Abstract
Many persons infected with hepatitis C virus (HCV) are unknown to the healthcare system because they may be asymptomatic for years, have not been tested for HCV infection, and only seek medical care when they develop liver-related complications. We analyzed data from persons who tested positive for past or current HCV infection during participation in the National Health and Nutrition Examination Survey (NHANES) from 2001 through 2008. A follow-up survey was conducted 6 months after examination to determine (1) how many participants testing positive for HCV infection were aware of their HCV status before being notified by NHANES, (2) what actions participants took after becoming aware of their first positive test, and (3) participants’ knowledge about hepatitis C. Of 30,140 participants tested, 393 (1.3%) had evidence of past or current HCV infection and 170 (43%) could be contacted during the follow-up survey and interviewed. Only 49.7% were aware of their positive HCV infection status before being notified by NHANES, and only 3.7% of these respondents reported that they had first been tested for HCV because they or their doctor thought they were at risk for infection. Overall, 85.4% had heard of hepatitis C; correct responses to questions about hepatitis C were higher among persons 40-59 years of age, white non-Hispanics, and respondents who saw a physician after their first positive HCV test. Eighty percent of respondents indicated they had seen a doctor about their first positive HCV test result.
Conclusion
These data indicate that fewer than half of those infected with HCV may be aware of their infection. The findings suggest that more intensive efforts are needed to identify and test persons at risk for HCV infection.
The estimated number of persons with chronic hepatitis C virus (HCV) infection increased from 2.7 million during 1988-19941 to 3.2 million during 1999-2002.2 Many infected persons, however, are unknown to the healthcare system because they may be asymptomatic for years, have not been tested for HCV, and only seek medical care for their infection when complications occur as part of the natural progression of untreated infection.3 Currently, HCV infection is the etiology most frequently associated with newly diagnosed chronic liver disease,4 and the effect on healthcare utilization is high. The number of healthcare visits associated with hepatitis C increased in ambulatory care settings during 1992-20035 and has remained at a high level through at least 2006.6
Ideally, infected persons would have medical management, both to prevent further liver damage and to limit transmission to others.7 Few infected persons, however, receive antiviral treatment,6,8 in large part because they may be unaware of their infection.8 Understanding the characteristics of persons who are unaware of their infection can help target appropriate education, but unfortunately, little is known about such individuals. In addition, little is known about the implementation of management guidelines by providers.
In this study, we analyzed data from participants who tested positive for past or current HCV infection and were interviewed as part of the Hepatitis C Follow-up Survey during the National Health and Nutrition Examination Survey (NHANES) conducted from 2001 through 2008. Three primary objectives of the follow-up survey were (1) to determine the percentage of participants testing positive for past or current HCV infection who were aware of their HCV status before being notified by NHANES, (2) to learn what actions participants took after becoming aware of their first positive test result, regardless of when they were made aware of the first positive test result, and (3) to find out what participants or the parents of participants <18 years in age knew about hepatitis C.
Participants and Methods
Survey
The NHANES, conducted by the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS), obtains nationally representative data on the health and nutritional status of the noninstitutionalized, civilian population of the United States. The NHANES uses a complex, stratified, and multistage probability sampling design and collects information from approximately 5,000 persons per year using standardized household interviews, physical examinations, and testing of biologic samples. More detailed information on the survey design for the NHANES, including approval from the institutional review board for data collection and analysis, is available from the survey documentation.9
Participants 6 years of age or older who tested positive for antibody to hepatitis C virus (anti-HCV) were sent a report of findings (ROF) letter informing them or the parents of participants <18 years of age of their HCV test result and encouraging them to follow up with a healthcare provider. The letter also provided information about HCV transmission, effect on the liver, and effect on general health. In addition, beginning in 2005-2006, serum samples from participants with a positive or indeterminate result for anti-HCV were tested for hepatitis C RNA (HCV-RNA); starting in 2007, participants with an indeterminate test result for anti-HCV and a positive HCV-RNA also were sent an ROF letter. Because a primary aim of the follow-up survey was to assess what actions participants took after becoming aware of their first positive test result, attempts to administer a follow-up telephone questionnaire to all those who were sent an ROF letter began 6 months after examination (approximately 4-5 months after the ROF letter was mailed) to allow participants time to have initiated or implemented actions after notification. Persons ≥18 years of age were interviewed directly; an adult proxy provided information for participants who were <18 years of age and for individuals unable to answer the questions themselves. The HCV Follow-up Questionnaire (available at: www.cdc.gov/nchs/nhanes/nhanes2003-2004/questexam03_04.htm) was mentioned in the informed consent and also in the ROF letter.
Bilingual (i.e., English and Spanish) trained interviewers contacted eligible participants by telephone for the interview. Participants who lived in households with no telephones were sent a letter asking them to call a toll-free number to answer a few questions about their hepatitis C results. Participants with communication or cognitive difficulties that made it impossible to respond to the questionnaire, and for whom a parent or guardian was not available to complete the interview, were excluded.
