Abstract
Recent studies indicate that the basolateral amygdala, like the neocortex and hippocampus, receives GABAergic inputs from the basal forebrain in addition to the well-established cholinergic inputs. Since the neuronal targets of these inputs have yet to be determined, it is difficult to predict the functional significance of this innervation. The present study addressed this question in the rat by employing anterograde tract tracing combined with immunohistochemistry at the light and electron microscopic levels of analysis. Amygdalopetal axons from the basal forebrain mainly targeted the basolateral nucleus (BL) of the amygdala. The morphology of these axons was heterogeneous and included GABAergic axons that contained vesicular GABA transporter protein (VGAT). These axons, designated type 1, exhibited distinctive large axonal varicosities that were typically clustered along the length of the axon. Type 1 axons formed multiple contacts with the cell bodies and dendrites of parvalbumin-containing (PV+) interneurons, but relatively few contacts with calretinin-containing and somatostatin-containing interneurons. At the ultrastructural level of analysis, the large terminals of type 1 axons exhibited numerous mitochondria and were densely packed with synaptic vesicles. Individual terminals formed broad symmetrical synapses with BL PV+ interneurons, and often formed additional symmetrical synapses with BL pyramidal cells. Some solitary type 1 terminals formed symmetrical synapses solely with BL pyramidal cells. These results suggest that GABAergic neurons of the basal forebrain provide indirect disinhibition, as well as direct inhibition, of BL pyramidal neurons. The possible involvement of these circuits in rhythmic oscillations related to emotional learning, attention, and arousal is discussed.
Keywords: substantia innominata, anterograde tract tracing, immunohistochemistry, electron microscopy, disinhibition, vesicular GABA transporter
The basal forebrain (BF) contains a diffuse array of cholinergic and noncholinergic neurons that extend through a continuous region which includes the medial septal area, diagonal band of Broca, ventral pallidum, and substantia innominata (SI) (Mesulam et al., 1983; Woolf, 1991; Gritti et al., 1997, 2003). This complex has topographically organized connections with different forebrain regions including the hippocampus, neocortex, and basolateral amygdala (Mesulam et al., 1983; Záborszky et al., 1999). The BF is important for attention and learning, and has been implicated in several neurological diseases, including Alzheimer’s disease (Everitt and Robbins, 1997).
One important subtype of noncholinergic BF neuron that has been extensively studied is the GABAergic corticopetal neuronal population (Freund and Buzsáki, 1996; Semba, 2000; Sarter and Bruno, 2002). These BF cells, which can be identified using either GABAergic markers or antibodies to the calcium-binding protein parvalbumin (Freund, 1989; Gritti et al., 2003), innervate primarily interneurons in both the hippocampus and neocortex (Freund and Gulyás, 1991; Freund and Meskenaite, 1992; Freund and Buzsáki, 1996). The axons of BF GABAergic corticopetal neurons (type 1 axons) have a very distinctive morphology. In contrast to the cholinergic axons (type 2 axons), which have small axon terminals, type 1 axons have very large, clustered terminals that form multiple synapses with cortical interneurons. Via this innervation BF GABAergic neurons indirectly disinhibit assemblies of cortical pyramidal cells and act together with cholinergic BF neurons to generate rhythmic oscillatory activity (Freund and Buzsáki, 1996; Tóth et al., 1997; Buzsáki, 2002; Borhegyi et al., 2004).
A recent study utilizing retrograde tract tracing combined with immunohistochemistry demonstrated that the basolateral amygdala, like cortical regions, receives projections from parvalbumin-positive GABAergic neurons in the BF that are mainly located in the ventral pallidum and SI (Mascagni and McDonald, 2009). However, since the neuronal targets of these inputs have yet to be determined, it is difficult to predict the functional significance of this innervation. The present study addressed this question by employing anterograde tract tracing combined with double-and triple-labeling immunohistochemistry at the light and electron microscopic levels of analysis. These studies indicate that the BF GABAergic inputs to the amygdala provide a particularly robust innervation of parvalbumin interneurons in the basolateral nucleus, but a much weaker innervation of other interneuronal subpopulations. Unlike the innervation of the hippocampus by BF GABAergic neurons, there was also a moderate innervation of pyramidal projection neurons in the basolateral amygdala. Surprisingly, many of the inputs to pyramidal cells were made by the same axon terminals that innervated parvalbumin interneurons.
EXPERIMENTAL PROCEDURES
Injections of anterograde tracers and tissue preparation
Adult male Sprague-Dawley rats (250–350 g; Harlan, Indianapolis, IN, USA) were anesthetized with sodium pentobarbital (50 mg/kg) and placed in a stereotaxic head holder (Stoelting, Wood Dale, IL, USA) for injections of either biotinylated dextran amine (BDA; mol.wt. 10,000; Invitrogen, Eugene, OR, USA) or Phaseolus vulgaris leucoagglutinin (PHA-L; Vector Laboratories Inc., Burlingame, CA, USA) into the SI and/or ventral pallidum (VP) of the BF. Injection coordinates were obtained from an atlas of the rat brain (Paxinos and Watson, 1997). Unilateral or bilateral iontophoretic injections of BDA (4–5% in 0.01 M phosphate buffer [PB], pH 7.3) or PHA-L (2.5% in 0.01 M PB, pH 7.8) were made via glass micropipettes (50 µm inner tip diameter) using a Midgard high voltage current source set at 5.0 µA (7 s on, 7 s off, for 20–30 min for each injection). Injections were made using either one pipette aimed at the center of the BF, or two pipettes at different mediolateral coordinates. Two injections were made along each pipette track at different dorsoventral coordinates separated by 0.5–1.0 mm. Micropipettes were left in place for 5 min following the last injection along each pipette track to prevent the tracers from flowing up the track and involving more dorsally located structures.
After a 5-day survival for BDA injections, or a 10–14-day survival for PHA-L injections, rats for light or confocal microscopy were perfused intracardially with phosphate buffered saline (PBS; pH, 7.4) containing 0.5% sodium nitrite (50 ml), followed by 4.0% paraformaldehyde in PBS for 20–30 min. Following perfusion, these brains were removed and postfixed for 3.5 h in 4.0% paraformaldehyde. Rats to be used for electron microscopy were perfused intracardially with PBS containing 0.5% sodium nitrite (50 ml), followed by an acrolein/paraformaldehyde mixture (2.0% paraformaldehyde-3.75% acrolein in PB for 1 min, followed by 2.0% paraformaldehyde in PB for 30 min). Following removal, acrolein-fixed brains were postfixed in 2.0% paraformaldehyde for 1 h. All brains were sectioned on a vibratome in the coronal plane at either 50 µm (for light microscopy) or 60 µm (for electron microscopy). Sections from acrolein-fixed brains were rinsed in 1.0% borohydride in PB for 30 min and then rinsed thoroughly in several changes of PB for 1 h. All sections were processed for immunohistochemistry in the wells of tissue culture plates.
Light microscopic dual-labeling immunoperoxidase preparations
A sequential two-color immunoperoxidase technique was used in nine rats (four injected with BDA and five injected with PHA-L) to analyze possible contacts of BF afferents with different interneuronal subpopulations in the basolateral amygdala. Previous studies have shown that there are two major cell classes in the basolateral amygdala, pyramidal neurons and nonpyramidal neurons. Although these cells do not exhibit a laminar or columnar organization, their morphology, synaptology, electrophysiology, and pharmacology are remarkably similar to their counterparts in the cerebral cortex (McDonald, 1982, 1984, 1992; Carlsen and Heimer, 1988; Washburn and Moises, 1992; Rainnie et al., 1993; Paré et al., 2003; Sah et al., 2003; Muller et al., 2005, 2006, 2007). Thus, pyramidal neurons in the basolateral amygdala are projection neurons with spiny dendrites that utilize glutamate as an excitatory neurotransmitter, whereas most nonpyramidal neurons are spine-sparse interneurons that utilize GABA as an inhibitory neurotransmitter. Recent dual-labeling immunohistochemical studies suggest that the basolateral amygdala, like the neocortex (Kubota et al., 1994; Kubota and Kawaguchi, 1997), contains at least four distinct subpopulations of GABAergic interneurons that can be distinguished on the basis of their content of calcium-binding proteins and peptides (Kemppainen and Pitkänen, 2000; McDonald and Betette, 2001; McDonald and Mascagni, 2001, 2002; Mascagni and McDonald, 2003). The innervation of the three main interneuronal subpopulations (parvalbumin (PV+), somatostatin (SOM+), and calretinin (CR+) neurons) by BF axons was investigated in the present study.
Sections through the injection sites and amygdala in BDA injected rats were incubated in 0.5% Triton X-100 in PBS for 3 h, and ABC reagent in PBS (Vector Laboratories Inc.) for 16 h at 4 °C. Nickel-enhanced DAB (3,3′-diaminobenzidine hydrochloride; Sigma Chemical Co., St. Louis, MO, USA) was then used as a chromogen to generate a black reaction product (Hancock, 1986). Three series of sections through the amygdala were subsequently rinsed in PBS and incubated overnight at 4 °C in one of the following three primary antibodies for distinct interneuronal subpopulations (McDonald and Mascagni, 2001, 2002; Mascagni and McDonald, 2003): (1) mouse anti-parvalbumin (1:2000; Sigma Chemical Co.), (2) mouse anti-calretinin (1:4000; Millipore, Billerica, MA, USA), or (3) mouse anti-somatostatin (1:1000, SOMA-8 monoclonal antibody obtained from Alison Buchan, University of British Columbia). All antibodies were diluted in 1% normal goat serum in PBS with 0.5% Triton X-100. Sections were then incubated in goat anti-mouse IgG (1:100; Sternberger Monoclonals, Inc., Lutherville, MD, USA) for 1 h, rinsed three times (10 min each) in PBS, followed by mouse peroxidase-antiperoxidase (PAP) complex (1:100; Sternberger Monoclonals, Inc.) for 1 h, and then reacted with nonintensified DAB to produce a brown reaction product.
Sections through the injection sites and amygdala in PHA-L injected rats were incubated in a rabbit anti-PHA-L antibody (1: 1000; Vector Laboratories Inc.) for 16 h at 4 °C and then processed for the avidin-biotin immunoperoxidase technique using a rabbit Vectastain ABC kit (Vector Laboratories Inc.). Nickel-enhanced DAB was used as a chromogen to generate a black reaction product. Three series of sections through the amygdala were subsequently rinsed in PBS and incubated overnight at 4 °C in one of the three primary antibodies for different interneuronal subpopulations (see above). After rinsing, sections were incubated in an ABC blocking solution (avidin/biotin blocking kit, Vector Laboratories, Inc.), and rinsed three times (10 min each), before being incubated in biotinylated goat anti-mouse secondary antibody (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h. All sections were then processed for the avidin-biotin immunoperoxidase technique using a Vectastain ABC reagent (Vector Laboratories Inc.), and then reacted with nonintensified DAB to produce a brown reaction product.
