Abstract
Although uterine leiomyomata (also known as fibroids or myomas) affect the reproductive health and well-being of approximately 25% of premenopausal women, risk factors are poorly understood. Elevated diastolic blood pressure may increase fibroid risk through uterine smooth muscle injury, not unlike atherosclerosis. The authors prospectively examined the relation between diastolic blood pressure and incidence of clinically detected leiomyomata. The sample included 104,233 premenopausal nurses from 14 US states enrolled in the Nurses’ Health Study II. Participants, aged 25–42 years, had intact uteri and no history of cancer or fibroids at enrollment in 1989. During the 827,348 woman-years of follow-up (1989–1999), 7,466 incident diagnoses of uterine leiomyomata, confirmed by ultrasound or hysterectomy, were reported. With a multivariable Cox proportional hazards model, the relative risk of self-reported ultrasound- or hysterectomy-confirmed uterine leiomyomata according to diastolic blood pressure in 1989 and time-varying antihypertensive use was estimated. With adjustment for age, race/ethnicity, body mass index, and reproductive history covariates, for every 10-mmHg increase in diastolic blood pressure, the risk of fibroids rose 8% (5–11%) and 10% (7–13%) among nonusers and users of antihypertensive medications, respectively. Elevated blood pressure has an independent, positive association with risk for clinically detected uterine leiomyomata among premenopausal women. Investigating this association may suggest possible pathways to prevent fibroids.
Keywords: blood pressure, cohort studies, hypertension, leiomyoma, uterine diseases, uterine neoplasms
Uterine leiomyomata (also known as fibroids or myomas) are the most common pelvic neoplasm in women (1–3). Epidemiologic studies demonstrate that these hormone-dependent, benign tumors follow a woman’s reproductive life cycle, increasing in risk with age up until the fifth decade followed by a precipitous decline at menopause (4, 5). Annually, fibroids account for 33 percent of all hysterectomies (n = 200,000–300,000) and hospital expenditures of $1.2 billion in the United States (6–9). While fibroids rarely progress to malignancy, in symptomatic cases they can lead to multiple gynecologic problems, such as pelvic pain, infertility, menstrual abnormalities, and spontaneous abortion, significantly affecting the quality of life among women (3, 10–15). Although these benign tumors represent a significant public health concern, the epidemiology of uterine leiomyomata is poorly understood.
A total of 20–25 percent of reproductive-age women have clinically symptomatic fibroids (14, 16). The true population prevalence of fibroids, however, is probably underestimated because of the unknown distribution of subclinical tumors (3). Studies screening randomly selected women using ultrasonography (17–19) or pathologic examination of uteri (20) have reported uterine leiomyomata prevalence values ranging from 5.4 to 77 percent. In one such study, over 50 percent of premenopausal women with ultrasound evidence for tumor had no previous diagnosis (17); therefore, our present understanding of fibroids is informed by the distribution of clinically symptomatic tumors. Purported risk factors include age (1, 7, 21), African-American ethnicity (12, 17, 21, 22), obesity (1, 23–26), diet (27), excessive radiation (28, 29), family history (19, 30–36), age at menarche (4, 37–40), and infertility (22, 37, 40). Factors believed to be protective include smoking (1, 5, 22, 41, 42) and increasing parity (1, 25, 37, 39, 40, 43–45) in some, but not all, studies.
African-American women have a higher fibroid prevalence in random screened samples (17) and 3.2 times the rate of new clinical diagnoses (21). They are also disproportionately affected by symptomatic fibroids (7, 12, 17, 21, 46), with nearly twice the incidence of hysterectomies for symptomatic uterine leiomyomata (3.8 vs. 1.6 per 1,000 woman-years) (7).
Hypertension has been associated with uterine leiomyomata risk through anecdotal reports (47–52), as well as retrospective (53, 54), cross-sectional (55), and case-control (56) studies. Research linking hypertension and hysterectomy (57–59) may indirectly implicate fibroids. Faerstein et al. (56) suggest that hypertension represents a “proatherogenic” state that enhances risk for fibroid development and/or growth in uterine smooth muscle in a manner analogous to atherosclerotic changes in arterial smooth muscle. Elevated blood pressure may cause smooth muscle cell injury and/or cytokine release and thereby increase the risk of uterine fibroid onset or growth, in a process analogous to atherosclerosis. Prior studies have focused on hypertension rather than on blood pressure levels.
Presently, the relation between blood pressure level and fibroid risk is unknown. Elevated systolic and diastolic blood pressures are each associated with the development of atherosclerosis, in a continuous, graded fashion (60, 61). Several lines of research suggest that diastolic blood pressure may be a better indicator than systolic blood pressure of cardiovascular risk among younger subjects (62–66). We examined the relation between baseline diastolic blood pressure and uterine leiomyomata incidence over the 10 years of follow-up in a prospective cohort of premenopausal women and the heterogeneity of this relation across racial/ethnic groups.
MATERIALS AND METHODS
The Nurses’ Health Study II is an ongoing prospective cohort study that began in September 1989 with 116,678 registered female nurses, aged 25–42 years, from 14 US states. The intent of the study is to explore factors that increase chronic disease risk, morbidity, and mortality among women. The mail survey is conducted biennially to collect information on diet, physical activity, pregnancies, oral contraceptive use, and chronic disease history. Response rates for the biennial questionnaires have historically been greater than 90 percent.
The study population for this analysis was restricted to premenopausal women with intact uteri. At baseline in 1989, participants with a history of uterine leiomyoma prior to September 1989 (n = 5, 288) who reported blood pressure unknown or not checked in the past 2 years (n = 834), who were postmenopausal (n = 2,270), who had a hysterectomy (n = 3,005), who reported diagnosis of any cancer other than nonmelonoma skin cancer (n=1,009), or who had no follow-up after 1993 (n = 1,675) were excluded. After the exclusions, 104,233 women remained for analysis.
Incident cases were defined as participants who reported a first diagnosis of “uterine fibroids” confirmed by ultrasound or hysterectomy on questionnaires in 1993, 1995, 1997, or 1999. If a participant reported both a hysterectomy and a diagnosis of uterine fibroids confirmed by ultrasound/ hysterectomy in the same time interval, the diagnosis was further classified as hysterectomy confirmed. If no hysterectomy was reported in the same time interval as a diagnosis confirmed by ultrasound/hysterectomy, the diagnosis was classified as ultrasound confirmed. Marshall et al. (21) performed a validation study among 243 Nurses’ Health Study II participants who reported a new diagnosis of fibroids detected by ultrasound or hysterectomy after 1989 on the 1993 questionnaire (100 White and 143 Black women) to assess the accuracy of self-report. The validation study revealed that the self-reported values had good agreement with the medical records, and confirmation rates were 92 percent and 94 percent among Black and White participants, respectively (21).
