Abstract
Background
Lactate clearance is a standard resuscitation goal in patients in non-traumatic shock but has not been investigated adequately as a tool to identify trauma patients at risk of dying. Our objective was to determine if trauma patients with impaired lactate clearance have a higher 24-hour mortality rate than patients whose lactate concentration normalizes.
Methods
A retrospective chart review identified patients who were admitted directly from the scene of injury to an urban trauma center between 2010 and 2013 and who had at least one lactate concentration measurement within 24 hours. Transfers, patients without lactate measurement, and those who were dead on arrival were excluded. Of the 26,545 screened patients, 18,304 constituted the initial lactate measurement population and 3,887 were the lactate clearance cohorts.
Results
Initial lactate had an area-under-the-receiver operating curve of 0.86 and 0.73 for mortality at 24 hours and in-hospital, respectively. An initial concentration ≥3 mmol/L had sensitivity of 0.86 and specificity of 0.73 for mortality at 24 hours. The mortality rate among patients with elevated lactate concentrations (n=2381, 5.6±2.8 mmol/L) that did not decline to <2.0 mmol/L in response to resuscitative efforts (mean second measurement, 3.7±1.9 mmol/L) was nearly seven times higher (4.1% vs 0.6% [p<0.001]) than among those with an elevated concentration (n=1506, 5.3±2.7 mmol/L) that normalized (1.4±0.4 mmol/L). Logistic regression analysis showed that failure to clear lactate was associated with death more than any other feature (OR=7.4; CI, 1.5–35.5), except having an Injury Severity Score >25 (OR=8.2; CI, 2.7–25.2).
Conclusions
Failure to clear lactate is a strong negative prognostic marker after injury. An initial lactate measurement combined with a second measurement for high-risk individuals might constitute a useful method of risk-stratifying injured patients.
Keywords: lactate, trauma, mortality, resuscitation
Introduction
Estimating depth of shock and adequacy of resuscitation is at the cornerstone of the care of badly injured patients. Vital signs are often used as an initial measure of hemodynamic stability, but we have known for many years that they might grossly underestimate the depth of shock1. Other measures such as base deficit and central venous oxygen saturation are far more accurate than vital signs2,3.
In 1993, Abramson et al4 demonstrated that the ability to clear lactate to normal was the most accurate predictor of outcome following critical injury. Their study revealed a stepwise increase in the mortality rate as the time to lactate clearance increased. All patients whose lactate concentration fell to normal within 24 hours survived.
In the past 20 years, trauma resuscitation techniques have changed dramatically. Hypotensive resuscitation—allowing patients to remain with a lower-than-normal blood pressure until hemorrhage is controlled—is now common practice in major American trauma centers. Several prospective randomized trials have demonstrated its efficacy5,6. In addition, damage control resuscitation, limiting the volume of crystalloid administered, and using blood and plasma early have been demonstrated to improve outcome7,8.
Lactate, the metabolic byproduct of anaerobic metabolism9, should be a sensitive marker of shock and resuscitation. High lactate levels at the time of patient presentation have been shown to predict death in studies of sepsis as well as trauma10–13, and is now used as a marker of resuscitation14,15. Base deficit also correlates with volume resuscitation and survival in trauma16–18. However, lactate has been shown to predict mortality as well, or better than, base deficit in several studies19–21, including injured patients with normal base deficits22. Serum lactate is an ideal biomarker because the technology is inexpensive23, the test is fast24, and there are multiple ways to obtain equivalent samples25–28. Many studies that examined the utility of lactate clearance have used in-hospital mortality as the endpoint29–34, yet death in the hospital can be caused by many events unrelated to the adequacy of resuscitation. Lactate has a half-life of 15–30 minutes in healthy subjects35, suggesting lactate levels may change rapidly in response to resuscitative efforts. Given validated point-of-care devices37–40, if lactate was shown to predict early death, we could use lactate in the prehospital arena to enhance triage protocols41. The objective of this work is to show that lactate clearance is a better marker of early death (within 24 hours after admission) than a marker of in-hospital mortality.
Methods
Population and Setting
We retrospectively reviewed the trauma registry at the R Adams Cowley Shock Trauma Center, University of Maryland, for the period of January 1, 2010, to December 31, 2012. At our institution, patients with acute complications of previous injuries (e.g. soft tissue infections in paraplegics), medical emergencies (e.g. myocardial infarction, acute stroke, etc.), and those with non-traumatic causes of shock (e.g. sepsis, ruptured abdominal aortic aneurysm, bowel infarction) are not recorded in the trauma registry. We reviewed the records of all patients admitted within the study dates and excluded those who 1) did not have a lactate measurement within 24 hours after arrival, 2) were transferred to the trauma center from other facilities, or 3) died within 15 minutes after arrival. We excluded files that contained corrupted data or had obvious data entry errors.
