Abstract
Background
Chagas cardiomyopathy is a chronic sequela of infection by the parasite, Trypanosoma cruzi. Advanced cardiomyopathy is associated with a high mortality rate, and clinical characteristics have been used to predict mortality risk. Though multiple biomarkers have been associated with Chagas cardiomyopathy, it is unknown how these are related to survival.
Objectives
Our study aimed to identify biomarkers associated with mortality in individuals with severe Chagas cardiomyopathy in an urban Bolivian hospital.
Methods
The population included individuals with and without T. cruzi infection recruited in an urban hospital in Santa Cruz, Bolivia. Baseline characteristics, ECG findings, medications, and serum cardiac biomarker levels (BNP, NTproBNP, CKMB, troponin I, MMP-2, MMP-9, TIMP-1, TIMP-2, TGFb1, and TGFb2) were ascertained. Echocardiograms were preferentially performed on those with cardiac symptoms or electrocardiogram abnormalities. Participants were contacted by phone approximately 1 year after initial evaluation; deaths were reported by family members. Receiver operating characteristic curves were used to optimize cut-off values for each marker. For markers with area under curve > 0.55, Cox proportional hazards models were performed to determine the hazards ratio (HR) and 95% confidence interval (CI) for the association of each marker with mortality.
Results
The median follow-up time was 14.1 months (interquartile range 12.5- 16.7 months). Of 254 individuals with complete cardiac data, 220 (87%) had follow-up data. Of 50 patients with severe Chagas cardiomyopathy, 20 (40%) had died. Higher baseline levels of BNP (HR[95% CI]:3.1 [1.2, 8.4]), NTproBNP (4.4[1.8,11.0]), CKMB (3.3[1.3, 8.0]), and MMP-2 (4.2[1.5, 11.8]) were significantly associated with subsequent mortality.
Conclusions
Severe Chagas cardiomyopathy is associated with high short-term mortality. BNP, NTproBNP, CKMB and MMP2 have added predictive value for mortality, even in the presence of decreased ejection fraction and other clinical signs of congestive heart failure.
Keywords: Chagas disease, Cardiomyopathy, Biomarkers, Mortality
Introduction
Chagas disease is caused by the protozoan Trypanosoma cruzi. Chagas disease has traditionally been a disease of rural Latin American communities where poor housing promotes human contact with infected vectors. However, Chagas disease can now be found in urban centers both in and outside of Latin America due to massive emigration from rural endemic areas, with an estimated 300,000 infected people in the United States alone (1). Vector elimination programs have successfully decreased transmission of Chagas disease, but Bolivia remains the country with the highest prevalence of Chagas disease in the world (2).
In the decades following acute infection, 20-30% of individuals will develop cardiac manifestations of disease, including conduction system abnormalities, arrhythmias, and late in the course of disease, congestive heart failure. Advanced heart failure of any etiology is associated with shortened survival, but multiple investigations have shown that heart failure from Chagas disease carries a particularly poor prognosis (3) (4) (5) (6) (7).
Observational studies suggest that antitrypanosomal treatment may improve the prognosis and decrease progression in T. cruzi-infected individuals with no or early signs of Chagas cardiomyopathy. This hypothesis is currently under study in the BENEFIT trial (clinicaltrials.gov/NCT00123916). However, once present, cardiac structural damage is not reversible, and access to advanced heart failure therapies such as left ventricular assist devices, heart transplants, and implantable cardiac defibrillators is limited in communities that are not even able to meet the need for simpler devices such as pacemakers (8). Therefore, identifying biomarkers that are predictive of those at highest risk for fatal outcomes of CCM could allow limited resources to be targeted to those most in need (9).
Clinical findings characteristic of advanced cardiomyopathy and congestive heart failure (NYHA class III or IV, large cardiothoracic ratio on chest radiography, and varied electrocardiogram findings including atrial fibrillation, multiple premature ventricular complexes, ventricular conduction deficits, low voltage, and pathologic Q waves and low QRS voltage) are well known as predictors of mortality in Chagas disease (10-12) (13) (14, 15) (9). Other less consistently identified risk factors include older age and male sex (11) (12) (14) (13) (16) (17). In recent retrospective study including only individuals with severe cardiomyopathy, low ejection fraction did not show further predictive value for mortality within this severely ill group (18) (19).
We measure 10 serum biomarkers in this study, Brain Natriuretic Peptide (BNP), N-terminal brain natriuretic peptide (NTproBNP), Troponin-1, Creatine kinase-MB (CKMB), matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, and transforming growth factor beta (TGFb)1 and TGFb2. Prior work has shown that serum cardiac markers including BNP, NTproBNP, CKMB, MMP-2, and TIMP-2 are associated with mortality in heart failure unrelated to Chagas disease (20-23). The association of TIMP-1 with mortality is controversial (21, 24) (25) (22). To date, only BNP had been shown to predict mortality in Chagas cardiomyopathy (26), (27), (28). The objective of the current analysis was to evaluate the additive predictive value of serum biomarkers within the Chagas disease patient group already known to be at high risk of short-term mortality based on clinical and echocardiographic signs of heart failure.
Materials and Methods
Ethics Statement
The study protocol was approved by the Insititutional Review Boards of Universidad Catolica Boliviana (Santa Cruz, Bolivia), PRISMA (Lima, Peru), and Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland). All participants provided written informed consent.