The diagnosis date was set to the midpoint of the interval in which it was first reported. Therefore, incidence is defined as the first diagnosis by ultrasound or hysterectomy, as opposed to the onset of a fibroid tumor, which cannot be ascertained. Nurses’ Health Study II participants were not systematically screened for fibroids through gynecologic ultrasound. Time at risk was defined as the number of months between the return of the 1989 questionnaire and May 1999, death, onset of menopause, diagnosis of cancer, or date of uterine leiomyomata diagnosis (whichever came first). Women who reported a new diagnosis confirmed by pelvic examination only or who lacked information on the method of diagnosis were not included as cases because of the uncertainty of the diagnosis. Those who reported a new diagnosis confirmed by pelvic examination alone were unable to contribute person-time during the reported interval but were allowed to reenter the analysis at a later date if they had a hysterectomy or ultrasound confirmation.
Diastolic blood pressure was self-reported in 1989 through nine response categories divided into approximately 10-unit intervals of millimeters of mercury. Respondents were instructed to report their current usual blood pressure if checked within the past 2 years. Self-reported hypertension was found to be reliable in a validation study conducted in 1983 among women in a similar cohort— Nurse’s Health Study I (67).
Antihypertensive medication use was assessed in four of the five biennial questionnaires: 1989, 1993, 1995, and 1997. Respondents were asked three questions in 1989 regarding current medications that they used regularly: specifically, whether they used any antihypertensive medication, furosemide-like diuretics, or thiazide diuretics. On later questionnaires they were asked whether, over the past 2 years, they regularly used thiazide diuretics and if they used any other medication to treat hypertension. As we lacked information on antihypertensive medication use in 1991, study participants were considered to be using antihypertensives in 1991 if they reported antihypertensive use on both the 1989 and 1993 questionnaires.
Regarding statistical analyses, participants were assigned to categories of diastolic blood pressure and antihypertensive medication use in 1989. We examined the baseline distribution of risk factors for uterine leiomyomata according to category of diastolic blood pressure and antihypertensive medication use in 1989, with direct age standardization to the study population in 5-year age categories. Antihypertensive medication use was updated on the basis of information from biennial questionnaires, allowing us to stratify 1989 diastolic blood pressure by updated use of antihypertensives.
Person-months of follow-up were assigned to groups according to exposure status at baseline and updated for subsequent time periods. The incidence rates of uterine fibroids, confirmed by ultrasound or hysterectomy, for specific blood pressure categories were computed by dividing the number of events by the person-time at risk in that category.
Multivariable Cox proportional hazards regression models were used to estimate incidence rate ratios while controlling for multiple risk factors using the SAS PROC PHREG procedure (SAS Institute, Inc., Cary, North Carolina). Relative risks are adjusted for the following variables: age (months), time period (months), age at menarche (≤10, 11, 12, 13, 14, 15, ≥16 years of age), age at first oral contraceptive use (never, 13–16, 17–20, 21–24, ≥25 years), body mass index (continuous measure in kg/m2), time since last pregnancy lasting at least 6 months (nulliparous, 1–7, 8–15, ≥16 years), age at first birth (<25, ≥25 years of age), marital status (ever/never), racial/ethnic group (Asian, Black, Latina, White, other), menstrual cycle length and regularity (regular, <40 days and irregular, ≥40 days and irregular, no menstrual periods or missing), and infertility (tried to become pregnant for 1 year without success). With the exceptions of marital status, age at menarche, and race/ethnicity, which were measured at baseline, all covariates were updated every 2 years with the Anderson-Gill data structure as described by Therneau (68). To control for confounding by age, calendar time, and any possible two-way interactions between these two time scales, analyses were stratified jointly by age in months at start of follow-up and calendar year of the current questionnaire cycle (69). Results for the total case group and for hysterectomy-confirmed cases are presented separately. Women with a diastolic blood pressure of less than 65 mmHg in 1989 and who did not use antihypertensive medications were used as the referent group. All relative risks are presented with 95 percent confidence intervals, and reported p values are two sided. Chi-square tests were used for group comparisons. To test for linear trends, all participants in each category were assigned the midpoint value for that category, which was then analyzed as an ordered categorical variable (70). Those in the lowest category of diastolic blood pressure (<65 mmHg) were assigned a value of 60 mmHg, and those in the category of more than 105 mmHg were assigned a value of 110 mmHg. In subanalyses conducted using months of hypertension as the exposure, the diagnosis of hypertension was set to the midpoint of the interval when it was reported.
Interactions between diastolic blood pressure and several covariates (body mass index, race/ethnicity, infertility workup, annual examination) were tested using the likelihood ratio test, which compared nested models with and without multiplicative interaction terms (71). The interaction between body mass index and blood pressure was represented by a cross-product interaction term. The interaction between racial/ethnic group and blood pressure was tested using dummy-coded race/ethnicity categories multiplied by continuous blood pressure.
RESULTS
In 1989, approximately 70 percent of the women in the Nurses’ Health Study II cohort reported a diastolic blood pressure of less than 75 mmHg, and 3 percent of the cohort used antihypertensive medications. Table 1 presents the age-standardized distributions of baseline characteristics of the study population by diastolic blood pressure group. There were several differences in risk factors across blood pressure groups, most importantly, in body mass index and race/ethnicity.
TABLE 1.
Age-adjusted distribution of potential risk factors for uterine leiomyomata according to diastolic blood pressure category at baseline among 104,233 premenopausal women, Nurses’ Health Study II, 1989*
| Systolic blood pressure (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No antihypertensive medication | Antihypertensive medication | |||||||
| <65 | 65–74 | 75–84 | 85–89 | ≥90 | <75 | 75–84 | 85–89 | ≥90 | |
| No. of women (%) | 22,718 (22) | 48,850 (47) | 24,094 (23) | 3,570 (3) | 1,886 (2) | 998 (0.96) | 766 (0.73) | 584 (0.56) | 767 (0.74) |
| Mean age (years) (SD†) | 33.4 (4.8) | 33.7 (4.6) | 34.5 (4.7) | 35.1 (4.7) | 35.7 (4.4) | 35.8 (4.4) | 36.8 (4.1) | 37.1 (4.2) | 36.9 (4.1) |
| Mean body mass index (kg/m2) (SD) | 22 (3) | 23 (4) | 25 (5) | 29 (7) | 29 (8) | 24 (5) | 28 (7) | 30 (8) | 31 (8) |
| Ever married (%) | 88 | 87 | 84 | 80 | 81 | 84 | 79 | 75 | 78 |
| Age at menarche <12 years (%) | 21 | 23 | 25 | 31 | 32 | 28 | 36 | 37 | 35 |
| Ever pregnant (%) | 81 | 79 | 75 | 72 | 72 | 75 | 70 | 69 | 74 |
| Age at first birth <25 years (%) | 27 | 26 | 27 | 28 | 28 | 37 | 31 | 29 | 31 |
| Mean time (months) since last birth (SD)‡ | 63 (55) | 64 (57) | 68 (61) | 74 (63) | 73 (63) | 85 (62) | 82 (65) | 84 (64) | 83 (63) |
| History of infertility (%) | 17 | 17 | 17 | 18 | 19 | 18 | 19 | 18 | 26 |
| Never oral contraceptive use (%) | 17 | 17 | 18 | 19 | 20 | 13 | 17 | 19 | 20 |
| Current oral contraceptive use (%) | 13 | 14 | 15 | 13 | 14 | 13 | 15 | 11 | 10 |
| First oral contraceptive use before age 17 years (%) | 6 | 5 | 5 | 5 | 6 | 9 | 8 | 7 | 7 |
| ≥40-day and irregular menstrual cycle (%) | 7 | 7 | 7 | 8 | 9 | 8 | 10 | 9 | 9 |
| White (%) | 91 | 91 | 91 | 90 | 87 | 92 | 88 | 90 | 84 |
| Black (%) | 1 | 2 | 2 | 3 | 4 | 1 | 5 | 4 | 8 |
| Latina (%) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 |
| Asian (%) | 2 | 2 | 2 | 2 | 3 | 1 | 1 | 1 | 2 |
Percentages shown are directly standardized to the age distribution of the study population at baseline in 5-year intervals.