In both BDA and PHA-L injected brains, sections through the amygdala were mounted on gelatinized slides, dried overnight, dehydrated in alcohols, cleared in xylene, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA). Sections through injection sites were counterstained with Cresyl Violet or Pyronin Y (a pink Nissl stain) before coverslipping. Injection sites were mapped using an Olympus BX51 microscope under brightfield illumination. Basal forebrain areas were identified using an atlas of the rat brain (Paxinos and Watson, 1997). The effective injection site (the area from which tracer is incorporated into neurons for anterograde transport) was defined as the area that contained BDA or PHA-L labeled perikarya (Gerfen et al., 1989).
Triple-labeling immunofluorescence preparations
Light and electron microscopy of immunoperoxidase preparations revealed that BF afferents to the basolateral amygdala contacted mainly PV+ interneurons, and to a lesser extent pyramidal cells. A triple-labeling immunofluorescence technique was used in four rats to determine if BF axons forming contacts with basolateral amygdalar interneurons or pyramidal cells were GABAergic. Pyramidal cells were selectively labeled using antibodies to the alpha subunit of CaMK (McDonald et al., 2002), whereas interneurons were labeled using an antibody to calbindin (CB), which labels the great majority of PV+ interneurons (as well as most SOM+ interneurons and some CCK+ interneurons; McDonald and Mascagni, 2001, 2002; Mascagni and McDonald, 2003). GABAergic axon terminals were labeled using an antibody to the vesicular GABA transporter (VGAT), which labels GABAergic axon terminals (Chaudhry et al., 1998).
PHA-L injection into the BF, tissue preparation, and immunoperoxidase localization of PHA-L injection sites was performed as described above. Sections through the amygdala were incubated overnight at 4 °C in a cocktail of goat anti-PHA-L (1:1500; Vector Laboratories Inc.), rabbit anti-VGAT (1:250; Millipore), and either mouse anti-CB (1:8000; Sigma) or mouse anti-CaMK antibodies (1:300; Sigma Chemical Co.). Sections were then rinsed in three changes of PBS (10 min each), and incubated in a cocktail of donkey anti-goat Alexa-488 (1:400, Invitrogen, Eugene, OR, USA), donkey anti-mouse Cy3 (1:400; Jackson ImmunoResearch Laboratories), and donkey anti-rabbit Cy5 antibodies (1:400; Jackson ImmunoResearch Laboratories) for 3 h at room temperature. These secondary antibodies were highly cross-adsorbed by the manufacturer to ensure specificity for primary antibodies raised in each species. All antibodies for the PHA-L/CB/VGAT preparations were diluted in a solution containing 3% normal donkey serum, 1% bovine serum albumin, and 0.4% Triton X-100 in PBS. All antibodies for the PHA-L/CaMK/VGAT preparations were diluted in a similar solution, but Triton X-100 was reduced to 0.04% (McDonald et al., 2002). Following incubation in the secondary antibodies, sections were rinsed in three changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories Inc.).
Sections were examined with a Zeiss LSM 510 Meta confocal microscope. Fluorescence of Alexa-488, Cy3, and Cy5 dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm, 543 nm, and 633 channels. Sections were first analyzed using a 20× objective to identify CB+ or CaMK+ neurons that were receiving contacts from PHA-L-labeled axons. These neurons/axons were then analysed using a 63× oil objective to determine if the PHA-L+ axons were VGAT+ (i.e. GABAergic). In most cases a z-series stack of three to eight optical sections (each of which was 1 µm thick) was compiled for each contacted neuron. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software. An additional series of control sections from each brain was processed as described above, but with one of the three primary antibodies omitted from the primary antibody cocktails in each control; no labeling was observed in the channel corresponding to the secondary antibody associated with the omitted primary antibody.
Electron microscopic dual-labeling immunoperoxidase preparations
Electron microscopic immunocytochemistry using a sequential dual immunoperoxidase method (Muller et al., 2006) was utilized in two rats to survey the innervation of PV+ neurons in the basolateral nucleus (BL) by type 1 axons. These rats received dual unilateral injections of PHA-L into the SI and VP of the basal forebrain as described above. Sections were cryoprotected in 30% sucrose in PB and freeze-thawed two times over liquid nitrogen in order to increase antibody penetration. Sections were then incubated overnight at 4 °C in a rabbit PHA-L primary antibody (1:750; Vector Laboratories, Inc.) in PBS containing 1% NGS, and processed using a rabbit ABC kit (Vector Laboratories, Inc.) with DAB as a chromogen. After rinsing, sections were incubated in a blocking solution (avidin/biotin blocking kit, Vector Laboratories, Inc.). Sections were then incubated overnight at 4 °C in rabbit anti-PV (1:4000; Millipore) to label PV+ neurons. Sections were then processed using a rabbit ABC kit (Vector Laboratories, Inc.). PV immunoreactivity was then visualized using a Vector Very Intense Purple (VIP) peroxidase substrate kit (Vector Laboratories, Inc.). This procedure yields a reaction product, which we term “PUR”, that appears purple in the light microscope, and granular or particulate in the electron microscope (Smiley et al., 1997; Van Haeften and Wouterlood, 2000; Muller et al., 2005). For electron microscopy, the penetration of the PUR reaction was similar to that of DAB immunoperoxidase, and the particulate reaction product was easily distinguishable from the dense, diffuse DAB immunoperoxidase reaction product.
Sections processed for electron microscopy were postfixed in 2% osmium tetroxide in 0.16 M sodium cacodylate buffer (pH 7.4) for 1 h, dehydrated in graded ethanols and acetone, and flat embedded in Polybed 812 (Polysciences, Warrington, PA, USA) in slide molds, between sheets of Aclar (Ted Pella, Redding, CA, USA). Selected areas of the BL that contained PV+ interneurons receiving multiple contacts from PHA-L labeled type 1 axons were remounted onto resin blanks. Serial silver thin-sections were collected on formvar-coated slot grids, stained with Uranyl Acetate and Lead Citrate, and examined with a JEOL-200CX electron microscope. Micrographs were taken with an AMT XR40 digital camera system (Advanced Microscopy Techniques, Danvers, MA, USA). For publication, figures were then assembled, labeled and their components’ tonal ranges adjusted and matched using Adobe Photoshop 6.0.
