Summary
Background
While laboratory aetiological diagnosis is considered the gold standard for diagnosis and management of sexually transmitted infections, syndromic management has been presented as a simplified and affordable approach for sexually transmitted infection management in limited resource settings.
Methods
Sexually transmitted infection signs and symptoms were collected using staff-administered computer-assisted personal interview and audio computer-assisted self-interview. Participants underwent a medical examination and laboratory testing for common sexually transmitted infections. The performance of syndromic management was assessed on the agreement between interviewing methods as well as accurate diagnosis.
Results
We screened 846 participants, of whom 88 (10.4%) received syndromic sexually transmitted infection diagnosis while 272 (32.2%) received an aetiological diagnosis. Agreement between syndromic and aetiological diagnoses was very poor (overall kappa = 0.09). The most prevalent sexually transmitted infection was herpes simplex virus type 2 and the percentage of persons with any sexually transmitted infection was higher among women (48.6%) than men (15.6%, p <0.0001). Agreement between audio computer-assisted self-interview and computer-assisted personal interview interviewing methods for syndromic diagnosis of sexually transmitted infections ranged from poor to good.
Conclusion
Our findings suggest that syndromic management of sexually transmitted infections is not a sufficient tool for sexually transmitted infection diagnosis in this setting; development and improvement of sexually transmitted infection diagnostic capabilities through laboratory confirmation is needed in resource-limited settings.
Keywords: Kenya, sexually transmitted infection, syndromic management, Kisumu
Introduction
The World Health Organization (WHO) estimates that more than 448 million incident cases of curable sexually transmitted infections (STIs) (syphilis, gonorrhoea, chlamydia and trichomoniasis) occur annually throughout the world. Addition of herpes simplex virus type 2 (HSV-2) to this tally will significantly increase this number.1–3 Sub-Saharan Africa continues to bear the greatest burden of these STIs.4–6 STIs are an important cause of morbidity and mortality among African populations, resulting in adverse birth outcomes, neonatal and infant infections, ectopic pregnancy, anogenital cancer, infertility, pelvic inflammatory disease and death.3,7–9 STIs may also facilitate the transmission of HIV by augmenting HIV infectiousness and HIV susceptibility via a variety of biological mechanisms.10–17 STI diagnosis and treatment needs to be prompt and accurate to effectively control their spread and to minimise associated morbidity and mortality.9
The proper management of STIs should include strategies for screening patients, partner notification, the administration of mono-dose and simplified therapies to improve compliance and increasing the accessibility of services.18 The introduction of new diagnostic tools and algorithms has improved STI management considerably. The WHO provides recommendations on these diagnostic tools and algorithms that different regions and countries may adapt.3,8 Aetiological diagnosis using laboratory confirmation is considered the gold standard for the diagnosis and management of STIs19; however, it is not always a practical strategy and may result in delayed treatment and increased loss to follow-up. Moreover, many health care facilities in developing countries lack the equipment and trained personnel required to perform aetiological diagnosis of STIs.
To overcome this problem, a syndrome-based approach to the management of STI patients has been developed and promoted in a large number of countries in the developing world as a simplified and affordable approach for the diagnosis of STIs. The syndromic management approach is based on the identification of consistent groups of clinical symptoms and easily recognised signs (syndromes), and the provision of treatment that will deal with the majority or the most serious, organisms responsible for producing a syndrome. The WHO developed a simplified algorithm to guide health workers in the implementation of syndromic management of STIs and in 2003, the WHO guidelines were revised to focus exclusively on syndromic management.8 Kenya, along with other countries, adapted these guidelines for the national programme.20,21
Ideally, syndromic management removes the need for laboratory testing and extra clinic visits for follow-up, which may result in treatment delays3 and to some extent the need for a physical examination.22 This approach is viewed as a practical strategy for use in resource-limited settings as it provides prompt treatment and helps avoid potential loss to follow-up observed in STI management involving laboratory-based diagnosis.23 Moreover, the practice may be necessary in cases where pathogen detection is difficult such as with upper genital tract infections.22
The literature has shown that the syndromic approach to the management of STIs can be effective16,24–26; however, most of these evaluations have focused on syndromic STI management within STI clinics. In this context, the probability of having an STI is relatively high because patients often self-select and present for evaluation with STI-specific signs and symptoms. By contrast, there are few evaluations of syndromic STI management within research contexts in which all study participants are routinely asked questions about STI-specific signs and symptoms. Because syndromic management is also carried out within a research context, it is important to determine if it is a sufficient tool for diagnosing STIs or whether it must be paired with laboratory testing.