SD, standard deviation.
Among parous women.
A total of 7,466 incident cases of uterine leiomyomata were diagnosed by ultrasound (n = 5,805) or hysterectomy (n = 1,661) during the 827,348 woman-years of follow-up (1989–1999). The 10-year cumulative incidence for uterine fibroids diagnosed by ultrasound or hysterectomy is 7 percent in this cohort of premenopausal women. As demonstrated in table 2, compared with the risk for women who had a diastolic blood pressure of less than 65 mmHg, the relative risk of uterine fibroids confirmed by ultrasound or hysterectomy increased as diastolic blood pressure rose, regardless of antihypertensive medication use. A statistically significant dose-response association remained between diastolic blood pressure categories greater than or equal to 65 mmHg and fibroid risk when adjusting for multiple risk factors (21, 23, 37), including age at menarche, body mass index, race/ethnicity, age at first birth, years since last term birth, history of infertility, marital status, first oral contraceptive use, and menstrual cycle irregularity (table 2). Cigarette smoking was not associated with uterine leiomyomata incidence and, therefore, was not included in the final model. In separate analyses of cases confirmed by hysterectomy, risk was strongly associated with diastolic blood pressure. In the fully adjusted model, every 10-mmHg increase in blood pressure led to an 8 percent (range: 5–11 percent) and 10 percent (range: 7–13 percent) increase in risk for ultrasound- or hysterectomy-confirmed fibroids among women untreated and treated with antihypertensive medications, respectively. The interaction between antihypertensive medication use and diastolic blood pressure in 1989 was not statistically significant (p = 0.60).
TABLE 2.
Relative risks of uterine leiomyomata confirmed by ultrasound or hysterectomy among premenopausal women according to diastolic blood pressure in 1989, Nurses’ Health Study II, 1989–1999*
| Diastolic blood pressure (mmHg) variable |
No. of woman-years |
No. of cases |
Age-adjusted relative risk† |
95% confidence interval |
Body mass index-adjusted relative risk |
95% confidence interval |
Multivariate relative risk‡ |
95% confidence interval |
|---|---|---|---|---|---|---|---|---|
| Ultrasound-or hysterectomy-confirmed cases | ||||||||
| While not using antihypertensives | ||||||||
| <65 mmHg§ | 184,117 | 1,346 | 1.00 | 1.00 | 1.00 | |||
| 65–74 mmHg | 391,658 | 3,419 | 1.17 | 1.10, 1.25 | 1.15 | 1.08, 1.23 | 1.15 | 1.08, 1.22 |
| 75–84 mmHg | 186,247 | 1,814 | 1.24 | 1.16, 1.34 | 1.19 | 1.11, 1.28 | 1.16 | 1.08, 1.24 |
| 85–89 mmHg | 25,600 | 312 | 1.52 | 1.34, 1.72 | 1.38 | 1.22, 1..57 | 1.30 | 1.14, 1.47 |
| ≥90 mmHg | 13,349 | 170 | 1.53 | 1.31, 1.80 | 1.40 | 1.19, 1.64 | 1.31 | 1.11, 1.54 |
| Relative risk/10-mmHg increase in diastolic blood pressure (%) | 14 | 11–16¶ | 10 | 7–13 | 8 | 5–11 | ||
| While using antihypertensives | ||||||||
| <75 mmHg | 6,715 | 74 | 1.22 | 0.96, 1.54 | 1.15 | 0.91, 1.46 | 1.07 | 0.84, 1.35 |
| 75–84 mmHg | 8,218 | 135 | 1.71 | 1.43, 2.04 | 1.55 | 1.29, 1.86 | 1.42 | 1.18, 1.70 |
| 85–89 mmHg | 5,576 | 95 | 1.79 | 1.45, 2.21 | 1.58 | 1.28, 1.95 | 1.44 | 1.17, 1.79 |
| ≥90 mmHg | 5,867 | 101 | 1.82 | 1.43, 2.23 | 1.59 | 1.29, 1.96 | 1.43 | 1.16, 1.76 |
| Relative risk/10-mmHg increase in diastolic blood pressure while using antihypertensives (%) | 15 | 12–17 | 12 | 9–14 | 10 | 7–13 | ||
| Hysterectomy-confirmed cases | ||||||||
| While not using antihypertensives | ||||||||
| <65 mmHg§ | 184,117 | 299 | 1.00 | 1.00 | 1.00 | |||
| 65–74 mmHg | 391,658 | 714 | 1.09 | 0.95, 1.25 | 1.06 | 0.93, 1.22 | 1.06 | 0.93, 1.22 |
| 75–84 mmHg | 186,247 | 389 | 1.15 | 0.99, 1.34 | 1.07 | 0.92, 1.25 | 1.06 | 0.91, 1.24 |
| 85–89 mmHg | 25,600 | 88 | 1.79 | 1.41, 2.27 | 1.55 | 1.21, 1.98 | 1.49 | 1.17, 1.91 |
| ≥90 mmHg | 13,349 | 33 | 1.22 | 0.85, 1.75 | 1.05 | 0.73, 1.51 | 1.01 | 0.70, 1.45 |
| Relative risk/10-mmHg increase in diastolic blood pressure (%) | 16 | 10–22 | 10 | 4–16 | 8 | 2–14 | ||
| While using antihypertensives | ||||||||
| <75 mmHg | 6,715 | 29 | 1.92 | 1.31, 2.82 | 1.76 | 1.19, 2.58 | 1.59 | 1.08, 2.34 |
| 75–84 mmHg | 8,218 | 45 | 2.26 | 1.65, 3.11 | 1.94 | 1.41, 2.68 | 1.79 | 1.30, 2.47 |
| 85–89 mmHg | 5,576 | 37 | 2.79 | 1.98, 3.94 | 2.29 | 1.61, 3.26 | 2.15 | 1.51, 3.06 |
| ≥90 mmHg | 5,867 | 27 | 1.90 | 1.30, 2.86 | 1.57 | 1.05, 2.35 | 1.46 | 0.98, 2.19 |
| Relative risk/10-mmHg increase in diastolic blood pressure while using antihypertensives (%) | 18 | 12–23 | 13 | 7–19 | 9 | 3–18 | ||
Person-years for all models 5 827,348.
Adjusted for age (months).