Patients began resuscitation with immediate vascular access and an initial bolus of intravenous crystalloid solution. All patients undergo standardized testing, including ultrasound and computed tomography imaging, at the time of admission. Testing includes an initial venous lactate level that is repeated six hours later. Blood products are immediately available as needed. Our massive transfusion protocol is activated if the patient’s has two successive systolic blood pressures less than 90 millimeters of mercury) or has received two units of uncross-matched blood, in combination with obvious blood loss, bleeding seen on imaging, penetrating torso injury, or if the patient plan will be managed in the operating room. Hypotensive and damage-control resuscitation strategies are used as the clinical picture dictates.
This study was approved by the institutional review board at the University of Maryland, Baltimore (HP-00050293).
Data Management
The study population included every patient who had at least one lactic acid measurement within the first 24 hours after admission. Within this population, we created two subgroups of patients who had at least two lactate measurements within the first 24 hours after arrival. The first subgroup, the High Clearance Group, comprised all patients who arrived with an elevated lactate concentration (≥3.0 mmol/L) that then normalized (<2.0 mmol/L). The second subgroup, the Poor Clearance Group, had an initially elevated lactate level (≥3.0 mmol/L) that did not normalize (≥2.0 mmol/L). Lactate measures <2.0 mmol/L are considered normal at our institution’s laboratory.
Data Analysis
We recorded Injury Severity Score (ISS)42, age, sex, race, and mechanism of injury. All between-group differences were evaluated using student’s t-test or chi-squared statistics and an alpha of 0.05. Using a cutoff of 3.0 mmol/L, we calculated the sensitivity and specificity of the initial lactate concentration to predict 24-hour mortality and in-hospital mortality. We also derived a receiver operating characteristic curve43 using the initial lactate level to predict 24-hour mortality and in-hospital mortality. We derived percent mortality for patients based on initial lactate and shock index (heart rate [beats per minute] / systolic blood pressure [mmHg])44. We obtained the demographics for the patients with a normal shock index and hyperlactemia for comparison to our lactate clearance cohorts.
We performed a multivariate logistic regression to compare the association of initial lactate concentration and lactate clearance with other commonly cited factors of poor outcome after traumatic injury (age, sex, ISS, and mechanism as well as blood pressure, Glasgow Coma Scale [GCS] score45, and heart rate on admission) on 24-hour mortality for patients with an initial lactate concentration ≥3.0 mmol/L. Delong’s method was used to compare the areas under receiver operating characteristic curves (AUC)46. SAS 9.2 software, Version 8 (SAS Institute, Cary, North Carolina), was used for all calculations.
Results
Study Population and Cohorts
Of the 26,454 patients for whom data were recorded in the trauma registry during the study period, 18,304 met the inclusion criteria (Figure 1). The High Clearance Group consisted of 1,506 patients and the Poor Clearance Group had 2,381 patients. In the initial population, 14,417 patients had only one lactate measurement or their initial lactate level was not elevated.
Figure 1.
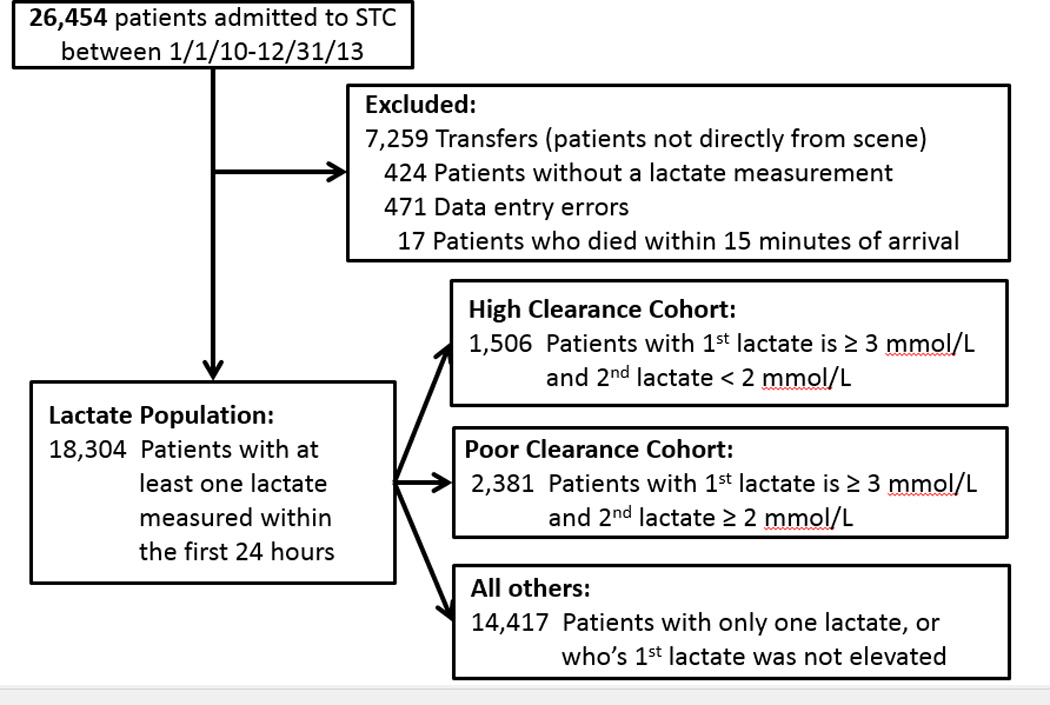
Consort diagram for lactate clearance population and cohort development.