In January 2007, the Kenya Medical Research Centre (KEMRI) in collaboration with the Centers for Disease Control and Prevention (CDC) initiated an HIV incidence cohort study to prepare for future community-based HIV vaccine or other prevention trials among young adults in Kisumu. We utilised data from the screening visit for this cohort to evaluate if the syndromic management of STIs is a sufficient tool for the diagnosis and management of STIs in a research setting and to compare the methods for the collection of symptom data.
Methods
Study population and procedures
Between January 2007 and March 2009, 1277 individuals were screened and 846 were enrolled into the Kisumu Incidence Cohort Study (KICoS), an observational prospective cohort study to estimate the incidence of HIV seroconversion and to identify determinants of successful recruitment and retention.27 Adults 18–34 years of age, residents of Kisumu, HIV-negative at baseline, not pregnant (women only), and reported having had sexual intercourse at least once in the past three months were eligible for study participation.
Demographic and behavioural information were collected from screened participants using both staff-administered computer-assisted personal interview (CAPI) and participant self-administered audio computer-assisted self-interview (ACASI). While STI signs were detected by a clinician and documented in CAPI, symptoms were collected through both ACASI and CAPI. In both interviews, participants were asked whether they had symptoms of vaginal or urethral discharge, lower abdominal or scrotal pain or genital ulcers. Following the CAPI interview, a clinician performed a physical examination for signs to match the collected symptoms. Blood, urine and vaginal swab samples were collected for laboratory testing for gonorrhoea, chlamydia, syphilis and HSV-2, regardless of symptoms or signs. In addition, rapid HIV testing with pre- and post-test counselling was also done for all participants. An appointment was scheduled two weeks thereafter to deliver laboratory STI results.
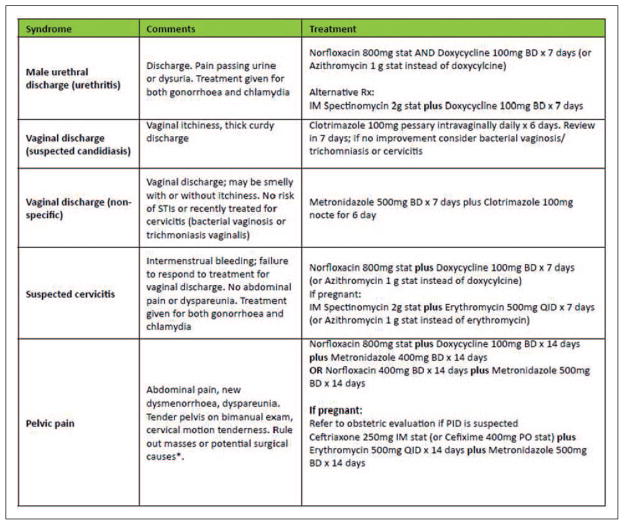
Participants were diagnosed at the screening visit and given immediate treatment using the Kenya national guidelines on syndromic management for STIs (Figure 1).21 Definitive treatment was offered as necessary when laboratory results became available. Participants receiving syndromic or definitive treatment also received contact treatment cards to give to their sexual partners. These contact cards had no identifying information and were coded with names of animals and fruits to correlate with STI syndromes or diagnoses. Sexual partners were required to present these cards to the clinic to receive anonymous presumptive treatment services.
Figure 1.
Summary of syndromic management of STIs.21
Syphilis testing was performed using BD Micro-Vue™ RPR (rapid plasma reagin) Card test and all reactive tests confirmed by Serodia® TP-PA Syphilis Test. HSV-2 serology was tested using KALON® HSV-2 IgG enzyme-linked immunoassay and infection with Chlamydia trachomatis or Neisseriae gonorrhoeae was evaluated by qualitative polymerase chain reaction using COBAS® AMPLICOR CT/NG (Roche). Real-time parallel rapid HIV testing was conducted using Trinity Biotech® Uni-Gold HIV-1/2, and Abbott Labs® Determine HIV-1/2; [tie breaker with Meridian Life Science® Bioline].
Ethical approval
This study was approved by the KEMRI Scientific Steering Committee and Ethical Review Committee and the CDC Institutional Review Board. All participants provided written informed consent to screen for eligibility and take part in the study in one of the three languages of their preference: English, Dholuo or Swahili. Participants received a standard transport reimbursement of KES 300 (USD 3.50). In addition, they received treatment for STIs and other common ailments as well as provision of condoms (male and female).