For the main NHANES survey, participants were interviewed in their homes to ascertain demographic characteristics, access to care, and health insurance coverage, using the Computer-Assisted Personal Interviewing (i.e., interviewer-administered) system. Having a usual source of medical care was determined by responses to the question: “Is there a place that {you/sampled person} usually {go/goes} when {you are/he/she is} sick or {you/s/he} need{s} advice about {your/his/her} health?”
Laboratory Testing
Qualitative determination of anti-HCV in blood serum or plasma was measured using direct solid-phase enzyme immunoassay with an anti-HCV screening enzyme-linked immunosorbent assay (ELISA) (Ortho CD VITROS Anti-HCV Immunodiagnostic System; Ortho Clinical Diagnostics, Raritan, NJ). Positive specimens were repeated in duplicate according to the same procedure. Repeatedly positive specimens were then tested using a confirmatory recombinant immunoblotting assay (RIBA) (Chiron RIBA Processor System, Chiron RIBA HCV 3.0 Strip SIA; Chiron Corporation, Inc., Emeryville, CA), an in vitro qualitative enzyme immunoassay for the detection of anti-HCV in human serum or plasma. RIBA-positive samples were reported as confirmed positive for anti-HCV, RIBA-negative samples were reported as negative for anti-HCV, and indeterminate results were reported as indeterminate. Beginning in 2005-2006, positive and indeterminate samples were further tested for HCV-RNA using the COBAS AMPLICOR HCV Test (version 2.0; Roche Diagnostics Corp., Indianapolis, IN), an in vitro nucleic acid amplification test for the quantitation of HCV-RNA in human serum or plasma on the COBAS AMPLICOR Analyzer.
Knowledge of Hepatitis C
To ascertain knowledge about hepatitis C, respondents were asked to respond “True” or “False” to a series of statements about hepatitis C transmission and its effect on health.
Statistical Analysis
NCHS analytic guidelines state that data from the Hepatitis C Follow-up Survey should not be used with sample weights to make national estimates because of small sample size and a response rate below 50%.10 Therefore, analyses were conducted in SAS 9.211 (SAS Institute, Cary, NC) using chi-square and, where appropriate (i.e., small cell sizes), Fisher’s exact tests. Percentages reported are percent of respondents, not population estimates. Results were considered statistically significant at the 0.05 level.
Data from four cycles (2001-2008) of the Hepatitis C Follow-up Survey were combined with demographic information, alcohol use data, and health insurance and healthcare utilization data. For some analyses, responses to knowledge questions were dichotomized to reflect whether the response given was correct according to the fact sheet sent with the ROF letter. Correct responses were coded as “correct”; responses that were incorrect as well as responses of “don’t know” and “refused” were coded as “not correct.” This recoding was done, in part, to avoid small cells in bivariate analyses testing for differences in the proportion of correct responses by factors such as age, gender, and health insurance coverage.
Results
Of the 43,655 persons ≥6 years of age sampled in the NHANES 2001-2008, a total of 34,365 (78.7%) were interviewed and 32,847 (95.6% of those interviewed) were examined. Serum samples were available for anti-HCV testing for 30,140 (91.8%) of those examined, and of these, 391 (1.3%) were anti-HCV positive. An additional 2 persons from 2007 to 2008 were anti-HCV indeterminate, but HCV-RNA positive, resulting in 393 persons who were eligible for the Hepatitis C Follow-up Survey. From these 393 eligible individuals, 168 full and two partial interviews were completed for a response rate of 43.3% (170 of 393). The major reason for not obtaining a survey was the inability to contact 191 (85.6%) of the 223 from whom survey responses could not be obtained. Characteristics of those eligible for the follow-up survey and of survey respondents and nonrespondents are shown in Table 1. Respondents were more likely than nonrespondents to be white non-Hispanic and to have health insurance. All subsequent results pertain only to the 170 individuals who tested positive for current or past HCV infection and responded to the follow-up survey; therefore, these results are not generalizable.
Table 1.
Characteristics of Hepatitis C Follow-up Survey Participants, NHANES Hepatitis C Follow-up Survey, 2001-2008
| Eligible for Follow-up (n = 393) |
Respondents (n = 170) |
Nonrespondents (n = 223) |
|||||
|---|---|---|---|---|---|---|---|
| Characteristics | n | Percent | n | Percent | n | Percent | P Value* |
| Age group (years) | |||||||
| <40 | 75 | 19.1 | 32 | 18.8 | 43 | 19.3 | |
| 40-49 | 148 | 37.7 | 59 | 34.7 | 89 | 39.9 | |
| 50-59 | 99 | 25.2 | 50 | 29.4 | 49 | 22.0 | |
| 60+ | 71 | 18.0 | 29 | 17.1 | 42 | 18.8 | NS |
| Male | 241 | 61.3 | 108 | 63.5 | 133 | 59.6 | NS |
| Race/ethnicity | |||||||
| White non-Hispanic | 171 | 43.5 | 87 | 51.2 | 84 | 37.7 | |
| Black non-Hispanic | 130 | 33.1 | 47 | 27.6 | 83 | 37.2 | |
| Hispanic/other/multiple | 92 | 23.4 | 36 | 21.2 | 56 | 25.1 | <0.05 |
| Educational attainment | |||||||
| Less than high school | 146 | 37.9 | 56 | 33.7 | 90 | 41.1 | |
| High school graduate/GED | 114 | 29.6 | 53 | 31.9 | 61 | 27.8 | |
| More than high school | 125 | 32.5 | 57 | 34.3 | 68 | 31.1 | NS |
| Poverty index | |||||||
| <1.0 (below poverty) | 123 | 33.0 | 44 | 27.0 | 79 | 37.6 | |
| 1.0-1.9 (near poverty) | 120 | 32.2 | 60 | 36.8 | 60 | 28.6 | |
| 2.0+ (above poverty) | 130 | 34.8 | 59 | 36.2 | 71 | 33.8 | NS |
| Have health insurance (yes) | 273 | 70.5 | 128 | 76.6 | 145 | 65.9 | <0.05 |
| Have a usual source of medical care (yes) |
339 | 86.3 | 150 | 88.2 | 189 | 84.8 | NS |
| More than 1 drink/day (yes) | 190 | 54.0 | 79 | 50.3 | 111 | 56.9 | NS |
NS = not significant at p < 0.05 level.