Study Population and data collection
Participants were drawn from a cross-sectional study of serum biomarkers for Chagas cardiomyopathy (23). Briefly, we recruited patients attending the inpatient and outpatient services, as well as visitors to San Juan de Dios Hospital, the largest general public hospital in Santa Cruz, from September 2012 to April 2013. Our aim was to recruit T. cruzi-infected and uninfected individuals spanning the spectrum from no heart disease to severe cardiomyopathy. Baseline demographic, electrocardiographic, echocardiographic, and laboratory data were collected as previously described (23). A total of 409 participants were recruited into the study, of whom 254 had full cardiac evaluations and were eligible to be included in the current analysis. The limiting factor was our capacity to perform echocardiograms, To ensure echocardiograms were performed on individuals in all severity stages, participants were grouped based on history of heart failure and presence of ECG abnormalities. Within each group, individuals were randomly chosen for an echocardiogram appointment until all available appointments were filled. One to three individual or household phone numbers were recorded at the time of baseline data collection. Study personnel attempted to contact all participants by phone from February 2014 to March 2014. Phone numbers were tried three times before coding the individual as lost to follow-up. If the individual was deceased, date of death and available information about the cause was collected from family members.
Clinical Classification
Participants were assigned cardiac disease severity stages based on electrocardiogram (ECG) and echocardiogram results at the time of enrollment (23). In the current analysis, participants with clinically severe disease who died before receiving an echocardiogram were excluded. Criteria for the stages were as follows:
Stage A: T. cruzi-infected (Tc +) with normal ECG (no abnormalities suggestive of Chagas heart disease) and normal echocardiogram
Stage B: T. cruzi-infected with characteristic ECG findings (RBBB, LBBB, LAFB, any AV conduction block, multiple PVCs, bradyarrythmias (HR <= 50), and/or atrial fibrillation) and normal echocardiogram
Stage C: T. cruzi-infected with mild to moderate systolic dysfunction (EF 40-54%)
Stage D: T. cruzi-infected with severe systolic dysfunction by ejection fraction (EF) < 40% and/or left ventricular end diastolic dilatation (LVEDD) >= 57 mm
T. cruzi-uninfected (Tc-) individuals assigned similar categories to provide healthy controls (Tc-, normal ECG) and Tc- individuals with heart failure (systolic dysfunction, severe LVEDD) for comparison.
Laboratory Methods
T. cruzi infection status was determined using two commercial IgG serological tests (enzyme-linked immunoassay and indirect hemaagglutination test); those with discordant results were tested by trypomastigote excreted-secreted antigens (TESA)-blot as described previously (23). Confirmed infection was defined by positive results by at least two assays.
We measured 10 serum biomarkers: BNP, NTproBNP, CKMB, troponin I, MMP-2, MMP-9, TIMP-1, TIMP-2, TGFb1, and TGFb2. Biomarker measurement was performed in sera by multiplex bead assays as previously described (23). Brain natriuretic protein, NTproBNP, CKMB, and troponin I were measured using the Milliplex Map Human Cardiovascular Disease Magnetic Bead Panel. Other biomarker levels were measured using Milliplex MAP Human MMP Magnetic Bead Panel 2, Milliplex Map Human TIMP Magnetic Bead Panel 1, and Milliplex MAP TGF-B1,2,3 Plex Magnetic Bead Panel (all kits from Millipore, Billerica, Massachusetts).
Statistical Analysis
Categorical variables are reported as percentages and compared using the Chi-square or Fisher exact test. Normality of variable distribution was determined by the Shapiro-Wilke test. Normal variables are reported by mean, standard deviation, and Student's T-test for comparisons. Continuous non-normal variables are reported by median and IQR and compared using Wilcoxon rank sum. Receiver operating characteristic (ROC) curves were created for each biomarker to optimize the cut-point that best discriminates death at follow-up. Markers with an area under the curve (AUC) value of >0.55 were selected for evaluation of their association with mortality, and a cut-point was defined based on the maximum sensitivity and specificity identified in the ROC analysis. Kaplan–Meier methods were used to estimate the proportion of participants surviving through follow-up. Univariate and multivariate Cox proportional hazards models were performed to determine the hazards ratio (HR) and 95% confidence interval (CI) for the association of each marker with mortality. Multivariate cox proportional hazards models were adjusted for age and sex. All statistical analyses were performed using Stata 12.0 with a two-tailed p <= 0.05 considered to be significant.
Results
Among the 254 individuals with full cardiac evaluations at baseline, follow-up information was available for 220 (87%) individuals, with no differences in completeness of follow-up by T. cruzi infection status or severity stage (Table 1). The median time to outcome was 424 days (14.0 months). Among T. cruzi-infected individuals, mortality was highest in Stage D (20/50; 40%) followed by Stage C (2/16; 13%). Individuals with Stage D disease at baseline had a significantly lower overall probability of survival as compared to individuals in earlier stages of cardiac disease across follow-up (p<0.001) (Figure 1). No deaths occurred among participants in Stage B. The single death in Stage A was due to a stroke in an 82 year-old man. In uninfected individuals, the only deaths occurred in Stage D, with a 50% (4/8) mortality rate. Stage D had the shortest time to outcome due to the deaths included. Among those with severe cardiac disease, follow-up information was available for 86% and 89% of T. cruzi-infected and uninfected individuals, respectively. Mortality was also high (50%) among uninfected individuals in Stage D, but the denominator was only 8, providing insufficient power for Stage D comparisons based on infection status. Therefore, further analyses focused on the 50 T. cruzi-infected Stage D patients with complete data. Among these patients, those who were still alive at the time of follow-up had a higher body mass index than those who died (Table 2). Other baseline characteristics at the time of recruitment, including age, sex, self-reported medical history, medication use, and ECG findings were not significantly different for those who died compared to those who were still alive.
