Abstract
The phosphatidylinositol-3-OH kinase [PI(3)K] pathway is frequently activated in human cancers and represents a rational target for therapeutic intervention. We have previously shown that enforced expression of Akt, which is a downstream effector of PI(3)K, could promote tumorigenesis and drug resistance in the Eμ-myc mouse lymphoma model, and that these tumors were particularly sensitive to inhibition of mammalian target of rapamycin (mTOR) with rapamycin when combined with conventional chemotherapy. We now show that reduced dosage of PTEN, a negative regulator of PI(3)K signaling, is sufficient to activate Akt, but has only a modest effect on lymphomagenesis in the same model. Nonetheless, loss of even one PTEN allele resulted in lymphomas that were resistant to conventional chemotherapy yet sensitive to rapamycin/chemotherapy combinations. These effects could be recapitulated by using RNA interference to suppress PTEN expression in lymphomas, which were previously established in the absence of PI(3)K lesions. Finally, the introduction of lesions that act downstream of mTOR (eIF4E) or disable apoptosis (Bcl-2 and loss of p53) into PTEN+/− lymphomas promoted resistance to rapamycin/chemotherapy combinations. Thus, whether activation of the PI(3)K pathway confers sensitivity or resistance to therapy depends on the therapy used as well as secondary genetic events. Understanding these genotype-response relationships in human tumors will be important for the effective use of rapamycin or other compounds targeting the PI(3)K pathway in the clinic.
Introduction
Tumorigenesis involves a series of genetic events that disrupt or alter signaling networks controlling proliferation and survival. The precise order of genetic alterations and their combinations that can confer malignant characteristics is variable, thereby producing heterogeneity in tumor behavior. As one example, increased oncogenic signals activate tumor suppressor programs, including apoptosis and senescence, and their disruption is an obligate requirement during tumorigenesis (1, 2). Disruption of apoptotic programs in tumor development can occur in different ways, for example through loss of tumor suppressor genes like ARF and p53, or increased activity of dominant oncogenes like Bcl-2 (3) and survival pathways like the phosphatidylinositol-3-OH kinase [PI(3)K] pathway or its effectors Akt and eIF4E (4–6). Importantly, some of the same pathways that block apoptosis during tumorigenesis also impinge on the apoptotic response to chemotherapeutic drugs. Thus, the nature of the genetic lesions incurred during tumorigenesis to disrupt apoptosis can influence treatment behavior to varying degrees (4, 7–10). Conversely, strategies to restore apoptosis to tumor cells, either by increasing proapoptotic signals, suppressing prosurvival signals, or by simultaneously achieving both, may prove effective for treating otherwise refractory tumors.
The PI(3)K pathway is implicated in cellular transformation and tumor development and contributes to the oncogenic activities of Ras and Bcr-abl [reviewed in ref. 11]. Concordantly, deregulation of this pathway is observed in many cancers, including lymphoma and leukemia, and most often involves inactivation of the negative regulator PTEN (refs. 12–14; reviewed in ref. 15). Also, PTEN heterozygous mice develop tumors in multiple tissues, sometimes in the absence of complete PTEN inactivation, indicating that in certain contexts PTEN can be haploinsufficient for tumor suppression (16–19). Activation of the PI(3)K pathway has myriad effects on cellular physiology by virtue of its ability to regulate effectors controlling translation, metabolism, and cell survival (20–25). Although it seems likely that all of these properties contribute to tumorigenesis and drug resistance, the ability of deregulated PI(3)K signaling to promote cell survival seems particularly important (4).
Owing to its “gain-of-function” mode of action, the PI(3)K pathway represents an attractive therapeutic target, and compounds targeting multiple components of the pathway are in preclinical and clinical development (26). One drug that targets PI(3)K signaling is rapamycin, which acts to inhibit specific mammalian target of rapamycin (mTOR) complexes, thereby modulating translation in response to survival signals, or nutrient or energy availability. Initially approved as an immunosuppressant, rapamycin and its analogues have antitumor activity in some preclinical models and are currently in clinical trials (4, 27–32). It is therefore important to identify mechanisms of sensitivity and resistance to these agents.
We have previously described the effects of aberrant Akt expression on tumorigenesis, chemotherapy responses, and rapamycin sensitivity in the Eμ-myc lymphoma model (4). Specifically, we have shown that Akt dramatically accelerated myc-induced tumorigenesis and promoted resistance to conventional chemotherapy. Rapamycin suppressed mTOR activity and synergized with chemotherapy in Akt-expressing lymphomas, leading to potent antitumor responses. Interestingly, eIF4E, a translational regulator acting downstream of mTOR, accelerated lymphomagenesis and promoted drug resistance in a manner comparable with Akt, suggesting that a substantial portion of the Akt survival signal is transmitted through deregulated translation. Here, we studied the effects of reduced PTEN activity in the same genetic context and address the problem of rapamycin resistance, because this genetic lesion is the most prominent lesion that produces Akt activation in human cancers. We further evaluated genetic determinants of sensitivity and resistance cytotoxic agents, rapamycin, and combinations of both.
Materials and Methods
Generation of mice
Eμ-myc mice (C57BL/6 strain) and PTEN+/−, ARF−/−, p53−/− mice were crossed, and their offsprings were genotyped as described (17, 33). The animals were monitored for development of lymphoma and associated leukemia by biweekly palpation and blood counts, respectively. Upon the appearance of well-palpable lymphomas, the tumors were harvested and either fixed in formalin for histologic evaluation, rendered single-cell suspensions and frozen in 10% DMSO, or transplanted directly into C57Bl/6 mice for treatment studies (4). Loss of heterozygosity at the PTEN locus in tumor cells was determined by allele-specific PCR following brief (24 hours) in vitro culture (17).