The general study population was predominantly Caucasian males, approximately 40 years of age, who were victims of motor vehicle crashes and had minor injuries (ISS<9) (Table 1). The High and Poor Clearance Groups had higher proportions of African-Americans, patients with penetrating trauma, and patients in higher ISS categories.
Table 1.
Demographics of total Lactate Clearance Population and Study Cohorts, Shock Trauma Center, Maryland, 2010–2012
| Lactate Population | High Clearance | Poor Clearance | |
|---|---|---|---|
| N (%) | 18,304 (100%) | 1506 (7.8%) | 2,381 (12.4%) |
| Age, mean (SD) | 41.3 (19.7) | 37.9 (17.9) | 39.9 (18.0) |
| Male Sex, % | 12,600 (68.8) | 1180 (78.4) | 1889 (79.3) |
|
Race, % | |||
| White | 10,679 (58.5) | 802 (53.4) | 1235 (52.1) |
| Black | 6,247 (34.3) | 629 (41.9) | 956 (40.3) |
| Hispanic | 502 (2.8) | 25 (1.7) | 68 (2.9) |
| Other | 814 (4.5) | 46 (3.1) | 112 (4.7) |
|
ISS | |||
| <9 | 9,639 (54.5) | 573 (38.6) | 596 (25.3) |
| 9<ISS<16 | 3,886 (22.0) | 420 (28.3) | 499 (21.2) |
| 16<ISS<25 | 2,206 (12.5) | 249 (16.8) | 440 (18.7) |
| ISS>25 | 1,958 (11.1) | 244 (16.4) | 820 (34.8) |
|
Mechanism | |||
| MVC | 8,576 (46.9) | 545 (36.2) | 1107 (46.5) |
| Falls | 4,493 (24.6) | 312 (20.7) | 413 (17.4) |
| Stabbing | 1,198 (6.6) | 173 (11.5) | 235 (9.9) |
| GSW | 951 (5.2) | 191 (12.7) | 280 (11.8) |
Initial Lactate Results
18,304 patients were enrolled, and initial lactate levels were reported (i.e., blood was drawn, the test was completed, and the value was made available to the health care provider in the electronic medical record) within a median 13.0 minutes after the patient’s arrival (Interquartile range [IRQ]=9–19 minutes). There were 4,576 patients with normal shock indices and hyperlactemia (Table 2) who had a 24-hour and in-hospital mortality of 1.55% and 4.02%, respectively. The demographics of this subpopulation (See Supplemental Digital Content) are similar to the Poor and High Clearance cohorts (Table 1). The (AUC) was 0.87 for mortality at 24 hours and 0.73 for in-hospital mortality (p-value<0.0001, Figure 2). A cut-off of 4.0 mmol/L had the best overall combination of sensitivity and specificity for mortality at 24 hours. Our pre-specified cut-off of 3.0 mmol/L had a considerably better sensitivity of 0.86, with a good specificity of 0.68, or a positive predictive value (PPV) of 3.70% and negative predictive value (NPV) of 99.72%. Positive and negative likelihood ratios (LR+ and LR−) for a cut-off of 3.0 mmol/L were 2.72 and 0.20, respectively47.
Table 2.
Comparison of Mortality by Shock Index and Initial Lactate. Shock Trauma Center, Maryland, 1/1/2010–12/31/12
| Lactate <=3 | Lactate >3 | ||
|---|---|---|---|
| Total patients | SI ≤ 0.9 | 12302 | 4576 |
| SI > 0.9 | 425 | 867 | |
| 24-Hour Mortality, N (%) | SI ≤ 0.9 | 29 (0.24) | 71 (1.55) |
| SI > 0.9 | 7 (1.65) | 57 (6.57) | |
| In-Hospital Mortality, N (%) | SI ≤ 0.9 | 138 (1.12) | 184 (4.02) |
| SI > 0.9 | 14 (3.29) | 107 (12.34) | |
Figure 2.
