Abstract
Purpose
We determined whether the pattern of low detectable prostate specific antigen during the first 3 years of followup after radical prostatectomy would predict subsequent biochemical recurrence.
Materials and Methods
An institutional database was queried to identify 1,136 patients who underwent open retropubic or robot-assisted radical prostatectomy between January 5, 1993 and December 29, 2008. After applying exclusion criteria we used serum prostate specific antigen and the prostate specific antigen pattern during the first 3 years of followup to divide 566 men into 3 groups, including 1) undetectable prostate specific antigen (0.03 ng/ml or less), 2) low detectable-stable prostate specific antigen (greater than 0.03 and less than 0.2 ng/ml, no 2 subsequent increases and/or prostate specific antigen velocity less than 0.05 ng per year) and 3) low detectable-unstable prostate specific antigen (greater than 0.03 and less than 0.2 ng/ml, 2 subsequent increases according to NCCN criteria and/or prostate specific antigen velocity 0.05 ng per year or greater). The primary end point was biochemical recurrence, defined as prostate specific antigen 0.2 ng/ml or greater, or receipt of radiation therapy beyond 3 years of followup.
Results
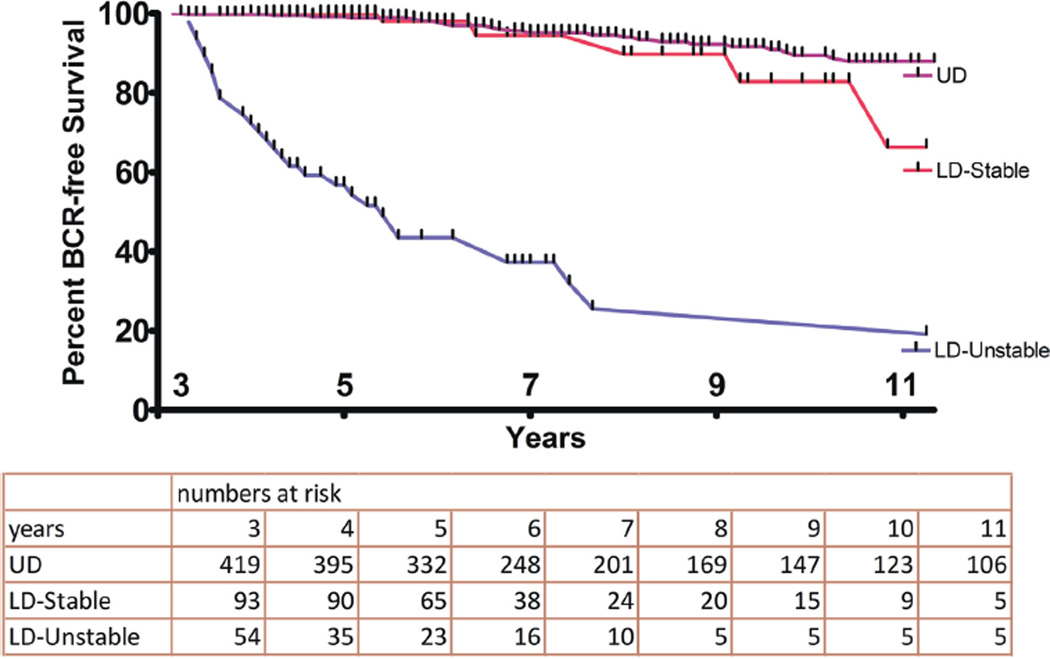
Seven-year biochemical recurrence-free survival was 95%, 94% and 37% in the undetectable, low detectable-stable and low detectable-unstable groups, respectively (log rank test p <0.0001). On multivariate analysis the prostate specific antigen pattern during 3 years postoperatively (undetectable vs low detectable-unstable HR 15.9 and vs low detectable-stable HR 1.6), pathological T stage (pT2 vs greater than pT2 HR 1.8), pathological Gleason score (less than 7 vs 7 HR 2.3 and less than 7 vs 8–10 HR 3.3) and surgical margins (negative vs positive HR 1.8) significantly predicted biochemical recurrence.