Electron microscopic analysis focused on the contacts formed by large PHA-L+ type 1 axon terminals with PV+ interneurons seen at the light microscopic level. Serial sections were analyzed and often followed on consecutive grids. Serial sections were helpful for determining the synaptic nature of contacts, and identifying multiple targets of individual PHA-L+ terminals. Synapses were identified using standard criteria: (1) parallel presynaptic and postsynaptic membranes exhibiting membrane thickenings, (2) a synaptic cleft containing dense material, and (3) clustered synaptic vesicles associated with the presynaptic membrane (Peters et al., 1991). Asymmetrical and symmetrical synapses were identified based on the presence or absence, respectively, of a prominent postsynaptic density and on the relative widths of their synaptic clefts. Whereas synaptic clefts of asymmetrical synapses are typically 20 nm wide, symmetrical synapses have a much narrower synaptic cleft that is only about 12 nm wide (Peters et al., 1991). Similar to many GABAergic symmetrical synapses in the cerebral cortex (e.g. Fig. 11–Fig. 9 of Peters et al., 1991), the postsynaptic densities of many symmetrical synapses formed by type 1 terminals in the BLa were very thin and created the appearance of a thickened postsynaptic membrane, rather than forming a discrete thickening below the postsynaptic membrane. Postsynaptic profiles were identified as perikarya, larger caliber (>1 µm) and smaller caliber (<1 µm) dendrites, and dendritic spines using established morphological criteria (Peters et al., 1991).
Fig. 11.
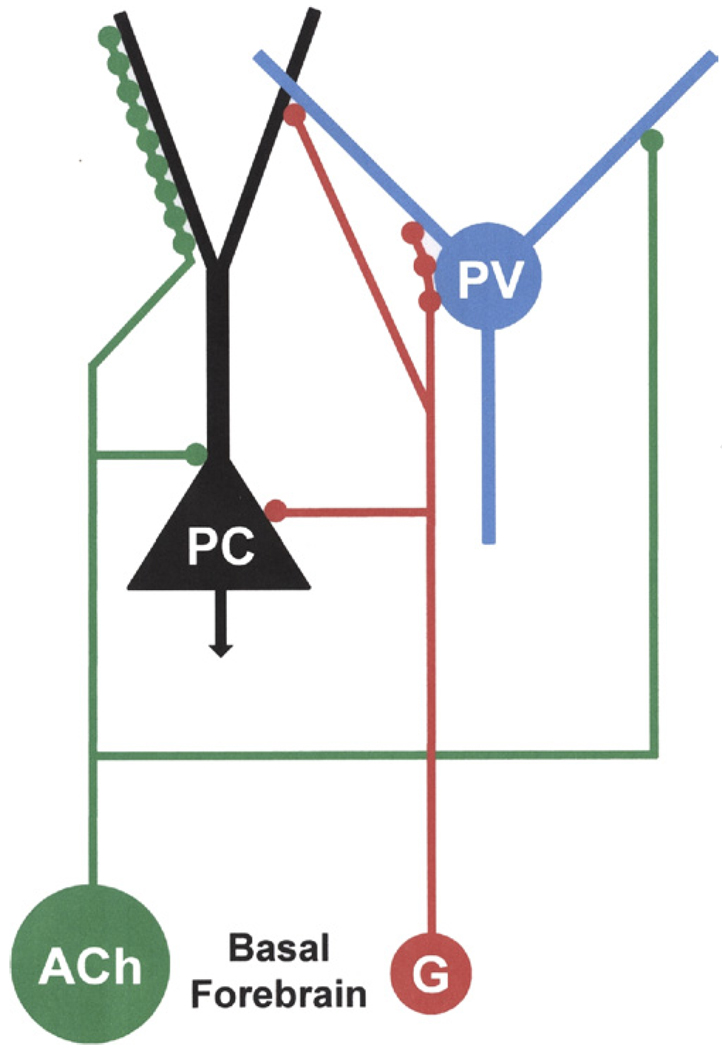
Diagram illustrating the innervation of pyramidal cells (PC) and parvalbumin-immunoreactive interneurons (PV) in the basolateral amygdala by basal forebrain GABAergic neurons (G) and cholinergic neurons (ACh). The results of the present study indicate that axons of BF GABAergic neurons (red) form multiple synaptic contacts with the perisomatic compartment of PV+ interneurons, but that some terminals form synapses with both PV+ interneurons and putative PCs, or only with PCs. A previous study demonstrated that BF cholinergic axons (green) mainly innervate the distal dendritic compartment of PCs, including dendritic spines, but that about 7% of the cholinergic axon terminals formed synapses with PV+ interneurons (Muller et al., in press). The difference in the sizes of the green (cholinergic) versus red (GABAergic) circles represents the difference in the relative numbers of these amygdalopetal BF neuronal subpopulations, with the GABAergic subpopulation constituting about 10–15% of the total population of amygdalopetal BF neurons (Mascagni and McDonald, 2009>), and the cholinergic subpopulation constituting about 75–80% (Carlsen et al., 1985; Záborszky et al., 1986). The reciprocal interconnections between basolateral amygdalar PCs and PV+ interneurons are not illustrated. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
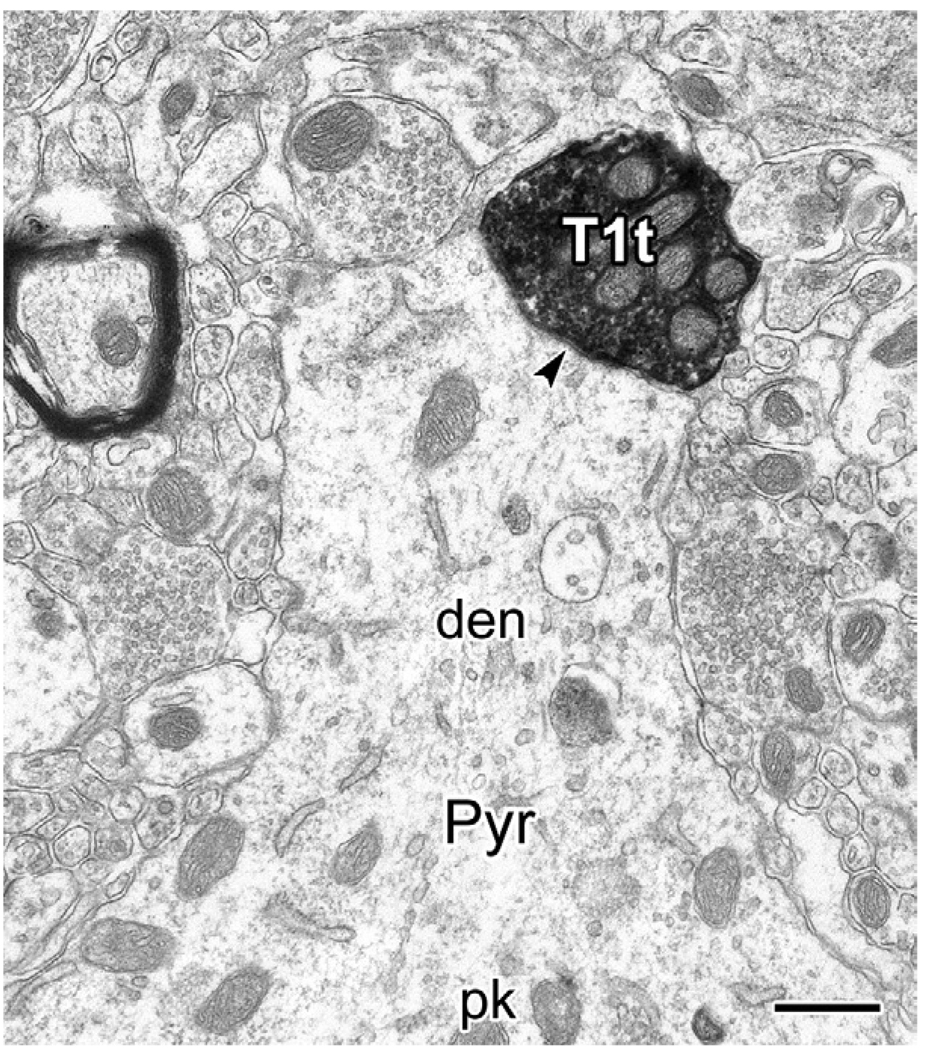
Fig. 9.
Electron micrographs of a PHA-L+ type 1 terminal (T1t) making synaptic contact (arrowhead) with the base of a proximal dendrite (den) emerging from an unlabeled putative pyramidal cell perikaryon (Pyr, pk). Scale bar: 0.5 µm.
RESULTS
Light microscopic dual-labeling immunoperoxidase studies
Rats with injection sites involving substantial portions of the SI and/or VP had a high density of anterogradely labeled axons in the basolateral amygdala on the side of the injection (Fig. 1). In some cases these injections also spread into surrounding areas, including the ventral striatum, olfactory tubercle, bed nucleus of the stria terminalis, or lateral preoptic area. However, injections centered in each of these surrounding areas, with no involvement of the SI/VP, produced few if any labeled axons in the basolateral amygdala, although in most cases labeled axons were seen in the central nucleus. In rats with unilateral injections, no labeled axons were found in the contralateral amygdala.
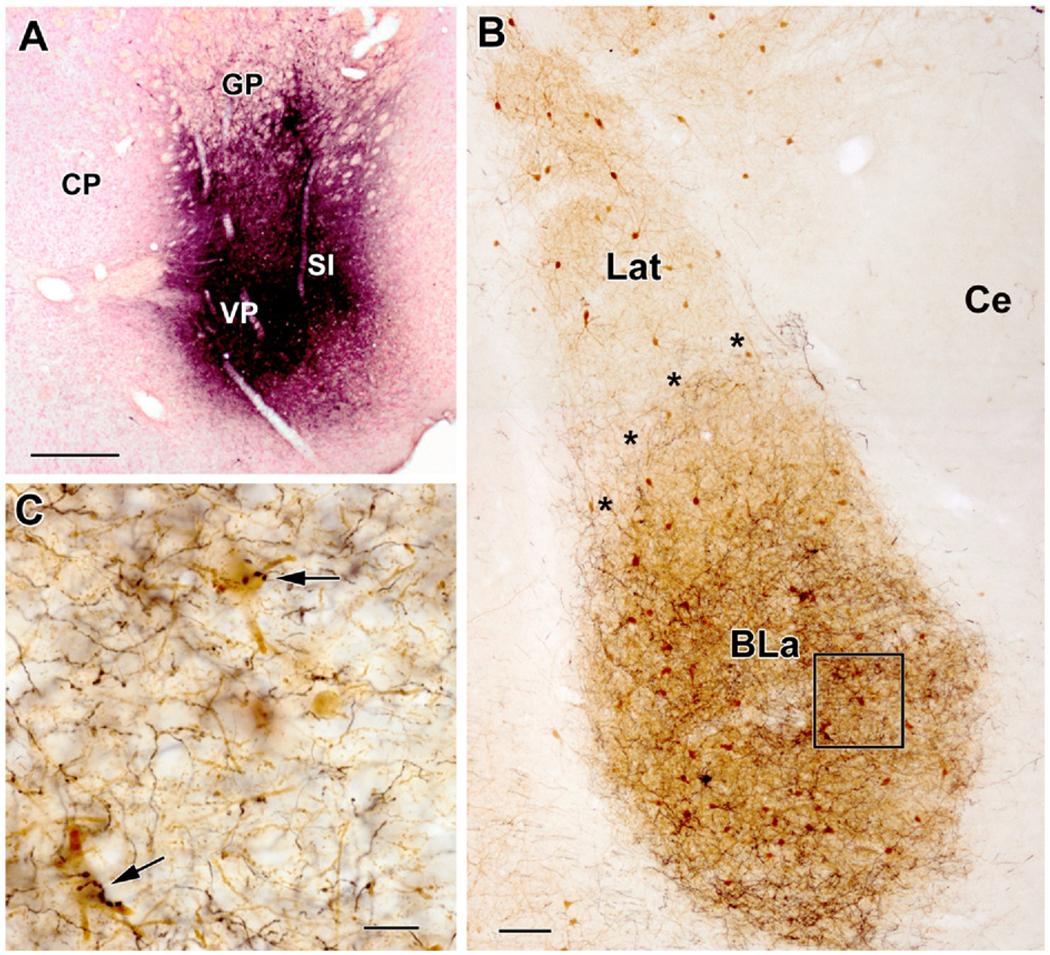
Fig. 1.
PHA-L injections into the basal forebrain mainly label axons in the basolateral amygdalar nucleus. (A) Representative dual injections of PHA-L into the caudal basal forebrain (BF) that involved the ventral pallidum (VP) and substantia innominata (SI) at the bregma −0.8 level (left is lateral; CP: caudatoputamen, GP: globus pallidus). (B) Parvalbumin-stained section through the basolateral amygdala (bregma −2.5 level) in the animal with the injections shown in (A). Parvalbumin-positive (PV +) structures are stained brown. PHA-L-labeled axons (black) are mainly confined to the anterior subdivision of the basolateral nucleus (BLa; asterisks mark the border of BLa and the lateral nucleus [Lat]). Few if any axons were seen in the lateral nucleus or central nucleus (Ce). Note that the dorsomedial quarter of the BLa has a much lower density of labeled axons than the rest of the BLa; anterograde tracer injections into more rostral portions of the amygdalopetal BF (bregma+0.2 to −0.3) produced the reverse pattern at this level, indicative of a rough topographical organization to BF afferents to the BLa. (C) Higher power view of the region in box (B). Note that the great majority of axons have small varicosities (type 2 axons), but two axons with large clustered varicosities (type 1 axons) innervate PV+ neurons via multiple contacts (arrows). Scale bars: (A) 500 µm; (B) 100 µm; (C) 20 µm. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
With the injection parameters used in this study, PHA-L injections were much larger than BDA injections and produced a higher density of labeled fibers in the amygdala. Some BDA-injected brains, but not PHA-L-injected brains, exhibited a small number of retrogradely labeled pyramidal cells in the basolateral amygdala. Although the labeling of the axonal collaterals of the latter cells could potentially contribute to the axonal labeling in the area surrounding these cells, the distribution and morphology of labeled axons (including type 1 axons, see below) appeared to be identical in both BDA (four cases) and PHA-L preparations (five cases). This suggests that little if any of the axonal staining in the BDA preparations was due to staining of axons of retrogradely labeled pyramidal cells. Nevertheless, all quantitative studies and all photomicrographs of immunoperoxidase material were done on brains with PHA-L injections. In addition, only PHA-L was used in the triple-labeling immunofluorescence and electron microscopic studies in this investigation.
With injections centered in caudal portions of the SI/VP (i.e. caudal to the crossing of the anterior commissure; Fig. 1A) the density of labeled axons was very high in the anterior subdivision of the BL (BLa; Fig. 1B, C), including its rostral pole, and in the rostral and lateral portions of the posterior subdivision of the BL (BLp). Anterograde labeling in the lateral and basomedial nuclei was very sparse. In contrast, with injections centered in rostral portions of the amygdalopetal basal forebrain (i.e. at or rostral to the crossing of the anterior commissure, where the VP, but not the SI, is present) the density of labeled axons was very high in the caudal three-fourths of BLa, especially in the dorsomedial portions of the nucleus, but very few axons were seen in its rostral pole. There was also a moderate density of labeled axons in the medial two-thirds of the BLp and adjacent rostroventral portion of the lateral nucleus with rostral basal forebrain injections. Only a few axons were observed in the basomedial nucleus and caudodorsal part of the lateral nucleus. Although this study focused on the basolateral amygdala, most injections into the SI/VP also produced some labeled axons in the central nucleus and cortical nuclei. Thus, these observations indicate that the BL is the main target of the SI/VP projection, and these afferents exhibit a rough topography.