Treatment studies
Treatment studies in mice were done as previously described (4). Briefly, 1 × 106 DMSO frozen or primary lymphoma cells were injected into the tail vein of 6- to 8-week-old female C57BL/6 mice. Upon the formation of palpable tumors, the animals were treated with rapamycin (4 mg/kg, i.p. ×5 days), doxorubicin (10 mg/kg, i.p.), cyclophosphamide (300 mg/kg, i.p.), or combinations. In combination studies, the cytotoxic agent was given on day 2 of the rapamycin protocol. Rapamycin (LC Labs, Woburn, MA) was initially dissolved in 100% ethanol, stored at −20°C, and further diluted in an aqueous solution of 5.2% Tween 80 and 5.2% PEG 400 (final ethanol concentration, 2%) immediately before use. Doxorubicin (Sigma, St. Louis, MO) and cyclophosphamide (Sigma) were dissolved in water. In treatment studies, chemosensitive and chemoresistant control lymphomas were Arf-null tumors arising in a Eμ-myc/Arf+/− background and p53-null lymphomas arising in a Eμ-myc/p53+/− background, respectively (8). Treatment responses were monitored by twice weekly palpation and blood smears stained with Giemsa (Fisher Diagnostics, Middletown, VA). A “complete remission” was defined as the absence of any detectable tumor and leukemia. Tumor-free survival was defined as the time between treatment and reappearance of lymphoma or leukemia (4). Tumor-free and overall survival data were analyzed in the Kaplan-Meier format using the log-rank (Mantel-Cox) test for statistical significance.
Histopathology
Samples were fixed for 24 hours in 10% buffered formalin and embedded in paraffin. Thin sections (5 μm) were stained with H&E according to standard protocols. Detection of PTEN (99552, 1:100, Cell Signalling, Danvers, MA), phosphorylated Akt (9275, 1:100, Cell Signalling), phosphorylated ribosomal S6 protein (2215, 1:100, Cell Signalling), and Ki67 (1:100, NovoCastra, Newcastle upon Tyne, United Kingdom) was by standard avidin-biotin immunoperoxidase method, using biotinylated goat or rabbit specific immunoglobulins (Vector Labs, Burlingame, CA) at 1:500 and avidin-biotin peroxidase complexes (1:25, Vector Labs). Diaminobenzidine was used as the chromogen and hematoxylin was used as counterstain. The apoptotic rate was analyzed by terminal deoxyribonucleotide transferase–mediated nick-end labeling assay (TUNEL) according to published protocols (34).
Fluorescence-activated cell sorting analysis
Tumor cell suspensions of representative tumors of each genotype were stained with the indicated monoclonal antibodies (PharMingen, San Diego, CA and CalTag, Burlingame, CA) conjugated with phycoerythrin, TRI-COLOR, or biotin, developed with streptavidin-allophycocyanin (PharMingen) and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) using CellQuest Pro software.
Western blotting
Immunoblots were done from whole-cell lysates as previously described (35). Fifty micrograms of protein per sample were resolved on SDS-PAGE gels and transferred to Immobilon-P membranes (Millipore, Billerica, MA). Antibodies against PTEN (a gift from M. Myers, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY), phosphorylated Akt (9275, 1:1,000, Cell Signalling), Akt (9272, 1:1,000, Cell Signalling), ribosomal S6 protein (2212, 1:1,000, Cell Signalling), phosphorylated ribosomal S6 (2215, 1:1,000 Cell Signalling), and α-tubulin (B-5-1-2, 1:5,000, Sigma) were used as probes and detected using enhanced chemiluminescence (ECL, Amersham, Piscataway, NJ; Lumilight, Roche, Nutley, NJ).
Competition experiments
Tumor cells, either Eμ-myc/ARF−/− or Eμ-myc/PTEN+/−, were transduced with retroviral vectors at a low multiplicity of infection to create mixed populations of cells containing or lacking each vector. The vectors were MSCV-GFP (the empty vector control), MSCV-bcl-2-IRES-GFP (10), MSCV-eIF4E-IRES-GFP (4), MSCV-shPTEN-SV40-GFP, MSCV-shPTEN-puroR-IRES-GFP, MSCV-p53C (36), or MSCV-p53D (LMP-p53D; ref. 37). For in vitro experiments, the resulting mixed populations of infected and uninfected tumor cells were propagated in standard medium in the presence or absence of rapamycin (1 μmol/L) and then analyzed for green fluorescent protein (GFP) content by flow cytometry. For the in vivo studies, the mixed populations were transplanted by tail-vein injection into nontransgenic female C57BL/6 animals; upon tumor formation, these mice were treated. The mice were typically sacrificed 48 hours later and single-cell suspensions of residual tumors were analyzed for GFP content.