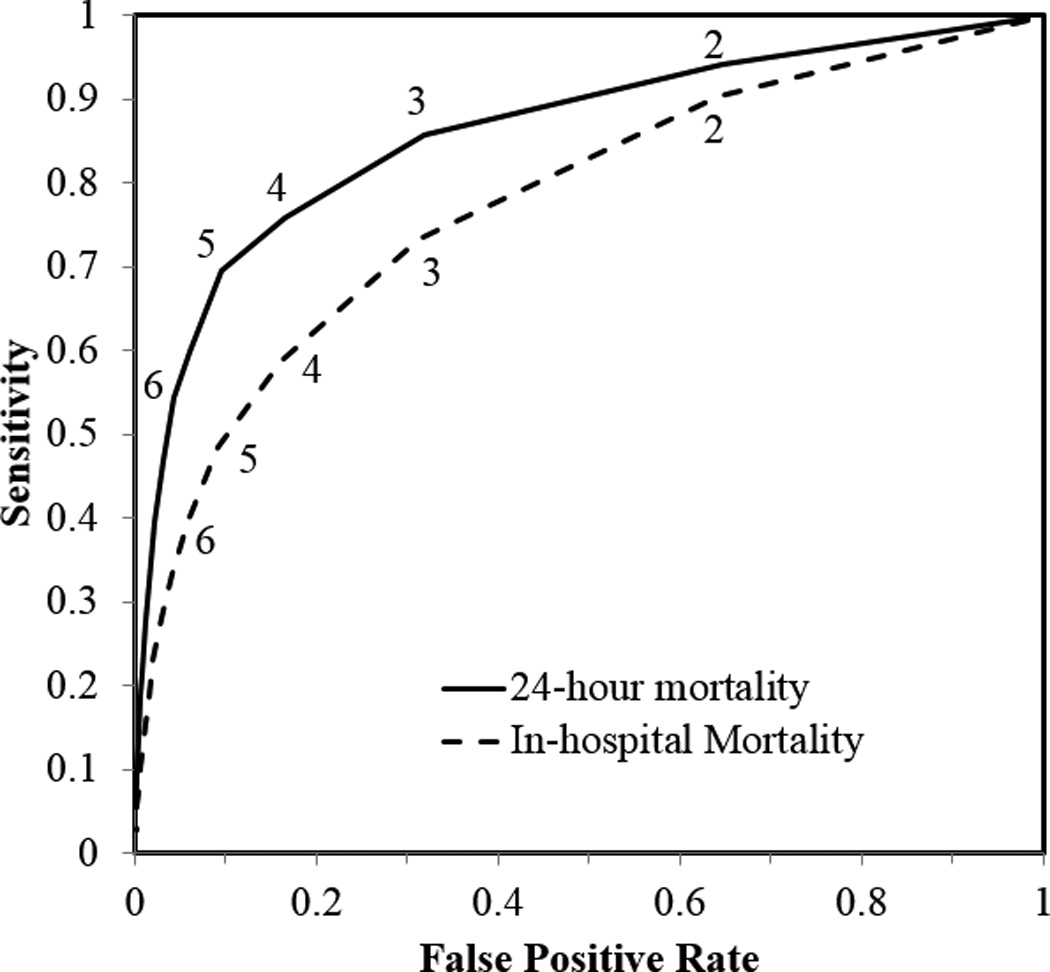
Receiver operating characteristic curve for initial lactate. Numbers on the curve denote the various cut-off values for lactate concentration (mmol/L). The AUC was 0.87 and 0.73 for death at 24 hours and in-hospital mortality, respectively
Lactate Clearance Group Analysis
The mean initial lactate concentrations for the Poor and High Lactate Clearance Groups were different statistically but not clinically (5.6±2.8 and 5.3±2.6, respectively [P=0.0002]) (Table 3). The second lactate measurement was reported a median of 251 minutes after arrival (IRQ=157–401 minutes). Compared with the High Clearance Group, the Poor Clearance Group was older (37.9±17.9 vs 39.9±18.0 years [P<0.001]) and had significantly worse injuries (ISS, 19.7±15.4 vs 13.0±11.5 [P<0.001]) and a nearly seven-fold higher 24-hour mortality rate (P<0.001).
Table 3.
