Abstract
Background
The Pediatric Oncology Group performed a pilot study to assess the feasibility of tandem high dose chemotherapy with stem cell rescue (HDC/SCR). We report here the results of this single arm trial of induction chemotherapy, local control measures (surgery and local radiation), and tandem HDC/SCR.
Procedure
Patients with high risk neuroblastoma underwent five cycles of induction chemotherapy and resection of primary tumors. PBSC were collected after Course 3 without exvivo manipulation. Myeloablative chemotherapy was performed in rapid sequence after induction chemotherapy and surgery. The ability of patients to complete both cycles of HDC/SCR was a primary endpoint. Transplant-related toxicity, progression-free survival (PFS) and overall survival (OS) were recorded.
Results
A total of 33 patients were enrolled. Twenty-two patients completed at least one HDC/SCR procedure and 17 patients completed both. Only one patient had insufficient stem cells collected for both transplants. There was one transplant-related death; engraftment was rapid and toxicity was as expected. The PFS of the 33 patients treated on this study is 24.2%±7.5% and OS is 36.4%±8.4% at 5 years. For patients who received at least one transplant PFS is 36.4%±11.0% and OS is 45.5%±11.2% at 5 years.
Conclusions
The treatment of high risk neuroblastoma with tandem HDC/SCR is feasible in terms of transplant-related mortality and the ability to collect adequate PBSC for 2 transplants. The outcomes from this intensified treatment have been used to design a Children's Oncology Group Phase III study testing the efficacy of tandem HDC/SCR.
Keywords: high risk neuroblastoma, Myeloablative therapy, tandem transplant, peripheral blood stem cell transplant, POG 9640
INTRODUCTION
Treatment of high risk neuroblastoma (NBL) remains a significant challenge in pediatric oncology. Improvements in event-free survival (EFS) in this disease have been demonstrated in both European [2] and North American trials [1,3] in which patients receive, after initial chemotherapy and surgery, high dose chemotherapy (HDC) followed by autologous bone marrow or peripheral blood stem cell rescue (SCR).
One such regimen, using tandem myeloablative procedures in rapid succession supported by peripheral blood stem cells (PBSC) after a uniform induction, used carboplatin, etoposide and cyclophosphamide in myeloablative doses followed 6 weeks later by melphalan and total body irradiation (TBI) in the second SCR procedure [8]. The Pediatric Oncology Group 9640 study was designed to establish the feasibility of performing tandem HDC/SCR using PBSC in a cooperative group setting and to obtain crude estimates of response and PFS in a tandem HDC/SCR regimen that did not include TBI.
We report here the results of this single arm trial of induction chemotherapy, local control measures (surgery and local radiation), and tandem HDC/SCR. The protocol also tested the ability of multiple centers to obtain adequate numbers of PBSC for tandem HDC/SCR in small children and involved three innovative aspects of study design: i) collection of PBSC early in therapy, presumably before any compromise of hematopoietic stem and progenitor cells had occurred from the induction chemotherapy cycles; ii) use of non-cross-reactive conditioning regimens for each cycle of HDC/SCR; and iii) rapid progression from the first to the second cycle of HDC/SCR. We also report the short-term toxicity outcomes and progression-free outcomes in this pilot group. Feasibility data from this pilot study helped form the basis for subsequent cooperative group studies of tandem HDC/SCR.
PATIENTS AND METHODS
Patient selection and evaluation
Patients with high risk NBL who were untreated, or those who had received a single course of chemotherapy for apparent intermediate risk disease which was later revealed to be high risk by molecular testing, were enrolled on this protocol through the Pediatric Oncology Group (now the Children's Oncology Group [COG]). Patients with high risk NBL included: 1) International Neuroblastoma Staging System (INSS) [9] Stage 2A/2B, older than 365 days of age with tumors that were MYCN amplified and unfavorable histology; 2) INSS Stage 3, ≥365 days with tumors that were MYCN amplified and/or unfavorable histology; 3) INSS Stage 3, 4 or 4S <365 days with tumors that were MYCN amplified; and 4) INSS Stage 4, ≥365 days of age. The protocol was activated on 4/1/98 and closed to accrual on 5/22/2000. Patients gave informed consent, and Institutional Review Board approval for treatment on this study was obtained at each center.