Measures
Syndromic diagnosis for urethritis/vaginitis/cervicitis was based on having urethral discharge and self-reported scrotal pain for men, and vaginal discharge and/or pruritus and self-reported lower abdominal pain for women. Genital ulcer disease (GUD) was based on reports of ulcers in the genitalia for both men and women. For ascribing syndromic diagnoses to aetiological diagnosis, urethritis, cervicitis, lower abdominal pain and scrotal pain were attributed to gonorrhoea and chlamydia, while vaginitis was attributed to Candida and trichomonas. GUD was ascribed to chancroid, syphilis and HSV-2. Syndromic diagnoses were based on signs and symptoms collected in CAPI but not ACASI, while aetiological diagnoses were based on laboratory testing.
Data analysis
Summary statistics were used to describe the sociodemographic characteristics of participants. We compared different groups with chi square statistics and calculated the syndromic and aetiological prevalence of STIs with 95% confidence intervals. We computed a kappa coefficient28,29 to evaluate the agreement between reporting symptoms of STIs between ACASI and CAPI as well as that of STI diagnoses by syndromic management versus a laboratory-based diagnosis. We also computed the positive and negative predictive values (NPVs) for STI diagnosis using laboratory-based aetiological diagnosis as the gold standard. Data analysis was performed using SAS version 9.2 (SAS, Cary, North Carolina, USA).
Results
Demographic characteristics
The 846 participants screened for enrolment in KICoS had a median age of 23 years; almost two-thirds (62.7%) were 20–24 years of age. Half (50.1%) were women, and the majority were Christians (81.4%), had never been married (61.2%), and had either secondary or post-secondary educational attainment levels (70%) (Table 1).
Table 1.
Demographic characteristics of participants completing KICoS screening in Kisumu, Kenya (2007–2008)
| Characteristic | n/Na | Percentage |
|---|---|---|
| Gender | ||
| Men | 422/846 | 49.9 |
| Women | 424/846 | 50.1 |
| Age group (years) | ||
| 18–19 | 105/846 | 12.4 |
| 20–24 | 530/846 | 62.7 |
| 25–29 | 149/846 | 17.6 |
| 30–34 | 62/846 | 7.3 |
| Marital status | ||
| Single/never married | 515/842 | 61.2 |
| Married/not married but living as married | 286/842 | 34.0 |
| Separated/divorced/widowed | 41/842 | 4.9 |
| Ethnic group or tribe | ||
| Luo | 709/845 | 83.9 |
| Luhya | 80/845 | 9.5 |
| Kisii | 33/845 | 3.9 |
| Kikuyu/maasai/other | 23/845 | 2.7 |
| Religion | ||
| Roman catholic | 318/846 | 37.6 |
| Protestant or other Christian | 370/846 | 43.8 |
| Muslim/nomiya/other | 132/846 | 15.6 |
| No religion | 25/846 | 3.0 |
| Highest education level | ||
| Never attended school/primary school | 254/842 | 30.2 |
| Secondary school | 311/842 | 36.9 |
| Post-secondary school | 277/842 | 32.9 |
| Occupation | ||
| Salaried worker | 22/841 | 2.6 |
| Self-employed/casual worker | 263/841 | 31.3 |
| Students, not otherwise employed | 279/841 | 33.2 |
| Other (includes farmer) | 54/841 | 6.4 |
| Not employed (includes homemaker) | 223/841 | 26.5 |
| Lifetime number of sexual partners | ||
| 0–1 partner | 111/808 | 13.7 |
| 2–3 partners | 269/808 | 33.3 |
| ≥ 4 partners | 428/808 | 53.0 |
Sample sizes fluctuate slightly for some variables due to missing data. Some percentages do not sum to 100 due to rounding.
STI prevalence
Overall, 10.4% (n = 88) of participants were diagnosed with an STI through the clinician-based syndromic diagnosis compared to 32.2% (n = 272) who received an aetiological STI diagnosis through laboratory confirmation. The prevalence of STIs was greater among women than men for both the syndromic diagnosis (17.2% vs. 3.6%, p <0.0001) and the aetiological diagnosis (48.6% vs. 15.6%, p <0.0001). Based on the aetiological diagnosis, HSV-2 was the most prevalent STI (29.1%) followed by chlamydia (2.8%), gonorrhoea (2.4%) and syphilis (1.7%). Lower abdominal/ scrotal pain was the most prevalent syndrome (5.9%) followed by vaginal/urethral discharge (5.3%) and GUD (1.7%). Among the 272 participants with a laboratory-diagnosed STI, 30 (11.0%) had two STIs while one (0.4%) had three STI co-infections at the time of diagnosis (Table 2). Overall, HIV prevalence was 14.5% with a disproportionate rate of infection among women (21.2%) compared to men (7.8%, p <0.0001).