Table 1. Overall study population recruited in the Hospital San Juan de Dios, Santa Cruz, Bolivia, with follow-up data and mortality by stage of disease and T. cruzi infection status.
| Mean age (SD) | Male N (%) | Inpatients | Successful Follow Up N (%) | Follow-up time, days Median (IQR) | Mortality N (%) | |
|---|---|---|---|---|---|---|
| Study population (N) | ||||||
| Screened for enrollment (409) | 57.7 (12.7) | 200 (48.9) | 119 (29.1) | NA | NA | NA |
| Completed cardiac evaluation (254) | 57.0 (12.7) | 123 (48.4) | 77 (30.3) | 220 (87) | 424 (374-501) | 27 (12) |
| T. cruzi infected (183) | 58.2 (12.3) | 92 (50.2) | 61 (33.3) | 160 (89) | 394 (352-475) | 23 (14) |
| T. cruzi uninfected (71) | 53.9 (13.2) | 31 (43.7) | 16 (22.5) | 60 (85) | 462 (384-504) | 4 (7) |
| Stage and T. cruzi infection status (N) | ||||||
| A (114) | ||||||
| T cruzi-infected (66) | 55.6 (12.7) | 29 (43.9) | 5 (7.6) | 60 (91) | 426 (382-497) | 1 (2) |
| Uninfected (48) | 53.2 (12.8) | 18 (37.5) | 3 (6.3) | 39 (81) | 469 (393-511) | 0 (0) |
| B (50) | ||||||
| T cruzi-infected (41) | 58.2 (12.7) | 19 (46.3) | 8 (19.5) | 34 (83) | 431 (385-509) | 0 (0) |
| Uninfected (9) | 53.8 (15.6) | 4 (44.4) | 3 (33.3) | 9 (100) | 495 (378-518) | 0 (0) |
| C (23) | ||||||
| T cruzi-infected (18) | 59.4 (12.6) | 8 (44.4) | 10 (55.6) | 16 (89) | 395 (367-488) | 2 (13) |
| Uninfected (5) | 51.4 (13.0) | 2 (40.0) | 3 (60.0) | 4 (80) | 480 (405-538) | 0 (0) |
| D (67) | ||||||
| T cruzi-infected (58) | 60.8 (11.0) | 36 (62.1) | 38 (65.5) | 50 (86) | 404 (257-483) | 20 (40) |
| Uninfected (9) | 59.1 (14.1) | 7 (77.8) | 7 (77.8) | 8 (89) | 360 (253-461) | 4 (50) |
“Follow-up time” based on either last date of contact alive or date of death
NA = not applicable; SD=standard deviation; IQR=interquartile range
Figure 1. Kaplan Meier Curves for T. cruzi-infected individuals by severity stage.
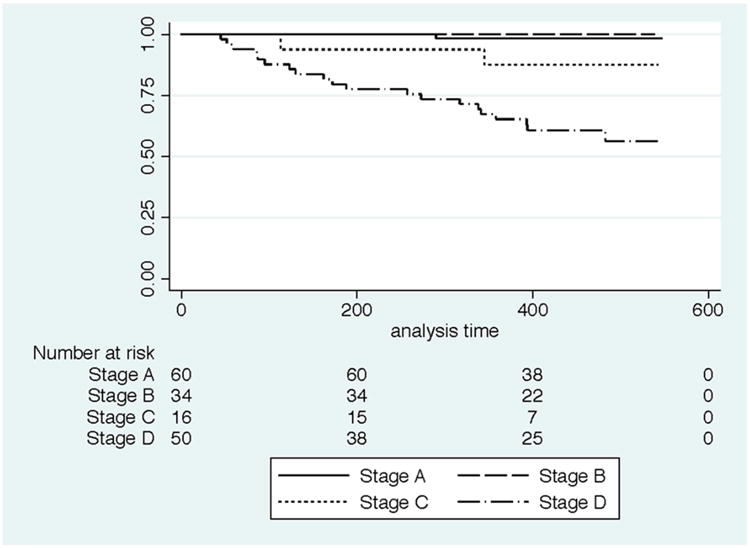
Table 2. Demographic information, clinical history, medications, ECG and echocardiographic findings for T. cruzi-infected Stage D at time of recruitment.