Conclusions
The combination of prostate specific antigen velocity and NCCN criteria for biochemical recurrence separated well men with low detectable prostate specific antigen after radical prostatectomy into those who required treatment and those who could be safely watched.
Keywords: prostate; neoplasm recurrence, local; prostatectomy; prostate-specific antigen; prognosis
Radical prostatectomy provides excellent long-term cure rates in most men with clinically localized disease.1 PSA is the most sensitive and widely used method to detect recurrence after RP. Increasing PSA after curative therapy without clinical or radiological evidence of disease is termed BCR. The incidence and behavior of BCR depend on its definitions.2 The NCCN divides men with BCR into 3 groups, including 1) those whose PSA fails to decrease to undetectable levels after RP (persistent disease), 2) those who achieve undetectable PSA after RP with a subsequent detectable PSA level that increases on 2 or more subsequent laboratory determinations (recurrent disease) and 3) those with low detectable, persistent PSA.3 However, exact definitions were not provided for the third group. PSA greater than 0.4 or greater than 0.2 ng/ml has been used in most studies as a BCR cutoff point.1,2 There is no consensus regarding treatment in men with detectable PSA less than 0.2 ng/ml.
As many as 40% of patients experience BCR after RP4 but the significance of BCR remains unclear. A reported 13% to 36% of patients with BCR experience clinical progression and 1.1% to 14% die of the disease.5 BCR precedes clinical recurrence in almost all patients.6 Those with BCR are at increased risk for subsequent metastasis and mortality.7 However, others reported that BCR correlated poorly with overall survival and expressed doubt about its clinical significance.8 About a third of patients with BCR receive secondary treatment9 but the best treatment in an individual with BCR remains controversial. Options for men with BCR include ADT, adjuvant or salvage XRT with or without ADT, or observation. Recent meta-analyses suggested that the treatment response rate for salvage XRT depends on pretreatment PSA and recommended initiating salvage XRT at the lowest possible PSA.10,11 On the other hand, early initiation of secondary treatment could lead to overtreatment since the natural history of BCR is prolonged and difficult to predict in an individual.
Shinghal at al described a subset of patients with detectable nonprogressive PSA recurrence after RP who did not show a progressive increase in serum PSA or clinical progression after 10 years of followup.12 Most of these men were characterized by late BCR (longer than 36 months after RP) and low PSA at BCR but no clinical or pathological characteristics were identified that predicted stable disease.
We hypothesized that men with low detectable and stable PSA should show the characteristics of men with undetectable PSA. To test this hypothesis we determined whether the pattern of low detectable PSA during the first 3 years of followup and/or clinicopathological characteristics were predictors of subsequent BCR.
MATERIALS AND METHODS
Patients
Institutional review board approval was obtained to query an institutional RP database to identify 1,136 patients who underwent ORP or RARP, performed by different surgeons between January 5, 1993 and December 29, 2008. Clinicopathological variables were populated retrospectively into a database until 2004, when data were collected and entered prospectively. Study exclusion criteria were fewer than 4 years of followup, preoperative ADT or XRT, lymph node metastasis, XRT or ADT, or PSA greater than 0.2 ng/ml within the first 3 years after RP, loss to followup and followup elsewhere due to the variable quality of PSA measurement. Serum PSA and the PSA pattern during the first 3 years of followup were used to divide the remaining 566 men into 3 groups, including 1) UD PSA (0.03 ng/ml or less), 2) LD-stable PSA (greater than 0.03 and less than 0.2 ng/ml, no 2 subsequent increases and/or PSAV less than 0.05 ng per year) and 3) LD-unstable PSA (greater than 0.03 and less than 0.2 ng/ml, 2 subsequent increases and/or PSAV 0.05 ng per year or greater). PSAV was calculated for 2 or more PSA values during 1 year or greater. PSAV thresholds less or greater than 0.05 ng per year did not improve the separation between the unstable and stable groups.