Results
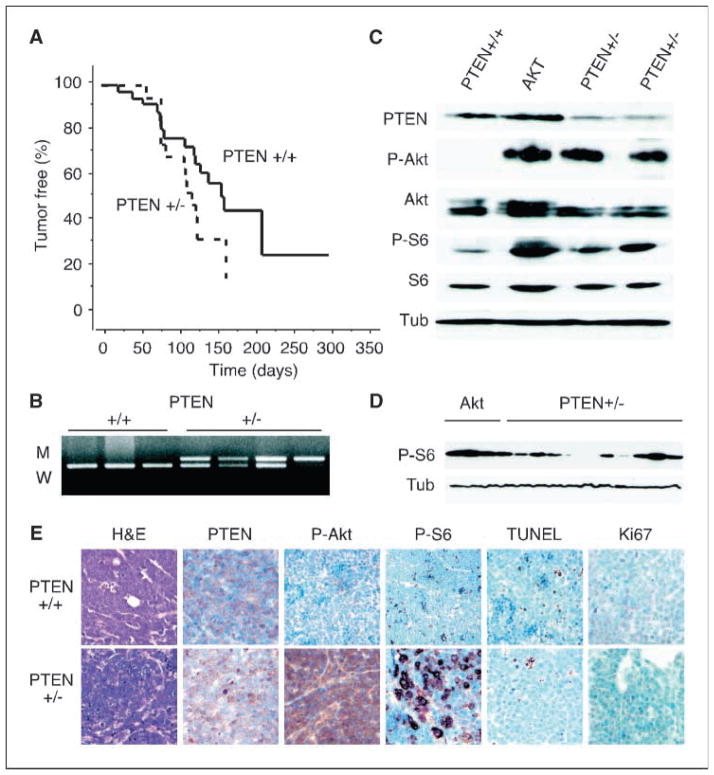
Given the high frequency of PTEN alterations in human tumors (15), we wished to address how PTEN heterozygosity would effect myc-driven tumorigenesis in vivo. To this end, we generated crosses of Eμ-myc to PTEN+/− mice and monitored the progeny for lymphoma development. Kaplan-Meier analysis comparing tumor latencies in Eμ-myc/PTEN+/− and their Eμ-myc/PTEN+/+ littermates revealed only a modest acceleration of disease onset in the heterozygous animals that did not achieve statistical significance (P = 0.06; Fig. 1A). Tumors in nontransgenic PTEN+/− animals were rare (data not shown). We analyzed DNA prepared from pure populations of tumor cells harvested from Eμ-myc/PTEN+/− animals by PTEN allele-specific PCR and found that the wild-type PTEN allele remained detectable in the majority of cases, with loss of heterozygosity only rarely observed (2 of 10 cases; Fig. 1B).
Figure 1. Effect of PTEN heterozygosity on Eμ-myc lymphomagenesis.
A, Kaplan-Meier plot comparing tumor latencies in Eμ-myc/PTEN+/+ (solid line, n = 33) and Eμ-myc/PTEN+/− (dashed line, n = 19) mice (P = 0.06). B, PCR to detect loss of heterozygosity at the PTEN locus (M, mutant allele, W, wild-type allele).
C, lysates prepared from untreated Eμ-myc tumors of the indicated genotypes and probed for PTEN, phosphorylated and total Akt (P-Akt and Akt), and ribosomal S6 protein (P-S6 and S6, respectively), and α-tubulin (Tub) as a loading control.
D, immunoblot for phosphorylated ribosomal S6 protein and tubulin in Eμ-myc tumors expressing Akt (lanes 1–3) or heterozygous for PTEN (lanes 4–14). E, representative microphotographs of Eμ-myc/PTEN+/+ or Eμ-myc/PTEN+/− tumors either H&E stained or immunohistochemically stained as indicated.
Immunoblotting analysis on lysates from primary tumors confirmed that PTEN protein expression was retained in Eμ-myc/PTEN+/− tumors. Nonetheless, these tumors displayed increased ratios of phosphorylated to total AKT and ribosomal S6 proteins, suggesting that loss of one PTEN allele was sufficient to deregulate the PI(3)K pathway (Fig. 1C). PI(3)K pathway activation was, however, more varied and typically to a lesser extend than observed in tumors expressing an activated form of Akt (Fig. 1D; see also ref. 19). Similar results were observed in tissue sections examined by immunohistochemistry (Fig. 1E). Consistent with their similar tumor latencies, lymphomas arising in PTEN+/+ and PTEN+/− animals showed similar rates of apoptosis and proliferation by TUNEL and Ki67 staining, respectively. Immunophenotypically, Eμ-myc/PTEN+/− tumors exhibited a mature B-cell phenotype, showing surface expression of B220 (CD45R), CD19, and surface immunoglobulin (data not shown). This immunophenotype was similar to that observed in Eμ-myc mice without loss of PTEN, but contrasts with the immature nonlineage determined phenotype of Eμ-myc lymphomas overexpressing AKT. Interestingly, overexpression of eIF4E, like PTEN loss, led to mature B-cell tumors (4). Thus, although loss of one PTEN allele can activate the PI(3)K pathway, its effect on lymphomagenesis is minimal. Because the PTEN−/− mouse is an embryonic lethal, we could not examine the effects of complete PTEN loss on lymphoma development using this model.
Given the minor effects of PTEN heterozygosity on tumor development, we wondered if this alteration would affect treatment response. We therefore transplanted primary tumors arising in Eμ-myc/PTEN+/− mice into multiple wild-type recipients. Upon tumor formation, we initiated therapy with either cyclophosphamide or doxorubicin and tracked its effect by biweekly tumor palpation and blood smears. Of note, in the absence of a cooperating oncogene (e.g., Akt or Bcl-2) or preexisting loss of tumor suppressors (e.g., PTEN), myc-driven tumors are heterogeneous with ARF and p53 being each inactivated in ~25% of tumors (38). We therefore chose to compare PTEN+/− tumors with genetically defined Eμ-myc lymphomas lacking either ARF or p53, which are chemosensitive and chemoresistant, respectively (8). Doxorubicin treatment at the maximum tolerated dose of 10 mg/kg induced complete responses in 90% of mice bearing Eμ-myc/ARF−/− tumors. By contrast, the response of Eμ-myc/PTEN+/− lymphomas was significantly worse and approached the poor response of p53-null lymphomas, with only 50% of mice achieving a complete remission and none lasting longer than 3 weeks (Fig. 2A). Cyclophosphamide therapy at the maximum tolerated dose of 300 mg/kg is more effective against Eμ-myc lymphomas and induced complete remissions in all animals irrespective of tumor genotype (Fig. 2B). However, both Eμ-myc/p53−/− and Eμ-myc/PTEN+/− tumors relapsed within 20 and 40 days, respectively, whereas half the animals bearing Eμ-myc/ARF−/− tumors did not relapse in the 100-day observation period. Thus, lymphomas with loss of only one PTEN allele display a resistance to common chemotherapeutic agents that is comparable with loss of p53 or overexpression of Akt (4, 39).