| Alive N=30 | Died N=20 | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 62.6 (11.2) | 60.3 (9.6) | 0.45 |
| Sex, male | 16 (53 %) | 14 (70 %) | 0.24 |
| BMI, mean (SD)* | 27.1 (4.4) | 24.4 (4.1) | 0.04 |
| Overweight or obese* | 20 (69 %) | 10 (50 %) | 0.18 |
| Inpatient at recruitment | 21 (70 %) | 10 (50 %) | 0.15 |
| Medical History by Self-Report** | |||
| Hypertension | 16 (53 %) | 7 (35 %) | 0.20 |
| Diabetes Mellitus | 3 (10 %) | 2 (10 %) | 1.00 |
| Coronary Artery Disease | 7 (23 %) | 2 (10 %) | 0.29 |
| Heart Failure | 22 (73 %) | 14 (70 %) | 1.00 |
| Pacemaker | 4 (13 %) | 2 (10 %) | 1.00 |
| Medications** | |||
| Beta Blocker | 6 (20 %) | 4 (20 %) | 1.00 |
| ACE inhibitor | 10 (33 %) | 10 (50 %) | 0.24 |
| Angiotensin II Receptor Blockers | 3 (10 %) | 1 (5 %) | 0.64 |
| Anticoagulant | 3 (10 %) | 2 (10 %) | 1.00 |
| Aspirin | 21 (70 %) | 10 (50 %) | 0.15 |
| Nitroglycerin | 1 (3 %) | 2 (10 %) | 0.56 |
| Amiodarone | 8 (27 %) | 3 (15 %) | 0.49 |
| Calcium Channel Blocker | 2 (7 %) | 1 (5 %) | 1.00 |
| Diuretics | 11 (37 %) | 8 (40 %) | 0.81 |
| Digoxin | 11 (37 %) | 5 (25 %) | 0.39 |
| Spironolactone | 5 (17 %) | 6 (30 %) | 0.27 |
| Any medication | 26 (87 %) | 16 (80 %) | 0.70 |
| ECG** | |||
| Isolated right bundle branch block | 2 (7 %) | 5 (25 %) | 0.10 |
| RBBB and LAFB | 3 (10 %) | 1 (5 %) | 0.64 |
| Left Bundle Branch Block | 4 (13 %) | 1 (5 %) | 0.33 |
| Atrial fibrillation or flutter | 9 (33 %) | 9 (45 %) | 0.28 |
| Multiple PVC | 5 (17 %) | 3 (15 %) | 0.60 |
| Low voltage | 1 (3 %) | 1 (5 %) | 0.65 |
| Normal ECG | 1 (3%) | 1 (5%) | 0.76 |
| Other Findings | |||
| NYHA III or IV | 15 (50 %) | 12 (60 %) | 0.49 |
| Ejection Fraction, median(IQR) | 27.5 (20-35) | 20 (20-30) | 0.13 |
| Segmental Score, median(IQR) | 2.0 (1.6-2.3) | 2.3 (1.8-2.4) | 0.09 |
BMI = body mass index; RBBB=right bundle branch block; LAFB=left anterior fascicular block; NYHA = New York Heart Association; PVC = premature ventricular contraction. Overweight and obese defined as BMI 25 - 29.9 and ≥ 30, respectively.
Weight data missing for one surviving participant
Adds up to >100% because some patients had more than one abnormality or drug.
The ROC analyses identified 4 serum markers (BNP, NTproBNP, CKMB and MMP-2) with AUCs above 0.55 (Table 3). The AUCs for the other biomarkers fell below the 0.55 cut-off (AUC[95% CI]: troponin 0.45[0.28, 0.61], MMP9 0.47[0.29, 0.65], TIMP1 0.48[0.29, 0.68], TIMP2 0.53[0.34, 0.71], TGFb1 0.51[0.33, 0.68] and TGFb2 0.51[0.33, 0.69]). Based on the optimal cut-offs in the ROC curves for each of these markers, Kaplan-Meier curves were constructed. All four biomarkers were elevated at the time of recruitment in individuals who died compared to those who survived (Figure 2). Elevations in these biomarker levels were significantly associated with mortality in Cox regression models analysis adjusted for age and sex (Table 3).
Table 3. Results of analyses of the association of candidate biomarkers with mortality among patients with advanced Chagas cardiomopathy (Stage D) using receiver operating characteristic curves and Cox regression models.
| Crude model | Adjusted model2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | AUC1 | Cut-off1 (pg/ml) | Status | Median (IQR) biomarker level in pg/ml | N (%) above cutoff | P | HR1 (95% CI) | P | HR (95% CI) |
| BNP3 | 0.61 | 184.1 | Alive | 109 (9-263) | 11 (37%) | 0.039 | 2.8 (1.1-7.2) | 0.025 | 3.1 (1.2-8.4) |
| Died | 267 (37-347) | 14 (70%) | |||||||
| NTproBNP3 | 0.66 | 765.1 | Alive | 425 (288-740) | 5 (16.7 %) | 0.002 | 4.1 (1.6-10.0) | 0.002 | 4.4 (1.8-11.0) |
| Died | 777 (423-1310) | 11 (55%) | |||||||
| CKMB3 | 0.56 | 8300 | Alive | 3994 (3447-7487) | 5 (17%) | 0.005 | 3.5 (1.6-8.5) | 0.009 | 3.3 (1.3-8.0) |
| Died | 7534 (2960-11,438) | 10 (50%) | |||||||
| MMP-23 | 0.72 | 103980 | Alive | 94,680 (75,549-111,884) | 9 (30%) | 0.008 | 4.0 (1.4-11.2) | 0.007 | 4.2 (1.5-11.8) |
| Died | 126,828 (96,148-164,256) | 14 (74 %)4 | |||||||
Area under the curve (AUC), cutoffs and hazard ratios (HRs) based on receiver operating characteristic (ROC) curves. Hazard ratios were only calculated for those with AUC greater than 0.55.
Model adjusted for age and sex.
BNP, Brain Natriuretic Peptide; NTproBNP, N-terminal brain natriuretic peptide; CKMB, Creatine kinase-MB; MMP2, Matrix metalloproteinase 2.
MMP2 data were missing for one individual who died
Figure 2.