Treatments
After diagnosis and enrollment on a centralized tissue-procurement protocol (POG 9047) to determine MYCN, ploidy and histology according to Shimada classification, informed consent was obtained. Enrollment on this study was required within 2 weeks of diagnosis. Patients underwent five cycles of induction therapy (Figure 1). Course 1 and 5 of therapy consisted of Cisplatin 40 mg/m2 in 125 ml/m2 NS containing 3 g/m2 mannitol, was infused IV over 1 hour on days 1-5 and Etoposide 100 mg/m2 over 1 hour every 12 hours × 6 doses, beginning just prior to cisplatin on days 2 through 4 of therapy. Course 2 therapy consisted of Vincristine 1.5 mg/m2 (max. dose 2 mg) IV push (or 0.05 mg/kg for weight <10 kg) on day 1, 8 and 15; Doxorubicin 60 mg/m2 IV over 1 hour in 150 ml/m2 D5 1/2 NS and Cyclophosphamide 2000 mg/m2 in 200 ml/m2 D5 1/2 NS with MESNA 400 mg/m2 over 1 hour IV on days 1 and 2. Course 3 consisted of Etoposide 75 mg/m2 over 1 hr in 200 ml/m2 D5 1/4 NS on Days 1-5 and Ifosfamide 2 g/m2 combined with MESNA 400 mg/m2 and given over 1 hr in 200 ml/m2 D5 1/2 NS. Posthydration was given with D5 1/2 NS at 150 ml/m2/hr and interrupted MESNA 400mg/m2 over 15 minutes at hours 4, 7, and 10 on days 1-3. Course 3 consisted of Etoposide 75 mg/m2 over 1 hr in 200 ml/m2 D5 1/4 NS on Days 1-5 and Ifosfamide 2 g/m2 combined with MESNA 400 mg/m2 and given over 1 hr in 200 ml/m2 D5 1/2 NS. Posthydration was given with D5 1/2 NS at 150 mg/m2/hr and interrupted MESNA 400mg/m2 over 15 minutes at hours 4, 7, and 10 on days 1-3. Chemotherapy doses were consistent based on weight and age. PBSC collection was performed after recovery from the third cycle of chemotherapy, but could be delayed until after the subsequent course when bone marrow morphology revealed absence of neuroblastoma. No exvivo manipulation (purging of tumor cells or selection of hematopoietic cells) was performed. Resection of the primary tumor, if not completed at diagnosis, was undertaken after completion of induction therapy and prior to HDC/SCR. Criteria for proceeding to transplant were absence of progressive disease, successful stem cell collection of at least 2.5×10 CD34+ cells/kg for each stem cell transplant, and acceptable organ function. A specific criterion for glomerular filtration rate was not a requirement. Each of the high dose treatments was fully myeloablative. Ablation (HDC) #1 began 6 days prior to stem cell infusion and included Etoposide 800 mg/m2/day IV as a continuous infusion (72 hrs total); Carboplatin 667 mg/m2 IV over 1 hour each day × 3 days; and Cyclophosphamide 60 mg/kg IV over 1 hour each day for 2 days plus MESNA 12 mg/kg at 0, 3 and 6 hours. There was a single rest day prior to stem cell infusion. Growth factor support was given with G-CSF 10 μg/kg/day IV daily until ANC > 1000/μl for 2 days consecutively. Ablation (HDC) #2 infused Thiotepa 900mg/m2/day over 2 hours for 3 days on days −7 to −5 followed by Cyclophosphamide 500mg/m2/dose over 1 hour IV every 8 hours for 12 total doses on Days −5 to −2 with MESNA 300mg/m2 and MESNA repeated 4 hours after each doses of Cyclophosphamide (Figure 1). Growth factor support was again given with G-CSF 10 μg/kg/day IV daily until ANC > 1000/μl for 2 days consecutively. The protocol recommended local irradiation for all patients after completion of the final episode of HDC/SCR. At Day +84 after the last HDC/SCR, patients received post consolidation therapy with 13-cis-retinoic acid at 80mg/m2 twice daily for 14 days of each 28 day cycle, for a total of six months provided they had no progression of disease on reevaluation and had recovered from toxicity of both HDC/SCR courses[10].