PHA-L labeled axons in the basolateral amygdala were morphologically heterogeneous (Figs. 1C and 2). At least three main types could be recognized. Type 1 axons exhibited large round or ovoid varicosities (ca. 1.5–2.5 µm in diameter) that were often clustered (Figs. 1C and 2A – D). Some clustered varicosities were associated with short side branches of the axons. Type 2 axons exhibited small fusiform or ovoid varicosities (ca. 0.5 µm in diameter) that were uniformly distributed (Figs. 1C and 2E). Type 3 axons had medium-sized round or ovoid varicosities (ca. 1.0 µm in diameter) that were fairly uniformly distributed (Fig. 2F). The morphology of the type 1 and type 2 axons closely resemble that of their namesakes in the hippocampus that are labeled by anterograde tracer injections into the medial septal region (Freund and Buzsáki, 1996). Axons of all types were highly branched and often one type of axon predominated in particular fields in the basolateral nucleus (Fig. 2), suggesting that either one BF neuron, or a small number of BF neurons of the same type, gave rise to the labeled axons. In general, the density of type 2 axons was much greater than either type 1 or type 3 axons (e.g. see Fig. 1C).
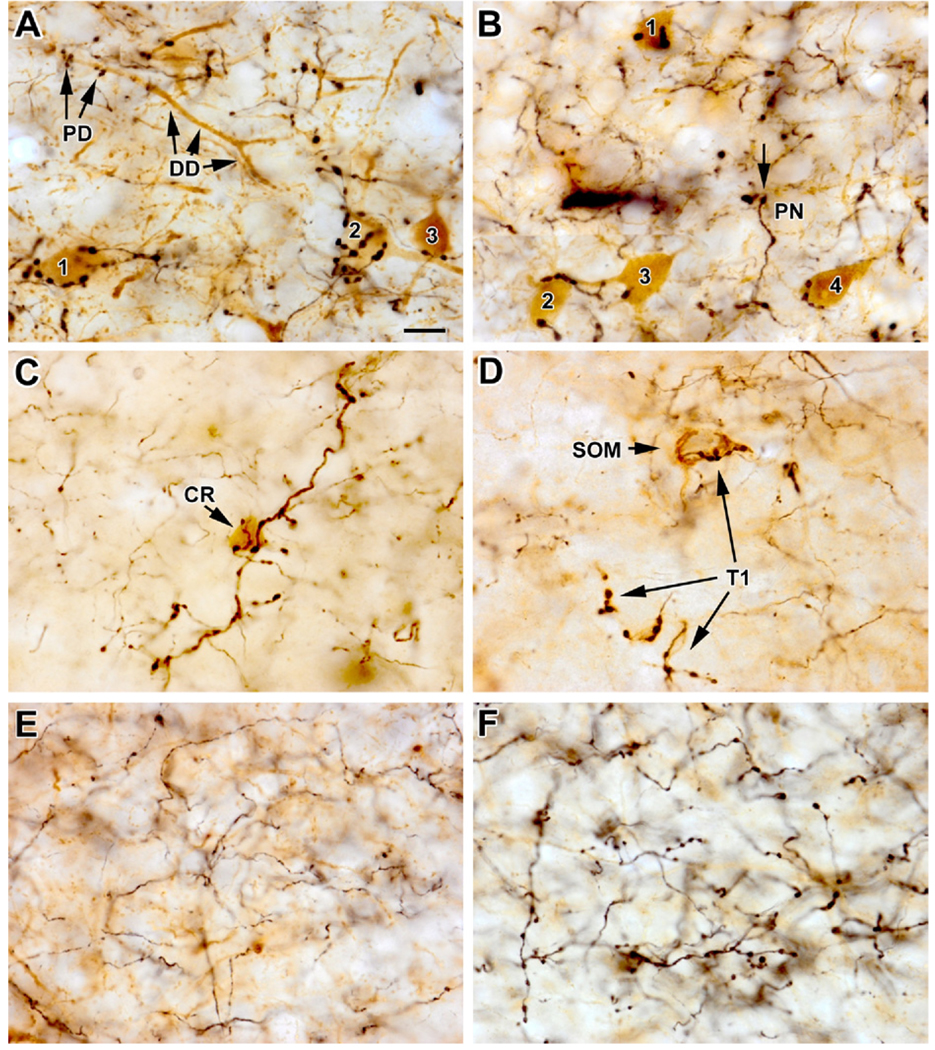
Fig. 2.
PHA-L labeled axons in the basolateral nucleus. (A) Photomontage of PHA-L-labeled type 1 axons (black) innervating PV+ interneurons (brown) in the BLa in a field dominated by these axons. Varicosities of type 1 axons are often large and clustered, and make multiple climbing-fiber-like contacts with the cell bodies and proximal dendrites of PV+ interneurons. Single large varicosities contacting PV+ structures are also seen. Note multiple contacts with three PV+ cell bodies in the lower half of the field (cells 1–3) and several PV+ dendrites in the upper half of the field. Also note multiple contacts with the proximal dendritic segment (PD), but not the distal dendritic segment (DD), of a diagonally-oriented PV+ dendrite. (B) Photomontage of PHA-L-labeled type 1 axons (black) innervating PV+ interneurons (brown) in the BLa in a different animal. Four PV + interneurons (cells 1–4) are innervated by one or more varicosities. Note that some large varicosities of putative Type 1 axons do not contact PV + structures. One such varicosity (arrow) is in close proximity to the soma of a putative pyramidal neuron (PN) that is surrounded by numerous, small, brown PV+ axon terminals. (C) Photomontage of a diagonally oriented type 1 axon (black) in a PHA-L/CR preparation. Large varicosities of this axon contact the soma of a CR+ neuron (brown). The remaining varicosities of this axon do not contact CR+ structures. The other axons in this field are type 2. (D) Photomontage of a several segments of type 1 axons (T1; black) in a PHA-L/SOM preparation. In the upper part of the field a SOM+ soma (brown) is contacted by several varicosities of a type 1 axon. In the lower part of the field the varicosities of type 1 axons do not contact SOM+ structures. (E) Type 2 axons (black) in the BLa in a preparation in which PV+ structures were stained brown. (F) Type 3 axons (black) in the BLa in a preparation in which PV+ structures were stained brown. Note that the varicosities are slightly smaller than most type 1 varicosities, and are more uniformly distributed along the axon. Scale bar: (A) 10 µm (B–F are at the same magnification). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Most of the type 1 axons in the BL made multiple climbing-fiber-like contacts with the somata and proximal dendrites of parvalbumin-positive (PV+) interneurons, especially in the BLa (Fig. 2A, B). In some cases these axons were in the form of pericellular baskets surrounding PV+ somata. Varicosities of type 1 axons occasionally made single contacts with distal dendrites of PV+ neurons. Very few type 1 fibers were observed in the lateral, basomedial, and cortical nuclei, and none were seen in other amygdalar nuclei. In order to determine the extent of innervation of PV+ neurons by type 1 axons, cell counts of PV neurons that received at least two contacts from individual type 1 axons were made in two rats in portions of the BLa that exhibited dense innervation by type 1 axons. These counts revealed that 69% (59/86) of PV neurons in these areas received multiple contacts, mainly with somata and proximal dendrites. The average number of contacts per neuron for these 59 cells was 5.56±3.21 (mean±SD). The most contacts seen with one neuron was 14. However, some varicosities of type 1 axons did not contact PV+ structures (Fig. 2B). Although single large varicosities contacting CR+ interneurons were sometimes observed, only a few baskets surrounding these neurons were seen (Fig. 2C). Likewise, only a small number of SOM + somata were seen to receive multiple contacts from type 1 axons (Fig. 2D). The great majority of type 2 and type 3 axons did not appear to contact PV+ (Fig. 2E, F), CR+, or SOM + neurons.
Triple-labeling immunofluorescence studies
Immunohistochemistry for the vesicular GABA transporter protein (VGAT) was performed in the BLa to determine if the type 1 axons identified in the immunoperoxidase experiments were GABAergic. Contacts of PHA-L-labeled type 1 fibers with CB+ interneurons or CaMK+ pyramidal cells were examined for VGAT immunoreactivity. PV antibodies were not used to label BLa interneurons in these experiments because BF GABAergic projection neurons contain PV (Freund, 1989; Gritti et al., 2003; Mascagni and McDonald, 2009), and it would be difficult to clearly distinguish presynaptic PV+ type 1 axons from their postsynaptic PV+ BLa interneuronal targets at the light microscopic level. Approximately 85% of PV+ interneurons in the BLa are CB+, constituting approximately 60% of all CB+ interneurons (McDonald and Betette, 2001).
The PHA-L injections into the basal forebrain and the distribution/morphology of PHA-L+ fibers in the BLa were similar to those described for the immunoperoxidase preparations. The distribution/morphology of CB+ interneurons and CaMK+ pyramidal cells in the BLa was identical to that described in previous studies (Kemppainen and Pitkänen, 2000; McDonald and Mascagni, 2001; McDonald et al., 2002), and there was no colocalization of PHA-L with either of these markers (Fig. 3). VGAT+ puncta were seen in high density in the BLa. Most of these puncta were small, but some were larger and approximated the size of type 1 axonal varicosities (Fig. 3). There was light punctate VGAT immunoreactivity in the somata of CB+ interneurons (which are GABAergic), but not in the somata of CaMK+ pyramidal cells.
Fig. 3.