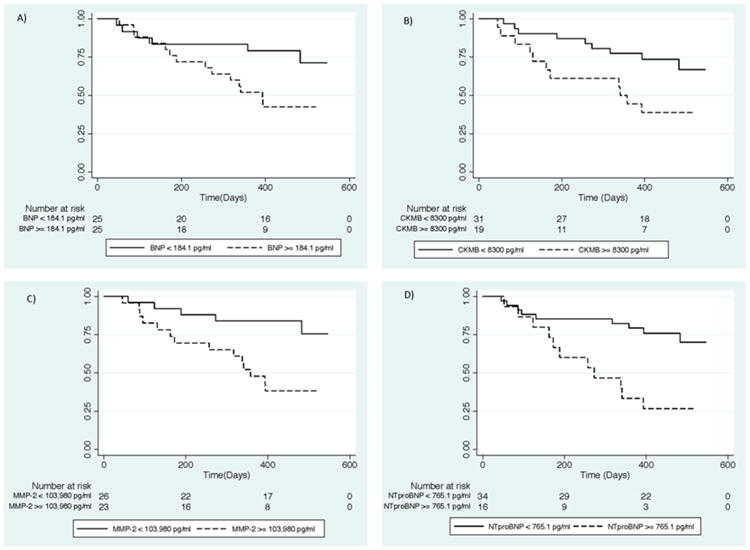
Kaplan Meier survival analyses stratified by biomarker results above and below the cut-offs chosen based on receiver operating characteristic curves. Survival of T. cruzi infected patients in Stage D by results of (A) Brain Natriuretic Peptide (BNP); (B) Creatine kinase-MB (CKMB); (C) Matrix metalloproteinase-2 (MMP2); (D) N-terminal brain natriuretic peptide (NTproBNP)
Discussion
Advanced Chagas cardiomyopathy is associated with very high short-term mortality (10-12). Both heart failure and sudden cardiac death are known causes of mortality in CCM. Consistent with the literature, we found the highest rates of mortality in patients in Stage D, the most severe stage of cardiomyopathy based on left ventricular dilatation and ejection fraction. Stage D T. cruzi infected individuals experienced 40% mortality over 14 months, similar to other studies of severe CCM (19). By restricting the analysis to patients in Stage D, we effectively examined the additive predictive value of the serum biomarkers beyond the clinical indicators already identified in many previous studies (10-12) (13) (14, 15) (9).
Of the 10 serum markers we examined in this analysis, four (BNP, NT-pro BNP, CKMB and MMP2) were significantly associated with mortality among patients with severe Chagas cardiomyopathy. Interestingly, troponin-1 in a conventional assay provided no additional predictive value in this study group. However, the sensitivity of the assay we used is much lower than the newly available high sensitivity troponin assays now being used in tertiary care centers in the United States (29).
BNP and NT-pro BNP were identified as strong predictors of mortality in our study. These markers are some of the most well characterized markers used to guide treatment and predict outcomes in heart failure. In heart failure from all etiologies BNP-guided therapy reduces all-cause mortality (30). Serum BNP levels were significantly positively correlated with 5-year mortality in elderly adults from Switzerland in those with and without identified cardiovascular disease (20). Furthermore, individuals with Chagas cardiomyopathy, even in the absence of systolic dysfunction, had higher BNP levels than healthy controls (26). For those with heart failure due to Chagas cardiomyopathy, BNP has previously been shown to be a strong predictor of mortality (27) (26) (28) (31). NT-pro BNP levels are correlated with severity of LV dysfunction in Chagas disease (32). Our results suggest that NT-pro BNP may be a better predictor of mortality than BNP, possibly related to the longer half-life of NT-pro BNP compared to BNP. While our data support using these markers as a predictor of mortality among those with known heart failure, their significance in predicting death among T. cruzi-infected individuals without heart failure is unknown.
CKMB is a well-known biomarker for acute cardiac muscle damage, and in the setting of myocardial infarction has a rapid evolution over hours to days (33-35). Elevated CKMB has been shown to be associated with poor prognosis and shortened survival after surgical interventions such as coronary artery bypass (36), but has not previously been examined as a prognostic marker in Chagas cardiomyopathy. We did not find a significant association of CKMB levels with Chagas cardiomyopathy stage in our previous analysis (23), but in this restricted sub-population, elevated CKMB levels predicted shortened survival. CKMB is likely acting as a non-specific marker of cardiac myocyte injury, and as in the setting of myocardial infarction (33), higher levels indicate more extensive damage and therefore worse prognosis.
We found elevated MMP2 levels to predict mortality in our study patients, suggesting that activation of the cardiac remodeling process is associated with negative outcomes in severe cardiomyopathy. This finding is consistent with studies of heart failure from other etiologies as well (21) (37) (38). In heart failure, MMP-2 mRNA expression and blood protein levels are increased (38). Higher levels of MMP-2 are markers of diastolic heart failure and predict poor outcomes (21) (37). Higher levels of MMP-2 and MMP-9 correlate with more severe Chagas heart disease (39). Elevated MMP-2 levels have been reported to be associated with mortality in acute experimental Chagas disease, but have not been previously studied for this purpose in chronic Chagas cardiomyopathy in humans (40). Interestingly, among patients with heart failure and high BNP levels, MMP-2 was a better predictor of mortality than BNP level alone, consistent with our findings (41).
Previously identified factors associated with longer survival include being overweight and being on beta-blockers (42) (43). We found an elevated BMI to be associated with survival, consistent with prior studies (44). Though not well explained, this may be influenced by a cachectic state that can occur in end stage disease. We did not find significant associations between medications and outcome, but this analysis may have been hindered by the limited number of individuals in our study on optimal heart failure medication regimens.
