Abstract
Most smokers who attempt to quit, lapse within the first week and are ultimately unsuccessful in their quit attempt. Nicotine withdrawal exacerbates cognitive and attentional problems and may be one factor in smoking relapse. The startle reflex response and prepulse inhibition (PPI) of the response are sensitive to arousal and early attentional dysregulation. The current study examined whether startle response and PPI are related to early smoking lapse, and if this differs in people with and without posttraumatic stress disorder (PTSD). Participants with (N = 34) and without (N = 57) PTSD completed a startle reflex and PPI assessment during (1) ad lib smoking (2) on the first day of abstinence during a quit attempt. Most (88%) participants lapsed within the first week of the quit attempt. PTSD status predicted shorter time to lapse. Larger startle magnitude and greater PPI predicted a longer duration before smoking lapse. When diagnostic groups were examined separately, greater PPI predicted a longer successful quit attempt only in participants with a PTSD diagnosis. The startle reflex response and PPI may provide an objective, neurophysiological evaluation of regulation of arousal and early attentional processes by nicotine, which are important factors in smoking cessation success.
Keywords: Posttraumatic stress disorder, nicotine, smoking cessation, prepulse inhibition, startle reflex response, smoking lapse
Introduction
The majority of smokers want to quit, and about half have made a quit attempt in the past 12 months [U.S. Department of Health and Human Services, 2014]. Unfortunately, few are successful in quitting. Only 4–6% of the smoking population succeeds in quitting smoking each year [Burns et al., 2000, Centers for Disease Control and Prevention, 2011]. In fact, most individuals who make a smoking cessation attempt lapse within the first or second week, and subsequently relapse [Doherty et al., 1995, Shiffman et al., 2000, Hughes et al., 2004]. Among non-psychiatric smokers who lapse within the first week of a quit attempt, more than 90% of them will fully relapse to smoking [Kenford et al., 1994]. Thus, it is very important to better understand the factors that impede smoking cessation and provoke a lapse, especially within the first two weeks of a quit attempt.
One factor frequently said to maintain smoking is that it helps control negative affect [Kassel et al., 2003]. Reduction of negative emotions is often cited as the single most common reason for lapsing [Shiffman, 1986], and ambulatory monitoring studies support the relationship between negative affect and relapse [Shiffman et al., 1996, Beckham et al., 2013]. Another factor that helps to maintain smoking is nicotine’s role in managing cognitive and attentional deficits. Nicotine’s attention-enhancing properties are well-known and extensively studied in both humans and animals [Bushnell et al., 2000]. Nicotine withdrawal during a quit attempt may exacerbate cognitive and attentional problems, which may contribute to smoking relapse [Heishman, 1998; 1999, Powell et al., 2010].
Prepulse inhibition (PPI) of the acoustic startle reflex response has a long history of use to evaluate sensorimotor gating of attention in humans and other animals [Graham, 1975]. The startle is a response occurs to an intense, sudden-onset stimulus, and PPI refers to a reduction in the magnitude of that response when a less intense stimulus (prepulse) occurs between 30–500 ms before the startling stimulus. A reduction in the size of the startle reflex response during prepulse trials compared to startle stimulus alone trials is considered an indication of early automatic attention regulation of environmental stimuli, though the size of PPI can also be affected by controlled processes through instructions [Braff et al., 2001]. Persons with difficulty regulating early attentional processes, such as persons with schizophrenia, exhibit reduced PPI [Braff et al., 2001].
PPI is sensitive to the attention-enhancing effects of nicotine. Smokers exhibit greater PPI after smoking than when abstinent [Kumari et al., 1996, Hutchinson et al., 2000, Duncan et al., 2001, Ashare and Hawk, 2012, Vrana et al., 2013], and an injection of nicotine increases PPI in healthy non-smokers and smokers [Kumari et al., 1997, Postma et al., 2006]. Further, smokers who are more dependent on nicotine (as measured by the Fagerström Test for Nicotine Dependence) exhibit less PPI during smoking withdrawal than less-dependent smokers [Kumari and Gray, 1999], implicating nicotine use in moderating sensorimotor gating deficits among smokers. Individuals with chronic schizophrenia have difficulty with sensorimotor gating as manifested in reduced PPI and other measures [Braff and Geyer, 1990], smoke at higher rates than the general population [De Leon and Diaz, 2005], and exhibit greater nicotine-related increase in PPI than do non-schizophrenics [Kumari et al., 2001, George et al., 2006, Woznica et al., 2009], raising the possibility that the smoking rate among individuals with schizophrenia is due in part to its enhancement of sensorimotor gating mechanisms. One may hypothesize that smokers who are more susceptible to attentional difficulties, as indexed by greater PPI deficit, may self-medicate in response to these cognitive deficits, and will have more trouble quitting smoking due to trouble tolerating cognitive deficits related to nicotine withdrawal [Kumari and Postma, 2005].