Abbreviation: GED, general educational development.
P value for chi-square test of difference between respondents and nonrespondents.
Table 2 shows responses to questions regarding whether the NHANES ROF letter was the first time the person had been told they had hepatitis C and whether the person had heard specifically of hepatitis C. Of those interviewed, only 84 (49.7%) responded that they had been told they had hepatitis C before receiving the letter. Awareness of HCV status was more than 2 times higher (57.0% versus 23.7%) among those who reported having health insurance coverage and 5 times higher (55.0% versus 10.0%) among those who had a usual source of medical care than among those who did not. In addition, those who were not previously aware of their infection were more likely to be younger than age 40. Of those who were previously aware of their HCV infection, approximately half had known that they had hepatitis C for more than 5 years, whereas 14.6% said they had known for about 1 year. When those who were aware of their HCV infection before receiving the ROF letter were asked why they were first tested for hepatitis C, only 3 (3.7%) said they or their doctor thought they were at risk for hepatitis C; nearly half (46.3%) said they had other blood work done for a routine physical that indicated possible liver disease. Additional reasons included blood donation (9.7%), symptoms (15.9%), other (18.3%), and don’t know (6.4%). Overall, 85.4% said they had heard of hepatitis C before receiving the ROF letter, but men and black non-Hispanics were less likely than women and those of other race/ethnic groups to have previously heard of hepatitis C.
Table 2.
Awareness of Hepatitis C and HCV Infection Status Before NHANES ROF Letter, NHANES Hepatitis C Follow-up Survey, 2001-2008
| Heard of Hepatitis C Before ROF Letter |
ROF Letter Was First Time Told Had Hepatitis C |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Yes % (n) | No % (n) | P Value | Yes % (n) | No % (n) | P Value |
| All respondents | 85.4 (141) | 14.6 (24) | – | 50.3 (85) | 49.7 (84) | – |
| Age group (years) | ||||||
| <40 | 86.7 (26) | 13.3 (4) | 71.0 (22) | 29.0 (9) | ||
| 40-49 | 82.8 (48) | 17.2 (10) | 42.4 (25) | 57.6 (34) | ||
| 50-59 | 87.5 (42) | 12.5 (6) | 44.0 (22) | 56.0 (28) | ||
| 60+ | 86.2 (25) | 13.8 (4) | NS | 55.2 (16) | 44.8 (13) | <0.05 |
| Sex | ||||||
| Male | 81.0 (85) | 19.0 (20) | 55.6 (66) | 44.4 (48) | ||
| Female | 93.3 (56) | 6.7 (4) | <0.05 | 41.0 (25) | 59.0 (36) | NS |
| Race/ethnicity | ||||||
| White non-Hispanic | 90.6 (77) | 9.4 (8) | 48.8 (42) | 51.2 (44) | ||
| Black non-Hispanic | 73.3 (33) | 26.7 (12) | 57.4 (27) | 42.6 (20) | ||
| Hispanic/other/multiple | 88.6 (31) | 11.4 (4) | <0.05 | 44.4 (16) | 55.6 (20) | NS |
| Have health insurance | ||||||
| Yes | 88.0 (110) | 12.0 (15) | 43.0 (55) | 57.0 (73) | ||
| No | 78.4 (29) | 21.6 (8) | NS | 76.3 (29) | 23.7 (9) | <0.001 |
| Have a usual source of medical care | ||||||
| Yes | 86.9 (126) | 13.1 (19) | 45.0 (67) | 55.0 (82) | ||
| No | 75.0 (15) | 25.0 (5) | NS | 90.0 (18) | 10.0 (2) | <0.001* |
NS = not significant at P < 0.05 level.
Fisher’s exact two-sided probability.