Demographic characteristics and mortality of patients in the High and Poor Clearance Cohorts, Shock Trauma Center, Maryland, 1/1/2010–12/31/12.
| High Clearance | Poor Clearance | p-values | |
|---|---|---|---|
| 1st Lactate (mmol/L), mean (SD) | 5.3 (2.6) | 5.6 (2.8) | <0.001 |
| 2nd Lactate (mmol/L), mean (SD) | 1.4 (0.4) | 3.7 (1.9) | <0.001 |
| Lactate Clearance (%) | 73.1 | 32.2 | <0.001 |
| ISS, mean (SD) | 13.0 (11.5) | 19.7 (15.4) | <0.001 |
| % male sex | 78.4 | 79.3 | 0.464 |
| Age, mean (SD) | 37.9 (17.9) | 39.9 (18.0) | <0.001 |
| % penetrating mechanism | 25.0 | 21.9 | 0.023 |
| % mortality at 24 hours | 0.6 | 4.1 | <0.001 |
| Admission SBP (mmHg), mean (SD) | 143.6 (25.8) | 137.0 (36.1) | <0.001 |
| Admission Heart Rate, mean (SD) | 96.6 (21.0) | 100.5 (26.5) | <0.001 |
| Admission GCS, mean (SD) | 13.6 (3.1) | 12.4 (4.2) | <0.001 |
| Patients (n) | 1506 | 2,381 |
Multivariate Analysis
The following factors were found to be predictive of death at 24 hours: critical injury (ISS>25, OR=8.2), negative lactate clearance (OR=7.4), initial lactate concentration >7 (OR=5.1), comatose presentation (GCS score=3, OR=4.3), hypotension on admission (OR=3.0), age >65 years (OR=2.7), and extreme tachycardia (heart rate >120, OR=2.0) (Table 4). Although none of the other lactate clearance groups shown in Table 4 reached statistical significance (the confidence intervals of the ORs contained 1), there is a trend in decreasing odds ratio as lactate clearance increases. The AUC for this multivariate model was 0.942 (95% confidence interval=0.923–0.960). Treating lactate clearance as a continuous variable did not significantly improve the predictive ability of the model (AUC=0.943 [0.934–0.953]) but it was still significantly associated with survival (OR=0.12, [0.07–0.21]). This suggests that each percentage point improvement in lactate clearance significantly improves the patient’s 24-hour survival.
Table 4.
Odds Ratios for classic clinical predictors of mortality in trauma, compared to lactate and lactate clearance in study cohort, Shock Trauma Center, Maryland, 1/1/2010–12/31/12.
| Adjusted OR for mortality at 24-hours (95%CI) | |
|---|---|
| Lactate clearance | |
| Negative* | 8.9 (1.9–42.4) |
| 0–20% | 3.23 (0.7–15.5) |
| 20–40% | 2.5 (0.5–12.0) |
| 40–60% | 1.2 (0.2–6.1) |
| 60–80% | 1.1 (0.2–5.2) |
| 80–100% | 1.0 (reference) |
| Initial Lactate (mg/L) | |
| 3.0–4.0 | 1.0 (reference) |
| 4.0–7.0 | 1.6 (0.8–3.1) |
| ≥7.0* | 5.7 (2.8–11.9) |
| ISS | |
| <9 | 1.0 (reference) |
| 9<ISS<16 | --- |
| 16<ISS<25 | 1.2 (0.3–4.8) |
| ISS≥25* | 8.2 (2.7–25.3) |
| Age (years) | |
| 17–45 | 1.0 (reference) |
| 45–65 | 1.2 (0.7–2.1) |
| 65+* | 2.9 (1.5–5.4) |
| Admission SBP (mmHg) | |
| <90* | 3.0 (1.5–5.9) |
| 90–120 | 1.5 (0.8–3.0) |
| 120–150 | 1.0 (reference) |
| ≥150 | 1.5 (0.8–2.7) |
| Admission Heart Rate (BPM) | |
| <60 | 2.0 (0.9–4.5) |
| 60–100 | 1.0 (reference) |
| 100–120 | 1.3 (0.7–2.5) |
| ≥120* | 2.0 (1.1–3.5) |
| Admission GCS | |
| 3–8* | 4.3 (2.4–7.7) |
| 9–13 | 1.6 (0.7–3.4) |
| 14–15 | 1.0 (reference) |
Statistically significant.
Discussion
The ability to optimally resuscitate patients is thought to be important in maximizing survival. The aforementioned study by Abramson and colleagues4, demonstrating that trauma patients’ ability to return lactate to a normal level was the best predictor of survival, used in-hospital death as its endpoint. Those deaths could have been related to complications such as multiple organ failure, deep venous thrombosis, or sepsis, not necessarily to the adequacy of resuscitation.
