were seen in the former. However, very few GAD+ terminals exhibited M2R-ir, and M2R/NPY+ terminals were common in both the lateral and basolateral nuclei. These discrepancies suggest that the NPY+ axons that exhibited M2R-ir may originate from neurons that are distinct from the M2R+ perikarya seen in the BLC. This is also consistent with a recent study which found that chemical ablation of NPY+ interneurons in the BLC did not result in a significant loss of NPY+ axons in the BLC (Truitt et al., 2009).
The vast majority of M2R+ puncta and processes in the neuropil of the BLC were not labeled by interneuronal markers in our preparations, and additional studies will be necessary to determine what structures they represent. Electron microscopic studies in the neocortex indicate that neuropilar M2R-ir is contained in distal dendrites and spines of presumptive pyramidal cells, as well as in axon terminals forming asymmetrical or symmetrical synapses (Mrzljak et al., 1993; Erisir et al., 2001). In preliminary electron microscopic studies of the anterior subdivision of the basolateral amygdalar nucleus we have observed a similar distribution of M2R-ir (Muller et al., 2011b). In both the cortex and BLC, the M2R+ axons forming asymmetrical (presumptive excitatory) synapses are most likely glutamatergic axons of thalamic or cortical afferents, or collaterals of local pyramidal cell axons (Sah et al., 2003). This is consistent with a study that found muscarinic suppression of glutamate release from cortical afferents in the BLC by multiple muscarinic receptor subtypes, including M2 receptors (Yajeya et al., 2000). Cholinergic neurons of the basal forebrain are one source of axons forming symmetrical synapses in the cortex (e.g., Turrini et al., 2001) and BLC (Carlsen and Heimer, 1986; Muller et al., 2011a). Since many of the cholinergic neurons in the amygdalopetal and corticopetal portions of the basal forebrain (i.e., Ch3-4 of Mesulam et al., 1983a) exhibit M2R-ir, it is possible that this receptor serves as an autoreceptor on these axons (Levey et al., 1995b). In fact, electron microscopic studies have confirmed that M2R-ir and vesicular acetylcholine transporter protein (VAChT, a marker for cholinergic neurons) immunoreactivity is colocalized in many axons forming symmetrical synapses in the hippocampus (Rouse et al., 1999, 2000). These results are consistent with studies which have implicated the M2 receptor in the inhibition of acetylcholine release in the hippocampus (Richards, 1990; McKinney et al., 1993; Stillman et al., 1993). The relative density of M2R+ neuropilar staining in the basolateral nucleus matches that seen using ChAT (Hellendall et al., 1986) or vesicular acetylcholine transporter protein (VAChT; Muller et al., 2011a) immunoreactivity as markers for cholinergic axons; all three markers are very dense in rostral portions of the nucleus, but less dense caudally. However, the high densities of M2R-ir in rostral portions of the lateral nucleus (see Figs. 1B, C) and the deep portion of the posterolateral cortical nucleus (Fig. 1C) are not matched by corresponding high densities of cholinergic axons. This suggests that M2R-ir is also contained in structures other than BF cholinergic inputs.
4.2 M2R in the centromedial amygdala and intercalated nuclei
Two areas in the centromedial amygdala exhibited robust neuropilar M2R: the medial subdivision of the central nucleus (CM) and the superficial half of the posterodorsal subdivision of the medial nucleus (Mpd). Examination of VAChT-ir in amygdalar sections used in a previous study in this lab (Muller et al., 2011a) revealed that the cholinergic innervation of the CM is fairly dense, while very few cholinergic axons were observed in the lateral subdivision of the central nucleus, which exhibited a paucity of M2R-ir. Thus, the density of the cholinergic innervation of the central nucleus is correlated with the density of M2R-ir. Since the CM gives rise to projections to hypothalamic and brainstem nuclei that are important for behavioral expression of fear conditioning (Pape and Pare, 2010), these findings suggest that the release of acetylcholine in this subnucleus may be critical for emotional learning. The robust neuropilar staining in the Mpd implies that M2R-mediated cholinergic modulation of this nucleus may play a role in neuroendocrine regulation via the projections of Mpd to the medial hypothalamus (Canteras et al., 1995).