PSA Measurement and Followup
Serum PSA was measured using the Hybritech® PSA assay and the PHOTON™ Era™ Immunoanalyzer with 0.03 ng/ml sensitivity since 1993, the Immuno 1™ Immunoanalyzer with 0.03 ng/ml sensitivity since 1999 and the Centaur Immunoassay analyzer (Siemens Healthcare, Erlangen, Germany) with 0.01 ng/ml sensitivity since 2004. Serum PSA was measured routinely 6 weeks after RP, every 6 months for 5 years and annually thereafter unless prostate cancer was organ confined and PSA was undetectable, in which case PSA was measured annually from years 1 to 5. Additional PSA levels were measured as clinically indicated. Digital rectal examination was performed at each annual or semiannual visit and additional tests were done according to NCCN guidelines. Indications for initiating secondary treatment varied during the years. However, all recommendations for care have been NCCN guideline compliant since 2003. BCR was defined as PSA 0.2 ng/ml or greater, or receipt of XRT after 3 years of followup. Systemic progression was defined as demonstrable metastasis on computerized tomography, magnetic resonance imaging or radionuclide bone scan and/or positive tissue biopsies outside the prostatic bed.
Statistical Analysis
Patient baseline characteristics are reported by PSA group using the mean, median and SD for continuous variables and frequencies, and relative frequencies for categorical variables. Comparisons were made between groups using the Kruskal-Wallis and Fisher exact (Freeman-Halton extension) tests for continuous and categorical variables, respectively. Postoperative BCR-free survival was summarized using standard Kaplan-Meier methods with between group comparisons made using the log rank test. Univariate Cox regression models were used to determine HRs. Patients were censored at last followup or death if BCR had not been attained. A multivariate Cox regression model was used to evaluate the association between BCR-free survival and PSA groups in the presence of other factors. Model variables were evaluated using HRs and model overall performance was summarized using the concordance index. All analysis was done using SAS®, version 9.3 with a significance level of 0.05.
RESULTS
A total of 570 men were excluded from study using a priori criteria. Preoperatively 124 men (11%) received ADT and 5 received XRT. Lymph node metastases were found in 20 men (2%). XRT was administered to 146 men (13%), ADT was initiated in 23 (2%) and PSA was greater than 0.2 ng/ml in 6 (0.5%) within the first 3 years after RP. Followup was done elsewhere in 120 patients (10%) and 126 (11%) had fewer than 4 years followup. Tables 1 and 2 list baseline demographic, clinical and pathological characteristics, and outcomes of the groups, respectively. Median followup was 82 months (range 38 to 224).