The survey contained a number of questions regarding follow-up with a doctor or other healthcare professional in response to the first positive hepatitis C test. “First positive test” can refer either to the NHANES test or to a previous positive test. Most respondents indicated that they had either seen a doctor or other healthcare professional about their hepatitis C result (77.5%) or had an appointment to do so (3.6%). Those who had already seen a doctor or other healthcare professional were more likely to have health insurance (80.6% versus 64.9%; P = 0.04) and to have a usual source of medical care (91.6% versus 76.3%; P = 0.01) than those who had not. Of 131 who had seen a doctor or other healthcare professional, just over half (51.6%) reported they were told they had hepatitis C and needed regular medical follow-up. Approximately one third (31.2%) reported they were told they tested positive for hepatitis C, but did not need to do anything or worry about it, and 12 (9.4%) indicated they had been told something else about their hepatitis C test result.
Of those who were told they had hepatitis C and needed regular medical follow-up (n = 66), 31 (47.0%) reported having had a liver biopsy performed. Those who reported having a biopsy did not differ by age, sex, race/ethnicity, health insurance status, or usual source of medical care status from those who did not report having a biopsy. When those who were told they had hepatitis C and needed regular medical follow-up were asked whether they had been told to avoid or limit alcoholic beverages in the future, 87.7% responded yes, but there were no differences in the percentage who had, on average, more than 1 drink per day during the past 12 months, based on whether they had been told to avoid or limit alcohol (49.9%) or not (50.0%).
Those who were told they had hepatitis C and needed regular medical follow-up were also asked a series of questions regarding treatment for hepatitis C. When asked whether they had been told that their hepatitis C should be treated with medication, such as interferon and ribavirin, just over half (52.9%) said yes, and of those, 61.8% indicated they were treated with these medications. Those who said they were told they should be treated did not differ by age, sex, race/ethnicity, or having health insurance or a usual source of medical care from those who said they were not told they should be treated.
The progression of respondents through their encounters with the healthcare system regarding their positive test results is shown in Fig. 1. One hundred and seventy of 393 survey-eligible individuals responded to the survey, 131 of those 170 had seen a physician or other healthcare professional about their first positive HCV test, 66 had been told medical follow-up was needed, and 22 were treated for their HCV infection.
Fig. 1.
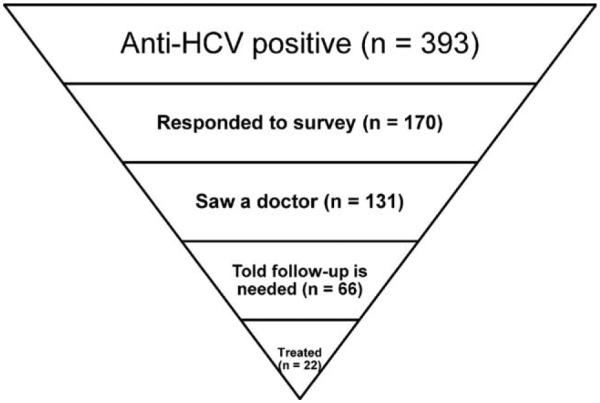
Progression of anti-HCV positive participants from NHANES report of findings letter to treatment for HCV.
Table 3 summarizes the assessment of respondents’ knowledge about hepatitis C. High proportions of respondents answered the knowledge questions correctly, with correct responses ranging from 57.1% to 95.7%. However, three of the eight questions regarding transmission of HCV had relatively lower proportions of correct responses: whether the virus could be transmitted by kissing, sexually, and vertically (i.e., mother-child). The question about vertical transmission had the highest proportion of “don’t know” responses.
Table 3.
Knowledge of Hepatitis C, NHANES Hepatitis C Follow-up Survey, 2001-2008 (n = 163*)
| Hepatitis C Knowledge Question | True % (n) | False % (n) | Don’t Know % (n) | Refused % (n) |
|---|---|---|---|---|
| If someone is infected with HCV, they will most likely carry the virus all their lives. | 74.8 (122) | 9.2 (15) | 16.0 (26) | 0.0 (0) |
| Infection with HCV can cause the liver to stop working. | 77.3 (126) | 3.7 (6) | 18.4 (30) | 0.6 (1) |
| Someone with hepatitis C can look and feel fine. | 90.2 (147) | 2.4 (4) | 6.8 (11) | 0.6 (1) |
| You can get hepatitis C by getting a blood transfusion from an infected donor. | 90.8 (148) | 1.2 (2) | 7.4 (12) | 0.6 (1) |
| You can get hepatitis C by shaking hands with someone who has hepatitis C. | 7.4 (12) | 84.6 (138) | 6.8 (11) | 1.2 (2) |
| You can get hepatitis C by kissing someone who has hepatitis C. | 9.8 (16) | 68.1 (111) | 20.9 (34) | 1.2 (2) |
| You can get hepatitis C by having sex with someone who has hepatitis C. | 63.8 (104) | 17.8 (29) | 17.2 (28) | 1.2 (2) |
| You can get hepatitis C by being born to a woman who had hepatitis C when she gave birth. |
57.1 (93) | 8.0 (13) | 33.7 (55) | 1.2 (2) |
| You can get hepatitis C by being stuck with a needle or sharp instrument that has hepatitis C–infected blood on it. |
95.7 (156) | 0.6 (1) | 3.1 (5) | 0.6 (1) |
| You can get hepatitis C by working with someone who has hepatitis C. | 10.4 (17) | 74.9 (122) | 13.5 (22) | 1.2 (2) |
| You can get hepatitis C by injecting illegal drugs, even if only a few times. | 84.7 (138) | 4.9 (8) | 9.8 (16) | 0.6 (1) |
Shading indicates correct response according to fact sheet sent with ROF letter.