As in the BLC, a numerically small subpopulation of M2R+ neurons was observed in the centromedial amygdala and intercalated nuclei. The spiny M2R+ neurons along the lateral and ventral borders of the lateral subdivision of the central nucleus closely resemble similar neurons described in Golgi preparations (McDonald, 1982; Millhouse, 1986). Golgi studies have demonstrated that this region is dominated by large numbers of densely-spiny neurons, but the present investigation revealed that only a very small number of them are M2R+. The present study indicates that some of these M2R+ neurons express SOM or NPY, but none express GAD. In the medial nucleus the most robustly-stained M2R+ neurons were a numerically small subpopulation that surrounded the anteroventral subdivision of the nucleus. The functional significance of these distinct M2R+ subpopulations in the central and medial nuclei remains to be determined.
The intercalated nuclei (IN) were noteworthy for a virtual absence of neuropilar M2R-ir. In addition, the small principal neurons of the INs were M2R-negative. The INs also contain a numerically small subpopulation of large neurons (Millhouse, 1986; McDonald and Augustine, 1993). In the present study some of the large neurons in the INs were M2R-negative, whereas others were large M2R+ aspiny neurons. The morphology of the latter, and their prevalence in the rostral pole of the IN complex, suggests that they correspond to a unique subpopulation of cholinergic neurons located within the rostral INs (Nitecka and Frotscher, 1989). It is possible that these neurons utilize M2Rs as presynaptic autoreceptors like other basal forebrain cholinergic neurons (see above).
The large M2R+ spiny neurons found along the borders of the INs (SPIN neurons) may correspond to the large spiny neurons described by Millhouse (1986) in Golgi-stained preparations. Although Millhouse described them as located within the INs, the cell body of the one neuron depicted (see Fig. 16 in Millhouse, 1986) appears to be located at the ventral border of the main IN in the rostral amygdala. Axons of Golgi-stained large spiny neurons project away from the INs in lateral or ventral directions, but beaded collaterals of these axons arborize both within and just outside the INs (Millhouse, 1986). Recently Ferraguti and colleagues have described a population of large neurons that surround the INs in a manner closely resembling that of the M2R+ SPIN neurons (see Figure 7 in Busti et al., 2011). These cells express the metabotrophic glutamate 1α receptor (mGlu1α). Moreover, these investigators have preliminary data that there may be at least two subpopulations of these cells, one of which is SOM-negative, and another that expresses SOM and NPY (Dr. Francesco Ferraguti, personal communication). The latter subpopulation would appear to correspond to the M2R+ SPIN neurons described in the present study. Moreover, these IN-related mGlu1α+ neurons are non-GABAergic, similar to SPIN neurons, and some of them are innervated by axons of IN principal neurons (Busti et al., 2011). Collectively, these findings suggest that there may be reciprocal connections between M2R+ SPIN neurons and IN principal neurons, and that these interconnected neuronal populations function as a unit. The small M2R-negative principal neurons of the rostral INs are GABAergic neurons that project to the basal forebrain and synapse with non-GABAergic presumptive cholinergic neurons (McDonald and Augustine, 1993; Paré and Smith, 1994). The expression of M2R-ir in SPIN neurons and the large aspiny neurons of the INs, as well as the expression of m1 immunoreactivity in the INs (McDonald and Mascagni, 2010), indicates that there are complex reciprocal interactions between the INs and the cholinergic neurons of the basal forebrain.
Research Highlights.