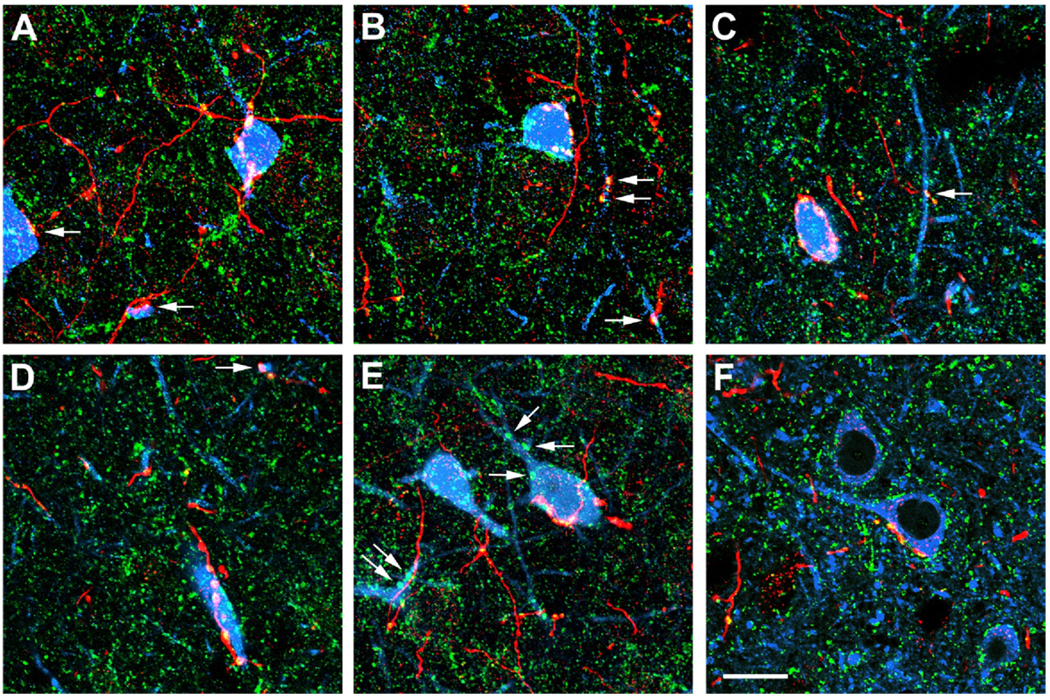
Triple immunofluorescence labeling for PHA-L, VGAT, and neuronal markers in the BLa. PHA-L+ axons are red, VGAT is green, and neuronal markers (CB in A–E; CaMK in F) are blue. Yellow indicates colocalization of PHA-L and VGAT (i.e. GABAergic axons). White indicates yellow axons overlapping with blue neurons. (A) A CB+ soma (blue, right) receives multiple contacts from a type 1 GABAergic axon (yellow/white). Additional contacts (arrows) with another CB+ soma (far left) and a dendrite (lower center) are also seen. (B) A CB+ soma (blue, center) receives multiple contacts from type 1 GABAergic axon terminals (yellow/white). Additional contacts are seen with a vertically oriented CB+ dendrite (arrows). (C) A CB+ soma (blue) receives multiple contacts from type 1 axon terminals (yellow) that form a basket around the cell. This image is from a z-series through a portion of the cell body; this CB+ soma actually received 10 contacts from type 1 GABAergic terminals. An additional contact is seen with a vertically oriented CB+ dendrite (arrow). (D) A large-caliber proximal CB+ dendrite (blue, lower center) receives five contacts from a type 1 GABAergic axon (yellow). Additional contacts are seen with a small-caliber CB+ dendrite (arrow). (E) A CB+ soma (blue, center) receives multiple contacts from a type 1 GABAergic axon (yellow). Several large, green, single-labeled VGAT varicosities (arrows) contact this CB+ neuron, and a dendrite of another CB+ neuron (far left). These varicosities may represent type 1 axons from GABAergic basal forebrain neurons that were located outside of the PHA-L injection site. (F) A CaMK+ soma (blue, center) receives multiple contacts from a type 1 GABAergic axon (yellow). Also note numerous small, green, single-labeled VGAT varicosities that contact the soma and apical dendrite (extending left) of this CaMK+ neuron, as well as the somata of other neighboring CaMK+ neurons in this field. These varicosities may represent GABAergic axons of local interneurons that have axon terminals that are smaller than those of type 1 GABAergic basal forebrain axons. Scale bar: (F) 20 µm (A–E are at the same magnification). For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
In the CB preparations it was common to observe large type 1 PHA-L+ terminals contacting somata or dendrites of CB+ interneurons (Fig. 3A – E). Individual PHA-L+ axons often made multiple contacts with these interneurons, but sometimes single contacts were seen, especially with dendrites, similar to what was observed in the peroxidase preparations. Virtually all of these type 1 PHA-L+ varicosities contacting CB+ structures were VGAT+, indicating that they were GABAergic axon terminals (Fig. 3A – E). In contrast to the CB preparations, very few type 1 PHA-L + axons were seen contacting CaMK+ pyramidal cells. In the latter instances type 1 PHA-L+/VGAT+ axonal varicosities forming multiple or single contacts with the somata or dendrites of CaMK+ pyramidal cells were observed (Fig. 3F).
Electron microscopic dual-labeling immunoperoxidase studies
As in the light microscopic and immunofluorescence preparations, rats processed for electron microscopy had PHA-L injection sites that involved substantial portions of the SI and VP. Light microscopic observations revealed that these injections produced a high density of labeled axons, including some type 1 axons with large varicosities that made multiple contacts with PV+ interneurons in the BLa. Areas with one or more of these innervated PV+ interneurons were selected for correlated electron microscopic analysis.
In the electron microscope, as in the light microscope, a variety of DAB-labeled PHA-L+ terminals were observed that measured approximately 0.5 µm to over 2 µm in diameter. Large type 1 axon terminals and their PV+ interneuronal targets identified at the light microscopic level were easily recognized at the electron microscopic level. Based on reconstruction through serial thin sections, these terminals were found to be ovoid, with their short axes measuring from 1.4 µm to approximately 2 µm, and their long axes measuring between 2 µm and 2.5 µm in diameter (Figs. 4–10). Type 1 terminals were densely packed with mitochondria and synaptic vesicles and were generally the most electron dense terminals in a given field (compare with a putative type 2 terminal in Fig. 7C). All contacts of type 1 terminals with PV+ interneurons observed at the light microscopic level were found to be symmetrical synapses when examined ultrastructurally. These axon terminals commonly exhibited more than one active zone, and individual active zones were often broad. The intervaricose segments varied from very slender and devoid of synaptic vesicles (Fig. 4B, D) to broad, packed with vesicles, and often making synaptic contact with the same profile as the adjacent terminal swellings (Figs. 4B, 6A, C and 7C), thus extending the synaptic area encompassed by two neighboring type 1 terminals.
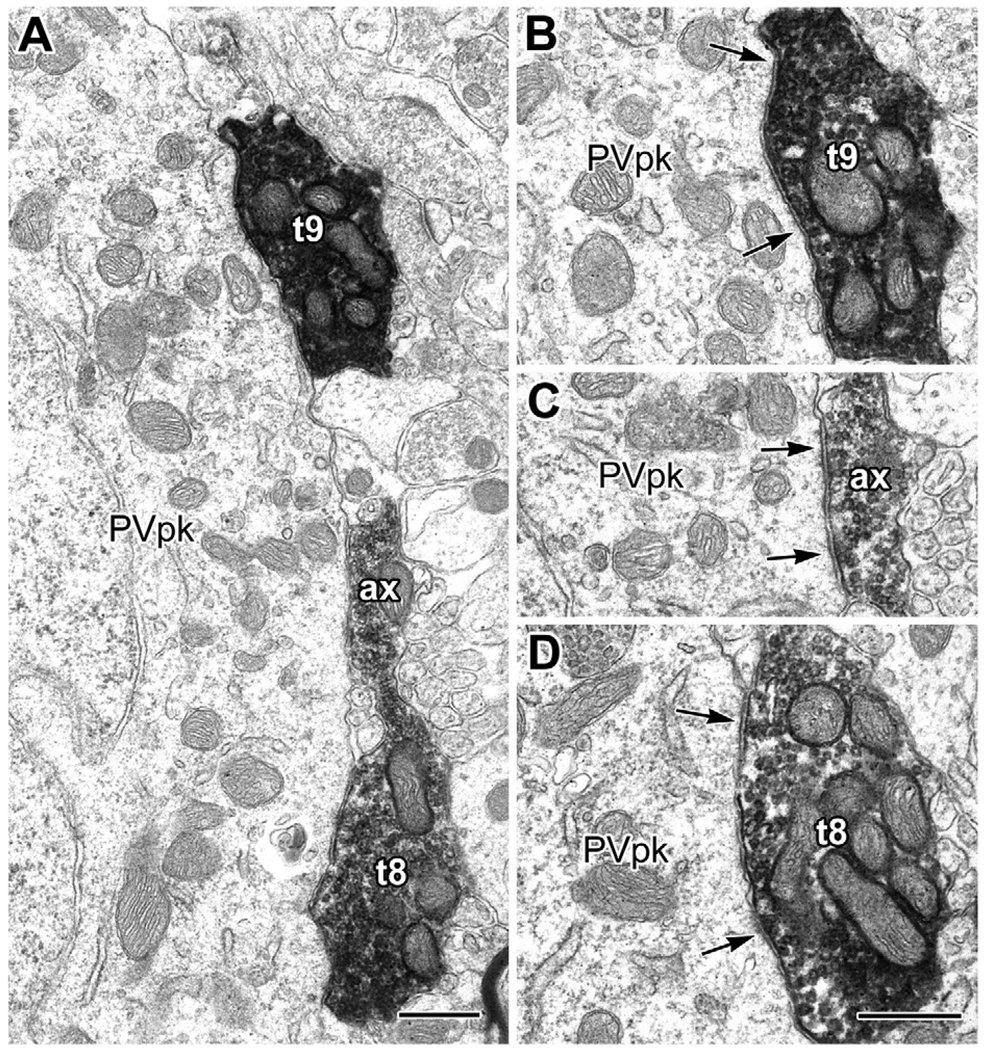
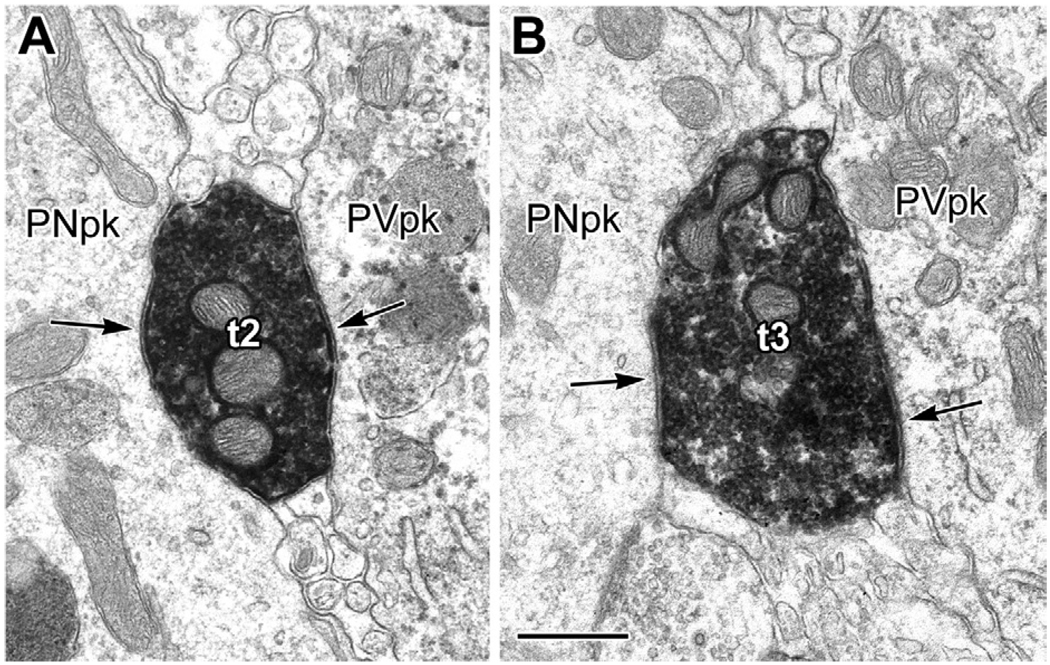
Fig. 4.