Multivariate relative risks are adjusted for age (months), calendar time (months), body mass index (continuous), age at menarche (≥10, 11, 12, 13,14, 15, ≥16 years), age at first oral contraceptive use (never, 13–16, 17–20, 21–24, ≥25 years), time since last birth (nulliparous, 1–7, 8–15, ≥16 years), age at first birth (<25/≥25 years), marital status (ever/never), racial/ethnic group (Black, White, Latina, Asian, other), menstrual cycle length and regularity (regular, <40 days and irregular, ≥40 days and irregular, no periods), and history of infertility (ever/never).
Referent category.
Relative risk/10-mmHg increase in diastolic blood pressure shown with range.
To evaluate potential diagnostic bias (that women evaluated for fibroids were more likely to have their blood pressure checked), we conducted additional analyses controlling for self-reported annual breast or pelvic examination and infertility evaluation in our multivariate model. Although both medical evaluation of infertility and annual breast or pelvic examinations were significant predictors of fibroids diagnosed by ultrasound or hysterectomy, they did not attenuate the strength of the relation between blood pressure and fibroid risk. In multivariate analyses stratified by these variables, the relation between diastolic blood pressure and uterine fibroids was not materially altered, and the likelihood ratio test was not significant (p = 0.27). Therefore, having had a recent gynecologic examination neither confounded nor modified the relation between diastolic blood pressure and uterine leiomyomata.
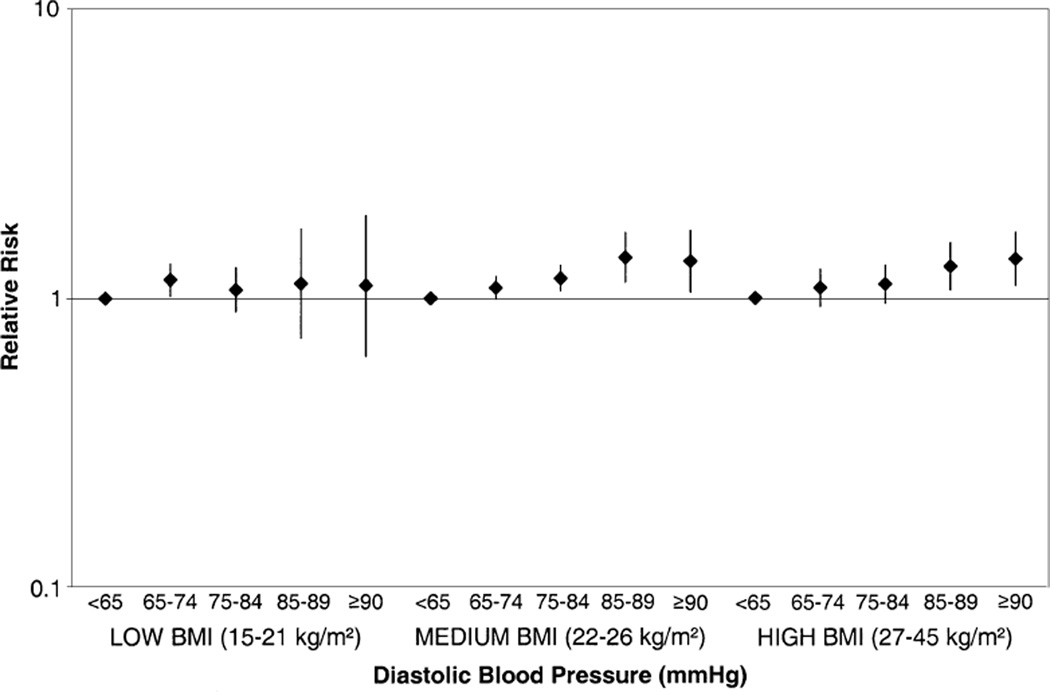
Recognizing that body mass index is also a strong, independent predictor of fibroid risk (23) and that both body mass index and blood pressure contribute to risk for atherosclerosis, we stratified the analysis by body mass index (figure 1). In body mass index-stratified multivariate analyses, the relative risks for continuous diastolic blood pressure were 1.004 (95 percent confidence interval (CI): 0.997, 1.012), 1.009 (95 percent CI: 1.005, 1.014), and 1.008 (95 percent CI: 1.003, 1.013) in the low (15–21 kg/m2), medium (22–26 kg/m2), and high (27–45 kg/m2) body mass index strata, respectively. However, the multiplicative interaction between continuous body mass index and continuous diastolic blood pressure was not statistically significant (p = 0.38).
FIGURE 1.
Relative risk of uterine leiomyomata (1989–1999) according to diastolic blood pressure (1989) and body mass index (BMI) (1989–1997), Nurses’ Health Study II. Multivariate relative risks are adjusted for age (months), calendar time (months), body mass index (continuous), age at menarche (≤10, 11, 12, 13, 14, 15, ≥16 years), age at first oral contraceptive use (never, 13–16, 17–20, 21–24, ≥25 years), time since last birth (nulliparous, 1–7, 8–15, ≥16 years), age at first birth (<25/≥25 years), marital status (ever/never), racial/ethnic group (Black, White, Latina, Asian, other), menstrual cycle length and regularity (regular, <40 days and irregular, ≥40 days and irregular, no periods), and history of infertility (ever/ never). Vertical bars, confidence intervals.
We repeated analyses using diagnosis of hypertension. In comparison with those who had no diagnosis of hypertension, the multivariate relative risks for uterine leiomyomata were 1.24 (95 percent CI: 1.13, 1.40) for all cases and 1.50 (95 percent CI: 1.26, 1.78) for hysterectomy-confirmed cases. Restricting the cohort to women who reported no diagnosis of hypertension in 1989 (n = 95,631), to determine whether more recent onset of hypertension predicted fibroid incidence, did not substantially affect the relative risk estimates. In this subanalysis, the multivariate relative risks for uterine leiomyomata were 1.17 (95 percent CI: 1.02, 1.36) and 1.33 (95 percent CI: 1.05, 1.68) for all cases and hysterectomy-confirmed cases, respectively.
In additional stratified analyses, we modeled the risk of uterine fibroids by years of hypertension (categorized as <2, 2–<5, 5–<10, ≥10 years) in comparison with those who had no diagnosis of hypertension (table 3). There appears to be a nonlinear relation between years of hypertension and fibroid risk. The relative risk for uterine leiomyomata increased with years since diagnosis of hypertension for the first 5–9 years and then leveled off. Finally, we did not observe any effect modification of the diastolic blood pressure-fibroid association in analyses stratified by years of hypertension (likelihood ratio test = 0.56).
TABLE 3.