Table 2.
Prevalence of STIs among persons screened for the Kisumu Incidence Cohort Study, Kisumu, Kenya, 2007–2008.
| Overall
|
Men
|
Women
|
Chi square p | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Syndromic prevalencea | |||||||
| Overall | 88 | 10.4 | 15 | 3.6 | 73 | 17.2 | <0.0001 |
| Vaginal/urethral discharge | 45 | 5.3 | 7 | 1.7 | 38 | 9.0 | <0.0001 |
| Genital ulcer disease | 14 | 1.7 | 7 | 1.7 | 7 | 1.7 | 1.0000 |
| Lower abdominal/scrotal pain | 50 | 5.9 | 6 | 1.4 | 44 | 10.4 | <0.0001 |
| Aetiological prevalenceb | |||||||
| Overall | 272 | 32.2 | 66 | 15.6 | 206 | 48.6 | <0.0001 |
| HSV-2 | 246 | 29.1 | 56 | 13.3 | 190 | 44.8 | <0.0001 |
| Gonorrhoea | 20 | 2.4 | 0 | 0.0 | 20 | 4.7 | <0.0001 |
| Chlamydia | 24 | 2.8 | 12 | 2.8 | 12 | 2.8 | 1.0000 |
| Syphilis | 14 | 1.7 | 3 | 0.7 | 11 | 2.6 | 0.0554 |
| Aetiological prevalence of STI co-infectionb,c | |||||||
| Two STIs | 30 | 11.0 | 5 | 7.6 | 25 | 12.1 | – |
| Three STIs | 1 | 0.4 | 0 | 0.0 | 1 | 0.5 | – |
Diagnoses based on the signs and symptoms collected through CAPI.
Diagnoses based on laboratory testing.
HSV-2 was one of the STIs in all co-infections.
STI: sexually transmitted infection; HSV-2: herpes simplex virus type 2.
Comparison of ACASI versus CAPI
The performance of syndromic management was assessed on both the agreement between interviewing methods as well as accurate diagnosis. The agreement between the ACASI and CAPI methods of interviewing for the syndromic diagnosis of STIs varied, with agreement on the diagnosis of GUD and vaginal/urethral discharge at kappas of 0.26 and 0.34, respectively, and for that of lower abdominal/scrotal pain at kappa of 0.65. More participants reported symptoms in ACASI compared to CAPI, with 8.1% reporting lower abdominal/scrotal pain in ACASI compared to 4.9% (p <0.0001) in CAPI. More participants also reported genital ulcers (7.0%) and vaginal/urethral discharge (4.6%) through ACASI than CAPI (2.3%, p <0.0001 and 1.4, p <0.0001, respectively) (Table 3).
Table 3.
Comparison between the symptoms collected through ACASI and CAPI among persons screened for the Kisumu Incidence Cohort Study, Kisumu, Kenya, 2007–2008.a
| Symptoms | ACASI
|
CAPI
|
Chi square p | Kappa coefficient (95% CI) | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Vaginal/urethral discharge | 38 | 4.6 | 12 | 1.4 | <0.0001 | 0.34 (0.20–0.48) |
| Genital ulcer | 58 | 7.0 | 19 | 2.3 | <0.0001 | 0.26 (0.10–0.43) |
| Lower abdominal/scrotal pain | 67 | 8.1 | 41 | 4.9 | <0.0001 | 0.65 (0.54–0.75) |
This analysis reflects a comparison of the symptoms (not signs) collected through ACASI and CAPI.
ACASI: audio computer-assisted self-interview; CAPI: computer-assisted personal interview; CI: confidence interval.
Performance of syndromic management
The agreement between the clinician-based syndromic diagnosis and the laboratory aetiological diagnosis had an overall kappa of 0.09. This was similar even when we dropped HSV-2 and syphilis from the model. The positive predictive value (PPV) of syndromic diagnosis was 50.6% overall, with syphilis being the lowest at 0.0% and HSV-2 being the highest at 63.6%. However, when we dropped HSV-2 and syphilis, the overall PPV dropped to 11.3%. The NPV was 67.2% overall, with HSV-2 being the lowest at 69.0% and syphilis being the highest at 98.3%. However, the overall NPV increased to 95.7% when we dropped HSV-2 and syphilis from the model (Table 4). HSV-2 was severely underdiagnosed using the syndromic management approach (1.4%) as compared to laboratory-based diagnoses (29.1%, p = 0.04), while gonorrhoea and chlamydia were overdiagnosed using syndromic management (9.5% for both) compared to laboratory-based diagnoses (2.4%, p = 0.008 and 2.8%, p = 0.27, respectively). The same number of syphilis diagnoses occurred with each method (n = 14), however, not among the same 14 participants (Table 4).