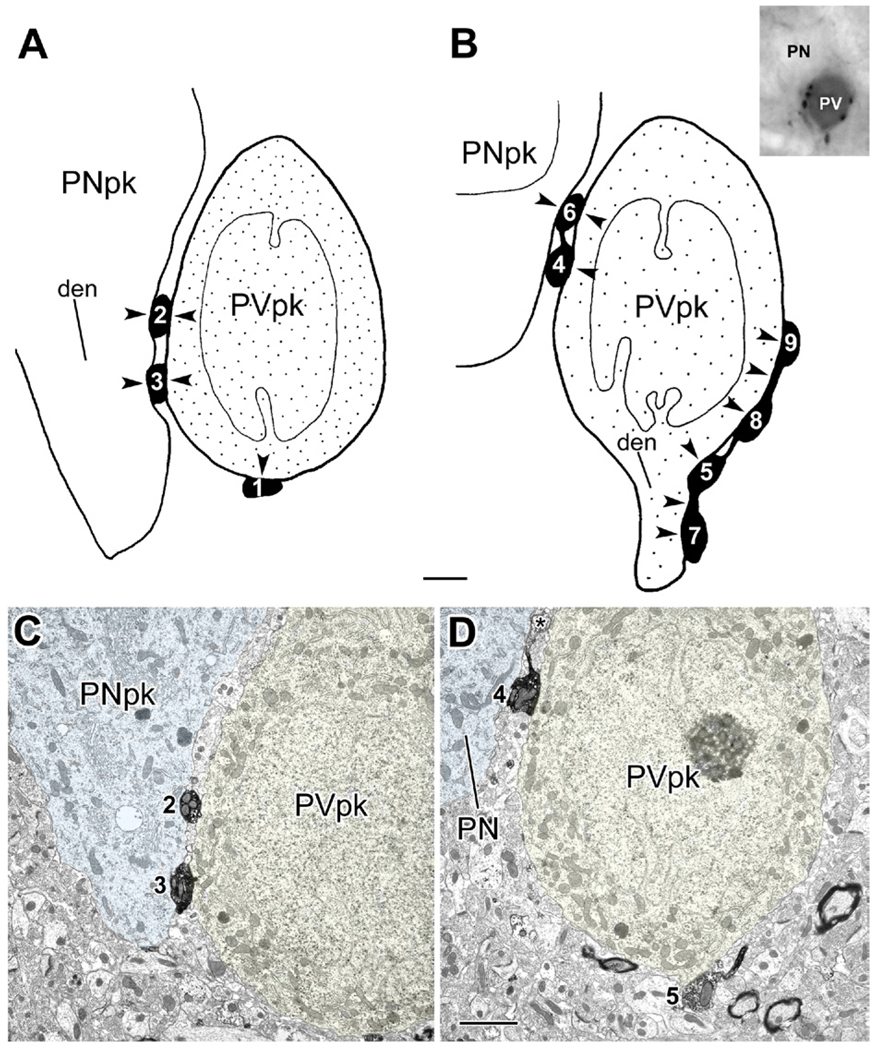
Synaptic contacts from type 1 PHA-L+ terminals with the perikarya of a PV-immunoreactive interneuron and an unlabeled putative pyramidal neuron (inset in B). (A, B) Schematic drawings, at two levels, of a single parvalbumin-immunoreactive interneuronal perikaryon (PVpk, stipple) in the BL, receiving synaptic inputs (arrowheads) from nine large (>1.5 µm in diameter) PHA-L+ terminals (black, with white numbers) from type 1 axons projecting from the basal forebrain. These nine large terminals were followed through 8 µm of serial thin sections and numbered in the order that they were encountered in the series. The drawings are derived from tracings of 10,000× electron micrographs (see panels C, D), with terminals 1–3 (A) encountered in the first 4 µm of the series and terminals 4–9 (B) encountered in the last 3 µm. The stipple in the PVpk indicates the granular PUR reaction product of PV immunoreactivity, which was sparser at greater depths in the series (sparser stippling in B). Terminals 2, 3, and 6 also make synaptic contacts (arrowheads) onto an adjacent unlabeled putative pyramidal neuron perikaryon (PNpk) or its proximal dendrite (den in A). In addition to exhibiting a pyramidal-shaped perikaryon, this neuron received only symmetrical synapses onto its soma, which is characteristic of pyramidal neurons, but not interneurons, in the BL. (B) The PVpk gives rise to a dendrite (den) as terminal 5 extends into terminal 7. The intervaricose segment between terminals 5 and 7 also makes synaptic contact with the PVpk (arrowhead). This is also true for the axonal segment between terminals 8 and 9 (arrowhead; Fig. 6). Inset: Light micrograph of the PV+ interneuron and adjacent pyramidal neuron flat-embedded in resin for thin sectioning. (C, D) Representative electron micrographs of the series that were traced to produce the drawings in (A, B). (C) Micrograph from the series represented by drawing (A). Terminals 2 and 3 each make synaptic contact (see Fig. 5) with both the PV+ interneuron (PVpk) and the putative pyramidal neuron (PNpk), but were found through serial sections not to be part of the same axon. (D) Micrograph from the series represented by drawing (B) (a thin section 2.8 µm further in the series than C). Terminal 4 makes synaptic contact onto the PVpk and appositional (not clearly synaptic) contact with the adjacent PN. Both terminals 4 and 5 give rise to slender axonal profiles that are devoid of synaptic vesicles. In this view terminal 6 (asterisk) is beginning to emerge from the axon leading from terminal 4. The slender axon leading from terminal 5 gives rise to terminal 8 later in the series. Scale bars: 2 µm. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
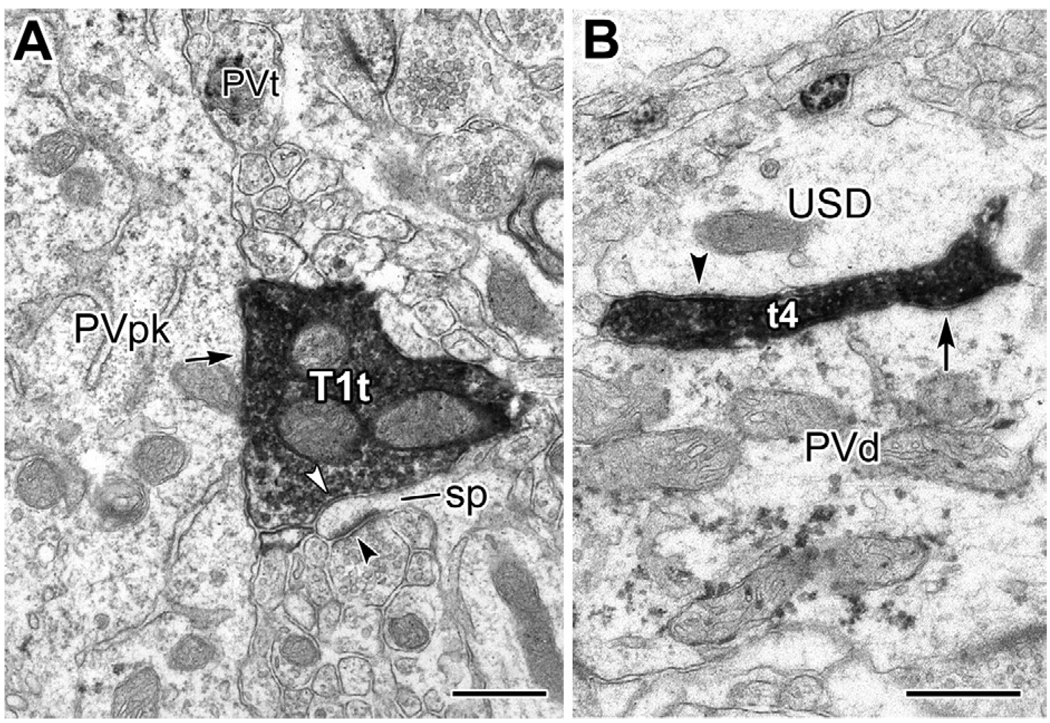
Fig. 10.
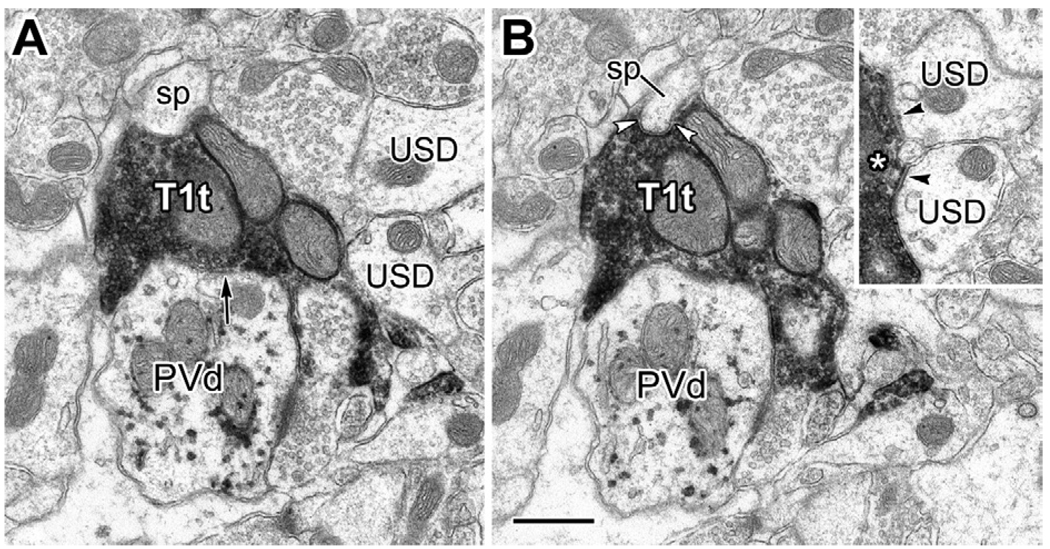
Electron micrographs of serial sections through a type 1 terminal (T1t) making synaptic contact with a PV+ dendrite (PVd; arrow in A) and a dendritic spine (sp, framed by white arrowheads in B). Inset: Five thin sections after the view in panel (A), the two unlabeled small-caliber dendrites in (A) (USD) also receive synaptic contacts from the T1t (black arrowheads; asterisk marks the right edge of T1t). Scale bar: 0.5 µm.
Fig. 7.