Table 1.
Baseline patient demographic, clinical and pathological characteristics
| UD | LD-Stable | LD-Unstable | Overall | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. pts (%) | 419 | (74) | 93 | (16) | 54 | (10) | 566 | ||
| Mean/median age at operation (range) | 60/60 | (39–74) | 58/58 | (41–78) | 58/57 | (44–77) | 60/60 | (39–78) | 0.003 |
| No. operation type (%): | |||||||||
| Open | 217 | (52) | 23 | (25) | 18 | (33) | 258 | (46) | <0.001 |
| Robotic | 202 | (48) | 70 | (75) | 36 | (67) | 308 | (54) | |
| Preop PSA (ng/ml): | |||||||||
| Mean (median) | 6.4 | (5.4) | 6 | (5.4) | 7.6 | (5.6) | 6.4 | (5.4) | 0.267 |
| No. less than 4.0 (%) | 76 | (18) | 23 | (25) | 6 | (11) | 105 | (19) | 0.215 |
| No. 4.0–10.0 (%) | 304 | (72) | 63 | (68) | 40 | (74) | 407 | (72) | |
| No. greater than 10.0 (%) | 39 | (9) | 7 | (7) | 8 | (15) | 54 | (10) | |
| No. clinical stage (%): | |||||||||
| Less than T1c | 4 | (1) | 4 | (1) | 0.164 | ||||
| T1c | 265 | (65) | 48 | (53) | 36 | (69) | 349 | (63) | |
| Greater than T1c | 140 | (34) | 43 | (47) | 17 | (31) | 200 | (36) | |
| Unknown | 10 | 2 | 1 | 13 | |||||
| No. preop Gleason sum (%): | |||||||||
| Less than 7 | 283 | (69) | 65 | (70) | 24 | (46) | 372 | (68) | 0.007 |
| 7 | 106 | (26) | 25 | (27) | 23 | (43) | 154 | (27) | |
| Greater than 7 | 19 | (5) | 3 | (3) | 6 | (11) | 28 | (5) | |
| Unknown | 11 | 1 | 12 | ||||||
| No. NCCN clinical risk group (%): | |||||||||
| Low | 249 | (63) | 58 | (64) | 23 | (43) | 330 | (61) | 0.034 |
| Intermediate | 117 | (30) | 26 | (29) | 20 | (38) | 163 | (30) | |
| High | 30 | (7) | 7 | (7) | 10 | (19) | 47 | (9) | |
| Unknown | 23 | 2 | 1 | 26 | |||||
| No. pathological stage (%): | |||||||||
| pT2 | 340 | (81) | 74 | (80) | 33 | (61) | 446 | (79) | 0.022 |
| Greater than pT2 | 79 | (19) | 19 | (20) | 21 | (39) | 120 | (21) | |
| No. pathological Gleason sum (%): | |||||||||
| Less than 7 | 209 | (50) | 41 | (44) | 17 | (32) | 267 | (47) | 0.052 |
| 7 | 191 | (46) | 47 | (51) | 31 | (57) | 269 | (48) | |
| Greater than 7 | 19 | (4) | 5 | (5) | 6 | (11) | 30 | (5) | |
| No. surgical margins (%): | |||||||||
| Neg | 359 | (86) | 79 | (85) | 38 | (70) | 476 | (84) | 0.021 |
| Pos | 59 | (14) | 14 | (15) | 16 | (30) | 89 | (16) | |
| Unknown | 1 | 1 | |||||||
| Median mos followup (range) | 85 | (38–224) | 69 | (45–183) | 84 | (48–218) | 82 | (38–224) | <0.001 |
Table 2.
Outcomes by PSA group
| UD | LD-Stable | LD-Unstable | Overall | |
|---|---|---|---|---|
| No. pts | 419 | 93 | 54 | 566 |
| No. BCR events (%) | 27 (6) | 7 (7) | 37 (68) | 71 (12) |
| No. BCR surgery type: | ||||
| ORP | 26 | 5 | 15 | 46 |
| RARP | 1 | 2 | 22 | 25 |
| No. XRT for PSA less than 0.2 ng/ml | 2 | 2 | 7 | 11 |