Figure 2.
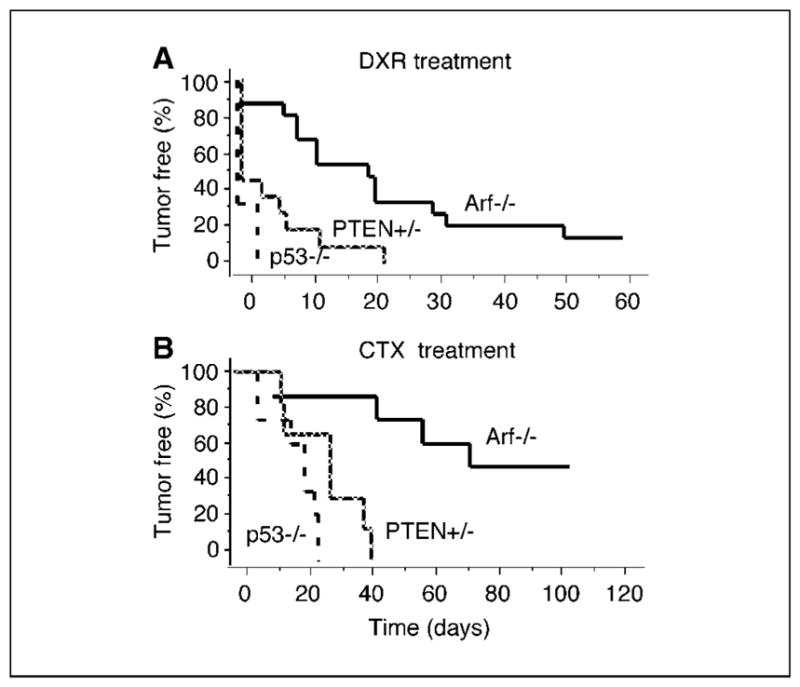
PTEN deficiency promotes chemoresistance in vivo. Kaplan-Meier plots detailing the time to relapse following treatment with doxorubicin (DXR; A) and cyclophosphamide (CTX; B). Mice bearing either chemosensitive Eμ-Myc/ARF−/− lymphomas (solid line; doxorubicin, n = 15; cyclophosphamide, n = 14), Eμ-Myc/PTEN+/− lymphomas (hatched line; doxorubicin, n = 11; cyclophosphamide, n = 6), or Eμ-myc/p53−/− lymphomas (dashed line; doxorubicin, n = 6; cyclophosphamide, n = 9) were monitored for tumor-free survival.
In Akt-expressing tumors, rapamycin potently blocks mTOR activity in vivo and sensitizes these tumors to apoptosis induced by cytotoxic therapy (4). We therefore tested whether rapamycin would have similar effects in PTEN+/− tumors. Immunoblotting of lysates from Eμ-myc/PTEN+/− cells confirmed low levels of PTEN expression and subsequent activation of the PI(3)K/Akt pathway (Fig. 3A). These experiments also documented the ability of rapamycin to inhibit mTOR activity in vivo, as phosphorylation of ribosomal S6 protein was reduced 6 hours after rapamycin treatment relative to an untreated control. Doxorubicin did not affect S6 phosphorylation, and neither treatment affected phosphorylation of Akt (Fig. 3A). Immunohistochemistry on parallel tumors harvested 18 hours after rapamycin treatment revealed persistent mTOR inhibition and undetectable phosphorylated S6 protein. However, at this dose and schedule, rapamycin therapy only produced a modest increase in apoptosis, similar to that produced by chemotherapy alone (Fig. 3B). Although the combination of doxorubicin and rapamycin produced no further reduction in S6 phosphorylation compared with rapamycin alone, the tumors displayed substantially more apoptosis. Thus, rapamycin induces lasting mTOR inhibition and enhances the apoptotic response to DNA damage in Eμ-myc/PTEN+/− tumors.
Figure 3.
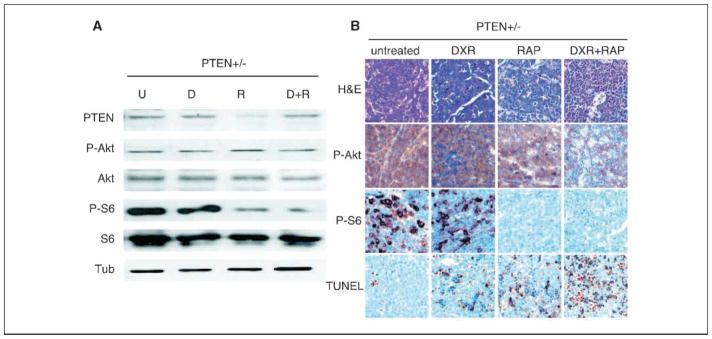
Rapamycin inhibits mTOR activity in vivo and promotes apoptosis in response to chemotherapy. A, lysates prepared from Eμ-myc/PTEN+/− tumors either left untreated (U) or 6 hours following in vivo treatment with doxorubicin (D), rapamycin (R), or doxorubicin and rapamycin (D + R), and probed for PTEN, phosphorylated and total Akt, and ribosomal S6 protein, and α-tubulin. B, representative micrographs of Eμ-myc/PTEN+/− tumors untreated or 18 hours after treatment with doxorubicin, rapamycin, or doxorubicin + rapamycin and stained with H&E or immunohistochemical probing detecting the phosphorylated forms of Akt and ribosomal S6 protein or TUNEL.