Total excludes 4 participants who were told by their doctor that they did not have hepatitis C based on follow-up testing and who were therefore not asked these questions.
Dichotomized responses (i.e., correct versus incorrect) to the knowledge questions were tested for differences in proportion with a correct response by age, sex, race/ethnicity, having health insurance, having a usual source of medical care, whether the respondent had heard of hepatitis C before receiving the ROF letter, whether the respondent had been aware of their HCV infection before receiving the ROF letter, and whether the respondent had visited a doctor or other healthcare professional about their first positive HCV test result. Significant differences were found by age, sex, and race/ethnicity (Table 4), by having heard of hepatitis C before receiving the ROF letter, having been aware of their HCV infection before receiving the ROF letter, and having visited a doctor or other healthcare professional about their first positive HCV test result (Table 5).
Table 4.
Influence of Age and Race/Ethnicity on Giving a Correct Answer to Hepatitis C Knowledge Questions, Hepatitis C Follow-up Survey Respondents, NHANES 2001-2008 (n = 163*)
| Age Group (Years) |
Race/Ethnicity |
||||||
|---|---|---|---|---|---|---|---|
| Hepatitis C Knowledge Question | <40 (% With Correct Answer) |
40-49 (% With Correct Answer) |
50-59 (% With Correct Answer) |
60+ (% With Correct Answer) |
WNH (% With Correct Answer) |
BNH (% With Correct Answer) |
Hispanic/Other (% With Correct Answer) |
| If someone is infected with HCV, they will most likely carry the virus all their lives. |
65.5† | 79.7 | 83.7 | 57.7 | 81.9† | 60.0 | 77.1 |
| Infection with HCV can cause the liver to stop working. | 89.7† | 78.0 | 81.6 | 53.8 | 84.3 | 66.7 | 74.3 |
| Someone with hepatitis C can look and feel fine. | 96.6|| | 89.8 | 95.9 | 73.1 | 94.0 | 82.2 | 91.4 |
| You can get hepatitis C by getting a blood transfusion from an infected donor. |
86.2 | 98.3 | 87.8 | 84.6 | 92.8|| | 82.2 | 97.1 |
| You can get hepatitis C by shaking hands with someone who has hepatitis C. |
96.6‡ | 91.5 | 81.6 | 61.5 | 92.8§ | 66.7 | 88.6 |
| You can get hepatitis C by kissing someone who has hepatitis C. |
75.9§ | 79.7 | 71.4 | 26.9 | 80.7§ | 46.7 | 65.7 |
| You can get hepatitis C by having sex with someone who has hepatitis C. |
65.5† | 72.9 | 65.3 | 38.5 | 60.2 | 68.9 | 65.7 |
| You can get hepatitis C by being born to a woman who had hepatitis C when she gave birth. |
62.1 | 64.4 | 55.1 | 38.5 | 56.6 | 53.3 | 62.9 |
| You can get hepatitis C by being stuck with a needle or sharp instrument that has hepatitis C-infected blood on it. |
96.6 | 98.3 | 95.9 | 88.5 | 100.0|| | 91.1 | 91.4 |
| You can get hepatitis C by working with someone who has hepatitis C. |
82.8† | 81.4 | 73.5 | 53.9 | 80.7 | 66.7 | 71.4 |
| You can get hepatitis C by injecting illegal drugs, even if only a few times. |
93.1 | 86.4 | 81.6 | 76.9 | 88.0‡ | 71.1 | 94.3 |
Hispanic/other includes Hispanic, all races other than WNH, or BNH and multiple races.
Abbreviations: WNH, white non-Hispanic; BNH, black non-Hispanic.
Total excludes 4 participants who were told by their doctor that they did not have hepatitis C based on follow-up testing and who were therefore not asked these questions.
P < 0.05 for difference in percentage with correct answer to question regard hepatitis C by factor status.
P < 0.01 for difference in percentage with correct answer to question regard hepatitis C by factor status.
P < 0.0001 for difference in percentage with correct answer to question regard hepatitis C by factor status.
Chi-square P < 0.05, but not reported as statistically significant, because it may not be valid as a result of small cell sizes and/or a zero cell.
Table 5.