| No. XRT | 13 | 4 | 24 | 41 |
| No. ADT | 4 | 1 | 2 | 7 |
| No. XRT/ADT | 1 | 0 | 1 | 2 |
| No. metastasis | 3 | 0 | 1 | 4 |
| No. death | 36 | 0 | 2 | 38 |
| No. prostate Ca death | 1 | 0 | 0 | 1 |
| Median followup (mos) | 85 | 69 | 84 | – |
| Median followup to PSA greater than 0.2 ng/ml (mos) | 84 | 96 | 51 | – |
| % 7-yr BCR-free survival | 95.1 | 94.5 | 37.3 | – |
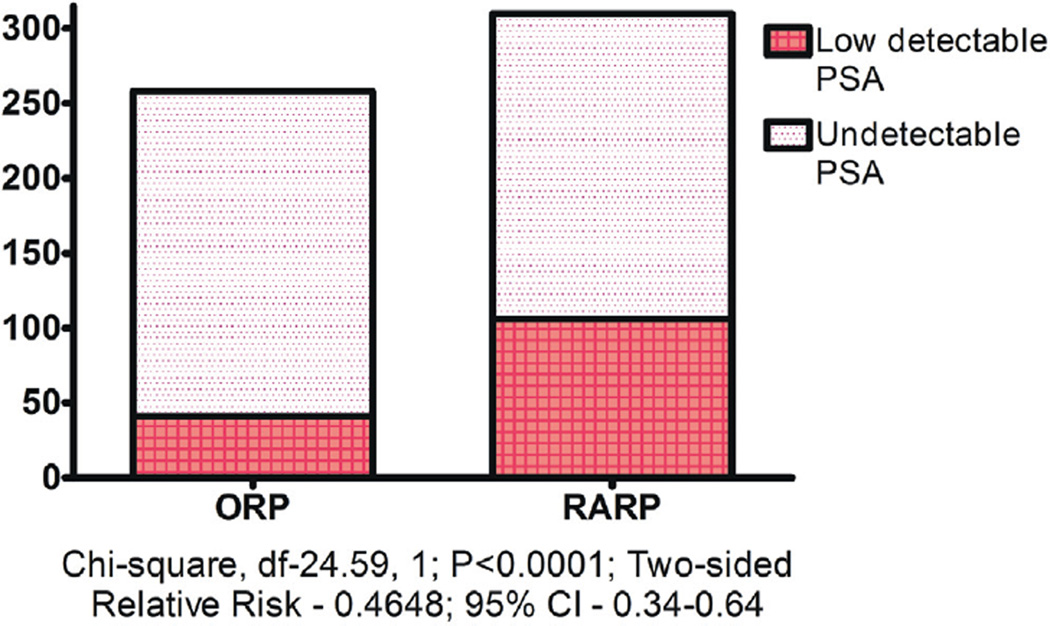
Seven-year BCR-free survival was 95%, 94% and 37% in the UD, LD-stable and LD-unstable groups, respectively (log rank test p <0.0001, fig. 1). The incidence of LD PSA during the first 3 years after RP was lower after ORP than after RARP (chisquare test p <0.0001, fig. 2).
Figure 1.
Kaplan-Meier plot of time to BCR
Figure 2.
Incidence of LD PSA during first 3 years after RP
Parameters associated with BCR after 3 years of followup were analyzed using Cox regression models. Univariate models revealed significant associations among the PSA groups for PSA kinetics, NCCN risk group, clinical and pathological Gleason grade, pathological T stage and surgical margin status (table 3). On multivariate analysis PSA group, pathological Gleason score, T stage and surgical margins were the only predictors of subsequent BCR (table 3).
Table 3.
Univariate and multivariate BCR models
| HR (95% CI) | p Value | ||
|---|---|---|---|
| Univariate | |||
| PSA group: | |||
| UD vs LD-unstable | 18.14 | (11.36–28.97) | <0.001 |
| UD vs LD-stable | 1.63 | (0.78–3.41) | |
| Pretreatment PSA (ng/ml): | |||
| Less than 4 vs 4–10 | 1.69 | (0.84–3.40) | 0.162 |
| Less than 4 vs greater than 10 | 2.29 | (0.98–5.36) | |
| Clinical Gleason sum: | |||
| Less than 7 vs 7 | 2.02 | (1.26–3.21) | <0.001 |
| Less than 7 vs 8–10 | 4.06 | (1.97–8.35) | |
| cT: | |||
| Less than T1c vs T1c | 0.50 | (0.07–3.60) | 0.652 |
| Less than T1c vs greater than T1c | 0.58 | (0.08–4.25) | |
| NCCN clinical risk group: | |||
| Low vs intermediate | 1.62 | (0.96–2.63) | <0.001 |
| Low vs high | 3.50 | (1.86–6.60) | |
| Surgery type (open vs robotic) | 1.12 | (0.66–1.89) | 0.672 |
| pT (pT2 vs greater than pT2) | 2.72 | (1.75–4.20) | <0.001 |
| Pathological Gleason sum: | |||
| Less than 7 vs 7 | 2.29 | (1.40–3.74) | <0.001 |
| Less than 7 vs 8–10 | 4.75 | (2.32–9.72) | |
| Surgical margin status (neg vs pos) | 2.36 | (1.49–3.78) | <0.001 |
| Multivariate | |||
| PSA group:* | |||
| UD vs LD-unstable | 15.97 | (9.85–25.9) | <0.001 |
| UD vs LD-stable | 1.65 | (0.79–3.45) | |
| pT (pT2 vs greater than pT2) | 1.76 | (1.13–2.76) | 0.013 |
| Pathological Gleason sum: | |||
| Less than 7 vs 7 | 2.32 | (1.39–3.85) | <0.001 |
| Less than 7 vs 8–10 | 3.37 | (1.62–7.03) | |
| Surgical margin status (neg vs pos) | 1.76 | (1.1–2.81) | 0.019 |
Concordance index 0.827 (95% CI 0.778–0.887).