Limitations of Study
The major limitation was the relatively small sample size, which impeded more in-depth analysis of T. cruzi-infected individuals, and precluded analysis of the uninfected group. In addition, those classified as having Stage D Chagas cardiomyopathy in this study were patients already presenting with clinical heart failure necessitating hospitalization. Therefore, this group may not be representative of all individuals with severe cardiomyopathy in Chagas disease. We had a thirteen percent loss to follow-up for the entire population, and a twelve percent loss to follow-up among those in group D. Individuals who were lost to follow-up may have had a higher mortality rate than those who remained in the study. We focused on individuals with advanced heart failure which may limit the generalizability of our results to less severe stages of Chagas disease. A longer follow-up period is necessary to investigate associations of biomarkers in early stages of disease with subsequent mortality. Finally, clinical data, such as the presence of co-morbid conditions and routine medications, were based on patient self-report and may have been subject to reporting biases based on the patient's overall health state and their comprehension of specific medical diagnoses. However, these data were collected at baseline and should not have biased the associations between biomarkers and subsequent mortality over the follow-up period.
Future Areas of Research
Longitudinal studies of longer duration would be valuable to examine a wider range of outcomes in a more representative group of Chagas disease patients. Biomarkers capable of identifying individuals at risk of cardiac progression (short of mortality) could enable targeted early interventions. A comparison between patients with Chagas and other cardiomyopathies could help elucidate common or specific pathways of pathogenesis. Finally, the biomarkers we identified may have additional predictive value when combined with existing risk stratification based on clinical cardiological evaluations (10) (45).
Conclusion
Chagas cardiomyopathy leads to significant morbidity and mortality in T. cruzi infected individuals. Insufficient information exists to anticipate which infected individuals will develop cardiomyopathy, and identify those at risk of fatal outcomes. We found that elevated serum levels of BNP, NT-pro BNP, CKMB, and MMP-2 were associated with an increased of death among individuals with advanced Chagas cardiomyopathy. Further research is needed to better qualify the trend of these markers throughout the different stages of the disease process.
Highlights.
Our study followed patients with severe Chagas cardiomyopathy who had a 40% mortality at a 1-2 year follow up.
For those with severe Chagas cardiomyopathy, the characteristics between those who died and those who survived to follow up were similar, except body mass index which was significantly higher among the survivors.
BNP, NT-pro BNP, CKMB, and MMP-2 were significantly higher in the subgroup that died compared to those who survived.
Acknowledgments
The members of the Chagas Disease Working Group in Bolivia and Peru include: Enzo Fortuny, Maurus Dorn, Lisbeth Ferrufino, Roxana Bravo Nevarro, Paola Roseliz Lurizi, Omar Gandarilla Montero, Walter Jesus Gomez, Margot Ramirez Jaldin, Eliana Saenza Vasquez, Nancy Chile Andrade, Noelia Angulo, Sandra Palma and Manuela Verastegui.
This study was supported by NIH Global Research Training Grant D43 TW006581 and discretionary funds awarded to RHG from Asociacion Benefica PRISMA (www.prisma.org.pe). The participation of Drs. Okamoto and Sherbuk was made possible by grants from the New York University School of Medicine International Health Program and the Milton Rosenbluth Foundation. The funding sources had no role in the study design, collection, analysis and interpretation of the data, preparation of the manuscript, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bern C, Montgomery SP. An estimate of the burden of Chagas disease in the United States. Clin Infect Dis. 2009;49(5):e52–4. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- 2.Andrade SG. The influence of the strain of Trypanosoma cruzi in placental infections in mice. Trans R Soc Trop Med Hyg. 1982;76(1):123–8. doi: 10.1016/0035-9203(82)90036-0. [DOI] [PubMed] [Google Scholar]
- 3.Pereira Nunes Mdo C, Barbosa MM, Ribeiro AL, Amorim Fenelon LM, Rocha MO. Predictors of mortality in patients with dilated cardiomyopathy: relevance of chagas disease as an etiological factor. Revista espanola de cardiologia. 2010;63(7):788–97. doi: 10.1016/s1885-5857(10)70163-8. Epub 2010/07/09. [DOI] [PubMed] [Google Scholar]
- 4.Bestetti RB, Otaviano AP, Fantini JP, Cardinalli-Neto A, Nakazone MA, Nogueira PR. Prognosis of patients with chronic systolic heart failure: Chagas disease versus systemic arterial hypertension. International journal of cardiology. 2013;168(3):2990–1. doi: 10.1016/j.ijcard.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Ayub-Ferreira SM, Mangini S, Issa VS, Cruz FD, Bacal F, Guimaraes GV, et al. Mode of death on Chagas heart disease: comparison with other etiologies. a subanalysis of the REMADHE prospective trial. PLoS Negl Trop Dis. 2013;7(4):e2176. doi: 10.1371/journal.pntd.0002176. Epub 2013/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas HF, Chizzola PR, Paes AT, Lima AC, Mansur AJ. Risk stratification in a Brazilian hospital-based cohort of 1220 outpatients with heart failure: role of Chagas' heart disease. Int J Cardiol. 2005;102(2):239–47. doi: 10.1016/j.ijcard.