We next asked whether the sensitization to apoptosis produced by rapamycin and chemotherapy would translate into a synergistic therapeutic effect in vivo. Indeed, cumulative analysis of “time-to-relapse” data from mice bearing Eμ-myc/PTEN+/− tumors revealed that the rapamycin/doxorubicin combination therapy induced complete remissions lasting at minimum 3 weeks and up to 45 days; by contrast, treatment with either drug alone achieved few responses and these were invariably short lived (rapamycin + doxorubicin versus rapamycin or doxorubicin: P = 0.0002; rapamycin versus doxorubicin: P = 0.5; Fig. 4A). The rapamycin/cyclophosphamide combination produced an even greater response and induced “cures” in almost 40% of animals (rapamycin + cyclophosphamide versus rapamycin or cyclophosphamide: P ~ 0.02; rapamycin versus cyclophosphamide: P = 0.07; Fig. 4B). This effect was highly specific for tumors with an activated PI(3)K pathway in that treatment of chemosensitive Eμ-myc/ARF−/− tumors with the same rapamycin and doxorubicin combination resulted in a reduced response compared with treatment with doxorubicin alone (P = 0.0002; Fig. 4C). Therefore, the synergistic antitumor activity of rapamycin-based combinations appears specific for tumors with PI(3)K/Akt pathway activating lesions. Surprisingly, sensitization to the effects of these agents requires loss of only one allele of PTEN.
Figure 4.
Rapamycin reverses chemoresistance in PTEN-deficient tumors in vivo. Kaplan-Meier analyses of tumor-free survival in Eμ-myc/PTEN+/− lymphomas following treatment with (A) doxorubicin (hatched line, n = 11), rapamycin (RAP; dashed line, n = 13) and doxorubicin + rapamycin (solid line, R + D, n = 13). B, following treatment with cyclophosphamide (n = 6, hatched line), rapamycin (n = 13, dashed line), or cyclophosphamide + rapamycin (C + R, n = 7, solid line). C, time to relapse following treatment of control tumors (Eμ-myc/ARF−/−) with doxorubicin (n = 15; hatched line), rapamycin (n = 11, dashed line), doxorubicin + rapamycin (D + R, n = 8, solid line).
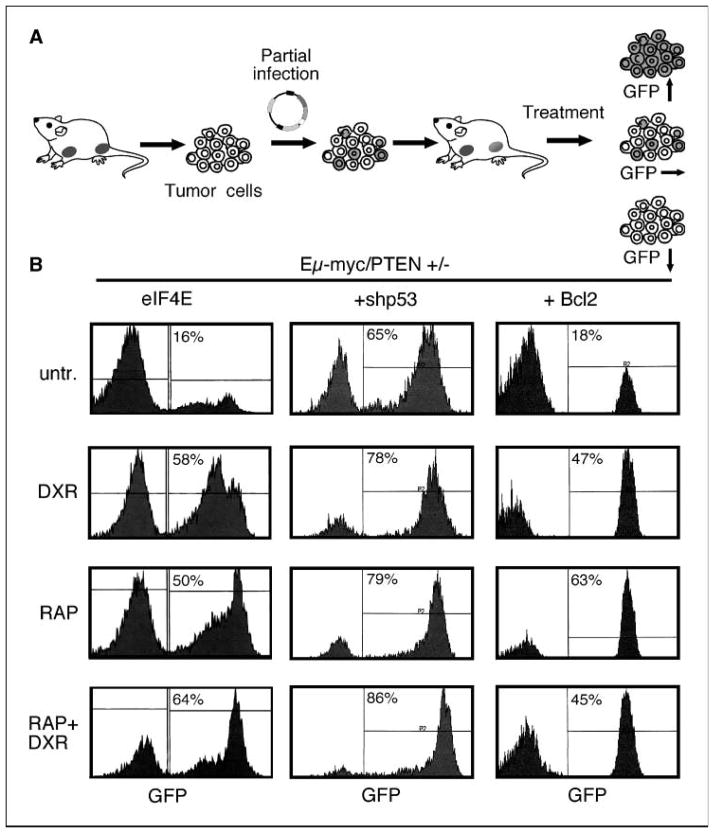
These findings raise further questions regarding rapamycin sensitivity: (a) whether additional genetic lesions affect rapamycin sensitivity in PTEN+/− tumors and (b) whether it is important for PI(3)K activation to occur early in tumorigenesis to induce sensitivity to rapamycin. To address these issues, we established an in vivo competition assay. This approach allows us to generate genetically defined mixed tumor populations and examine the relative competitiveness of each subpopulation in vivo (Fig. 5A). Specifically, we infect a fraction of tumor cells from a defined genotype with a retroviral vector coexpressing a transgene or RNA interference (RNAi) construct together with GFP, creating a mixed population of cells expressing or lacking the provirus. These populations are reintroduced into several recipient animals and, upon lymphoma manifestation, the tumor-bearing mice are either left untreated or treated with a drug or drug combination. Later, residual tumor cells are harvested and subjected to flow cytometry to determine the percentage of GFP-positive cells. Enrichment of GFP-positive cells following therapy (relative to the GFP fraction in untreated tumors) indicates that the construct enhances resistance to the therapy, whereas depletion of GFP suggests a sensitizing effect. Such internally controlled experiments provide a simple and sensitive assay to assess whether a particular lesion influences treatment response in vivo.
Figure 5.
Genetic modifiers of rapamycin sensitivity in vivo. A, schematic of the in vivo competition experimental design. B, representative fluorescence-activated cell sorting (FACS) analyses of GFP expression in Eμ-myc/PTEN+/− tumors expressing either eIF4E/GFP, shp53/GFP, or Bcl-2/GFP in a fraction of tumor cells harvested 48 hours after the indicated treatment.