Whereas prepulse inhibition is seen as indexing early attentional processes, the overall startle response is more complexly determined. Startle magnitude is larger (and onset latency shorter) in the context of increased attention and arousal, and while processing negative affect [Vrana et al., 1988, Witvliet and Vrana, 1995, Panayiotou et al., 2011]. Since nicotine increases attention and arousal, and withdrawal from nicotine increases negative affect, it is not surprising that nicotine and smoking do not consistently augment or reduce the strength of the startle reflex response: One study reported a trend toward smaller startle magnitude during abstinence compared to smoking [Postma et al., 2001], one study reported larger startle magnitude during abstinence [Ashare and Hawk, 2012], and at least eight studies reported no difference in startle response between smoking and abstinence conditions [e.g., [Duncan et al., 2001, Rissling et al., 2007, Vrana et al., 2013]]. Given startle magnitude’s sensitivity to attention/arousal and negative affect, both of which are factors in smoking lapse during a cessation attempt, startle magnitude is potentially related to success in a quit attempt. One study examined this by assessing startle magnitude while participants were smoking and on the first day of a quit attempt [Postma et al., 2001]. Participants who remained successfully abstinent for one month were characterized by larger startle magnitude while smoking. Successfully abstinent participants also exhibited a larger drop in magnitude from the smoking to the abstinence session; however, mean startle magnitude during the abstinence session remained larger for the abstinent participants than for participants who lapsed. While examination of a one-month cessation duration is laudable, these results are inconclusive given a small sample size and a gender balance uncharacteristic of smokers (total N=18, 13 female); in addition, the analysis was retrospective rather than predictive in that participants who were ultimately successful or unsuccessful in their quit attempt were divided into groups that were examined for their pre-quit startle response. Further research is needed to determine whether the startle reflex and PPI can reliably predict a successful smoking cessation attempt.
High smoking rates, and difficulty in successfully quitting, are of particular concern in populations with psychopathology [Beckham et al., 1995, Beckham et al., 1997, Lasser et al., 2000]. Perhaps not coincidentally, these same groups also often exhibit documented attentional difficulties and abnormalities in their PPI and startle response [Braff and Geyer, 1990, Grillon et al., 1998]. People with PTSD are of particular interest in this regard, because in addition to documented high smoking rates, attention regulation difficulties, and PPI deficits, the diagnostic criteria defining the disorder includes hypervigilance and exaggerated startle response [American Psychiatric Association, 2013]. Thus a startle response and PPI assessment may be particularly informative in evaluating the likelihood of a successful smoking cessation attempt in this group.
In the current study male and female smokers with and without a PTSD diagnosis agreed to make a cessation attempt aided by two smoking cessation counseling sessions. Identical startle magnitude and PPI assessment sessions were held during ad lib smoking and on the first day of abstinence, and variables from these assessments were used to predict the length of the cessation attempt. It was predicted that, following Postma et al. [Postma et al., 2001], a larger startle magnitude during smoking and abstinence would be associated with a more successful quit attempt. Further, because of the role nicotine plays in the maintenance of attention, it is predicted that greater prepulse inhibition will be associated with a more successful quit attempt. Because of the high rate of smoking in people with PTSD, and the roles that attention and arousal dysregulation play in this disorder, the value of the startle reflex and PPI in predicting a successful quit attempt will be examined in people with and without PTSD.