Table 4.
Comparison between syndromic and aetiological diagnoses among persons screened for the Kisumu Incidence Cohort Study, Kisumu, Kenya, 2007–2008
| STI | Syndromic diagnosisa
|
Aetiological diagnosisb
|
Chi square p | Kappa coefficient (exact 95% CI) | PPV (exact 95% CI) | NPV (exact 95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Overall | 83 | 10.6 | 272 | 34.2 | 0.0021 | 0.09 (0.03, 0.15) | 50.6 (39.4–61.8) | 67.2 (63.6–70.7) |
| Overall minus HSV-2 and syphilis | 80 | 9.5 | 42 | 5.0 | 0.0128 | 0.09 (−0.0001, 0.1764) | 11.3 (5.3, 20.3) | 95.7 (94.0, 97.0) |
| HSV-2 | 11 | 1.4 | 246 | 31.5 | 0.0425 | 0.03 (−0.0013, 0.06) | 63.6 (30.8–89.1) | 69.0 (65.6–72.2) |
| Gonorrhoea | 80 | 9.5 | 20 | 2.4 | 0.0078 | 0.09 (−0.0004, 0.17) | 7.5 (2.8–15.6) | 98.2 (97.0–99.0) |
| Chlamydia | 80 | 9.5 | 24 | 2.8 | 0.2734 | 0.03 (−0.04, 0.11) | 5.0 (1.4–12.3) | 97.4 (96.0–98.4) |
| Syphilis | 14 | 1.7 | 14 | 1.7 | 1.0000 | −0.02 (−0.02, −0.01) | 0.0 (0.0–23.2) | 98.3 (97.2–99.1) |
Diagnoses based on the signs and symptoms collected through CAPI.
Diagnoses based on laboratory testing.
PPV: positive predictive value; NPV: negative predictive value; HSV-2: herpes simplex virus type 2; CI: confidence interval; STI: sexually transmitted infection.
Partner notification and treatment
A total of 54 participants, eight men and 46 women, were issued contact tracing cards following their syndromic management diagnosis. Among these participants, only one partner showed up for contact evaluation and treatment. Of the 272 participants issued with contact tracing cards following aetiological diagnosis, one partner presented for evaluation and treatment.
Discussion
These data show that the aetiological prevalence of STIs is very high among persons screened for KICoS. HSV-2 was the most prevalent STI, found across all STI co-infections observed in the study. The majority of STI infections (75.7%) were found in women, consistent with findings from other studies conducted in the same general geographical location.5,30–32 Overall, the majority of STI diagnoses were missed on syndromic diagnosis alone, indicating that laboratory tests are still needed to diagnose and confirm infection in this setting. HSV-2 was severely underdiagnosed using the syndromic management approach, while gonorrhoea and chlamydia were overdiagnosed.
There may be several reasons why the syndromic approach to the management of STIs was not effective in our study. First, most of the evaluations that have shown success using syndromic management were performed within STI clinics.16,24–26 In this context, the probability of having an STI is relatively high because patients often self-select and present for evaluation with STI-specific signs and symptoms. By contrast, persons who screen and enrol in research studies provide a better representation of the burden of disease and prevalence of both symptomatic and asymptomatic infection among the general population. STI clinics thus still present one of the most appropriate avenues for the use of syndromic diagnosis as it captures symptomatic acute STIs such as gonorrhoea and chlamydia as well as active HSV-2 and syphilis, which arguably have more impact on morbidity/mortality and the need to rely on laboratory studies to diagnose latent STI such as latent HSV and syphilis. In general, it seems optimal that the syndromic management algorithms overdiagnose chlamydia/gonorrhoea which likely results in fewer missed treatment opportunities.
The high prevalence of asymptomatic infection for STIs has been well documented in literature.33–35 Asymptomatic infection is problematic because patients not having symptoms are less likely to seek medical care; and among those who do, they are less likely to get tested for the STIs. In order for the syndromic management approach to be effective, health care providers must have a basis for diagnosing and treating disease through symptoms and/or signs. In our study, STI diagnoses that were missed through syndromic management appeared to reflect asymptomatic infection or lack of disclosure of symptoms among study participants.