There is a high density of neuropilar M2 immunoreactivity in the amygdala, especially in rostral portions of the basolateral amygdala
A subset of M2+ nonpyramidal neurons that express GABA, somatostatin, and neuropeptide Y were observed in the lateral nucleus and adjacent external capsule
Large spiny and large aspiny neurons associated with the intercalated nuclei were M2R+
Many neuropeptide Y containing axons in the basolateral amygdala were M2R+
Discrete subpopulations of M2R+ neurons were also seen in the central and medial nuclei
Acknowledgments
The authors would like to thank Dr. John H. Walsh (CURE/Digestive Diseases Research Center, Antibody/RIA Core, NIH Grant #DK41301, Los Angeles, CA) for the donation of the mouse anti-CCK antibody and Dr. Alison Buchan (University of British Columbia) for the donation of the mouse anti-somatostatin antibody. The authors would like to thank Dr. Jay F. Muller for his comments on an earlier version of this manuscript. This work was supported by NIH grant R01-DA027305.
ABBREVIATIONS
- BLC
Basolateral nuclear complex
- CCK
Cholecystokinin
- ChAT
Choline acetyltransferase
- CR
Calretinin
- GAD
Glutamic acid decarboxylase
- IN
Intercalated nuclei
- M2R
m2 muscarinic cholinergic receptor
- NPY
Neuropeptide Y
- PV
Parvalbumin
- SI
Substantia innominata
- SOM
Somatostatin
- SPIN neuron
spiny peri-intercalated nuclear neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alheid GF, deOlmos JS, Beltramino CA. The Rat Nervous System. San Diego: Academic Press; 1995. Amygdala and Extended Amygdala; pp. 495–578. [Google Scholar]
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute J, Pinto A, Paré D. Muscarinic control of long-range GABAergic inhibition within the rhinal cortices. J Neurosci. 2007;27:4061–4071. doi: 10.1523/JNEUROSCI.0068-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977;120:435–444. doi: 10.1016/0006-8993(77)90397-3. [DOI] [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Ma ko M, Kaufmann W, Sätzler K, Singewald N, Capogna M, Ferraguti F. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31:5131–5144. doi: 10.1523/JNEUROSCI.6100-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J Comp Neurol. 1986;244:121–136. doi: 10.1002/cne.902440110. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the medial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- Chaudhuri JD, Hiltunen M, Nykänen M, Ylä-Herttuala S, Soininen H, Miettinen R. Localization of M2 muscarinic receptor protein in parvalbumin and calretinin containing cells of the adult rat entorhinal cortex using two complementary methods. 2005;131:557–566. doi: 10.1016/j.neuroscience.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Cortés R, Palacios JM. Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res. 1986;362:227–238. doi: 10.1016/0006-8993(86)90448-8. [DOI] [PubMed] [Google Scholar]
- Cortés R, Probst A, Palacios JM. Quantitative light microscopic autoradiographic localization of cholinergic muscarinic receptors in the human brain: forebrain. Neuroscience. 1987;20:65–107. doi: 10.1016/0306-4522(87)90006-6. [DOI] [PubMed] [Google Scholar]
- De Olmos JS, Ingram WR. The projection field of the stria terminalis in the rat brain. J Comp Neurol. 1972;146:303–330. doi: 10.1002/cne.901460303. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol. 2008;507:1748–1762. doi: 10.1002/cne.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert FJ, Roeske WR, Yamamura HI. Molecular biology, pharmacology, and brain distribution of subtypes of the muscarinic receptor. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 111–124. [Google Scholar]
- Erisir A, Levey AI, Aoki C. Muscarinic receptor M(2) in cat visual cortex: laminar distribution, relationship to gamma-aminobutyric acidergic neurons, and effect of cingulate lesions. J Comp Neurol. 2001;441:168–185. doi: 10.1002/cne.1405. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Girgis M. Acetylcholinesterase enzyme localization in the amygdala: a comparative histochemical and ultrastructural study. Acta Anat (Basel) 1980;106:192–202. doi: 10.1159/000145181. [DOI] [PubMed] [Google Scholar]