Trauma resuscitation has evolved considerably since 1992. Newer techniques such as hypotensive resuscitation and damage control are enabling patients who would have died in past to now survive. It is unclear whether these changes have salutary effects on late deaths. Holcomb and associates demonstrated that patients who receive high ratios of plasma and platelets relative to the number of red cells transfused are much less likely to bleed to death but more likely to die from late complications such as traumatic brain injury8
In the series reported in this article, ISS, inability to clear lactate, mental obtundation at the time of presentation, initial lactate concentration, hypotension on admission, age, and heart rate at admission all predicted early death. Of these variables, only the ability to clear lactate to normal can be modified. Patients whose lactate concentration did not fall to normal had a nearly seven-fold increase in the 24-hour mortality rate compared with those whose lactate levels declined. The trend toward decreasing mortality as lactate clearance improved suggests that clearing lactate to normal is highly advantageous.
Admittedly, the patients with high lactate levels that did not decrease were more severely injured and older than those in whom the lactate level did return to normal. However, after adjusting for other risk factors, our multivariate analysis demonstrated that failure to clear lactate was more predictive of death than age, hypotension, tachycardia, and mental obtundation. Only injury anatomy (ISS> 25) had a greater predictive value. Our model further showed that each incremental increase in lactate clearance was associated with an improvement in 24-hour survival (OR=0.12, 95%CI=0.07–0.21).
As a compliment to the work done by Odom and colleagues33, this is the largest study to examine if lactate clearance predicts 24-hour mortality following injury. The more recent dates of our study make it more relevant in the era of damage control surgery and damage control resuscitation. In addition, our data set was quite complete: our data would represent more than 95% of the patients directly transported to our center. This is also the largest study to show that the initial lactate concentration is useful in predicting death after trauma. Patients with initial lactate levels >7 mmol/L had a five-fold greater risk of death after 24 hours. There was a large group of patients (n=4,567, 25.0%) with elevated initial lactates and normal shock indices, and the mortality of this group was high (1.55% at 24-hours and 4.02% in-hospital, respectively). We found the mathematically ideal cut off for initial lactate to be 4.0 mmol/L, which is remarkably similar to data from patients with sepsis13–15. We found that a value of 3 mmol/L yielded similar sensitivity but better specificity (0.86 and 0.68, respectively) compared with data generated by Pal and colleagues (sensitivity, 0.85, and specificity, 0.38)29.
Our focus on 24-hour mortality might explain the increased specificity of the initial lactate concentration, as it eliminates confounding clinical scenarios that could influence patient outcome (e.g., hospital-acquired infections). Our decision was supported by our results: our in-hospital mortality AUC was 0.73 – similar to what had been reported by other investigators26,31,41 – yet the AUC for initial lactate concentration predicting 24-hour mortality was higher: 0.86. Given the results of this study, and the short half-life of lactate, this study suggests that clinicians should check the lactate levels of injured patients early and often.
In our trauma center, lactate levels can be determined quickly and, in this study, were available to clinicians approximately 20 minutes after patient presentation. Thus, the initial concentration is a rapidly available screening test that is useful in identifying patients at high risk for early death. In parallel, a normal initial lactate level should be reassuring, indicating that the risk of death is quite low.
For the current investigation, we did not extract registry data regarding patients’ initial treatment strategy. Our receiving physicians commonly employ hypotensive resuscitation as well as damage control resuscitation. It seems that patients with initially elevated lactate concentrations would be ideal candidates for those approaches. Once hemorrhage is controlled, efforts should be directed toward normalizing the lactate level as quickly as possible. A repeat measurement should be requested early in the resuscitation. Patients whose levels are not falling require particular diligence to ensure that hemorrhage is controlled and resuscitative efforts are optimized.
Limitations
As with any retrospective study, this investigation is subject to sources of unmeasured bias. Clinicians determined at the point of care which patients should or should not have a second lactate measurement. As a result, we do not have a second lactate reading on all patients, and the timing of the second measurement is quite variable. Additionally, although we attempted to account for other factors that predict death after trauma, residual confounding might still have occurred.
Conclusions
After injury, an initially elevated serum lactate concentration predicts mortality. Each incremental improvement in lactate clearance enhances 24-hour survival. The inability to reduce lactate concentration toward normal increases the risk of death within the first 24 hours of hospitalization. Serial measurements of the lactate concentration are important markers of the adequacy of resuscitation. No single value has been identified as “perfect,” but it appears that serial lactate measurements early after resuscitation are an important factor in treatment strategies for critically injured patients.