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Vilas Boas LG, Bestetti RB, Otaviano AP, Cardinalli-Neto A, Nogueira PR. Outcome of Chagas cardiomyopathy in comparison to ischemic cardiomyopathy. International journal of cardiology. 2013;167(2):486–90. doi: 10.1016/j.ijcard.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Clark EH, Sherbuk J, Okamoto E, Jois M, Galdos-Cardenas G, Vela-Guerra J, et al. Hyperendemic Chagas disease and the unmet need for pacemakers in the Bolivian Chaco. PLoS Negl Trop Dis. 2014;8(6):e2801. doi: 10.1371/journal.pntd.0002801. Epub 2014/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes MC, Carmo AA, Rocha MO, Ribeiro AL. Mortality prediction in Chagas heart disease. Expert review of cardiovascular therapy. 2012;10(9):1173–84. doi: 10.1586/erc.12.111. Epub 2012/10/27. [DOI] [PubMed] [Google Scholar]
- 10.Rassi A, Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101–8. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 11.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Salas LA, Klein E, Acquatella H, Catalioti F, Davalos VV, Gomez-Mancebo JR, et al. Echocardiographic and Clinical Predictors of Mortality in Chronic Chagas' Disease. Echocardiography (Mount Kisco, NY) 1998;15(3):271–8. doi: 10.1111/j.1540-8175.1998.tb00607.x. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 13.Leite LR, Fenelon G, Simoes A, Jr, Silva GG, Friedman PA, de Paola AA. Clinical usefulness of electrophysiologic testing in patients with ventricular tachycardia and chronic chagasic cardiomyopathy treated with amiodarone or sotalol. Journal of cardiovascular electrophysiology. 2003;14(6):567–73. doi: 10.1046/j.1540-8167.2003.02278.x. Epub 2003/07/24. [DOI] [PubMed] [Google Scholar]
- 14.Salles G, Xavier S, Sousa A, Hasslocher-Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas' disease: results of a long-term follow-up study. Circulation. 2003;108(3):305–12. doi: 10.1161/01.CIR.0000079174.13444.9C. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa RA, Pericchi LR, Carrasco HA, Escalante A, Martinez O, Gonzalez R. Prognostic indicators of chronic chagasic cardiopathy. International journal of cardiology. 1991;30(2):195–202. doi: 10.1016/0167-5273(91)90095-7. Epub 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco HA, Parada H, Guerrero L, Duque M, Duran D, Molina C. Prognostic implications of clinical, electrocardiographic and hemodynamic findings in chronic Chagas' disease. Int J Cardiol. 1994;43(1):27–38. doi: 10.1016/0167-5273(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 17.Basquiera AL, Sembaj A, Aguerri AM, Omelianiuk M, Guzman S, Moreno Barral J, et al. Risk progression to chronic Chagas cardiomyopathy: influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart. 2003;89(10):1186–90. doi: 10.1136/heart.89.10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acquatella H. Echocardiography in Chagas heart disease. Circulation. 2007;115(9):1124–31. doi: 10.1161/CIRCULATIONAHA.106.627323. [DOI] [PubMed] [Google Scholar]
- 19.Rassi DD, Vieira ML, Arruda AL, Hotta VT, Furtado RG, Rassi DT, et al. Echocardiographic Parameters and Survival in Chagas Heart Disease with Severe Systolic Dysfunction. Arquivos brasileiros de cardiologia. 2014;102(3):245–52. doi: 10.5935/abc.20140003. Parametros Ecocardiograficos e Sobrevida na Cardiopatia Chagasica com Disfuncao Sistolica Importante. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallen T, Landahl S, Hedner T, Nakao K, Saito Y. Brain natriuretic peptide predicts mortality in the elderly. Heart. 1997;77(3):264–7. doi: 10.1136/hrt.77.3.264. Epub 1997/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George J, Patal S, Wexler D, Roth A, Sheps D, Keren G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. American heart journal. 2005;150(3):484–7. doi: 10.1016/j.ahj.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Bhalla V, Georgiopoulou VV, Azeem AA, Marti CN, Cole RT, Laskar SR, et al. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, and heart failure outcomes. International journal of cardiology. 2011;151(2):237–9. doi: 10.1016/j.ijcard.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto EE, Sherbuk JE, Clark EH, Marks MA, Gandarilla O, Galdos-Cardenas G, et al. Biomarkers in Trypanosoma cruzi-infected and uninfected individuals with varying severity of cardiomyopathy in Santa Cruz, Bolivia. PLoS neglected tropical diseases. 2014;8(10):e3227. doi: 10.1371/journal.pntd.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frantz S, Stork S, Michels K, Eigenthaler M, Ertl G, Bauersachs J, et al. Tissue inhibitor of metalloproteinases levels in patients with chronic heart failure: an independent predictor of mortality. European journal of heart failure. 2008;10(4):388–95. doi: 10.1016/j.ejheart.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Franz M, Berndt A, Neri D, Galler K, Grun K, Porrmann C, et al. Matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, B(+) tenascin-C and ED-A(+) fibronectin in dilated cardiomyopathy: potential impact on disease progression and patients' prognosis. International journal of cardiology. 2013;168(6):5344–51. doi: 10.1016/j.ijcard.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Moreira Mda C, Wang Y, Heringer-Walther S, Wessel N, Walther T. Prognostic value of natriuretic peptides in Chagas' disease: a head-to-head comparison of the 3 natriuretic peptides. Congestive heart failure (Greenwich, Conn) 2009;15(2):75–81. doi: 10.1111/j.1751-7133.2009.00051.x. Epub 2009/04/22. [DOI] [PubMed] [Google Scholar]