Relative risks of uterine leiomyomata confirmed by ultrasound or hysterectomy among premenopausal women according to years since diagnosis of hypertension, Nurses’ Health Study II, 1989–1999
| Years since hypertension diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| None | <2 years (<24 months) | 2–<5 years (24–59 months) | 5–<10 years (60–119 months) | ≥10 years (≥120 months) | |||||
| Relative risk |
95% confidence interval |
Relative risk |
95% confidence interval |
Relative risk |
95% confidence interval |
Relative risk |
95% confidence interval |
||
| Ultrasound-or hysterectomy-confirmed cases | |||||||||
| Model 1: age adjusted | 1.00 | 1.37 | 1.15, 1.63 | 1.59 | 1.39, 1.82 | 1.48 | 1.29, 1.70 | 1.47 | 1.30, 1.67 |
| Model 2: age and body mass index adjusted | 1.00 | 1.25 | 1.05, 1.50 | 1.44 | 1.28, 1.65 | 1.33 | 1.16, 1.53 | 1.34 | 1.19, 1.52 |
| Model 3: multivariate adjusted* | 1.00 | 1.19 | 1.00, 1.42 | 1.36 | 1.18, 1.56 | 1.24 | 1.08, 1.43 | 1.25 | 1.10, 1.42 |
| Hysterectomy-confirmed cases | |||||||||
| Model 1: age adjusted | 1.00 | 1.77 | 1.28, 2.45 | 1.91 | 1.47, 2.47 | 2.16 | 1.69, 2.75 | 1.92 | 1.54, 2.39 |
| Model 2: age and body mass index adjusted | 1.00 | 1.56 | 1.13, 2.17 | 1.65 | 1.27, 2.14 | 1.86 | 1.45, 2.38 | 1.68 | 1.34, 2.11 |
| Model 3: multivariate adjusted* | 1.00 | 1.48 | 1.07, 2.06 | 1.56 | 1.20, 2.04 | 1.75 | 1.36, 2.25 | 1.65 | 1.32, 2.07 |
Multivariate relative risks are adjusted for age (months), calendar time (months), body mass index (continuous), age at menarche (≥10, 11, 12, 13, 14, 15, ≥16 years), age at first oral contraceptive use (never, 13–16, 17–20, 21–24, ≥25 years), time since last birth (nulliparous, 1–7, 8–15, ≥16 years), age at first birth (<25/≥25 years), marital status (ever/never), racial/ethnic group (Black, White, Latina, Asian, other), menstrual cycle length and regularity (regular, <40 days and irregular, ≥40 days and irregular, no periods), and history of infertility (ever/never).
We also evaluated the extent to which the association between diastolic blood pressure and risk of incident fibroids was consistent across racial/ethnic groups. Stratifying by race/ethnicity, we found that the association between diastolic blood pressure and fibroids was of similar magnitude among White, non-White Latina, Asian, and Black women. Relative to White women, the global test for a multiplicative interaction between diastolic blood pressure and Black race/ethnicity was not significant by the likelihood ratio test (p = 0.41). In a multivariate model without blood pressure terms, the relative risk for Black women compared with White women was 2.31 (95 percent CI: 2.04, 2.63). Inclusion of terms for diastolic blood pressure and antihypertensive medication use did not materially change the relative risk attributed to Black race/ethnicity (relative risk = 2.27, 95 percent CI: 2.00, 2.58). The relative risk for uterine fibroids among Latina women in comparison with non-Latina White women was 1.22 (95 percent CI: 1.02, 1.45) in the multivariate model that did not include blood pressure. The relative risk was essentially unchanged (relative risk = 1.22, 95 percent CI: 1.03, 1.46) with inclusion of terms for diastolic blood pressure and antihypertensive medication use. In comparison with White women, women of Asian descent did not have a statistically significant increase in risk of fibroids (relative risk = 1.06, 95 percent CI: 0.89, 1.25).
Finally, all of the above analyses were repeated using systolic blood pressure in 1989, stratified by updated antihypertensive medication use. Women with a systolic blood pressure less than or equal to 104 mmHg in 1989 who did not use antihypertensive medications were used as the referent group. A similar dose-response relation was found for high normal and elevated systolic blood pressure and uterine fibroid risk. In the fully adjusted model, the risk of fibroids rose 6 percent (4–8 percent) and 4 percent (2–6 percent) per 10-mmHg increase in systolic blood pressure for women not using and using antihypertensive medication, respectively.
DISCUSSION
To our knowledge, this is the first prospective study of the relation among diastolic (and systolic) blood pressure, antihypertensive medication use, and risk for clinically symptomatic uterine fibroid tumors. These prospective data demonstrate a dose-response relation between diastolic blood pressure and fibroid incidence, with higher blood pressure associated with increased fibroid risk. For each 10-mmHg increase in blood pressure, the multivariate relative risk was elevated 8 percent (range: 5–11 percent) and 10 percent (range: 7–13 percent) among antihypertensive medication nonusers and users, respectively, indicating a sizeable association with blood pressure that is independent of body mass index, oral contraceptive use, and reproductive history. Hypertensive women were 24 percent (range: 11–41 percent) more likely to report fibroids compared with normotensive women. Finally, risk for fibroids increased with duration of hypertension.
These results are consistent with cross-sectional data (55) and with data from a recent case-control study (56). Faerstein et al. (56) found that subjects with hypertension were 1.7 times (95 percent CI: 1.0, 2.8) more likely to have diagnosed fibroids and that risk increased with time since diagnosis of hypertension.
These data support the idea that atherogenesis is a significant component of a multifactorial etiology of uterine fibroid development and/or growth. In 1975, Moss and Benditt (72) first proposed an analogy between atherosclerotic plaque and uterine leiomyomata. Hemodynamic stress (resulting from hypertension) may cause arterial smooth muscle cell injury leading to endothelial dysfunction, increased permeability, migration of smooth muscle cells, and fibrous plaque/fibroid formation. Uterine smooth muscle cells may be injured by a parallel process, which initiates fibroid formation. Fibroids are postulated to arise from uterine myometrium, uterine arteries, or connective tissue (16). Excessive injury to the uterine endometrial lining may promote the monoclonal expansion of uterine smooth muscle cells (4). Observed abnormalities in the structure and function of uterine vasculature in the presence of leiomyomata (73) invite the possibility that direct atherosclerotic injury to uterine blood vessels (74) may play a role. Existing evidence suggests similarities between atherosclerotic plaques and smooth muscle tumor cells in uterine leiomyomata: 1) both appear to have a monoclonal origin; 2) they behave similarly in culture; 3) during toxemia of pregnancy, lipids accumulate in uterine cells, not unlike atheroscleroma (56, 72, 75); and 4) they both display a similar tendency to become fibrotic and calcified (72).
Fibroid tumors are distinguished by an accumulation of extracellular matrix and fibrous connective tissue (76). The overproduction of extracellular matrix, a central component of uterine leiomyomata pathophysiology, may also be related to elevated blood pressure. Transforming growth factor β1 is upregulated in response to tissue injury (77), enhances extracellular matrix production, and reduces its degradation (76, 78). In leiomyomata tissue, transforming growth factor β1 is overexpressed, mitogenic, and fibrogenic (76, 79). Mechanical stress (79), hormones (76), and angiotensin II (80) may activate transforming growth factor β1. Hemodynamic stress, due to elevated blood pressure, may trigger a proinflammatory process that initiates transforming growth factor β1 release and thereby induces the accumulation of extracellular matrix and fibrosis.