Influence of Selected Factors on Giving a Correct Answer to Hepatitis C Knowledge Questions, Hepatitis C Follow-up Survey Respondents, NHANES 2001-2008 (n = 163*)
| Factor |
||||||
|---|---|---|---|---|---|---|
| Heard of Hepatitis C Before ROF Letter |
Knew You Were HCV+Before ROF Letter |
Seen a Doctor About First Positive HCV Test |
||||
| Hepatitis C Knowledge Question | Yes (% With Correct Answer) |
No (% With Correct Answer) |
Yes (% With Correct Answer) |
No (% With Correct Answer) |
Yes (% With Correct Answer) |
No (% With Correct Answer) |
| If someone is infected with HCV, they will most likely carry the virus all their lives. |
77.9† | 59.3 | 80.0 | 69.9 | 79.2† | 60.5 |
| Infection with HCV can cause the liver to stop working. | 81.6‡ | 55.6 | 83.8 | 71.1 | 81.6† | 63.2 |
| Someone with hepatitis C can look and feel fine. | 93.4‡ | 74.1 | 91.2 | 89.2 | 92.0 | 84.2 |
| You can get hepatitis C by getting a blood transfusion from an infected donor. |
91.2 | 88.9 | 91.3 | 90.4 | 91.2 | 89.5 |
| You can get hepatitis C by shaking hands with someone who has hepatitis C. |
85.3 | 81.4 | 90.0 | 79.5 | 87.2 | 76.3 |
| You can get hepatitis C by kissing someone who has hepatitis C. |
68.8 | 59.3 | 70.0 | 66.3 | 72.0 | 55.3 |
| You can get hepatitis C by having sex with someone who has hepatitis C. |
62.5 | 70.4 | 61.2 | 66.3 | 64.0 | 63.1 |
| You can get hepatitis C by being born to a woman who had hepatitis C when she gave birth. |
53.7 | 74.1 | 48.8† | 65.1 | 54.4 | 65.8 |
| You can get hepatitis C by being stuck with a needle or sharp instrument that has hepatitis C–infected blood on it. |
96.3 | 92.6 | 95.0 | 96.4 | 96.8 | 92.1 |
| You can get hepatitis C by working with someone who has hepatitis C. |
75.0 | 74.1 | 80.0 | 69.9 | 86.4 | 79.0 |
| You can get hepatitis C by injecting illegal drugs, even if only a few times. |
86.8 | 74.1 | 86.2 | 83.1 | 82.4§ | 50.0 |
Total excludes 4 participants who were told by their doctor that they did not have hepatitis C based on follow-up testing and who were therefore not asked these questions.
P < 0.05 for difference in percentage with correct answer to question regarding hepatitis C by factor status.
P < 0.01 for difference in percentage with correct answer to question regarding hepatitis C by factor status.
P < 0.0001 for difference in percentage with correct answer to question regarding hepatitis C by factor status.
Table 4 should be interpreted as follows: for the first question (“If someone is infected with hepatitis C virus, they will most likely carry the virus all their lives”), 65.5% of those 40 or younger, 79.7% of those 40-49, 83.7% of those 50-59, and 57.7% of those 60 or older gave a correct response to the question. The percentage of correct responses differed by both age and race/ethnicity for some questions. Because age did not differ statistically significantly across race/ethnic groups for respondents, age-standardized analyses were not performed.
Regarding differences by age, those who were 60 or older were significantly less likely to respond correctly to all questions except those regarding vertical transmission and transmission by blood transfusion, needle stick, and injection drug use. Those who were 40 or younger were less likely to have given a correct response to the question regarding whether an infected person is likely to carry HCV all their life. For one question (“Someone with hepatitis C can look and feel fine”), the difference is not reported as significant, in spite of a chi-square P < 0.05, because the test may not be valid as a result of a large number of small cells.
Significant differences in the proportion of correct responses were also found for some of the knowledge questions by race/ethnicity. The proportion who responded correctly to the question about vertical transmission was low for all race/ethnic groups. Black non-Hispanics were less likely to respond correctly to the questions regarding carrying HCV for life, as well as transmission by shaking hands with or kissing an HCV-infected person or by injection drug use. As a group, Hispanics, those of other races, and those who reported multiple races were also less likely to have given a correct response regarding transmission by kissing. For the questions regarding transmission by blood transfusion and by needle stick, differences are not reported as significant, in spite of a chi-square P < 0.05, because the test may not be valid as a result of a large number of small cells (e.g., blood transfusion) or to a zero cell (e.g., needle stick). Male and female respondents differed only on the question regarding transmission by blood transfusion, with 98.3% of females having given a correct response, compared to 86.5% of males (Fisher’s exact two-sided test; P = 0.008).
Table 5 shows the percent of respondents with a correct response to each of the knowledge questions based on having heard of hepatitis C before receiving the ROF letter, having been aware of their HCV infection before receiving the ROF letter, and having visited a doctor or other healthcare professional about their first positive HCV test result. Thus, for the first question (“If someone is infected with hepatitis C virus, they will most likely carry the virus all their lives”), approximately three quarters of those who had heard of hepatitis C before receiving the ROF letter and approximately 60% of those who had not heard of hepatitis C before receiving the ROF letter gave a correct response to the question.
Respondents who had heard of hepatitis C before receiving the ROF letter were significantly more likely than those who had not previously heard of hepatitis C to respond correctly to three of the questions: “If someone is infected with hepatitis C virus, they will most likely carry the virus all their lives”; “Infection with the hepatitis C virus can cause the liver to stop working”; and “Someone with hepatitis C can look and feel fine.” Respondents who reported having seen a doctor or other healthcare professional about their first positive HCV test result were more likely to respond correctly to the first two of those three questions plus the question regarding transmission by injection drug use than those who had not. Respondents who knew they were HCV positive before the ROF letter were significantly less likely than those who were unaware they were HCV positive to have responded correctly to the question regarding vertical (i.e., mother-child) transmission.