- 27.Lima-Costa MF, Cesar CC, Peixoto SV, Ribeiro AL. Plasma B-type natriuretic peptide as a predictor of mortality in community-dwelling older adults with Chagas disease: 10-year follow-up of the Bambui Cohort Study of Aging. American journal of epidemiology. 2010;172(2):190–6. doi: 10.1093/aje/kwq106. [DOI] [PubMed] [Google Scholar]
- 28.Heringer-Walther S, Moreira MC, Wessel N, Saliba JL, Silvia-Barra J, Pena JL, et al. Brain natriuretic peptide predicts survival in Chagas' disease more effectively than atrial natriuretic peptide. Heart. 2005;91(3):385–7. doi: 10.1136/hrt.2003.026856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Antonio M, Lupon J, Galan A, Vila J, Zamora E, Urrutia A, et al. Head-to-head comparison of high-sensitivity troponin T and sensitive-contemporary troponin I regarding heart failure risk stratification. Clinica chimica acta; international journal of clinical chemistry. 2013;426:18–24. doi: 10.1016/j.cca.2013.08.014. Epub 2013/08/28. [DOI] [PubMed] [Google Scholar]
- 30.Troughton RW, Frampton CM, Brunner-La Rocca HP, Pfisterer M, Eurlings LW, Erntell H, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. European heart journal. 2014;35(23):1559–67. doi: 10.1093/eurheartj/ehu090. Epub 2014/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro AL, Marcolino MS, Prineas RJ, Lima-Costa MF. Electrocardiographic abnormalities in elderly chagas disease patients: 10-year follow-up of the Bambui Cohort Study of Aging. Jounal of the American Heart Association. 2014;3(1):e000632. doi: 10.1161/JAHA.113.000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbosa MM, Nunes Mdo C, Ribeiro AL, Barral MM, Rocha MO. N-terminal proBNP levels in patients with Chagas disease: a marker of systolic and diastolic dysfunction of the left ventricle. Eur J Echocardiogr. 2007;8(3):204–12. doi: 10.1016/j.euje.2006.03.011. Epub 2006/05/03. [DOI] [PubMed] [Google Scholar]
- 33.Hedstrom E, Astrom-Olsson K, Ohlin H, Frogner F, Carlsson M, Billgren T, et al. Peak CKMB and cTnT accurately estimates myocardial infarct size after reperfusion. Scandinavian cardiovascular journal : SCJ. 2007;41(1):44–50. doi: 10.1080/14017430601071849. Epub 2007/03/17. [DOI] [PubMed] [Google Scholar]
- 34.Malasky BR, Alpert JS. Diagnosis of myocardial injury by biochemical markers: problems and promises. Cardiology in review. 2002;10(5):306–17. doi: 10.1097/00045415-200209000-00007. Epub 2002/09/07. [DOI] [PubMed] [Google Scholar]
- 35.Rajappa M, Sharma A. Biomarkers of cardiac injury: an update. Angiology. 2005;56(6):677–91. doi: 10.1177/000331970505600605. Epub 2005/12/06. [DOI] [PubMed] [Google Scholar]
- 36.Mediratta N, Chalmers J, Pullan M, McShane J, Shaw M, Poullis M. In-hospital mortality and long-term survival after coronary artery bypass surgery in young patients. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013;43(5):1014–21. doi: 10.1093/ejcts/ezs459. Epub 2012/11/10. [DOI] [PubMed] [Google Scholar]
- 37.Zile MR, Desantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, et al. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circulation Heart failure. 2011;4(3):246–56. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. International journal of cardiology. 2011;151(1):18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 39.Bautista-Lopez NL, Morillo CA, Lopez-Jaramillo P, Quiroz R, Luengas C, Silva SY, et al. Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J. 2013;165(4):558–66. doi: 10.1016/j.ahj.2013.01.001. Epub 2013/03/30. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez FR, Lalu MM, Mariano FS, Milanezi CM, Cena J, Gerlach RF, et al. Increased activities of cardiac matrix metalloproteinases matrix metalloproteinase (MMP)-2 and MMP-9 are associated with mortality during the acute phase of experimental Trypanosoma cruzi infection. The Journal of infectious diseases. 2008;197(10):1468–76. doi: 10.1086/587487. [DOI] [PubMed] [Google Scholar]
- 41.Ho YL, Lin YH, Lee CM, Hsu RB, Ting HT, Chou NK, et al. Prognostic significance of adipocytokines and extracellular matrix activity in heart failure patients with high B-type natriuretic peptide. Clinical biochemistry. 2009;42(13-14):1407–12. doi: 10.1016/j.clinbiochem.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Bestetti RB, Otaviano AP, Cardinalli-Neto A, da Rocha BF, Theodoropoulos TA, Cordeiro JA. Effects of B-Blockers on outcome of patients with Chagas' cardiomyopathy with chronic heart failure. International journal of cardiology. 2011;151(2):205–8. doi: 10.1016/j.ijcard.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Theodoropoulos TA, Bestetti RB, Otaviano AP, Cordeiro JA, Rodrigues VC, Silva AC. Predictors of all-cause mortality in chronic Chagas' heart disease in the current era of heart failure therapy. International journal of cardiology. 2008;128(1):22–9. doi: 10.1016/j.ijcard.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 44.Beleigoli AM, Ribeiro AL, Diniz Mde F, Lima-Costa MF, Boersma E. The “obesity paradox” in an elderly population with a high prevalence of Chagas disease: the 10-year follow-up of the Bambui (Brazil) Cohort Study of Aging. International journal of cardiology. 2013;166(2):523–6. doi: 10.1016/j.ijcard.2012.09.126. Epub 2012/10/13. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro AL, Cavalvanti PS, Lombardi F, Nunes Mdo C, Barros MV, Rocha MO. Prognostic value of signal-averaged electrocardiogram in Chagas disease. Journal of cardiovascular electrophysiology. 2008;19(5):502–9. doi: 10.1111/j.1540-8167.2007.01088.x. Epub 2008/02/13. [DOI] [PubMed] [Google Scholar]