Supplementary Material
Acknowledgments
We thank Brian J. Browne, MD, and the Department of Emergency Medicine at the University of Maryland for funding this project via a Resident Research Grant. We thank Kimberly M. Auman, MS, at the National Study Center for Trauma and Emergency Medical Systems for her assistance with database management. Dr. Dezman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of their analysis. The manuscript was copyedited by Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine at the University of Maryland School of Medicine.
This study was supported by a Resident Research Grant from the Maryland Emergency Medicine Network. Dr. Smith was supported by a grant from the U.S. National Institute on Alcohol Abuse and Alcoholism (R01AA18707). Dr. Hirshon was supported by a grant from the U.S. National Institute of Health Fogarty International Center (5D43TW007296).
Footnotes
The authors have no conflicts of interest to disclose.
Some of these findings were presented as an abstract at the 2014 Scientific Assembly of the American College of Emergency Physicians.
Author Contribution Statement
All of the authors were members of the research team and 1) participated in the conception, design, and interpretation of the data, 2) the drafting and revising of the manuscript, 3) and gave approval for the final manuscript to be submitted.
Contributor Information
Angela C. Comer, Email: acomer@stapa.umm.edu.
Gordon S. Smith, Email: gssmith@som.umaryland.edu.
Mayur Narayan, Email: mnarayan@umm.edu.
Thomas M. Scalea, Email: tscalea@umm.edu.
Jon Mark Hirshon, Email: jhirshon@umaryland.edu.
References
- 1.Basel KJ, Guse C, Gentilello LM, Nirula R. Heart rate: Is it truly a vital sign? J Trauma. 2007;62:812–817. doi: 10.1097/TA.0b013e31803245a1. [DOI] [PubMed] [Google Scholar]
- 2.Scalea TM, Holman M, Fuortes M, Baron B, Phillips TF, Goldstein AS, Sclafani SJA, Shaftan GW. Central venous blood oxygen saturation: an early accurate measurement of volume status during hemorrhage. J Trauma. 1998;28:725–732. [PubMed] [Google Scholar]
- 3.Davis JW, Parks SN, Kaups KL, Gladen HE, O’Donnell-Nicol S. Admission base deficit predicts transfusion requirements and risk of complication. J Trauma. 1996;41:769–771. doi: 10.1097/00005373-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35:584–589. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Bickell WH, Wall MJ, Jr, Pepe PE, Martin RP, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 6.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins GJ, Spinella PC, et al. Increased plasma and platelets to red blood cell ratios improves outcomes in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi: 10.1186/2110-5820-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 13.Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):R25. doi: 10.1186/cc8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JW, Shackford SR, Mackersie RC, Hoyt DB. Base deficit as a guide to volume resuscitation. J Trauma. 1988;28(10):1464–1467. doi: 10.1097/00005373-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Davis JW, Shackford SR, Holbrook TL. Base deficit as a sensitive indicator of compensated shock and tissue oxygen utilization. Surg Gynecol Obstet. 1991;173(6):473–476. [PubMed] [Google Scholar]
- 18.Rutherford EJ, Morris JA, Jr, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33(3):417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Mikulaschek A, Henry SM, Donovan R, Scalea TM. Serum Lactate Is Not Predicted by Anion Gap or Base Excess after Trauma Resuscitation. Journal of Trauma-Injury Infection & Critical Care. 1996;40(2):218–224. doi: 10.1097/00005373-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185(5):485–491. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Murray J, Berne T, Demetriades D, Belzberg H. Diagnosis of acid-base derangements and mortality prediction in the trauma intensive care unit: the physiochemical approach. J Trauma. 2005;58(2):238–243. doi: 10.1097/01.ta.0000152535.97968.4e. [DOI] [PubMed] [Google Scholar]
- 22.Martin MJ, FitzSullivan E, Salim A, Brown CVR, Demetriades D, Long W. Discordance between lactate and base deficit in the surgical intensive care unit: which one do you trust? Am J Surg. 2006;191(5):625–630. doi: 10.1016/j.amjsurg.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37(10):2827–2839. doi: 10.1097/CCM.0b013e3181a98899. [DOI] [PubMed] [Google Scholar]
- 24.Aduen J, Bernstein WK, Khastgir T, Miller J, Kerzner R, Bhatiani A, Lustgarten J, Bassin AS, Davison L, Chernow B. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA. 1994;272(21):1678–1685. [PubMed] [Google Scholar]