Discussion
Based on the sample of individuals who responded to the Hepatitis C Follow-Up Survey after having tested positive for past or current HCV infection during NHANES 2001-2008, we found that 49.7% were not aware they were infected with HCV before receiving notification from NHANES; more than 80% saw a doctor or other healthcare professional about their first positive HCV test or had an appointment to do so, and for most of the 11 knowledge questions, approximately 75% of respondents provided a correct answer about hepatitis C and its transmission. Of those who were aware of their positive HCV infection status before being notified by NHANES, only 3.7% reported that they had first been tested for HCV because they or their doctor thought they were at risk for this infection. Overall, 85.4% of those who were infected had heard of hepatitis C before receiving the ROF letter; correct responses to specific questions about hepatitis C were higher among persons 40-59 years of age, white non-Hispanics, and those who saw a physician regarding their first positive HCV test.
Approximately one half of the respondents had not been aware of their HCV status before receiving the ROF letter. We found that those 40-59 years of age were more likely to be aware of their HCV status than were those who were either younger or older. This is encouraging, because the burden of HCV disease is highest among those 40-59 years of age. Respondents who were not previously aware of their infection were more likely to lack health insurance coverage and a usual source of medical care. This suggests that screening efforts for HCV that work through the healthcare system may not be successful in reaching many HCV-infected individuals because of lack of health insurance coverage and/or lack of a usual source of medical care.
Only 3.7% of those who were previously aware of their HCV status reported that they had first been tested because they or their doctor thought that they were at risk for hepatitis C. Nearly half reported they had first been tested based on results from a routine physical. These findings are consistent with patterns in primary care12 and suggest a lack of effectiveness in the current risk-based screening strategy. Risk-based screening might fail because providers may not elicit complete risk-factor histories13 or patients deny risk behaviors.14 Regardless of the reason, our results suggest that the current risk-based screening is not being implemented for a large number of infected individuals during their encounters with the healthcare system. This is particularly important, because 70%-85% of HCV-infected individuals are asymptomatic, making serological testing the major avenue by which their infection will be discovered.
Approximately one third of respondents who had seen a doctor or other healthcare professional about their positive HCV test result reported that they were told they had tested positive for hepatitis C, but did not need to do anything or worry about it. This message would be appropriate for anti-HCV-positive individuals with either resolved or previously treated infections; however, 23 of 29 such individuals with HCV-RNA results available were, in fact, HCV-RNA positive when tested during the NHANES. There are a number of reasons why patients who were HCV-RNA positive when tested by NHANES may have reported being told they did not need to do anything or worry about their positive HCV test result, including the following: The respondent misunderstood or misreported what they were told; a negative HCV-RNA test result was obtained at follow-up because those chronically infected with HCV can have intermittent viremia; an individual had been treated and reinfected; or their physician did not know how to manage a chronically infected case. Each of these latter reasons suggests the need for patient and provider education to ensure that correct messages are given and understood.
It is encouraging to note that more than 80% of respondents had either already seen a doctor or healthcare professional about their first positive test results or had an appointment to do so. However, having health insurance coverage or a usual source of medical care affected whether a person testing positive for HCV had seen a doctor or other healthcare professional. Figure 1 highlights a dramatic decline in the number of patients at each stage as they progress from seeing a physician about their positive test results to treatment for HCV infection. Only 12.9% (22 of 170) from this sample were treated for their infection. In contrast, facility-based studies suggest treatment rates closer to 30%-40%.15 With the approval of new medications (www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm151488.htm), treatment rates for HCV are expected to improve and monitoring their impact will be essential.
Our finding that approximately three quarters of the respondents knew the correct answer to most of the knowledge questions is encouraging. We found that incorrect responses to a number of questions were significantly associated with black non-Hispanic race/ethnicity and older or younger age than 40-59 years, two factors that were also associated with survey non-response (black non-Hispanic), having heard of hepatitis C before the ROF letter (black non-Hispanic), and being unaware of their HCV infection before the ROF letter (younger age), thus suggesting these same groups for targeted prevention efforts. We cannot know whether the source of the knowledge was the fact sheet that accompanied the ROF letter (either because they had read and learned from it or had it at hand during the interview), a healthcare provider, or some other source. However, because the interview was conducted 4-5 months after receipt of the fact sheet and letter, it is less likely that respondents would have the fact sheet at hand. Furthermore, one of the questions with a lower frequency of correct responses was regarding vertical transmission of HCV, a topic included in the fact sheet.
Two other questions had a relatively low frequency of accurate responses: whether HCV could be transmitted sexually or by kissing an infected person. The first of these, sexual transmission, may require a more specific question to accurately assess knowledge. For example, sexual transmission of HCV among men who have sex with men with human immunodeficiency virus (HIV) infection has been documented, whereas risk of transmission among monogamous non-HIV-infected heterosexual partners is rare or nonexistent.16 The lower frequency of correct responses to transmission from kissing an infected person might be a result of the fact that this was not explicitly stated on the fact sheet or may reflect a lack of understanding about HCV transmissibility.