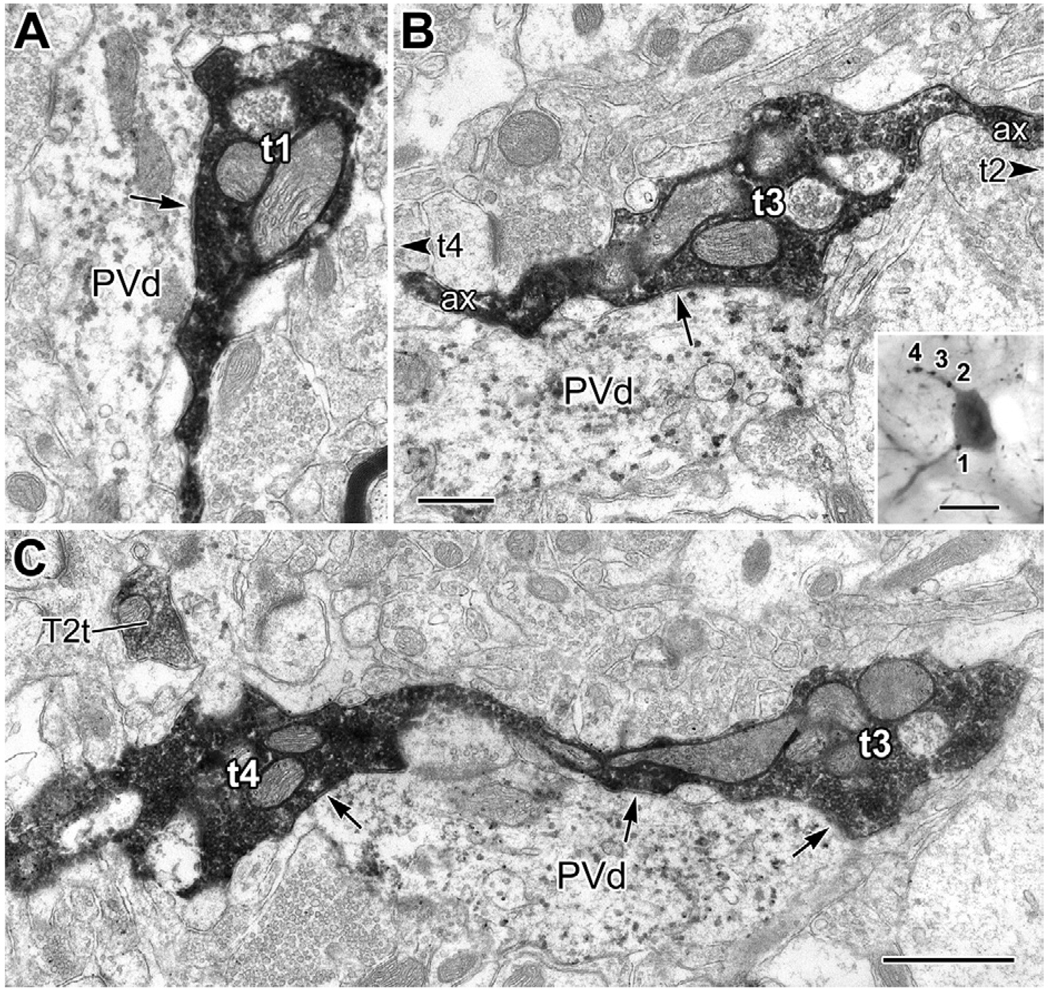
Synaptic contacts from type 1 PHA-L+ terminals onto the proximal dendrites of a PV+ interneuron (inset in B). (A) The proximal end of the lower dendrite of the PV+ interneuron (PVd) receives synaptic input (arrow) from a large PHA-L+ terminal (t1: terminal #1, inset in B). (B) Serial section analysis revealed that terminals 2–4 (inset) belonged to the same axon. In this view, the upper proximal dendrite (PVd) of the PV+ interneuron (inset) receives synaptic input from t3 (arrow). The connecting axon (ax), and the direction where t2 and t4 are located, are indicated (arrowheads). Inset: Light micrograph of the PV+ interneuron receiving contacts from four large terminals (1–4), belonging to type 1 axons projecting from the basal forebrain, flat-embedded in resin for thin sectioning. (C) Three thin sections away from the view in panel (B), t3 and t4 are shown to be linked by their axon. Three sites of synaptic input (arrows) to the PV+ proximal dendrite (PVd), confirmed in serial sections, are indicated. A small PHA-L+ terminal from a putative type 2 basal forebrain axon (T2t) is seen nearby for comparison. Scale bars: 0.5 µm in (B) (for A and B); 1 µm in (C); 20 µm in the inset.
Fig. 6.
Electron micrographs of terminals 8 and 9 (t8, t9) and the axonal segment bridging them (ax) that make synaptic contact onto the PV + interneuron from Fig. 4B, D. (A) Lower magnification view of t8, t9, and the axonal segment between them. (B, C) Synaptic contacts from t9 (B) and its axonal extension (ax, in C) onto the PV+ interneuron (PVpk) shown in Fig. 4 (arrows bracket a broad synaptic contact in (B), and indicate two active zones in C). Both (B, C) were taken from two thin sections further in the series than the view in (A). (D) Synaptic contacts (arrows) from t8 onto the PVpk, taken from three thin sections before the view in (A). Scale bars: 0.5 µm.
Serial section analysis was performed on type 1 terminals that contacted eight PUR-labeled PV+ interneurons. These PV+ interneurons were contacted by a total of 30 type 1 terminals and were surveyed from three samples, representing two experiments. Some type 1 axons mainly contacted the somata of PV+ neurons (Figs. 4–6), whereas others targeted dendrites (Fig. 7). Some of the type 1 terminals contacting individual PV+ neurons were clearly from different axons (Figs. 4A and 7A) and others were interconnected as part of the same axon (Figs. 4B, 6, and 7C).
Most type 1 terminals surveyed made other synaptic contacts in addition to those onto PV+ structures (Figs. 4, 5, 8, and 10). Of the 27 (out of 30) type 1 terminals that were substantially reconstructed through serial thin sections, 18 (67%) formed synapses with additional neuronal profiles, forming a total of 33 additional synapses. Of these 33 synapses, three (9%) were with PV+ dendrites that were not associated with the PV+ neuron initially analyzed, 15 (45%) were with dendritic spines (Fig. 8A), 11 (33%) were with small-caliber unlabeled dendritic shafts (Fig. 8B); and four (12%) were with putative pyramidal cell perikarya (Figs. 4 and 5). The latter neurons had pyramidal-shaped perikarya and received only symmetrical synapses onto their somata, which is characteristic of pyramidal neurons, but not interneurons, in the BL (Muller et al., 2005, 2006). Since most dendritic spines originate from distal dendrites of pyramidal neurons (Muller et al., 2006), these counts indicate that many of the additional synapses were formed with the distal dendritic compartment of pyramidal cells.
Fig. 5.
(A, B) Electron micrographs of PHA-L+ terminals 2 and 3 (t2, t3) shown in Fig. 4A, C, making synaptic contacts (arrows) onto the PV + interneuron (PVpk) and putative pyramidal neuron (PNpk). Scale bar: 0.5 µm.
Fig. 8.
Type 1 axons frequently form synapses with multiple targets, including dendritic spines of putative pyramidal cells. (A) Electron micrograph of one of three type 1 terminals (T1t) forming synapses with a lightly labeled PV+ interneuron (PVpk, arrow). In addition, this T1t makes synaptic contact with a dendritic spine (sp, white arrowhead), which also receives an asymmetrical synapse (black arrowhead) from an unlabeled terminal. Serial sections confirmed that the T1t and a nearby PV+ terminal (PVt) make synaptic contact with the PVpk. (B) Electron micrograph of the edge of terminal 4 (t4) from Fig. 7. In addition to synaptic contacts onto the proximal dendrite of a PV+ interneuron (arrow; see also Fig. 7C), t4 makes synaptic contact with an adjacent unlabeled small-caliber dendrite (USD, arrowhead). Scale bars: 0.5 µm.
Additional nonquantitative surveys of one sample from each experiment found other examples of type 1 terminals forming synapses with putative pyramidal cell proximal dendrites (Fig. 9), as well as other unlabeled dendrites and spines, sometimes in addition to forming synapses with PV+ dendrites (Fig. 10).
DISCUSSION
This study demonstrates that the axons of basal forebrain inputs to the basolateral amygdala are morphologically heterogeneous, and include GABAergic type 1 axons that exhibit clusters of large terminals. As in the hippocampus and neocortex, these distinctive type 1 terminals make symmetrical synaptic contacts, and are characterized by numerous mitochondrial profiles and large numbers of synaptic vesicles. The main postsynaptic targets of type 1 axon terminals in the basolateral amygdala are parvalbumin-immunoreactive interneurons, but many individual terminals make synaptic contacts with additional structures, including the somata and dendrites of putative pyramidal cells.
Consistent with a retrograde tract tracing study (Ottersen, 1980), the results of the present study indicate that the BL is the main target of the BF projections to the rat amygdala. Projections to the BL were also observed with injections of anterograde tracers into the ventral pallidum (Haber et al., 1985) or SI (Grove, 1988) in previous investigations, but these studies provided no information regarding the morphology, neurotransmitters, or neuronal targets of labeled axons. The great majority of labeled axons in the present study were type 2 axons with small varicosities. The morphology and density of these axons as seen with both light and electron microscopy closely matched that of cholinergic axons in the BL (Wainer et al., 1984; Carlsen and Heimer, 1986; Nitecka and Frotscher, 1989; Li et al., 2001; Muller et al., in press). Axons of similar morphology in the hippocampus and neocortex labeled by injections of anterograde tracers into the BF are also thought to be cholinergic (Freund and Antal, 1988; Henny and Jones, 2008). Type 3 axons in the BL had varicosities that were intermediate in size between type 1 and type 2 varicosities. Since varicosities of similar size and morphology in the neocortex are immunoreactive for vesicular glutamate transporter protein 2 (VGLuT2; Henny and Jones, 2008), it is tempting to speculate that type 3 axons in the BL may be glutamatergic.
This is the first study to determine the neuronal targets of amygdalopetal BF GABAergic projection neurons (BFGNs). The distinctive morphology of type 1 BF axons in the BL, with large clustered varicosities forming contacts with interneurons, is identical to that of type 1 axons in the hippocampus (Freund and Antal, 1988; Freund and Buzsáki, 1996) and neocortex (Freund and Gulyás, 1991; Freund and Meskenaite, 1992). Postembedding immunocytochemical studies at the ultrastructural level have demonstrated that type 1 axons in the hippocampus and neocortex contain GABA (Freund and Buzsáki, 1996; Freund and Gulyás, 1991; Freund and Meskenaite, 1992). Likewise, the finding in the present study that type 1 axons in the BL are immunoreactive for VGAT indicates that they are GABAergic, as in the prefrontal cortex (Henny and Jones, 2008). Cortical type 1 terminals make symmetrical synapses, as expected for GABAergic terminals, and are characterized by numerous mitochondrial profiles, large numbers of synaptic vesicles, large synaptic contact areas, and multiple synaptic release sites. Type 1 terminals in the BL were identical to those in the cortex and were morphologically distinct from putative PHA-L-labeled type 2 terminals (see Fig. 7C). As discussed in detail by Eyre and coworkers (2007) for the hippocampus, there are obvious functional correlates of these unique morphological features of type 1 terminal synapses. The large synaptic surface area, multiple release sites, and large synaptic vesicle pool indicate reliable, large amplitude inhibitory events, and the ability for sustained, high frequency release of GABA which is correlated with the rhythmic bursting properties of BFGNs (Eyre et al., 2007). The abundant mitochondria in type 1 terminals are obviously important for supplying energy for such active synapses.