Fig 1.
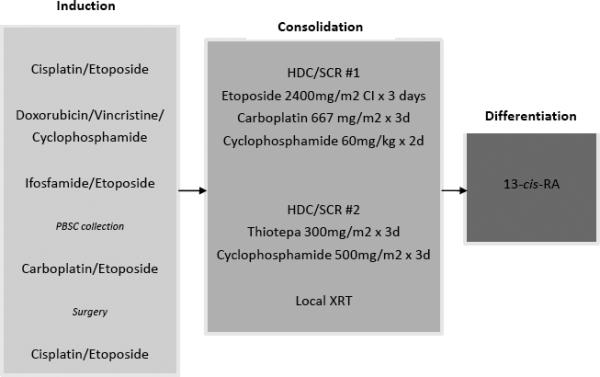
Schema of induction, consolidation and post-consolidation therapy. Abbreviations: Cl, continuous infusion over 24 hours; PBSC, peripheral blood stem cell; XRT, radiotherapy; 13-cis-RA, 13-cis-retinioc acid.
Statistical analysis
Descriptive statistical analysis was performed to assess patient characteristics. PFS and OS were calculated using the method of Kaplan and Meier, with confidence intervals per Greenwood's formula [11]. For PFS, time to event was calculated from time of study enrollment to the first occurrence of progression/relapse of primary tumor or death, or time to last contact if no event occurred. For OS, the time to event was defined as the time from study enrollment to death, or time to last contact if alive. PFS curves were compared using the log-rank test. Transplant related mortality (TRM) was defined as death from any cause within 100 days post-transplant.
For patients who received at least one transplant, secondary intent-to-treat survival analyses were performed, with PFS and OS times starting from the date of first transplant (without regard to whether one or two transplants were performed). Despite the inherent bias of analyzing patients who have survived long enough to receive a transplant, the purposes of these analyses were to provide results of the intent to deliver two transplants, and to allow comparisons to other studies in which PFS and OS begin from the date of transplant [10].
RESULTS
Patient characteristics
Thirty-three patients with high risk NBL were treated according to this protocol. The patient characteristics are detailed in Table 1. The median age at diagnosis was 30 months (range: 6 months–8 years). Two patients were less than 12 months of age and 4 patients were between 12 and 18 months of age. All of the patients <12 months had MYCN amplified disease, as did 1 of the 4 in the 12-18 month range. There were two patients with Stage 3 disease, one of whom had a MYCN amplified tumor. Ninety-four percent of the patients (n=31) had Stage 4 disease and MYCN amplification was observed in 34% of the tumors analyzed.
Table I.
Clinical and Biological Characteristics of Patients Enrolled on 9640 (N=33).