- Hájos N, Papp EC, Acsády L, Levey AI, Freund TF. Distinct interneuron types express m2 muscarinic receptor immunoreactivity on their dendrites or axon terminals in the hippocampus. Neuroscience. 1998;82:355–376. doi: 10.1016/s0306-4522(97)00300-x. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hellendall RP, Godfrey DA, Ross CD, Armstrong DM, Price JL. The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol. 1986;249:486–498. doi: 10.1002/cne.902490405. [DOI] [PubMed] [Google Scholar]
- Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:389–395. doi: 10.1007/s00210-008-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judas M, Sestan N, Kostovi I. Nitrinergic neurons in the developing and adult human telencephalon: transient and permanent patterns of expression in comparison to other mammals. Microsc Res Tech. 1999;45:401–419. doi: 10.1002/(SICI)1097-0029(19990615)45:6<401::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol 2000. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Martin LJ, Walker LC, Richardson RT, DeLong MR, Price DL. Topographic, non-collateralized basal forebrain projections to amygdala, hippocampus, and anterior cingulate cortex in the rhesus monkey. Brain Res. 1988;463:133–139. doi: 10.1016/0006-8993(88)90535-5. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, Gash DM. Telencephalic cholinergic system of the New World monkey (Cebus apella): morphological and cytoarchitectonic assessment and analysis of the projection to the amygdala. J Comp Neurol. 1989;279:528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective Coexpression of Multiple Chemical Markers Defines Discrete Populations of Neocortical GABAergic Neurons. Cereb Cortex. 2011 Jan 10; doi: 10.1093/cercor/bhq252. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Levey AI, Stormann TM, Brann MR. Bacterial expression of human muscarinic receptor fusion proteins and generation of subtype-specific antisera. FEBS Lett. 1990;275:65–69. doi: 10.1016/0014-5793(90)81440-y. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Hersch SM, Wiley RG, Heilman CJ. Light and electron microscopic study of m2 muscarinic acetylcholine receptor in the basal forebrain of the rat. J Comp Neurol. 1995a;351:339–356. doi: 10.1002/cne.903510303. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995b;15:4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, Muly EC, Rainnie DG, McDonald AJ. Immunohistochemical characterization of parvalbumin-containing interneurons in the monkey basolateral amygdala. Neuroscience. 2009;158:1541–1550. doi: 10.1016/j.neuroscience.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Potter LT. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience. 1986;19:551–564. doi: 10.1016/0306-4522(86)90280-0. [DOI] [PubMed] [Google Scholar]
- Mash DC, White WF, Mesulam MM. Distribution of muscarinic receptor subtypes within architectonic subregions of the primate cerebral cortex. J Comp Neurol. 1988;278:265–274. doi: 10.1002/cne.902780209. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985;82:6344–638. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208:401–418. doi: 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500:37–45. doi: 10.1016/0006-8993(89)90297-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ, Payne DR, Mascagni F. Identification of putative nitric oxide producing neurons in the rat amygdala using NADPH-diaphorase histochemistry. Neuroscience. 1993;52:97–106. doi: 10.1016/0306-4522(93)90185-i. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Augustine JR. Neuropeptide Y and somatostatin-like immunoreactivity in the monkey amygdala: distribution, morphology, and differential coexistence. Neuroscience. 1995;66:959–982. doi: 10.1016/0306-4522(94)00629-j. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette R. Parvalbumin containing neurons in the rat basolateral amygdala: morphology and colocalization of calbindin D-28k. Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and gamma-aminobutyric acid in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Neuronal localization of m1 muscarinic receptor immunoreactivity in the rat basolateral amygdala. Brain Struct Funct. 2010;215:37–48. doi: 10.1007/s00429-010-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McKinney M, Miller JH, Aagaard PJ. Pharmacological characterization of the rat hippocampal muscarinic autoreceptor. J Pharmacol Exp Ther. 1993;264:74–78. [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983a;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983b;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS. Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 1993;90:5194–5198. doi: 10.1073/pnas.90.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Comp Neurol. 2011a;519:790–805. doi: 10.1002/cne.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011b. Ultrastructural analysis of m1 and m2 muscarinic cholinergic receptor immunoreactivity in the rat basolateral amygdala. Online. (In Press) [Google Scholar]