Because few evaluations of syndromic STI management within research contexts routinely ask all study participants questions about STI-specific signs and symptoms, we have a limited ability to compare our findings with other studies. Among the studies that have been done in research settings, some algorithms have been shown to work, including those for urethral discharge and GUD, while others have performed poorly, such as those for chlamydia and gonorrhoea.36–39 These findings suggest that laboratory screening of STIs should occur regardless of reported symptoms or condom use. Prevention efforts should focus on increasing awareness of asymptomatic STI infection among providers and the community. Left untreated, these infections could be co-factors in the transmission of HIV as well as contributors to increased morbidity and mortality3,7–9
Ensuring that appropriate STI screening occurs requires non-judgmental risk assessments of all patients. Although the syndromic diagnosis is based on the signs and symptoms collected through the CAPI, we included ACASI responses in this analysis to compare the symptoms that were reported through ACASI to those reported through CAPI. We hypothesised that persons would be more forthcoming with sharing information pertaining to sexual behaviour and risk through the self-administered ACASI, as compared to clinician-based CAPI. This was found to be true as more participants reported symptoms in ACASI as compared to CAPI. This might present an opportunity for capturing more syndromic STI cases that would otherwise be lost as individuals might not be forthcoming when faced by a clinician or another staff asking for them.
There have been significant changes and improvements in STI testing technologies and diagnostic capabilities in the past decade; however, further work is needed to develop and improve upon point-of-care tests (POCT) for STIs.40,41 The implementation of POCTs at healthcare facilities would be ideal as they have fast processing times and would allow patients to receive their test results and the necessary treatment in the same visit, resulting in lower rates of loss to follow-up. Further, a POCT for STIs would support the WHO sexually transmitted diseases diagnostics initiative (WHO SDI). This initiative has identified criteria for an STI POCT as being: affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to end-users.42
An essential component of STI control is partner treatment. This has been implemented through ‘partner notification’, which involves notifying the partner of their exposure to an STI followed by a recommendation for the partner to seek evaluation and treatment, if necessary.43 The primary goals of partner notification are identifying and treating new cases of STIs and providing prophylactic treatment to individuals who have had an exposure.44 In developed countries, the responsibility of notifying the patient has typically fallen on trained public health professionals; however, this method is costly and labour-intensive and many developing countries lack the resources and infrastructure necessary for successful implementation.45 Instead, the most common alternative notification method is ‘patient referral’ where providers encourage patients to notify their own sexual partners and refer them for treatment. In our study, the number of partners that presented for evaluation and treatment was very low, indicating poor rates of partner notification and/or partners coming for treatment. In developing countries and resource-limited settings, strategies such as strengthened education and counselling services, especially toward women in maternal health or family planning clinics, contact cards, educational materials, follow-up and financial incentives may be necessary to increase partner notification.45
There are several limitations to consider when interpreting our findings. First, our participants may not be representative of the Kisumu population, since they were volunteers for a research study and were recruited through convenience sampling. However, HIV prevalence found at the screening visit was nearly identical to local population estimates from national surveillance data, suggesting that the study participants may be representative of the local community.32 Second, as with any self-reported behavioural surveys, response bias may have been present. We tried to control for this through the use of ACASI, which has been reported to facilitate more accurate responses to sensitive behavioural questions than face-to-face interviews.46,47 Third, the aetiological diagnosis of chlamydia and gonorrhoea used an assay for active disease; however, the HSV-2 and syphilis serology may have misclassified recent acquisition as it also classifies people as positive even if their infection was remote and is inactive.48–51 Further, the misclassification of symptom status could have occurred if the reported symptoms were unrelated to gonorrhoea, chlamydia, syphilis or HSV-2 but to other organisms that were not tested for example mycoplasma genitalium, trichomonas vaginalis, etc.
These data show that more participants reported symptoms in ACASI as compared to CAPI. This presents a challenge on the applicability of this methodology in research and non-research settings as the current algorithms require for a clinician-based evaluation of signs in addition to the reported symptoms.3,8 Further, the syndromic management of STIs is not a sufficient tool for STI diagnosis in this setting; laboratory tests are still needed to diagnose and confirm infection. Further, work is needed to develop and improve upon STI diagnostic capabilities in resource-limited settings, address asymptomatic infection, and improve STI management including partner notification and referral in research settings. This will improve point-of-care testing with reduced turnaround time of tests and patient retention.
Acknowledgments
The authors are grateful to the study participants as well as Kayla Laserson, Katrina Kretsinger, Peter McElroy, Charles Vitek, Alan Greenberg, Laurence Slutsker, Kevin DeCock and John Vulule for their contribution to study design and protocol development, Clement Zeh for his expertise in laboratory analyses, and Deborah A Gust for input on an earlier version of this manuscript. This paper is published with the approval of the Director of the Kenya Medical Research Institute.