| Characteristic | N (%) | MYCN status | ||
|---|---|---|---|---|
| Amplified | Not Amplified | Unknown | ||
| Age | ||||
| <12 mo | 2 (6%) | 2 | 0 | 0 |
| 12-18 mo | 4 (12%) | 1 | 3 | 0 |
| > 18 mo | 27 (82%) | 7 | 16 | 4 |
| Stage | ||||
| 3 | 2 (6%) | |||
| Favorable Histology | 0 | 0 | 0 | |
| Unfavorable Histology | 1 | 1 | 0 | |
| 4 | 31 (94%) | |||
| Favorable Histology | 0 | 4 | 0 | |
| Unfavorable Histology | 6 | 11 | 0 | |
| Unknown Histology | 3 | 2 | 4 | |
| MYCN gene copy number | 33 | 10 (30%) | 19 (58%) | 4 (12%) |
| Number of transplants | ||||
| 0 | 11 (33%) | 5 | 5 | 1 |
| 1 | 5 (15%) | 2 | 2 | 1 |
| 2 | 17 (52%) | 3 | 12 | 2 |
Protocol Completion
The ability of patients on a cooperative group protocol to receive both planned cycles of HDC/SCR was a major feasibility endpoint of this protocol. Eleven of the 33 patients (33%) did not undergo any transplant on study. Reasons for this included progressive disease (5), inability to collect adequate numbers of PBSC for tandem HDC/SCR in one infant (1), surgical complications leading to death (1), bone marrow as preferred source of stem cells (1), adverse reaction to etoposide (1), and withdrawal of parental consent (2). Five of the 22 patients who received the first HDC/SCR (23%) did not complete the second. Reasons for patients not proceeding to second transplant included death after HDC/SCR #1 (1), renal insufficiency (1), parental choice (2), and insurance issues (1). For the 17 patients who received both transplants, the median time between treatments (Day 0 to Day 0) was 45 days (range 28–66 days).
The median infused dose at SCR #1 was approximately 4×106 total CD34+ cells/kg. The median total CD34+ cells/kg infused at SCR #2 was approximately 5×106 CD 34 + cells/kg. There were no failures to engraft and no patients received late stem cell infusions. Time to an ANC of 500 cells per mm3 was achieved at a median of 12 days (range 0–33 days) after HDC/SCR #1 and 11 days after HDC/SCR #2. Platelet engraftment defined as a non-transfused platelet count greater than 50,000 per μl, was achieved at a median of 18 days after HDC/SCR #1 and 28 days after HDC/SCR #2 (Table 2).
Table II.
Engraftment results for patients after 1st and 2nd transplants
| Engraftment Days to: | Transplant 1 | Transplant 2 | ||
|---|---|---|---|---|
| N | Median (Range) | N | Median (Range) | |
| ANC 500 | 21 | 12 (0–33) | 17 | 11 (0–16) |
| ANC 100 | 19 | 11 (1–35) | 15 | 11 (0–44) |
| Platelets ≥ 20K | 18 | 16 (1–23) | 14 | 18 (2–78) |
| Red Cell Independence | 17 | 13 (1–36) | 16 | 11.5 (6–107) |
| Platelet Independence | 19 | 16 (7–41) | 17 | 15 (11–78) |
| Platelets ≥ 50K | 18 | 18 (1–41) | 16 | 28 (3–119) |
Toxicity
Toxicities that were Grade 3 or greater were reported during transplants #1 or #2 and no unexpected toxicity was noted. Patients experienced hematopoietic toxicity with neutropenia, anemia and thrombocytopenia. Stomatitis was reported in 23% of patients after HDC/SCR #1 and in 47% after HDC/SCR #2. Elevated transaminases were reported at 23% after HDC/SCR #1, with none reported after HDC/SCR #2. Infection rates were similar for HDC/SCR #1 and #2 among proved and unproved agents (9.1% and 11.8% respectively for unknown infections and 4.5% and 11.8% respectively for bacteremia). Mild veno-occlusive disease (VOD) occurred in 1 of 22 patients after HDC/SCR#1 and in 1 of 17 patients after HDC/SCR #2. A total of 39 HSC/SCR cycles were completed with only one TRM. This patient death occurred after HDC/SCR #1 and was due to multi-system organ failure at 37 days following transplant.