We first wished to determine whether deregulation of translational initiation downstream of mTOR would promote resistance to rapamycin/chemotherapy combinations in PTEN+/− tumors. eIF4e is a translation factor that is inhibited by 4E-BPs, which, in turn, are negatively regulated by mTOR-mediated phosphorylation (40). We therefore infected a subset of Eμ-myc/PTEN+/− tumor cells with a vector encoding eIF4E. As occurs in Akt-expressing lymphomas (4), eIF4E-expressing cells were enriched in PTEN+/− tumors 48 hours following rapamycin treatment or rapamycin/doxorubicin combinations (Fig. 5B, left), indicating that eIF4E enhances resistance to this drug combination. Furthermore, despite their initial poor response to conventional chemotherapy, PTEN+/− tumor cells expressing eIF4E were also enriched following treatment with doxorubicin alone. Apparently, deregulation of translation beyond that produced by PTEN heterozygosity alone can further enhance chemoresistance (Fig. 5B, left).
Although PI(3)K lesions can contribute to sensitization to rapamycin therapy, the extent of this sensitization may be influenced by factors that act downstream (e.g., eIF4E) or in parallel to the oncogenic signaling pathway itself. For example, some studies suggest that rapamycin/chemotherapy combinations may require p53 tumor suppressor function, although the importance of p53 to rapamycin-based therapies seems variable (41–43). In our lymphoma model, mTOR inhibition, particularly in combination with chemotherapy, is associated with apoptosis induction, suggesting that an intact apoptotic program might be required for an optimal antitumor response. To determine whether p53 and/or apoptosis contribute significantly to the therapeutic responses in PTEN+/− tumors, we transduced short hairpin RNAs (shRNA) capable of suppressing p53 (36, 37), or a retrovirus overexpressing Bcl-2, into PTEN+/− lymphomas, and did in vivo competition assays. As expected, cells expressing p53 shRNAs were more resistant to doxorubicin, confirming the effectiveness of RNAi in this context (Fig. 5B, middle). More importantly, lymphoma cells expressing p53 shRNAs were also enriched following treatment with either rapamycin or combined treatment with doxorubicin and rapamycin, implying p53 function is also needed for optimal responses to these agents. Similar results were obtained in lymphoma populations harboring Bcl-2-expressing cells, which were more resistant to all three therapies (Fig. 5B, right; refs. 10, 44). Thus, genetic lesions acting downstream of mTOR or affecting parallel signaling networks can modulate rapamycin sensitivity in tumors in vivo.
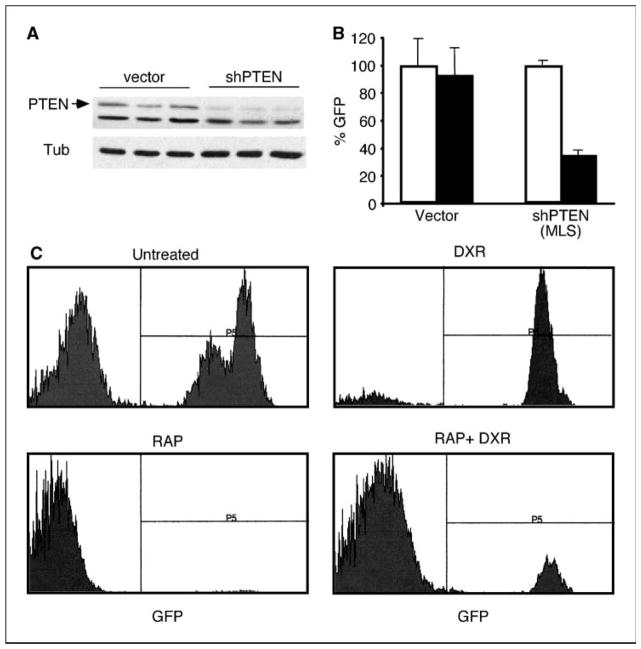
In human cancer, PTEN inactivation is often a late event and associated with advanced disease (45). In the PTEN+/− tumors we studied, reduced PTEN expression and elevated PI(3)K signaling were presumably present during tumorigenesis (46). To determine whether acute ablation of PTEN in an established tumor could produce similar effects, we used RNAi to suppress PTEN function in ARF-deficient tumor cells, which show no evidence of PI(3)K pathway deregulation and are not sensitive to rapamycin. We generated a PTEN shRNA that could achieve a significant, albeit incomplete, knockdown of PTEN in pure populations of infected cells (Fig. 6A). Mixed populations of Eμ-myc/ARF−/− cells harboring this shPTEN construct or a vector control were treated with rapamycin in vitro. Seven days later, the cultures were subjected to flow cytometry to examine the percentage of GFP-expressing cells. Cells expressing PTEN shRNAs showed a marked competitive disadvantage, which was not observed in cells harboring the control vector (Fig. 6B). This implies that acute suppression of PTEN sensitizes cells to rapamycin, at least in vitro.
Figure 6.
PTEN knockdown sensitizes established tumors to rapamycin in vitro and in vivo. A, immunoblot of lysates prepared from separate batches of Ba/F3/p210 cells transduced with either a control vector (vector; lanes 1–3) or an RNAi vector targeting PTEN (shPTEN; lanes 4–6), probed with antibodies against PTEN and tubulin as loading control. B, in vitro competition experiment. Summary of FACS analyses of GFP expression in Eμ-myc/ARF−/− tumors partially transduced with an RNAi-GFP vector targeting PTEN (shPTEN) or a control vector-GFP (Vector) and either left untreated (white columns) or treated with 1 μmol/L rapamycin for 7 days (black columns), data are normalized to the untreated control (100%). Columns, mean; bars, SD. C, FACS analysis of Eμ-myc/ARF−/− tumors partially transduced with an RNAi-GFP construct targeting PTEN (shPTEN) and harvested 48 hours after the indicated treatments.