Approximately 15% of respondents had not heard of hepatitis C before receiving the ROF letter; this proportion was higher for men and black non-Hispanics, among whom the burden of HCV disease is higher, and for persons lacking health insurance or a usual source of medical care. We think it is noteworthy that having previously heard of hepatitis C did not vary by age group. These findings may serve as a roadmap for education programs to prevent infection, because there is currently no vaccine available for HCV. Clearly, more work is needed to bring this disease of public health importance to the attention of the U.S. population, especially those in the subgroups most affected by the disease. The 2010 Institute of Medicine report identified a lack of education about HCV among the public and among healthcare providers as an important barrier to controlling the HCV epidemic in the United States.17 The CDC plans to expand efforts to educate both the public and providers; continued monitoring of the effect of education on prevention is warranted.
As with all studies, there are limitations to consider when interpreting these findings. First, NHANES data are generalizable to the U.S. noninstitutionalized civilian population and exclude the homeless, persons living in correctional institutions, and other group quarters (e.g., student dormitories, military recruits). Second, although the Hepatitis C Follow-up Survey is nested within the NHANES, the data from the follow-up survey cannot be used to generate population estimates because of the small number of respondents and low response rate. Frequencies for some questions may be affected by differences in characteristics of respondents and nonrespondents. In addition, the small sample size limited our power to detect statistically significant differences between subgroups. Third, the data are self-reported and therefore subject to the usual biases associated with such data (e.g., recall bias), including possibly not understanding questions regarding medical information, such as whether they have had a particular medical procedure performed or what they were told by a healthcare provider. Finally, the sample consisted of persons who were positive for anti-HCV, whether currently infected or not; thus, treatment would not have been indicated in all those who received an ROF letter—however, 91 of 115 with HCV-RNA results available were HCV-RNA positive when tested during the NHANES, suggesting chronic infection.
In summary, we report results for a sample of NHANES participants who responded to a follow-up survey after having tested positive for past or current HCV infection from 2001 through 2008, which, to our knowledge, is the only survey of such individuals to be conducted as part of a national population-based study. These data indicate that fewer than half of those infected with HCV may be aware of their infection. The findings suggest that more intensive efforts are needed to identify and test those at risk for HCV infection and the need to educate patients and providers about appropriate interaction on prevention decisions and actions.
Abbreviations
- anti-HCV
antibody to hepatitis C virus
- CDC
Centers for Disease Control and Prevention
- ELISA
enzyme-linked immunosorbent assay
- HCV
hepatitis C virus
- HCV-RNA
hepatitis C RNA
- HIV
human immunodeficiency virus
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- RIBA
recombinant immunoblotting assay
- ROF
report of findings
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflict of interest: Nothing to report.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Seef LB. Natural history of chronic hepatitis C. HEPATOLOGY. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Bell BP, Manos MM, Zaman A, Terrault N, Thomas A, Navarro VJ, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. quiz, 2737. [DOI] [PubMed] [Google Scholar]
- 5.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Cheung R, Mannalithara A, Singh G. Utilization and antiviral therapy in patients with chronic hepatitis C: analysis of ambulatory care visits in the US. Dig Dis Sci. 2010;55:1744–1751. doi: 10.1007/s10620-010-1147-z. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 8.Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. HEPATOLOGY. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention About the National Health and Nutrition Examination Survey (NHANES) 2009 Available at: www.cdc.gov/nchs/about/major/nhanes/intro mec.htm. Accessed on March 8, 2011. [Google Scholar]
- 10.Centers for Disease Control and Prevention Documentation, Codebook and Frequencies: Hepatitis C Follow-up Questionnaire, Analytic Notes. Available at: www.cdc.gov/nchs/nhanes/nhanes2007–2008/HCQ_E.htm Accessed on July 15, 2010. [Google Scholar]
- 11.SAS Institute, Inc. SAS/STAT User’s Guide, Version 9. SAS Institute, Inc.; Cary, NC: 2009. [Google Scholar]
- 12.Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. Am J Gastroenterol. 2003;98:639–644. doi: 10.1111/j.1572-0241.2003.07331.x. [DOI] [PubMed] [Google Scholar]
- 13.Navarro VJ, St Louis TE, Bell BP. Identification of patients with hepatitis C virus infection in New Haven County primary care practices. J Clin Gastroenterol. 2003;36:431–435. doi: 10.1097/00004836-200305000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Schuckman H, Hazelett S, Powell C, Steer S. A validation of self-reported substance use with biochemical testing among patients presenting to the emergency department seeking treatment for backache, headache, and toothache. Subst Use Misuse. 2008;43:589–595. doi: 10.1081/JA-200030572. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:465–479. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 16.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? HEPATOLOGY. 2010;52:1497–1505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 17.IOM (Institute of Medicine) Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. National Academies Press; Washington, DC: 2010. Available at: www.nap.edu/catalog/12793.html. Accessed on March 8, 2011. [PubMed] [Google Scholar]