- Nitecka L, Frotscher M. Organization and synaptic interconnections of GABAergic and cholinergic elements in the rat amygdaloid nuclei: single- and double-immunolabeling studies. J Comp Neurol. 1989;279:470–488. doi: 10.1002/cne.902790311. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol. 1994;344:33–49. doi: 10.1002/cne.903440104. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003a;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Power AE, McIntyre CK, Litmanovich A, McGaugh JL. Cholinergic modulation of memory in the basolateral amygdala involves activation of both m1 and m2 receptors. Behav Pharmacol. 2003b;14:207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1362. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Rao ZR, Shiosaka S, Tohyama M. Origin of cholinergic fibers in the basolateral nucleus of the amygdaloid complex by using sensitive double-labeling technique of retrograde biotinized tracer and immunocytochemistry. J Hirnforsch. 1987;28:553–560. [PubMed] [Google Scholar]
- Richards MH. Rat hippocampal muscarinic autoreceptors are similar to the M2 (cardiac) subtype: comparison with hippocampal M1, atrial M2, and ileal M3 receptors. Br J Pharmacol. 1990;99:753–761. doi: 10.1111/j.1476-5381.1990.tb13002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E. Cholinergic transduction. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven Press; New York: 1995. pp. 125–134. [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 1999;64:501–509. doi: 10.1016/s0024-3205(98)00594-3. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI. Localization of M(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett. 2000;284:182–186. doi: 10.1016/s0304-3940(00)01011-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. Infracortical interstitial cells concurrently expressing m2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex. Neuroscience. 1998;84:755–769. doi: 10.1016/s0306-4522(97)00524-1. [DOI] [PubMed] [Google Scholar]
- Smith TD, Annis SJ, Ehlert FJ, Leslie FM. N-[3H]methylscopolamine labeling of non-M1, non-M2 muscarinic receptor binding sites in rat brain. J Pharmacol Exp Ther. 1991;256:1173–1181. [PubMed] [Google Scholar]
- Spencer DG, Jr, Horváth E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Stillman MJ, Shukitt-Hale B, Kong RM, Levy A, Lieberman HR. Elevation of hippocampal extracellular acetylcholine levels by methoctramine. Brain Res Bull. 1993;32:385–389. doi: 10.1016/0361-9230(93)90204-o. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Bird ED. Acetylcholinesterase staining of the human amygdala. Neurosci Lett. 1985;54:313–318. doi: 10.1016/s0304-3940(85)80097-5. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience. 2001;105:277–285. doi: 10.1016/s0306-4522(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Muscarinic responses of rat basolateral amygdaloid neurons recorded in vitro. J Physiol. 1992;449:121–154. doi: 10.1113/jphysiol.1992.sp019078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. Projections to the limbic telencephalon. Brain Res Bull. 1984;13:751–784. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Van Denderen JC, Blijleven N, Van Minnen J, Hartig W. Two-laser dual-immunofluorescence confocal laser scanning microscopy using Cy2- and Cy5-conjugated secondary antibodies: unequivocal detection of co-localization of neuronal markers. Brain Res Brain Res Protoc. 1998;2:149–159. doi: 10.1016/s1385-299x(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Yajeya J, De La Fuente A, Criado JM, Bajo V, Sánchez-Riolobos A, Heredia M. Muscarinic agonist carbachol depresses excitatory synaptic transmission in the rat basolateral amygdala in vitro. Synapse. 2000;38:151–160. doi: 10.1002/1098-2396(200011)38:2<151::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]