In parallel experiments, the mixed populations of the tumor cells described above were transplanted into recipient animals for in vivo treatment. Consistent with the ability of deregulated PI(3)K signaling to promote doxorubicin resistance (see Fig. 2A), tumor cells harboring PTEN shRNAs were enriched to near purity within 48 hours of doxorubicin administration (Fig. 6C). By contrast, treatment with rapamycin or the rapamycin/doxorubicin combination dramatically depleted the shPTEN-expressing cell population from mixed tumors in vivo. Thus, RNAi-mediated depletion of PTEN in established tumors can sensitize tumor cells to rapamycin-based therapies in a manner that is similar to tumors arising in an Eμ-myc/PTEN+/− background. These data also provide the proof of concept for the feasibility of in vivo RNAi screens to identify modulators of drug response in vivo.
Discussion
The PI(3)K pathway is activated in many tumors, for example, through mutations that target the PI3KA, PTEN, Akt, or TSC1 or TSC2 genes (reviewed in refs. 11, 47). PI(3)K activation can produce diverse changes in cell physiology and inhibits apoptosis, indicating that PI(3)K pathway mutations may have broad consequences on tumorigenesis and treatment responses. Owing to its gain-of-function mode of action, the pathway is also a compelling target for new therapeutics. Nonetheless, whether mutations in different components of the PI(3)K pathway produce the same effects on tumor physiology is not clear. We had previously described the effects of Akt expression on lymphomagenesis and treatment response in the Eμ-myc lymphoma model, and observed a dramatic effect of Akt in promoting tumorigenesis, resistance to conventional therapy, and sensitivity to rapamycin/chemotherapy combinations (4). However, lesions that activate PI(3)K signaling at the level of PTEN are more common in human cancer (15), and so we wished to determine how suppression of PTEN would affect tumor phenotypes in the Eμ-myc system.
Although our previous studies showed that Akt could dramatically accelerate myc-induced lymphomagenesis, Eμ-myc transgenic mice harboring loss of one PTEN allele showed little, if any, acceleration and most tumors examined retained the wild-type PTEN allele. However, the PI(3)K pathway is clearly activated in PTEN+/− lymphomas, although to a lesser and more varied extent than in tumors expressing an activated form of Akt (Fig. 1D). Likely, tumorigenesis in the presence of Myc requires a high flux through the Akt pathway (19). It remains possible, however, that high levels of Akt may have broader consequences for tumorigenesis than PTEN loss, either through “off-target” effects on other signaling networks or by differentially affecting various feedback loops. In this regard, the PTEN knockout mouse dies during embryonic development, precluding study of a complete ablation of PTEN on tumorigenesis in these animals. More recently, conditional PTEN knockout mice have been developed, and at least in a glioma model, complete loss of PTEN mimics Akt action during tumorigenesis (48).
Despite the relatively minor effects of PTEN heterozygosity on tumorigenesis, we noted marked responses on the response of tumors to conventional or targeted therapy. Thus, loss of even one allele of PTEN was sufficient to produce chemoresistant tumors, a phenomenon also observed in lymphomas produced by over-expressing Akt. In principle, such resistance could represent a secondary consequence of other mutations arising in the PTEN+/− tumors, but we see that acute suppression of PTEN in an established tumor was sufficient to promote resistance to doxorubicin, and that rapamycin (which targets mTOR downstream of PTEN) restores drug-induced apoptosis to PTEN+/− tumors. Such a haploinsufficient effect of PTEN has been noted in tumorigenesis in a mouse prostate cancer model (19). Here, we see that reduced PTEN dosage can have a varied effect on tumor phenotypes, affecting treatment sensitivity to a greater degree than tumorigenesis in our model.
Mice harboring PTEN+/− lymphomas could be effectively treated using rapamycin/chemotherapy combinations, leading to durable responses that were similar to mice harboring Akt lymphomas. Also, as observed in mice harboring Akt lymphomas, mice with PTEN+/− lymphomas treated did not respond well to rapamycin therapy at our dose and schedule, despite the ability of the drug to efficiently inhibit mTOR activity. Nevertheless, previous studies indicate that PTEN loss can be sufficient to confer rapamycin sensitivity in some contexts (28, 29, 31), and we see that suppression of PTEN confers a selective disadvantage to tumor cells treated with rapamycin in culture. Thus, chemotherapy combinations are not required for rapamycin to have an antitumor effect, but potentiate the consequences of mTOR inhibition. Together, these data indicate that even modest changes in flux through the PI(3)K pathway can have profound effects on treatment behavior.
Precisely how rapamycin and chemotherapy synergize to produce antitumor responses in Eμ-myc lymphomas or other cancers remains to be determined, but our studies provide insights into its mode of action. One factor seems to be the ability of rapamycin to inhibit translation, as enforced expression of the translation initiation factor eIF4E in PTEN+/− tumors enhanced resistance to chemotherapy or rapamycin/chemotherapy combinations. Another factor seems to be the presence of a PI(3)K pathway lesion, leading to the deregulation of PI(3)K signaling. Thus, tumors overexpressing Akt (4) or with reduced PTEN expression were sensitive to this combination and, at the same dose and schedule, ARF-deficient tumors [which show no evidence for PI(3)K involvement] showed a worse response to the combined therapy than to chemotherapy alone. Interestingly, these lesions did not have to be present during tumorigenesis, because acute suppression of PTEN in established tumors using RNAi could enhance rapamycin sensitivity.