Funding
This research was funded by Centers for Disease Control and Prevention, Office of Infectious Diseases, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of HIV/AIDS Prevention, Atlanta, Georgia.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention or the Department of Health and Human Services.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.WHO. Prevalence and incidence of selected sexually transmitted infections; Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis. Geneva: World Health Organisation; 2011. [Google Scholar]
- 2.WHO. Sexually transmitted infections. Geneva: World Health Organisation; 2011. [Google Scholar]
- 3.WHO. Sexually transmitted and other reproductive tract infections: a guide to essential practice. Geneva: World Health Organisation; 2005. [Google Scholar]
- 4.WHO. Global prevalence and incidence of selected curable sexually transmitted infections. Geneva: World Health Organisation; 2001. [Google Scholar]
- 5.Amornkul PN, Vandenhoudt H, Nasokho P, et al. HIV prevalence and associated risk factors among individuals aged 13–34 years in rural Western Kenya. PLoS One. 2009;4:e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Estimation of the incidence and prevalence of sexually transmitted infections. Geneva: World Health Organisation; 2002. [Google Scholar]
- 7.Buve A, Laga M, Piot P. Sexually transmitted diseases; Where are we now? Health Pol Plann. 1993;8:277–281. [Google Scholar]
- 8.WHO. Guidelines for the management of sexually transmitted infections. Geneva: World Health Organisation; 2003. [Google Scholar]
- 9.WHO. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015: breaking the chain of transmission. Switzerland: World Health Organisation; 2007. [Google Scholar]
- 10.Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1867–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 12.Gilson L, Mkanje R, Grosskurth H, et al. Cost-effectiveness of improved treatment services for sexually transmitted diseases in preventing HIV-1 infection in Mwanza Region, Tanzania. Lancet. 1997;350:1805–1809. doi: 10.1016/S0140-6736(97)08222-6. [DOI] [PubMed] [Google Scholar]
- 13.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet. 1999;353:535–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 14.Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361:645–652. doi: 10.1016/s0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Sexually transmitted infections prevalence study methodology: guidelines for the implementation of STI prevalence surveys. Switzerland: World Health Organisation; 1999. [Google Scholar]
- 16.Mayaud P, Mosha F, Todd J, et al. Improved treatment services significantly reduce the prevalence of sexually transmitted diseases in rural Tanzania: results of a randomized controlled trial. Acquir Immune Defic Syndr. 1997;11:1873–1880. doi: 10.1097/00002030-199715000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catchpole M. Sexually transmitted infections: control strategies. Br Med J. 2001;322:1135–1136. doi: 10.1136/bmj.322.7295.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Workowski KA, Levine WC, Wasserheit JN. U.S Centers for disease control and prevention guidelines for the treatment of sexually transmitted diseases: an opportunity to unify clinical and public health practice. Ann Intern Med. 2002;137:255–262. doi: 10.7326/0003-4819-137-4-200208200-00010. [DOI] [PubMed] [Google Scholar]
- 20.National AIDS and STI Control Programme. Kenya national guidelines for syndromic management of sexually transmitted infections. Nairobi: National AIDS and STD Control Programme; 1994. [Google Scholar]
- 21.Ministry of Health (MOH) [Kenya] National manual for the management of HIV-related opportunistic infections and conditions. Nairobi: MOH; 2008. [Google Scholar]
- 22.Donovan B. Sexually transmissible infections other than HIV. Lancet. 2004;363:545–556. doi: 10.1016/S0140-6736(04)15543-8. [DOI] [PubMed] [Google Scholar]
- 23.Low N, Broutet N, Adu-Sarkodie Y, et al. Global control of sexually transmitted infections. Lancet. 2006;368:2001–2016. doi: 10.1016/S0140-6736(06)69482-8. [DOI] [PubMed] [Google Scholar]
- 24.Mbofana FS, Brito FJ, Saifodine A, et al. Syndromic management of sexually transmitted diseases at primary care level, Mozambique. Sex Transm Infect. 2002;78:1–2. doi: 10.1136/sti.78.1.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolday D, G-Mariam Z, Mohammed Z, et al. Risk factors associated with failure of syndromic treatment of sexually transmitted diseases among women seeking primary care in Addis Ababa. Sex Transm Infect. 2004;80:393–394. doi: 10.1136/sti.2003.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickering JM, Whitworth JAG, Hughes P, et al. Aetiology of sexually transmitted infections and response to syndromic treatment in southwest Uganda. Sex Transm Infect. 2005;81:488–493. doi: 10.1136/sti.2004.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chege W, Pals SL, McLellan-Lemal E, et al. Baseline findings of an HIV incidence cohort study to prepare for future HIV prevention clinical trials in Kisumu, Kenya. J Infect Dev Ctries. 2012;6:870–880. doi: 10.3855/jidc.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crewson PE. Reader agreement studies. Am J Roentgenol. 2005;184:1391–1397. doi: 10.2214/ajr.184.5.01841391. [DOI] [PubMed] [Google Scholar]