Four patients (1%) experienced systemic progression during followup and 38 (7%) died, including 1 of prostate cancer. Therefore, there was insufficient power to estimate median systemic progression-free and cancer specific survival. Of the patients 11 (8%) were treated with XRT before PSA reached 0.2 ng/ml, including 7 in the LD-unstable PSA group.
DISCUSSION
The most commonly reported measure of prostate cancer control after RP has been BCR. However, BCR does not always translate into clinical progression due to the heterogeneous natural history of BCR.1,13,14 BCR precedes systemic relapse in almost all patients and men with BCR are at increased risk for additional treatment,9 which is administered in an attempt to prevent metastasis and death.7
Most reports and guidelines have used PSA greater than 0.2 or greater than 0.4 ng/ml as a cutoff point for BCR.1,2 ASTRO (American Society for Radiation Oncology)/AUA (American Urological Association) guidelines for adjuvant and salvage XRT after RP define BCR as detectable or increasing PSA that is 0.2 ng/ml or greater after RP with a second confirmatory level of 0.2 ng/ml or greater.15 NCCN defines BCR as PSA that fails to decrease to undetectable levels after RP or undetectable PSA after RP with a subsequent detectable PSA level that increases on 2 or more subsequent laboratory determinations.3 Thus, NCCN guidelines may consider additional treatment after RP for detectable PSA when PSA is less than 0.2 ng/ml. Each guideline recommends that adjuvant radiotherapy be considered in patients with adverse pathological findings at RP (seminal vesicle invasion, positive surgical margins or extraprostatic extension) regardless of PSA level.
To our knowledge this is first study to address treatment in men with PSA less than 0.2 ng/ml after RP. We describe patients in whom PSA became detectable during the first 3 years after RP and did not exceed 0.2 ng/ml. We used NCCN criteria for BCR and PSAV to divide men with low detectable PSA into groups, including LD-unstable and LD-stable. These definitions were stronger predictors of BCR (HR 15.9) than pathological Gleason score, stage or surgical margin status on multivariate analysis. The LD-stable group included patients with adverse pathological features but the followup course was benign and BCR-free survival was similar to that in the UD group (94% and 95%, respectively, table 1). In contrast, only 37% patients in the LD-unstable group remained free of BCR at 7 years of followup. Shinghal at al previously reported that patients with detectable, nonprogressive PSA recurrence after RP could be observed safely with rigorous PSA kinetics followup before recommending adjuvant therapy, imaging or anastomotic biopsy.12 However, no clinical or pathological characteristics were identified that could predict stable disease.
The LD-unstable group appeared to have a high frequency of residual cancer that was the source of PSA but in the LD-stable group the source of PSA remains uncertain. An explanation of detectable PSA in that group is nonprostatic expression. PSA released from sources other than prostate tissue may interfere with the diagnosis of prostate cancer recurrence.16 This risk may increase when ultrasensitive PSA assays are used. The Lepor group reported that ultrasensitive PSA nadirs are independent predictors of BCR.17,18 However, others reported that ultrasensitive assays may be more likely to detect PSA that is not of prostate origin and may inappropriately increase the frequency of detectable PSA.16,19
Another possibility is that some patients may have residual benign prostate tissue post-operatively.20 Up to 53% of urethral stump and 38% of bladder neck biopsy specimens showed benign glands after ORP.20,21 The incidence may be higher in men who underwent RARP because of more precise dissection during RARP, especially if bladder neck sparing or veil of Aphrodite nerve sparing approaches were used.22 In our study RARP was associated with a higher incidence of LD PSA than ORP (34% or 106 patients vs 16% or 41, fig. 2). Widespread adoption of the robotic platform for RP may increase the incidence of low detectable PSA after RP.