Risk of relapse and survival analysis
As of late 2009, 24 of the 33 patients had died. Causes of death included neuroblastoma (21), (late) infection (1), surgical complications (1), and one TRM from multi-system organ failure hypotension. One patient experienced a second malignancy with AML/monosomy 7; the patient ultimately died of AML. The median follow-up time for patients who did not experience an event is 7.7 years (range 5.4–8.2 years). The overall 5-year PFS and OS on this protocol are 24.2%±7.5% and 36.4%±8.4%, respectively (n=33; Table 2; Figure 2). Among the 22 patients who received at least one course of HDC/SCR, 8 (36%) were alive and relapse-free as of last contact in 2006 for 7 patients and 2004 for 1 patient. PFS and OS at 5 years from the time of first transplant are 36.4%±11.0% and 45.5%±11.2%, respectively.
Figure 2.
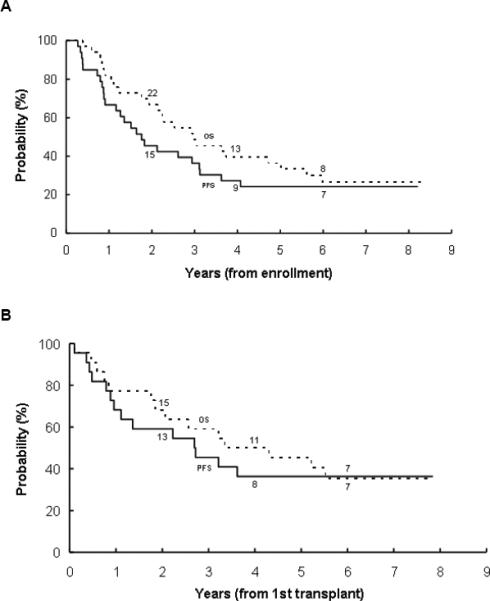
(A) Progression-free survival (PFS) and overall survival (OS) from the time of study enrollment of 33 patients with high-risk neuroblastoma. (B) PFS and OS from time of first HDC/SCR for 22 patients who underwent at least one HDC/SCR procedure. The number at risk at the start of year 2, 4, and 6 are given along the curves.
All but one of the 11 patients who did not receive at least one HDC/SCR on protocol had died. There was no evidence that MYCN amplification and stage at diagnosis influenced PFS (P=0.9119 and P=0.2840, respectively) or OS (P=0.6647 and P=0.4201, respectively).
DISCUSSION
The Pediatric Oncology Group conducted a study to assess the feasibility of delivering tandem cycles of HDC/SCR in a cooperative group setting. This study, conducted in 1998-2000, was one of the first cooperative group experiences involving collection of peripheral blood stem cells in pediatrics. At a time when apheresis methodologies were just beginning to include smaller patients, we documented the feasibility of stem cell collection in a multi-center trial, with every center able to collect adequate stem cells for two transplants in all but one patient. These feasibility data contributed to the decision to switch from bone marrow support to PBSC support in subsequent North American high risk NBL trials. In addition, we found the treatment to be well tolerated, with only one transplant-related death during HDC/SCR. Toxicities were as expected and time to engraftment was rapid for both cycles. Five patients (23%) failed to receive a second HDC/SCR after undergoing the first, with 2 of these related to toxicity and 3 related to non-medical reasons (parent preference, insurance limitations). This established a baseline for design of two subsequent NBL trials in the Children's Oncology Group, including a second pilot study and the ongoing randomized Phase III trial.
The shift to the use of PBSC rather than bone marrow, now routine, was an important innovation and a major challenge in a group of patients whose median age was under 3 years. An earlier European trial attempting tandem BMT using bone marrow as the stem cell source was judged not feasible due to a 24% TRM rate during transplant [13]. Use of PBSC provides significant advantages over BM in the autologous setting. Recovery of counts with PBSC occurs more rapidly, which is important both in terms of reducing the risk of infection and allowing rapid sequence tandem HDC/SCR. A potential limitation of this trial at its inception was the concern that PBSC were potentially contaminated with tumor cells and there was no manipulation of the stem cell product to reduce tumor burden. However, a recently completed randomized study completed by the Children's Oncology Group suggests that subjecting peripheral blood stem cell collections to tumor-cell purging, utilizing immunomagnetic antibody against neuroblastoma antigens, does not improve upon outcomes [14].