- 29.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:4. [PubMed] [Google Scholar]
- 30.Buvé A, Carael M, Hayes RJ, et al. The multicentre study on factors determining the differential spread of HIV in four African cities: summary and conclusions [The Multicentre Study of Factors Determining the Different Prevalences of HIV in sub-Saharan Africa] Acquir Immune Defic Syndr. 2001;15:S127–S131. doi: 10.1097/00002030-200108004-00014. [DOI] [PubMed] [Google Scholar]
- 31.Goh BT. Syphilis in adults. Sex Transm Infect. 2005;81:448–452. doi: 10.1136/sti.2005.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National AIDS and STI Control Programme. Kenya AIDS indicator survey 2007: final report. Nairobi: Ministry of Health, Kenya; 2009. p. 357. [Google Scholar]
- 33.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502–509. doi: 10.1016/s0091-7435(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 34.Grosskurth H, Todd J, Mwijarubi E, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 35.Cohen DA, Nsuami M, Martin DH, et al. Repeated school-based screening for sexually transmitted diseases: a feasible strategy for reaching adolescents. Pediatrics. 1999;104:1281–1285. doi: 10.1542/peds.104.6.1281. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson D, Connolly A-M, Harrison A, et al. Sexually transmitted disease syndromes in rural South Africa: results from health facility surveillance. Sex Transm Dis. 1998;25:20–23. doi: 10.1097/00007435-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Moodley P, Sturm PDJ, Connolly C, et al. Identification of women at high STD risk among STD clinic attendees: implications for STD programmes. Int J STD AIDS. 2003;14:526–531. doi: 10.1258/095646203767869138. [DOI] [PubMed] [Google Scholar]
- 38.Vuylsteke B. Current status of syndromic management of sexually transmitted infections in developing countries. Sex Transm Infect. 2004;80:333–334. doi: 10.1136/sti.2004.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Carlo A. Sexually transmitted diseases syndromic approach: urethral discharge. G Ital Dermatol Venereol. 2012;147:389–394. [PubMed] [Google Scholar]
- 40.Kane B. Point-of-care testing: instant gratification? Ann Intern Med. 1999;130:870–872. doi: 10.7326/0003-4819-130-10-199905180-00102. [DOI] [PubMed] [Google Scholar]
- 41.Peeling RW, Holmes KK, Mabey D, et al. Rapid tests for sexually transmitted infections (STIs): the way forward. Sex Transm Infect. 2006;82:v1–v6. doi: 10.1136/sti.2006.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO/OMS. World Health Organization sexually transmitted diseases diagnostics initiative. Geneva: WHO/ OMS; 2001. [Google Scholar]
- 43.Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, Syphilis, Gonorrhea, and Chlamydial infection. Morb Mortal Wkly Rep. 2008;57:1–63. [PubMed] [Google Scholar]
- 44.Hogben M. Partner notification for sexually transmitted diseases. Clin Infect Dis. 2007;44:S160–S174. doi: 10.1086/511429. [DOI] [PubMed] [Google Scholar]
- 45.Njeru E, Eldridge G, Ngugi E, et al. STD partner notification and referral in primary level health centers in Nairobi, Kenya. Sex Transm Dis. 1995;22:231–235. doi: 10.1097/00007435-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Waruru AK, Nduati R, Tylleskär T. Audio computer- assisted self-interviewing (ACASI) may avert socially desirable responses about infant feeding in the context of HIV. BMC Med Inform Decis Making. 2005;5:7. doi: 10.1186/1472-6947-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oloo I, Gust DA, Shinde S, et al. Effect of gender of the recorded voice on responses to sensitive sexual behavior questions: use of audio computer-assisted self interview (ACASI) in Kisumu, Kenya. Field Methods. 2012;24:367–381. [Google Scholar]
- 48.Young H. Guidelines for serological testing for syphilis. Sex Transm Infect. 2000;76:403–405. doi: 10.1136/sti.76.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald A, Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis. 2002;35:S173–S182. doi: 10.1086/342104. [DOI] [PubMed] [Google Scholar]
- 51.Ashley RL. Performance and use of HSV type-specific serology test kits. Herpes. 2002;9:38–45. [PubMed] [Google Scholar]