Methods and Materials
Participants
Participants were 39 smokers (24 women) with PTSD and 61 smokers (26 women) with no current PTSD diagnosis who had participated in a study examining predictors of smoking lapse among smokers with PTSD [Beckham et al., 2013]. This study was approved by the local Institutional Review Board and all participants gave informed consent prior to participation. Study eligibility criteria included smoking at least 10 cigarettes daily for the past year, willingness to make a smoking cessation attempt, and age between 18–65 years. Participants who met criteria for current alcohol or other substance abuse or dependence, a current psychotic disorder (including schizophrenia), or bipolar disorder with active manic symptoms were excluded from the both the PTSD and control groups. Additionally, potential participants were excluded from either group if they used non-cigarette forms of nicotine (e.g., cigars, pipes, chewing tobacco), had major unstable medical problems or major respiratory disorders, or used bupropion or benzodiazepines [Grillon and Baas, 2003]. Participants were included in the current analysis if they completed both laboratory startle assessment sessions described below. Trauma history and PTSD diagnosis were evaluated using the Clinician-Administered PTSD Scale [CAPS; [Blake et al., 1995]] and the Trauma Life Events Questionnaire [Kubany et al., 2000]. The Structured Clinical Interview for DSM-IV Diagnosis [First et al., 1994] was administered to assess other Axis I disorders (kappa across 14 raters for diagnoses was .94).
Procedure
Following a screening session, participants completed two identical startle reflex response and prepulse inhibition (PPI) assessments separated by a mean of 24.5 days (range 12 – 106). Between the two assessments, participants completed a week of electronic diary (ED) monitoring and two smoking cessation counseling sessions based on the NCI Fresh Start program [Lando et al., 1990]. The first (“smoking”) assessment occurred during ad lib smoking and the second (“abstinent”) assessment occurred on the participant’s smoking quit date, with overnight abstinence (from 10 pm the night before) verified by a reduction of expired carbon monoxide based on a formula that takes into account baseline CO levels [Rose and Behm, 2004]. Following the quit date, participants completed another week of ED monitoring and returned to the laboratory every other day for verification of smoking abstinence by providing expired carbon monoxide (CO) and saliva to be tested for cotinine level.
Participants were asked to record lapses on the ED as they occurred. As a check against potential missed readings, we also evaluated several other sources of information. Each night, participants were asked whether they had a smoking lapse that day on the assessment completed just before going to sleep. Six participants reported smoking abstinence but were determined to have lapsed based on cotinine >10 ng/ml. Their lapse time was assigned based on their first CO reading that exceeded > 9 ppm during laboratory visits (n=2) or the midpoint of the week during which cotinine indicated smoking (n=4). Participants were paid $750 for their complete participation, including $25 per day for ED monitoring; up to $45 during the post-quit week for good ED monitoring adherence, and $25 at each of the post-quit visits for remaining abstinent by self-report and CO reading.
Assessment of acoustic startle response
Each startle session included 24 total startle trials: six startle-only trials and six trials each with the startle preceded by a prepulse at 60 ms, 120 ms, and 240 ms stimulus onset interval. Sessions began with a startle-alone trial, and proceeded in one of three pseudorandom orders. Intertrial intervals averaged 25 (range = 17 – 51) seconds. Electromyographic (EMG) activity of the right orbicularis oculi muscle was used as an index of the startle response. The signal was bandpass-filtered (1.0 Hz to 5 kHz) with a notch filter at 60 Hz and digitized at 500 Hz. The startle (100 dB [A], 50 ms) and prepulse (70 dB [A], 20 ms) stimuli were broadband white noise with instantaneous rise time presented binaurally through Sony model MDR-V600 headphones. Participants were instructed to relax and keep their eyes open while they heard noises over the headphones.
Raw EMG data was digitally filtered (28 Hz – 500 Hz), integrated (root mean square method), and scored using AcqKnowledge Software. Responses were considered valid if the trial had a stable baseline with no artifact and a deflection was observed beginning within 20–120 ms of startle onset. Response magnitude was calculated by subtracting mean baseline EMG for the 25 ms prior to startle onset from response peak. Participants needed to have a minimum of two valid data points at each level of startle and prepulse condition (e.g., startle alone, 60 ms, 120 ms, and 240 ms) for each session to be included in analyses. This left 91 (34 PTSD) participants for analyses. PPI was calculated as ((A−B)/A) × 100, where A = the startle only mean and B = the prepulse mean, so that larger numbers indicate greater prepulse inhibition. To eliminate outliers, PPI values more than two standard deviations from the mean were coded as missing data.