Men in whom persistent but stable postoperative PSA is due to residual prostate cancer should be distinguished from those in whom detectable PSA is due to benign prostate tissue or extraprostatic sources. Data from 3 randomized clinical trials demonstrated that adjuvant XRT benefited men with high risk pathological features at RP23–26 and improved overall survival.27 A recent meta-analysis recommended that salvage XRT be delivered at the lowest possible PSA.10,11 Treating all men with any detectable PSA could lead to overtreatment and increase the incidence of treatment related toxicity. Overtreatment may increase further since many urology groups have integrated intensity modulated radiation therapy in their practice.28
Overtreatment was not observed in this study because decisions to deliver salvage XRT were not based on a single PSA test. A total of 11 patients received XRT before PSA became 0.2 ng/ml, including 7 in the LD-unstable PSA group. If XRT had been administered using NCCN criteria for BCR, more than 90% of the men in the LD-stable group could have been overtreated. If ASTRO/AUA definitions for BCR had been used, men in the LD-unstable group would have experienced delayed XRT. XRT should not be delayed when salvage XRT is indicated since BCR-free survival increases when pre-XRT PSA levels are lower.10 Dividing men with low detectable PSA into LD-stable and LD-unstable groups may allow earlier identification of those destined to experience relapse after RP and improve treatment results while avoiding the toxicity associated with unnecessary salvage XRT.
Fewer than a third of patients with BCR after RP experienced systemic recurrence.1 In those with progression BCR predates metastatic disease progression by an average of 8 years and prostate cancer specific mortality by 13 years. A favorable cohort of men for disease recurrence (PSA less than 0.2 ng/ml within the first 3 years after RP) was selected for study. Therefore, a limited number of events of clinical progression (3 UD cases and 1 LD-unstable case) and cancer specific mortality (1 UD case) were observed during the median followup of 7 years (table 2). Men with LD PSA represent a special group who have a protracted disease recurrence course after RP.
Metastasis specific and cancer specific survival was not estimated due to the short followup. Men with prostate cancer are often older than 60 years so that competing causes of mortality may obscure the ability of BCR to predict death from prostate cancer.29 Men have been reported to be as likely to die of a competing cause as of prostate cancer within 15 years of BCR.30 Therefore, estimating life expectancy is crucial in this patient cohort before additional treatment is recommended.
This study has some limitations. This is a retrospective series, which may have introduced selection bias with time. Patients were included in the analysis who underwent ORP and RARP performed by different surgeons who used different surgical techniques, including different apical and bladder neck dissection techniques, and different criteria for nerve sparing. PSA assays and recommendations for treatment differed during the study course. Patients were assigned to a PSA group based on PSA values and kinetics measured during the first 3 years of followup. Therefore, most patients at high risk had undergone additional treatment. Of those studied 5% had Gleason sum greater than 7 and 21% had greater than pT2 disease. These findings do not apply to patients at high risk, who should be considered for immediate adjuvant/salvage XRT. Finally, much longer followup is required to determine the impact of low detectable PSA on metastasis specific and cancer specific survival.
CONCLUSIONS
Treatment of patients with low detectable PSA should be individualized. Men in whom PSA becomes detectable at low levels after RP can be divided into 2 groups using a combination of PSAV and NCCN criteria for BCR. Men with LD-unstable PSA experience BCR and can start salvage XRT at a lower tumor volume. However, men with LD-stable PSA do not often experience disease progression and can be followed safely. Men with LD-stable PSA after RP can/should avoid the anxiety, toxicity and costs associated with additional treatment.
Acknowledgments
Study received institutional review board approval.
Abbreviations and Acronyms
- ADT
androgen deprivation therapy
- BCR
biochemical recurrence
- LD
low detectable
- NCCN®
National Comprehensive Cancer Network®
- ORP
open retropubic RP
- PSA
prostate specific antigen
- PSAV
PSA velocity
- RARP
robot-assisted RP
- RP
radical prostatectomy
- UD
undetectable
- XRT
radiation therapy
Footnotes
Financial interest and/or other relationship with NCCN, Genomic Health Scientific Advisory Board, AndroBioSys, Medivation MTA and Simulated Surgical Systems.