Although a PI(3)K pathway lesion may confer sensitivity to rapamycin-based therapies, our data indicate that other pathways also influence rapamycin responses. Thus, disruption of p53 or over-expression of Bcl-2 in PTEN+/− tumors produced tumor cells that were more resistant to rapamycin/chemotherapy combinations. Interestingly, the role of p53 in influencing rapamycin responses is controversial, with studies differing on whether p53-deficient cells are more sensitive or resistant to rapamycin therapy in vitro (41–43, 49, 50). One recent report indicates that the antitumor activity of combination therapy with rapamycin and cisplatin depends on an intact p53 response in cultured tumor cells (43), and our study indicates that p53 is important for other rapamycin/chemotherapy combinations in vivo. However, the role of p53 in affecting rapamycin-based therapies is not restricted to combinations involving cytotoxic drugs, as we see that disruption of p53 can produce a selective advantage to tumor cells treated with rapamycin alone. This suggests that p53 may influence how a tumor cell responds to mTOR inhibition, and is consistent with our observation that p53 mutations promote resistance to imatinib in a mouse leukemia model initiated by Bcr-abl, an imatinib target (51). However, these observations do not rule out p53-independent modes of action, and conceivably the relative contributions of p53 and apoptotic mechanisms to rapamycin action may depend on the cellular context. Understanding the precise molecular contexts whereby PI(3)K pathway lesions promote sensitivity to mTOR inhibition and the parallel pathways that can modulate its effects will be important for the effective use of mTOR inhibitors in the clinic.
Acknowledgments
Grant support: Grant CM030062 and a Special fellowship of the Leukemia and Lymphoma Society (H.G. Wendel), grant CA87497 (S.W. Lowe), a grant from Canadian Institutes of Health Research (J. Pelletier), and a Leukemia and Lymphoma Society of America Specialized Center of Research program (S.C. Kogan and S.W. Lowe).
We thank Dr. P.P. Pandolfi (Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY) for providing us with the PTEN heterozygous animals, Lisa Bianco and the Cold Spring Harbor Laboratory animal facility, and members of the Lowe laboratory for advice and discussion.
References
- 1.Lowe SW, Ruley HE. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–45. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 2.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 3.Bissonnette RP, Echeverri F, Mahboubi A, Green DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–4. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 4.Wendel HG, De Stanchina E, Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 5.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 6.Orsulic S, Li Y, Soslow RA, Vitale-Cross LA, Gutkind JS, Varmus HE. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1:53–62. doi: 10.1016/s1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–7. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–46. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nat Med. 2000;6:1029–35. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- 11.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 12.Sakai A, Thieblemont C, Wellmann A, Jaffe ES, Raffeld M. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–5. [PubMed] [Google Scholar]
- 13.Nakahara Y, Nagai H, Kinoshita T, et al. Mutational analysis of the PTEN/MMAC1 gene in non-Hodgkin’s lymphoma. Leukemia. 1998;12:1277–80. doi: 10.1038/sj.leu.2401099. [DOI] [PubMed] [Google Scholar]
- 14.Min YH, Eom JI, Cheong JW, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17:995–7. doi: 10.1038/sj.leu.2402874. [DOI] [PubMed] [Google Scholar]
- 15.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 16.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–11. [PubMed] [Google Scholar]
- 17.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Teruya-Feldstein J, Behrendt N, et al. Genetic analysis of Pten and Tsc2 functional interactions in the mouse reveals asymmetrical haploinsufficiency in tumor suppression. Genes Dev. 2005;19:1779–86. doi: 10.1101/gad.1314405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 21.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–6. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 22.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 23.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 24.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13:75–8. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 25.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 26.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, Frankel A, Radvanyi LG, Penn LZ, Miller RG, Mills GB. Rapamycin enhances apoptosis and increases sensitivity to cisplatin in vitro. Cancer Res. 1995;55:1982–8. [PubMed] [Google Scholar]
- 28.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–9. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–34. [PubMed] [Google Scholar]
- 30.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–5. [PubMed] [Google Scholar]
- 31.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98:10320–5. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 33.Adams JM, Harris AW, Pinkert CA, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–8. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 34.Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–4. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 35.de Stanchina E, McCurrach ME, Zindy F, et al. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 1998;12:2434–42. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemann MT, Fridman JS, Zilfou JT, et al. An epiallelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- 37.Dickins RA, Hemann MT, Zilfou JT, et al. Probing tumor phenotypes using stable and regulated synthetic micrRNA precursors. Nat Genet. 2005;37:1163–5. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 38.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–69. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt CA, Wallace-Brodeur RR, Rosenthal CT, McCurrach ME, Lowe SW. DNA damage responses and chemosensitivity in the E mu-myc mouse lymphoma model. Cold Spring Harb Symp Quant Biol. 2000;65:499–510. doi: 10.1101/sqb.2000.65.499. [DOI] [PubMed] [Google Scholar]
- 40.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 41.Hosoi H, Dilling MB, Shikata T, et al. Rapamycin causes poorly reversible inhibition of mTOR and induces p53-independent apoptosis in human rhabdomyosarcoma cells. Cancer Res. 1999;59:886–94. [PubMed] [Google Scholar]
- 42.Xing D, Orsulic S. A genetically defined mouse ovarian carcinoma model for the molecular characterization of pathway-targeted therapy and tumor resistance. Proc Natl Acad Sci U S A. 2005;102:6936–41. doi: 10.1073/pnas.0502256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 44.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 45.Whang YE, Wu X, Suzuki H, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci U S A. 1998;95:5246–50. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein IB. Cancer Addiction to oncogenes—the Achilles heal of cancer. Science. 2002;297:63–4. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 47.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–90. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 48.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356–68. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang S, Shu L, Easton J, et al. Inhibition of mammalian target of rapamycin activates apoptosis signal-regulating kinase 1 signaling by suppressing protein phosphatase 5 activity. J Biol Chem. 2004;279:36490–6. doi: 10.1074/jbc.M401208200. [DOI] [PubMed] [Google Scholar]
- 50.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol Cell. 2003;11:1491–501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 51.Wendell HG, de Stanchina E, Cepero E, et al. Loss of p53 impedes the antileukemic response to BCR-ABL inhibition. Proc Natl Acad Sci U S A. 2006;103:7444–9. doi: 10.1073/pnas.0602402103. [DOI] [PMC free article] [PubMed] [Google Scholar]