Over the past decade, consolidation therapy with stem cell rescue for patients with initial responses to chemotherapy has become the standard of care for high risk neuroblastoma in the United States and Europe, largely based upon results from cooperative group randomized trials comparing outcomes with this approach with chemotherapy only [12]. However, questions regarding the optimal choice of myeloablative regimens, the role of tandem or multiple regimens, and the interaction between induction therapy and choice of consolidation therapies remain of great interest. In this study, HDC/SCR was performed using carboplatin/etoposide/cyclophosphamide followed in rapid sequence by thiotepa/cyclophosphamide. Alternative consolidation regimens in HDC/SCR studies include melphalan (as a single agent) [2], busulphan/melphalan [17] and carboplatin/etoposide/melphalan (CEM) [14]. A recent and compelling report from the ENSG suggests that, in a randomized comparison of patients who achieved a good response with a platinum-intensive induction regimen, busulphan/melphalan is associated with an improved event-free survival compared with CEM [17].
There is a developing body of data to support use of multiple-cycle HDC/SCR for consolidation therapy in NBL. In a retrospective analysis of 546 patients with advanced neuroblastoma from the European Bone Marrow Transplantation Solid Tumor Registry, a 5-year PFS of 24% was reported for 436 patients who underwent a single HDC/SCR, compared to 33% for 110 patients who underwent some variation of a tandem HDC/SCR procedure, a difference that was statistically significant (P=0.05) [4]. A multiple cycle HDC/SCR study was conducted by Kletzel et al. from Children's Memorial in Chicago, using three HDC/SCR regimens in sequence. Of 25 patients in the published report, 19 completed HDC/SCR #2, 17 went on to HDC/SCR #3, and only one late TRM was observed. Six of the patients also received at least one course of anti-GD2 monoclonal antibody as well. The EFS in this group of patients at 3 years was 57% [7]. The largest phase II experience to date utilized a tandem HDC/SCR regimen including TBI in the second consolidation [6]. This study has been updated, showing a 3-year EFS from diagnosis of 55%. Five-and 7-year EFS were 47% and 45%, respectively, demonstrating stable, long-term survival in a subset of these high risk patients [8].
This study has several limitations, including a small sample size, lack of followup information beyond five years, lack of biological correlates of response and minimal residual disease measurement, and lack of analysis of cost of tandem (vs. single) HDC/SCR. The induction therapy was novel, building on prior POG experience [15,16], and contained only five cycles of chemotherapy during induction, utilized carboplatin and ifosfamide during induction, and limited the exposure to anthracyclines to a single doxorubicin-containing cycle. Our small sample size precludes efficacy analysis of the induction regimen and a more refined outcome analysis looking at response to induction and subsequent outcomes.
In summary, the treatment of high risk neuroblastoma with tandem HDC/SCR is feasible in terms of transplant-related mortality, ability to collect adequate PBSC for 2 transplants, and recovery between tandem transplants.
Table III.
Overall Survival (OS) and Progression-Free Survival (PFS) of Patients Treated with HDC/SCR.
| N | 3yr (± SE) | 5yr (± SE) | |
|---|---|---|---|
| Survival from time of diagnosis for all patients | |||
| PFS | 33 | 36.4% (±8.4%) | 24.2% (±7.5%) |
| OS | 48.5% (±8.7%) | 36.4% (±8.4%) | |
| Survival from time of first transplant for patients who received at least one transplant | |||
| PFS | 22 | 45.5% (±10.6%) | 36.4% (±11.0%) |
| OS | 59.1% (±10.5%) | 45.5% (±11.2%) | |
Acknowledgements
The authors thank the physicians, nurses and clinical research associates of all participating centers for their outstanding clinical care of these patients, and their data management and reporting. The Children's Oncology Group combined the Pediatric Oncology Group and the Children's Cancer Group into one consortium in 2000 and continues to strive to cure and prevent childhood cancer. Supported by the COG NIH Chairman's Grants U10 CA98543, U10 CA98413 and POG Grants U10 CA29139 and U10 CA57745. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
Supported in part by funds from the Sanford and WW Smith Trusts (SAG) and the Friends-for-Life Foundation (LD).