Atherosclerotic risk factors, such as hyperinsulinemia, may elevate fibroid risk (5) by stimulating uterine leiomyomata cell growth (81) and mitosis (56) or by altering ovarian hormone regulation. Smoking is an atherosclerotic risk factor that has been inversely associated with uterine leiomyomata risk in other studies (1, 5, 25, 39, 42), although it was not predictive in our analyses. Although inconsistent among premenopausal women, a relation between smoking and estrogen bioavailability has been observed (82–85). Therefore, the antiestrogenic effects of smoking on fibroid risk (41) may outweigh its proatherogenic effects.
It is possible that fibroids may elevate blood pressure. Large fibroids may compress the urinary tract due to mass effect. If so, does the stronger association between diastolic blood pressure and hysterectomy-confirmed cases of fibroids support the reverse causation hypothesis? Larger fibroids may be more likely to be both clinically symptomatic and subsequently treated and confirmed by hysterectomy. However, the prospective evidence provided by this study suggests that elevated blood pressure precedes confirmatory diagnoses of uterine leiomyomata. Furthermore, fibroid risk increases with increasing time since diagnosis of hypertension. Nonetheless, because many fibroids are asymptomatic, it is impossible in this study to establish conclusively that the elevated blood pressure preceded the development of the fibroid.
The primary limitation of this study, shared with all previous studies, is the inability to determine fibroid date of onset. Another limitation is our reliance upon self-reported blood pressure. Neither hypertension nor blood pressure has been validated in this cohort. However, self-reported hypertension was validated and is a good predictor of cardiovascular events among the older Nurses’ Health Study I participants (86). In general, participants of the Nurses’ Health Study cohorts have proven quite accurate in their self-reported data. One advantage of this study is its prospective design; blood pressure was related to fibroid diagnosis in the following 2 years. As there is no reason to suspect that self-reported blood pressure is systematically overreported among women destined to receive future diagnoses of fibroids, any inaccuracies in self-reported blood pressure are likely only to have biased our estimates of the association of blood pressure with uterine leiomyomata toward the null.
A more serious threat to validity is potential detection bias. Women with elevated blood pressure may be more likely to seek regular medical care or may be encouraged to have gynecologic visits, therefore having a higher likelihood of being diagnosed with fibroids. However, the medical record validation study by Marshall et al. (23) found that nearly two thirds of women reporting a fibroid diagnosis also reported symptoms consistent with uterine leiomyomata, which would diminish the magnitude of differential detection, if present. Women who are pregnant, infertile, or using oral contraceptives are more likely to have medical contact and/or to undergo procedures that would lead to incidental detection of fibroids (37). However, all these women are very likely to have had their blood pressure measured. Because we included only those women whose blood pressure had been measured in the previous 2 years, it is unlikely that incidental detection of high blood pressure during an obstetric/gynecologic visit would affect these findings. Finally, controlling for annual breast and/or pelvic examinations and medical evaluation of infertility in additional models did not impact the relation between blood pressure and uterine fibroid risk.
Physicians may choose alternative methods, such as the bimanual examination, for the clinical evaluation of fibroids; however, there is no reason to believe this would vary by diastolic blood pressure level. If elevated blood pressure was associated with symptoms that influenced the detection of leiomyomata, then overestimation of the incidence rate ratio may have occurred. Settnes and Jorgensen (59) hypothesized that hypertension may cause menorrhagia, a common symptom of fibroids. However, there is no evidence to support a relation between hypertension and menorrhagia among premenopausal women (87, 88). Residual confounding by body mass index is unlikely, because we modeled this variable in multiple ways with no significant changes in the relative risk. Additional analyses including fibroids confirmed by pelvic examination only (n = 2,994) did not alter our results.
In summary, these prospective analyses demonstrate a strong and independent association between blood pressure and fibroid risk. Although uterine fibroids are the most common gynecologic tumor and the second largest indication for hysterectomies annually, mechanisms underlying their development remain poorly understood. Reasonable next steps include the following: 1) attempting to replicate these findings using a cohort study with frequent, standardized screening for fibroids and blood pressure; 2) investigating whether enhanced control of blood pressure and/or early detection of hypertension reduces the incidence of and complications associated with uterine fibroids; and 3) conducting a clinical trial to investigate shrinkage of fibroids among women treated with antihypertensive therapy compared with those untreated, using ultrasonography. While currently the most effective therapies available for symptomatic fibroids are surgical (73), this research provides reasonable indication to explore novel approaches to medical management of fibroids, which may reduce associated morbidity and surgery (89). These results offer evidence for a temporal and dose-response association between blood pressure and fibroids, they extend previous work on hypertension and fibroids, and they provide new evidence regarding the impact of proatherogenic chronic disease processes on the reproductive health and the quality of life for premenopausal women.
Abbreviation
- CI
confidence interval
REFERENCES
- 1.Ross R, Pike MC, Vessey MP, et al. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J. 1986;293:359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollenhoven B. Introduction: the epidemiology of uterine leiomyomas. Baillieres Clin Obstet Gynaecol. 1998;12:169–176. doi: 10.1016/s0950-3552(98)80059-x. [DOI] [PubMed] [Google Scholar]
- 3.Newbold RR, DiAugustine RP, Risinger JI, et al. Advances in uterine leiomyoma research: conference overview, summary, and future research recommendations. Environ Health Perspect. 2000;108:769–773. doi: 10.1289/ehp.00108s5769. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SF, Horiszny JA, Leppert P. Epidemiology of uterine leiomyomas. With an etiologic hypothesis. J Reprod Med. 1995;40:595–600. [PubMed] [Google Scholar]
- 5.Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108:821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- 6.Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance—United States, 1980–1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]