- 25.Weil MH, Michaels S, Rackow EC. Comparison of blood lactate concentrations in central venous, pulmonary artery, and arterial blood. Crit Care Med. 1987;15(5):489–490. [PubMed] [Google Scholar]
- 26.Younger JG, Falk JL, Rothrock SG. Relationship between arterial and peripheral venous lactate levels. Acad Emerg Med. 1996;3(7):730–734. doi: 10.1111/j.1553-2712.1996.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher EJ, Rodriguez K, Touger M. Agreement between peripheral venous and arterial lactate levels. Ann Emerg Med. 1997;29(4):479–483. [PubMed] [Google Scholar]
- 28.Fauchère JC, Bauschatz AS, Arlettaz R, Zimmermann-Bär U, Bucher HU. Agreement between capillary and arterial lactate in the newborn. Acta Paediatr. 2002;91(1):78–81. doi: 10.1080/080352502753458003. [DOI] [PubMed] [Google Scholar]
- 29.Pal JD, Victorino GP, Twomey P, Liu TH, Bullard MK, Harken AH. Admission serum lactate levels do not predict mortality in the acutely injured patient. J Trauma. 2006;60(3):583–587. doi: 10.1097/01.ta.0000205858.82575.55. [DOI] [PubMed] [Google Scholar]
- 30.Dunne JR, Tracy JK, Scalea TM, Napolitano LM. Lactate and base deficit in trauma: does alcohol or drug use impair their predictive accuracy? J Trauma. 2005;58(5):959–966. doi: 10.1097/01.ta.0000158508.84009.49. [DOI] [PubMed] [Google Scholar]
- 31.Callaway DW, Shapiro NI, Donnino MW, Baker C, Rosen CL. Serum lactate and base deficit as predictors of mortality in normotensive elderly blunt trauma patients. J Trauma. 2009;66(4):1040–1044. doi: 10.1097/TA.0b013e3181895e9e. [DOI] [PubMed] [Google Scholar]
- 32.Régnier MA, Raux M, Le Manach Y, Asencio Y, Gaillard J, Devilliers C, Langeron O, Riou B. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012;117(6):1276–1288. doi: 10.1097/ALN.0b013e318273349d. [DOI] [PubMed] [Google Scholar]
- 33.Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI, Talmor D. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg. 2013;74(4):999–1004. doi: 10.1097/TA.0b013e3182858a3e. [DOI] [PubMed] [Google Scholar]
- 34.Parsikia A, Bones K, Kaplan M, Strain J, Leung PS, Ortiz J, Joshi AR. The predictive value of initial serum lactate in trauma patients. Shock. 2014;42(3):199–204. doi: 10.1097/SHK.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 35.Almenoff PL, Leavy J, Weil MH, Goldberg NB, Vega D, Rackow EC. Prolongation of the half-life of lactate after maximal exercise in patients with hepatic dysfunction. Crit Care Med. 1989;17(9):870–873. doi: 10.1097/00003246-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Plowman SA, Smith DL. Exercise Physiology for Health Fitness and Performance. Lippincott: Williams & Wilkins; 2013. Feb 25, [Google Scholar]
- 37.Brinkert W, Rommes JH, Bakker J. Lactate measurements in critically ill patients with a hand-held analyser. Intensive Care Med. 1999;25(9):966–969. doi: 10.1007/s001340050990. [DOI] [PubMed] [Google Scholar]
- 38.Saunders AC, Feldman HA, Correia CE, Weinstein DA. Clinical evaluation of a portable lactate meter in type I glycogen storage disease. J Inherit Metab Dis. 2005;28(5):695–701. doi: 10.1007/s10545-005-0090-1. [DOI] [PubMed] [Google Scholar]
- 39.Ridenour RV, Gada RP, Brost BC, Karon BS. Comparison and validation of point of care lactate meters as a replacement for fetal pH measurement. Clin Biochem. 2008;41(18):1461–1465. doi: 10.1016/j.clinbiochem.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 40.Goyal M, Pines JM, Drumheller BC, Gaieski DF. Point-of-care testing at triage decreases time to lactate level in septic patients. J Emerg Med. 2010;38(5):578–581. doi: 10.1016/j.jemermed.2007.11.099. [DOI] [PubMed] [Google Scholar]
- 41.Vandromme MJ, Griffin RL, Weinberg JA, Rue LW, 3rd, Kerby JD. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage? J Am Coll Surg. 2010;210(5):861–869. doi: 10.1016/j.jamcollsurg.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Baker SP, O'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 43.Hanley JA, McNeil BJ. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 44.Cannon CM, Braxton C, Kling-Smith M, Mahnken J, Carlton E, Moncure M. Utility of the Shock Index in Predicting Mortality in Traumatically Injured Patients. Journal of Trauma-Injury Infection & Critical Care. 2009;67(6):1426–1430. doi: 10.1097/TA.0b013e3181bbf728. [DOI] [PubMed] [Google Scholar]
- 45.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 46.DeLong ER, DeLong DM, Clarke-Person DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 47.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17(8):646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