Footnotes
The authors have no conflicts of interest.
REFERENCES
- 1.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J.Clin.Oncol. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard J, Cotterill BA, Germond SM, Imeson J, de Kraker J, Jones DR. High dose melphalan in the treatment of advances neuroblastoma: Results of a randomized trial (ENSG-1) by the European Neuroblastoma Study Group. Ped. Blood and Cancer. 2005;44(4):348–357. doi: 10.1002/pbc.20219. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, O'Leary MC, Ramsay NK, et al. Role of myeloablative therapy in improved outcome for high risk neuroblastoma: review of recent Children's Cancer Group results. Eur.J.Cancer. 1995;31A:572–575. doi: 10.1016/0959-8049(95)00015-b. [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Ladenstein R, Lasset C, et al. 1070 myeloablative megatherapy procedures followed by stem cell rescue for neuroblastoma: 17 years of European experience and conclusions. European Group for Blood and Marrow Transplant Registry Solid Tumour Working Party. Eur.J.Cancer. 1997;33:2130–2135. doi: 10.1016/s0959-8049(97)00324-9. [DOI] [PubMed] [Google Scholar]
- 5.Grupp SA, Stern JW, Bunin N, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J.Clin.Oncol. 2000;18:2567–2575. doi: 10.1200/JCO.2000.18.13.2567. [DOI] [PubMed] [Google Scholar]
- 6.Grupp SA, Stern JW, Bunin N, et al. Rapid-sequence tandem transplant for children with high-risk neuroblastoma. Med.Pediatr.Oncol. 2000;35:696–700. doi: 10.1002/1096-911x(20001201)35:6<696::aid-mpo46>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Kletzel M, Katzenstein HM, Haut PR, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J.Clin.Oncol. 2002;20:2284–2292. doi: 10.1200/JCO.2002.06.060. [DOI] [PubMed] [Google Scholar]
- 8.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J.Clin.Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 9.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J.Clin.Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 10.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13cisretinoic acid. Children's Cancer Group. N.Engl.J.Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 12.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13cisretinoic acid: a children's oncology group study. J.Clin.Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philip T, Ladenstein R, Zucker JM, et al. Double megatherapy and autologous bone marrow transplantation for advanced neuroblastoma: the LMCE2 study. Br.J.Cancer. 1993;67:119–127. doi: 10.1038/bjc.1993.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreissman SG, Villablanca JG, Seeger RC, et al. A randomized phase III trial of myeloablative autologous peripheral blood stem cell (PBSC) transplant (ASCT) for high-risk neuroblastoma (HR-NB) employing immunomagnetic purged (P) versus unpurged (UP) PBSC: A Children's Oncology Group study. J Clin Oncol (Meeting Abstracts) 2008;26(15)(suppl):10011. [Google Scholar]
- 15.Castleberry RP, Cantor AB, Green AA, et al. Phase II investigational window using carboplatin, iproplatin, ifosfamide, and epirubicin in children with untreated disseminated neuroblastoma: a Pediatric Oncology Group study. J.Clin.Oncol. 1994;12:1616–1620. doi: 10.1200/JCO.1994.12.8.1616. [DOI] [PubMed] [Google Scholar]
- 16.Kretschmar CS, Kletzel M, Murray K, et al. Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: a pediatric oncology group study. J.Clin.Oncol. 2004;22:4119–4126. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 17.Ladenstein RL, Poetschger U, Luksch R, et al. Busulphan-melphalan as a myeloablative therapy (MAT) for high-risk neuroblastoma: Results from the HR-NBL1/SIOPEN trial. 2011 ASCO Annual Meeting. Abstract 2. Presented. 2011 Jun 5; [Google Scholar]