REFERENCES
- 1.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Amling CL, Bergstralh EJ, Blute ML, et al. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146. [PubMed] [Google Scholar]
- 3.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:1081. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 4.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Boorjian SA, Tollefson MK, Thompson RH, et al. Natural history of biochemical recurrence after radical prostatectomy with adjuvant radiation therapy. J Urol. 2012;188:1761. doi: 10.1016/j.juro.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Leibovici D, Spiess PE, Agarwal PK, et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer. 2007;109:198. doi: 10.1002/cncr.22372. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal PK, Sadetsky N, Konety BR, et al. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112:307. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 8.Jhaveri FM, Zippe CD, Klein EA, et al. Biochemical failure does not predict overall survival after radical prostatectomy for localized prostate cancer: 10-year results. Urology. 1999;54:884. doi: 10.1016/s0090-4295(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SS, Lubeck DP, Sadetsky N, et al. Patterns of secondary cancer treatment for biochemical failure following radical prostatectomy: data from CaPSURE. J Urol. 2004;171:215. doi: 10.1097/01.ju.0000100087.83112.23. [DOI] [PubMed] [Google Scholar]
- 10.Ohri N, Dicker AP, Trabulsi EJ, et al. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012;48:837. doi: 10.1016/j.ejca.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King CR. Adjuvant radiotherapy after prostatectomy: does waiting for a detectable prostate-specific antigen level make sense? Int J Radiat Oncol Biol Phys. 2011;80:1. doi: 10.1016/j.ijrobp.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 12.Shinghal R, Yemoto C, McNeal JE, et al. Biochemical recurrence without PSA progression characterizes a subset of patients after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:380. doi: 10.1016/s0090-4295(02)02254-9. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Humphreys EB, Mangold LA, et al. Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J Urol. 2006;176:1404. doi: 10.1016/j.juro.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Boorjian SA, Thompson RH, Tollefson MK, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59:893. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Olsson AY, Bjartell A, Lilja H, et al. Expression of prostate-specific antigen (PSA) and human glandular kallikrein 2 (hK2) in ileum and other extraprostatic tissues. Int J Cancer. 2005;113:290. doi: 10.1002/ijc.20605. [DOI] [PubMed] [Google Scholar]
- 17.Shen S, Lepor H, Yaffee R, et al. Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. J Urol. 2005;173:777. doi: 10.1097/01.ju.0000153619.33446.60. [DOI] [PubMed] [Google Scholar]
- 18.Malik RD, Goldberg JD, Hochman T, et al. Three-year postoperative ultrasensitive prostate-specific antigen following open radical retropubic prostatectomy is a predictor for delayed biochemical recurrence. Eur Urol. 2011;60:548. doi: 10.1016/j.eururo.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Elgamal AA, Ectors NL, Sunardhi-Widyaputra S, et al. Detection of prostate specific antigen in pancreas and salivary glands: a potential impact on prostate cancer overestimation. J Urol. 1996;156:464. doi: 10.1097/00005392-199608000-00040. [DOI] [PubMed] [Google Scholar]
- 20.Shah R, Bassily N, Wei J, et al. Benign prostatic glands at surgical margins of radical prostatectomy specimens: frequency and associated risk factors. Urology. 2000;56:721. doi: 10.1016/s0090-4295(00)00775-5. [DOI] [PubMed] [Google Scholar]
- 21.Lepor H, Chan S, Melamed J. The role of bladder neck biopsy in men undergoing radical retropubic prostatectomy with preservation of the bladder neck. J Urol. 1998;160:2435. doi: 10.1097/00005392-199812020-00013. [DOI] [PubMed] [Google Scholar]
- 22.Savera AT, Kaul S, Badani K, et al. Robotic radical prostatectomy with the “Veil of Aphrodite” technique: histologic evidence of enhanced nerve sparing. Eur Urol. 2006;49:1065. doi: 10.1016/j.eururo.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 23.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 25.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 26.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 27.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell JM. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 29.Albertsen PC, Hanley JA, Gleason DF, et al. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280:975. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 30.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]