In cortical regions the types of interneurons innervated by type 1 axons varies depending on region and species. Thus, in the rat hippocampus all major interneuronal sub-populations are innervated (Freund and Buzsáki, 1996). In the rat neocortex the main subpopulations innervated are CB+ and SOM+ interneurons, and a small number of PV+ interneurons (Freund and Gulyás, 1991; Henny and Jones, 2008). However, in the cat neocortex the main subpopulations innervated are SOM+ and PV+ subtypes (Freund and Meskenaite, 1992). Since distinct subpopulations of cortical interneurons exhibit distinct innervation patterns of neighboring pyramidal cells (PCs) and interneurons (Freund and Buzsáki, 1996), these differences in type 1 axonal innervation suggest differential modulation of cortical circuits depending on the types of interneurons receiving BF GABAergic inputs in any one cortical region. The results of the present study indicate that PV+ interneurons are the main subpopulation innervated by GABAergic type 1 axons in the BL. Similar to the hippocampus (Freund and Antal, 1988), about two-thirds of PV+ interneurons in the BL received multiple inputs from type 1 axons in BL areas that exhibited dense type 1 axonal input. Since the injection sites in each animal involved only limited portions of the entire extent of the amygdalopetal BF region, it is likely that a much higher percentage of PV+ interneurons actually receive multiple inputs from GABAergic type 1 axons. Previous anatomical and physiological studies suggest that there may be separate subpopulations of PV+ interneurons in the BL with different postsynaptic targets (Rainnie et al., 2006; Muller et al., 2006; Woodruff and Sah, 2007). It remains to be determined if all of these subpopulations are innervated by type 1 axons.
One notable difference between type 1 terminals in the hippocampus and BL is that virtually all of these terminals in the hippocampus form only one synapse (Eyre et al., 2007), whereas two-thirds of type 1 terminals forming synapses with interneurons in the BL formed at least one additional synapse with a different structure. Many of the latter targets appeared to be PCs, especially their dendritic spines. Likewise, occasional VGAT+PHA-L-labeled type 1 axons were observed forming contacts with CaMK+ PCs in immunofluorescence preparations. These data are consistent with an electrophysiological study that found evidence for short-latency inhibition of both PCs and presumptive interneurons in the BL upon stimulation of the SI/VP region (Mello et al., 1992). There is also evidence that a small number of GABAergic type 1 BF axons innervate PC dendrites in the neocortex (Freund and Gulyás, 1991; Henny and Jones, 2008).
If it is assumed that most unlabeled spines and pyramidal perikarya targeted by PHA-L labeled type 1 terminals in the present EM study belong to PCs, then about one-third of the postsynaptic targets of type 1 terminals (19 of 60 synapses formed by reconstructed type 1 terminals) may be PCs. This fraction would increase to one-half (30/60) if the unlabeled dendritic shafts targeted by type 1 terminals belong to PCs. However, PCs make up about 85% of the total neuronal population in the BLa, whereas PV+ interneurons constitute about 6% (i.e. 40% of the interneurons). Since PCs in the BLa outnumber PV+ interneurons by 14:1, these data suggest that there is pronounced tendency for type 1 terminals to innervate PV+ interneurons, which is consistent with our light microscopic observations.
The BL receives one of the densest cholinergic innervations in the CNS (Ben-Ari et al., 1977; Hellendall et al., 1986; Amaral and Bassett, 1989), and approximately 75–80% of BF neurons projecting to this region are cholinergic (Carlsen et al., 1985; Záborszky et al., 1986). Although only about 10–15% of BF amydalopetal neurons are BFGNs (Mascagni and McDonald, 2009), their robust perisomatic innervation of PV+ interneurons should allow their effects on amygdalar activity to be amplified via the extensive local axonal arborizations of these interneurons (Rainnie et al., 2006). The dense perisomatic innervation of numerous PCs by PV+ GABAergic interneurons (Muller et al., 2005; Rainnie et al., 2006; Woodruff and Sah, 2007a,b) suggests that amygdalopetal BFGNs could indirectly provide robust disinhibition of BL PCs, similar to that demonstrated in the hippocampus (Tóth et al., 1997). Electrophysiological studies have shown that long-term potentiation (LTP) in the lateral nucleus of the amygdala is tightly controlled by local GABAergic interneurons, and that dopamine facilitates LTP by causing disinhibition of PCs (Bissière et al., 2003). Disinhibition generated by the activation of amygdalopetal BFGNs could presumably have the same effect on LTP in the basolateral nucleus, potentially facilitating contextual fear conditioning dependent on hippocampal inputs to this nucleus (Maren and Fanselow, 1995).
Numerous studies have shown that ensembles of BFGNs exhibit burst firing at theta frequency, and that these inputs produce theta and gamma frequency oscillations in the hippocampus and neocortex (Borhegyi et al., 2004; Lin et al., 2006). This suggests that inputs to the cortex-like BL from this system may contribute to the generation of similar oscillations in the basolateral amygdala. These oscillations may play an important role in emotional arousal and emotional memory in the BL, similar to what is seen in the lateral nucleus (Paré and Collins, 2000; Pape et al., 2005). The two main targets of these BFGN inputs, PV+ interneurons and PCs, exhibit reciprocal perisomatic connections (McDonald et al., 1995; Rainnie et al., 2006; Woodruff and Sah, 2007a,b) which may further contribute to rhythmic oscillations, and electrophysiological studies in the BL have demonstrated counterphase firing of PCs and fast-firing (presumptive PV+) interneurons during theta rhythms (Paré and Gaudreau, 1996). As in the cortex, subpopulations of PV+ interneurons form interconnected networks (Muller et al., 2005; Woodruff and Sah, 2007a), and inputs to these networks could presumably induce gamma oscillations (Freund and Buzsáki, 1996). The two main cell types targeted by BFGN inputs in the BL also receive inputs from cholinergic BF neurons (Muller et al., in press; Fig. 11), which also exhibit burst firing at theta frequency (Lee et al., 2005). Thus, as in the hippocampus (Lee et al., 1994), both cholinergic and GABAergic inputs may contribute to the generation of theta rhythms in the BL. BFGN inputs to PCs might affect spike timing in these neurons in relation to oscillatory activity in the BL (Freund and Buzsáki, 1996). The recent finding that ensembles of putative BFGNs in the SI encode the motivational salience and hedonic valence of sensory stimuli (Lin and Nicolelis, 2008), and that neurons encoding these features are also found in the basolateral amygdala (Belova et al., 2007), suggests that BF-BL networks are important for emotional learning, attention, and arousal.
CONCLUSIONS
This study demonstrates that the basolateral amygdala, like the cerebral cortex, receives GABAergic inputs from the BF that provide a significant innervation of GABAergic interneurons. Unlike the hippocampus, but similar to the neocortex, particular interneuronal subtypes are targeted in the basolateral amygdala, especially the parvalbumin containing subpopulation. At both the light and electron microscopic level, the axonal morphology of BF GABAergic inputs to the basolateral amygdala is identical to those providing inputs to the cortex. Anatomical features of these axons indicate that they provide robust inhibition, which has been verified physiologically for the cortex. Unlike the hippocampus, axon terminals of BF GABAergic inputs to parvalbumin immunoreactive interneurons of the basolateral amygdala often synapse with multiple postsynaptic targets, including putative pyramidal cells. These results suggest that GABAergic neurons of the BF provide indirect disinhibition, as well as direct inhibition, of pyramidal neurons in the basolateral amygdala.
Acknowledgments
The authors thank Dr. Alison Buchan (University of British Columbia) for donating the SOMA-8 monoclonal antibody to somatostatin. This work was supported by National Institutes of Health Grant R01-DA027305.
Abbreviations
- BDA
biotinylated dextran amine
- BF
basal forebrain
- BFGN
basal forebrain GABAergic neuron
- BL
basolateral nucleus
- BLa
anterior subdivision of the basolateral nucleus
- BLp
posterior subdivision of the basolateral nucleus
- CB
calbindin
- CR
calretinin
- DAB
3,3’-diaminobenzidine hydrochloride
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PC
pyramidal cell
- PHA-L
Phaseolus vulgaris leucoagglutinin
- PUR
very intense purple (VIP) reaction product
- PV
parvalbumin
- SI
substantia innominata
- SOM
somatostatin
- VGAT
vesicular GABA transporter
- VP
ventral pallidum
REFERENCES
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977;120:435–444. doi: 10.1016/0006-8993(77)90397-3. [DOI] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Varga V, Szilágyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Záborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. A correlated light and electron microscopic immunocytochemical study of cholinergic terminals and neurons in the rat amygdaloid body with special emphasis on the basolateral amygdaloid nucleus. J Comp Neurol. 1986;244:121–136. doi: 10.1002/cne.902440110. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Eyre MD, Freund TF, Gulyas AI. Quantitative ultrastructural differences between local and medial septal GABAergic axon terminals in the rat hippocampus. Neuroscience. 2007;149:537–548. doi: 10.1016/j.neuroscience.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyás AI. GABAergic interneurons containing calbindin D28K or somatostatin are major targets of GABAergic basal forebrain afferents in the rat neocortex. J Comp Neurol. 1991;314:187–199. doi: 10.1002/cne.903140117. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. Gamma-aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–364. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hellendall RP, Godfrey DA, Ross CD, Armstrong DM, Price JL. The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol. 1986;249:486–498. doi: 10.1002/cne.902490405. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Res. 1997;752:175–183. doi: 10.1016/s0006-8993(96)01446-1. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsáki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Nishijo H, Wang Q, Uwano T, Tamura R, Ohtani O, Ono T. Light and electron microscopic study of cholinergic and noradrenergic elements in the basolateral nucleus of the rat amygdala: evidence for interactions between the two systems. J Comp Neurol. 2001;439:411–425. doi: 10.1002/cne.1359. [DOI] [PubMed] [Google Scholar]
- Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96:3209–3219. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Parvalbumin-immunoreactive neurons and GABAergic neurons of the basal forebrain project to the rat basolateral amygdala. Neuroscience. 2009;160:805–812. doi: 10.1016/j.neuroscience.2009.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ, Betette R. Parvalbumin containing neurons in the rat basolateral amygdala: morphology and colocalization of calbindin-(D-28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and gamma-aminobutyric acid in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- Mello LE, Tan AM, Finch DM. Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res. 1992;587:24–40. doi: 10.1016/0006-8993(92)91425-e. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. doi: 10.1002/cne.22550. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L, Frotscher M. Organization and synaptic interconnections of GABAergic and cholinergic elements in the rat amygdaloid nuclei: single- and double-immunolabeling studies. J Comp Neurol. 1989;279:470–488. doi: 10.1002/cne.902790311. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1361. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur J Neurosci. 2002;15:1867–1873. doi: 10.1046/j.1460-9568.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res. 2000;115:117–141. doi: 10.1016/s0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haeften T, Wouterlood FG. Neuroanatomical tracing at high resolution. J Neurosci Methods. 2000;103:107–116. doi: 10.1016/s0165-0270(00)00300-9. [DOI] [PubMed] [Google Scholar]
- Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: a correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308:69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12(10):4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007a;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol. 2007b;98:2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]