- 7.Wilcox L, Koonin LM, Pokras R, et al. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Xhao SZ, Wong JM, Arguelles LM. Hospitalization costs associated with leiomyoma. Clin Ther. 1999;21:563–575. doi: 10.1016/S0149-2918(00)88309-5. [DOI] [PubMed] [Google Scholar]
- 9.Carlson KJ, Nichols DH, Schiff I. Current concepts: indications for hysterectomy. N Engl J Med. 1993;328:856–860. doi: 10.1056/NEJM199303253281207. [DOI] [PubMed] [Google Scholar]
- 10.Coronado GD, Marshall LM, Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a populations-based study. Obstet Gynecol. 2000;95:764–769. doi: 10.1016/s0029-7844(99)00605-5. [DOI] [PubMed] [Google Scholar]
- 11.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women’s Health Study. II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566–572. doi: 10.1097/00006250-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kjerulff KH, Langenberg P, Seidman JD, et al. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–490. [PubMed] [Google Scholar]
- 13.Haney AF. Clinical decision making regarding leiomyomata: what we need in the next millenium. Environ Health Perspect. 2000;108:835–839. doi: 10.1289/ehp.00108s5835. [DOI] [PubMed] [Google Scholar]
- 14.Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–455. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 15.Wegienka G, Baird DD, Hertz-Picciotto I, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431–437. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- 16.Moore JG. Benign diseases of the uterine corpus. In: Hacker NF, Moore JG, editors. Essentials of obstetrics and gynecology. Philadelphia, PA: WB Saunders Company; 1998. pp. 412–420. [Google Scholar]
- 17.Day Baird D, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 18.Borgfeldt C, Andolf E. Transvaginal ultrasonographic findings in the uterus and the endometrium: low prevalence of leiomyoma in a random sample of women age 25–40 years. Acta Obstet Gynecol Scand. 2000;79:202–207. [PubMed] [Google Scholar]
- 19.Luoto R, Kaprio J, Rutanen EM, et al. Heritability and risk factors of uterine fibroids—the Finnish Twin Cohort Study. Maturitas. 2000;37:15–26. doi: 10.1016/s0378-5122(00)00160-2. [DOI] [PubMed] [Google Scholar]
- 20.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 21.Marshall L, Spiegelman D, Barbieri R, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 22.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153:1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Marshall L, Spiegelman D, Manson J, et al. Risk of uterine leiomyomata among premenopausal women in relation to body size and cigarette smoking. Epidemiology. 1998;9:511–517. [PubMed] [Google Scholar]
- 24.Okoronkwo MO. Body weight and uterine leiomyomas among women in Nigeria. West Afr J Med. 1999;18:52–54. [PubMed] [Google Scholar]
- 25.Lumbiganon P, Rugpao S, Phandhu-fung S, et al. Protective effect of depot-medroxyprogesterone acetate on surgically treated uterine leiomyomas: a multicentre case-control study. Br J Obstet Gynaecol. 1996;103:909–914. doi: 10.1111/j.1471-0528.1996.tb09911.x. [DOI] [PubMed] [Google Scholar]
- 26.Sato F, Nishi M, Kudo R, et al. Body fat distribution and uterine leiomyomas. J Epidemiol. 1998;8:176–180. doi: 10.2188/jea.8.176. [DOI] [PubMed] [Google Scholar]
- 27.Chiaffarino F, Parazzini F, La Vecchia C, et al. Diet and uterine myomas. Obstet Gynecol. 1999;94:395–398. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- 28.Wong F, Yamada M, Sasaki H, et al. Noncancer disease incidence in the atomic bomb survivors: 1958–1986. Radiat Res. 1993;135:418–430. [PubMed] [Google Scholar]
- 29.Kawamura S, Kasagi F, Kodama K, et al. Prevalence of uterine myoma detected by ultrasound examination in the atomic bomb survivors. Radiat Res. 1997;147:753–758. [PubMed] [Google Scholar]
- 30.Van Voorhis BJ, Romitti PA, Jones MP. Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J Reprod Med. 2002;47:663–669. [PubMed] [Google Scholar]
- 31.Vikhlyaeva E, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995;51:127–131. doi: 10.1016/0020-7292(95)02533-i. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz S, Voigt L, Tickman E, et al. Familial aggregation of uterine leiomyomata. Am J Epidemiol. 2000;151(suppl):S10. (Abstract). [Google Scholar]
- 33.Gross K, Morton CC. Genetics and the development of fibroids. Clin Obstet Gynecol. 2001;44:335–349. doi: 10.1097/00003081-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Briggs ND, John CT, Ufomadu L. Is fibroid familial? (Letter) Trop Doct. 1993;23:181. doi: 10.1177/004947559302300421. [DOI] [PubMed] [Google Scholar]
- 35.Sato F, Mori M, Nishi M, et al. Familial aggregation of uterine myomas in Japanese women. J Epidemiol. 2002;12:249–253. doi: 10.2188/jea.12.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treloar S, Martin NG, Dennerstein L, et al. Pathways to hysterectomy: insights from longitudinal twin research. Am J Obstet Gynecol. 1992;167:82–86. doi: 10.1016/s0002-9378(11)91632-9. [DOI] [PubMed] [Google Scholar]
- 37.Marshall LM, Spiegelman D, Goldman M, et al. A prospective study of reproductive factors and oral contraceeptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 38.Sato F, Miyake H, Nishi M, et al. Early normal menstrual cycle pattern and the development of uterine leiomyomas. JWomens Health Gend Based Med. 2000;9:299–302. doi: 10.1089/152460900318489. [DOI] [PubMed] [Google Scholar]
- 39.Samadi AR, Lee NC, Flanders WD, et al. Risk factors for selfreported uterine fibroids: a case-control study. Am J Public Health. 1996;86:858–862. doi: 10.2105/ajph.86.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parazzini F, Negri E, La Vecchia C, et al. Reproductive factors and risk of uterine fibroids. Epidemiology. 1996;7:440–442. doi: 10.1097/00001648-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull. 1996;52:58–73. doi: 10.1093/oxfordjournals.bmb.a011533. [DOI] [PubMed] [Google Scholar]
- 42.Parazzini F, Negri E, La Vecchia C, et al. Uterine myomas and smoking. Results from an Italian study. J Reprod Med. 1996;41:316–320. [PubMed] [Google Scholar]
- 43.Walker CL, Cesen-Cummings K, Houle C, et al. Protective effect of pregnancy for development of uterine leiomyoma. Carcinogenesis. 2001;22:2049–2052. doi: 10.1093/carcin/22.12.2049. [DOI] [PubMed] [Google Scholar]
- 44.Chen CR, Buck GM, Courey NG, et al. Risk factors for uterine fibroids among women undergoing tubal sterilization. Am J Epidemiol. 2001;153:20–26. doi: 10.1093/aje/153.1.20. [DOI] [PubMed] [Google Scholar]
- 45.Baird D, Danson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz SM. Invited commentary: studying the epidemiology of uterine leiomyomata—past, present, and future. Am J Epidemiol. 2001;153:27–29. doi: 10.1093/aje/153.1.27. [DOI] [PubMed] [Google Scholar]
- 47.Summers W, Watson RL, Wooldridge WH, et al. Hypertension, obesity and fibromyomata uteri, as a syndrome. Arch Intern Med. 1971;128:750–754. [PubMed] [Google Scholar]
- 48.Hocutt J. Uterine fibroids and hypertension. Del Med J. 1979;51:697–699. [PubMed] [Google Scholar]
- 49.Lowell H, Miall WE, Stewart DB. Arterial blood pressure in Jamaican women with and without fibroids. West Indian Med J. 1966;15:45–51. [PubMed] [Google Scholar]
- 50.Clinch J, Sweetnam P. Fibroids and hypertension. J Obstet Gynaecol Br Commonw. 1968;75:1052–1053. doi: 10.1111/j.1471-0528.1968.tb02880.x. [DOI] [PubMed] [Google Scholar]
- 51.Everett H, Scott RB. The possible etiologic role of gynecologic lesions in the production of hypertension. Am J Obstet Gynecol. 1942;44:1010–1025. [Google Scholar]
- 52.Moehlig R. Association of uterine fibromyomas with other clinical conditions. J Clin Endocrinol. 1942;2:219–222. [Google Scholar]
- 53.Aboyeji AP, Ijaiya MA. Uterine fibroids: a ten-year clinical review in Ilorin, Nigeria. Niger J Med. 2002;11:16–19. [PubMed] [Google Scholar]
- 54.Emembolu JO. Uterine fibromyomata: presentation and management in northern Nigeria. Int J Gynaecol Obstet. 1987;25:413–416. doi: 10.1016/0020-7292(87)90349-3. [DOI] [PubMed] [Google Scholar]
- 55.Luoto R, Rutanen E, Auvinen A. Fibroids and hypertension: a cross-sectional study of women undergoing hysterectomy. J Reprod Med. 2001;46:359–364. [PubMed] [Google Scholar]
- 56.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153:11–19. doi: 10.1093/aje/153.1.11. [DOI] [PubMed] [Google Scholar]
- 57.Koepsell TD, Weiss NS, Thompson DJ, et al. Prevalence of prior hysterectomy in the Seattle-Tacoma area. Am J Public Health. 1980;70:40–47. doi: 10.2105/ajph.70.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luoto R, Kaprio J, Reunanen A, et al. Cardiovascular morbidity in relation to ovarian function after hysterectomy. Obstet Gynecol. 1995;85:515–522. doi: 10.1016/0029-7844(94)00456-N. [DOI] [PubMed] [Google Scholar]
- 59.Settnes A, Jorgensen T. Hypertension and hysterectomy in Danish women. Obstet Gynecol. 1998;92:274–280. doi: 10.1016/s0029-7844(98)00171-9. [DOI] [PubMed] [Google Scholar]
- 60.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 61.Stamler J. Blood pressure and high blood pressure: aspects of risk. Hypertension. 1991;18:I95–I107. doi: 10.1161/01.hyp.18.3_suppl.i95. [DOI] [PubMed] [Google Scholar]
- 62.Benetos A, Thomas F, Bean K, et al. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med. 2002;162:577–581. doi: 10.1001/archinte.162.5.577. [DOI] [PubMed] [Google Scholar]
- 63.Kannel W, Gordon T, Schwartz MJ. Systolic versus diastolic blood pressure and risk of coronary heart disease: the Framingham Study. Am J Cardiol. 1971;27:335–345. doi: 10.1016/0002-9149(71)90428-0. [DOI] [PubMed] [Google Scholar]
- 64.Kannel W, Dawber TR, McGee DL. Perspectives on systolic hypertension: the Framingham study. Circulation. 1980;61:1179–1182. doi: 10.1161/01.cir.61.6.1179. [DOI] [PubMed] [Google Scholar]
- 65.Izzo JL, Jr, Levy D, Black HR. Clinical advisory statement. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021–1024. doi: 10.1161/01.hyp.35.5.1021. [DOI] [PubMed] [Google Scholar]
- 66.Franklin S, Martin GL, Kahn SA, et al. Does the relation of blood pressure to coronary heart disease change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 67.Colditz G, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 68.Therneau T. Extending the Cox model. In: Lin D, Fleming TR, editors. Proceedings of the First Seattle Symposium in Biostatistics: survival analysis. New York, NY: Springer Verlag; 1997. pp. 51–84. [Google Scholar]
- 69.Hertzmark E, Spiegleman D. The SAS MPHREG macro. Boston, MA: Channing Laboratory; 2001. [Google Scholar]
- 70.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 71.Hosmer DW, Lemeshow S. Applied logistic regression. New York, NY: John Wiley & Sons, Inc; 1989. [Google Scholar]
- 72.Moss N, Benditt EP. Human atherosclerotic plaque cells and leiomyoma cells. Am J Pathol. 1975;78:175–190. [PMC free article] [PubMed] [Google Scholar]
- 73.Nowak RA. Novel therapeutic strategies for leiomyomas: targeting growth factors and their receptors. Environ Health Perspect. 2000;108:849–853. doi: 10.1289/ehp.00108s5849. [DOI] [PubMed] [Google Scholar]
- 74.Crawford BS, Davis J, Harrigill K. Uterine artery atherosclerotic disease: histologic features and clinical correlation. Obstet Gynecol. 1997;90:210–215. doi: 10.1016/S0029-7844(97)00225-1. [DOI] [PubMed] [Google Scholar]
- 75.Haust M, Las Heras J, Harding P. Fat-containing uterine smooth muscle cells in “toxemia”: possible relevance to atherosclerosis? Science. 1977;195:1353–1354. doi: 10.1126/science.841334. [DOI] [PubMed] [Google Scholar]
- 76.Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 77.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 78.Kuwahara F, Kai H, Tokuda K, et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 79.Arici A, Sozen I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-beta1 in human myometrium and leiomyoma. Am J Obstet Gynecol. 2003;188:76–83. doi: 10.1067/mob.2003.118. [DOI] [PubMed] [Google Scholar]
- 80.Fukuda N, Hu WY, Kubo A, et al. Abnormal regulation of transforming growth factor-beta receptors on vascular smooth muscle cells from spontaneously hypertensive rats by angiotensin II. Hypertension. 1998;31:672–677. doi: 10.1161/01.hyp.31.2.672. [DOI] [PubMed] [Google Scholar]
- 81.Cramer SF, Robertson AL, Ziats NP, et al. Growth potential of human uterine leiomyomas: some in vitro observations and their implications. Obstet Gynecol. 1985;66:36–41. [PubMed] [Google Scholar]
- 82.Longcope C, Johnston CC., Jr Androgen and estrogren dynamics in pre- and post-menopausal women: a comparison between smokers and nonsmokers. J Clin Endocrinol Metab. 1988;67:379–383. doi: 10.1210/jcem-67-2-379. [DOI] [PubMed] [Google Scholar]
- 83.MacMahon B, Trichopoulos D, Cole P, et al. Cigarette smoking and urinary estrogens. N Engl J Med. 1982;307:1062–1065. doi: 10.1056/NEJM198210213071707. [DOI] [PubMed] [Google Scholar]
- 84.Westhoff C, Bentile G, Lee J, et al. Predictors of ovarian steroid secretion in reproductive-age women. Am J Epidemiol. 1996;144:381–388. doi: 10.1093/oxfordjournals.aje.a008939. [DOI] [PubMed] [Google Scholar]
- 85.Zumoff B, Miller EH, Heinz U, et al. The effect of smoking on serum progesterone, estradiol, and luteinizing hormone levels over a menstraul cycle in normal women. Steroids. 1990;55:507–511. doi: 10.1016/0039-128x(90)90089-t. [DOI] [PubMed] [Google Scholar]
- 86.Fiebach N, Hebert PR, Stampfer MJ, et al. A prospective study of high blood pressure and cardiovascular disease in women. Am J Epidemiol. 1989;130:646–654. doi: 10.1093/oxfordjournals.aje.a115386. [DOI] [PubMed] [Google Scholar]
- 87.Anastasiadis PG, Koutlaki NG, Skaphida PG, et al. Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol. 2000;21:180–183. [PubMed] [Google Scholar]
- 88.Reslova T, Tosner J, Resl M, et al. Endometrial polyps. A clinical study of 245 cases. Arch Gynecol Obstet. 1999;262:133–139. doi: 10.1007/s004040050241. [DOI] [PubMed] [Google Scholar]
- 89.Baird DD. Invited commentary: uterine leiomyomata—we know so little but could learn so much. Am J Epidemiol. 2004;159:124–126. doi: 10.1093/aje/kwh017. [DOI] [PubMed] [Google Scholar]