Data analyses
Survival analyses using Cox proportional hazard regression modeled time to smoking lapse. Based on previously established relationships among smoking cessation, age [Monso et al., 2001] and gender [Piper et al., 2010] were entered as covariates in all models. Based on the results of Beckham et al [Beckham et al., 2013], PTSD was also included in all models given that it was associated with shorter time to smoking lapse. The next steps in the model added startle response magnitude during startle alone trials in the smoking session, and then magnitude during startle alone trials in the abstinence session. Finally, the interaction between PTSD status and each of these two startle variables was added to the model. This model was run separately for startle response magnitude and prepulse inhibition of startle magnitude. For calculating correlations, days to lapse was set at seven days when no smoking lapse occurred.
Results
Participant Characteristics
Characteristics of the participants at baseline are presented in Table 1. PTSD and non-PTSD groups did not differ on demographic variables (age, gender, minority status, education, employment status, marital status, % military veterans) or baseline CO reading. However, compared to the group without PTSD, the group with PTSD reported more previous traumas, negative affect, past substance abuse or dependence, current depression and anxiety disorder diagnoses, and higher Davidson Trauma Scale (DTS) and Fagerström Test for Nicotine Dependence (FTND) scores. The PTSD group also had larger magnitude startle responses during each startle session and had a shorter time before a smoking lapse than the group without PTSD. Prepulse inhibition was significantly greater during the smoking than the abstinence session [Vrana et al., 2013].
As expected, the startle variables were correlated across the smoking and abstinence sessions, startle magnitude r = .68, prepulse inhibition r = .36, both p < .01. As previously reported [Vrana et al., 2013], older age was associated with smaller startle magnitude and less prepulse inhibition during the abstinence session only, both r = −.25, p < .05. None of the startle variables (startle magnitude or prepulse inhibition in the smoking or abstinence session) were significantly (p < .05) correlated with nicotine dependence (FTND). Independent sample t-tests also found that the startle variables were not significantly associated with gender, marital status, or status as a veteran.
Table 1.
Demographics and Clinical Characteristics (Mean and SD)
| PTSD (n=39) | W/out PTSD (n=61) |
Test Statistic | |
|---|---|---|---|
| Gender (female) | 61.5% (24) | 42.6% (26) | χ2=3.40, p=.06, ns |
| Age (years) | 41.7 (11.3) | 42.3 (10.4) | t=−.31, p=.76, ns |
| Minority | 59.0% (23) | 70.5% (43) | χ2=1.40, p=.24, ns |
| Married | 18.0% (7) | 14.8% (9) | χ2=0.18, p=.67, ns |
| Education | 12.6 (1.9) | 12.9 (2.9) | t=.56, p=.57, ns |
| Employed | 51.3% (20) | 55.7% (34) | χ2=0.19, p=.66 |
| Veteran | 25.6% (10) | 23.0% (14) | χ2=.09, p=.76, ns |
| # Trauma types with fear, helplessness, or horror | 9.3 (3.4) | 3.8 (3.4) | t=−7.82, p<.0001 |
| Current MDD diagnosis | 30.8% (12) | 1.6% (1) | χ2=17.8, p=.001 |
| Current other anxiety disorder | 30.8% (12) | 6.6% (4) | χ2=10.38, p=.001 |
| Past substance abuse or dependence | 92% (36) | 67.2 (41)% | χ2=8.4, p=.004 |
| FTND score | 6.1 (2.1) | 5.2 (2.0) | t=−2.02, p=.05 |
| Davidson Trauma Scale | 66.8 (25.0) | 19.1 (33.3) | t=−7.7, p=.0001 |
| Negative affect | 23.8 (8.7) | 15.7 (5.5) | t=−5.2, p=.0001 |
| Baseline CO reading | 25.5 (13.3) | 24.4 (11.3) | t=−0.44, p=.66 |
| Days to lapse | 1.8 (1.8) | 2.7 (2.5) | t=1.98, p=.05 |
| Startle mag (smoking) | .050 (.072) | .027 (.046) | t=−2.64, p=.009 |
| Startle mag (abstinence) | .041 (.049) | .031 (.076) | t=−2.45, p=.016 |
| %PPI (smoking) | 42.9 (67.2) | 42.1 (71.2) | t=−0.06, p=.96 |
| %PPI (abstinence) | 34.9 (68.8) | 33.4 (56.1) | t=−0.18, p=.86 |
Startle Response as a Predictor of Smoking Lapse
Startle magnitude
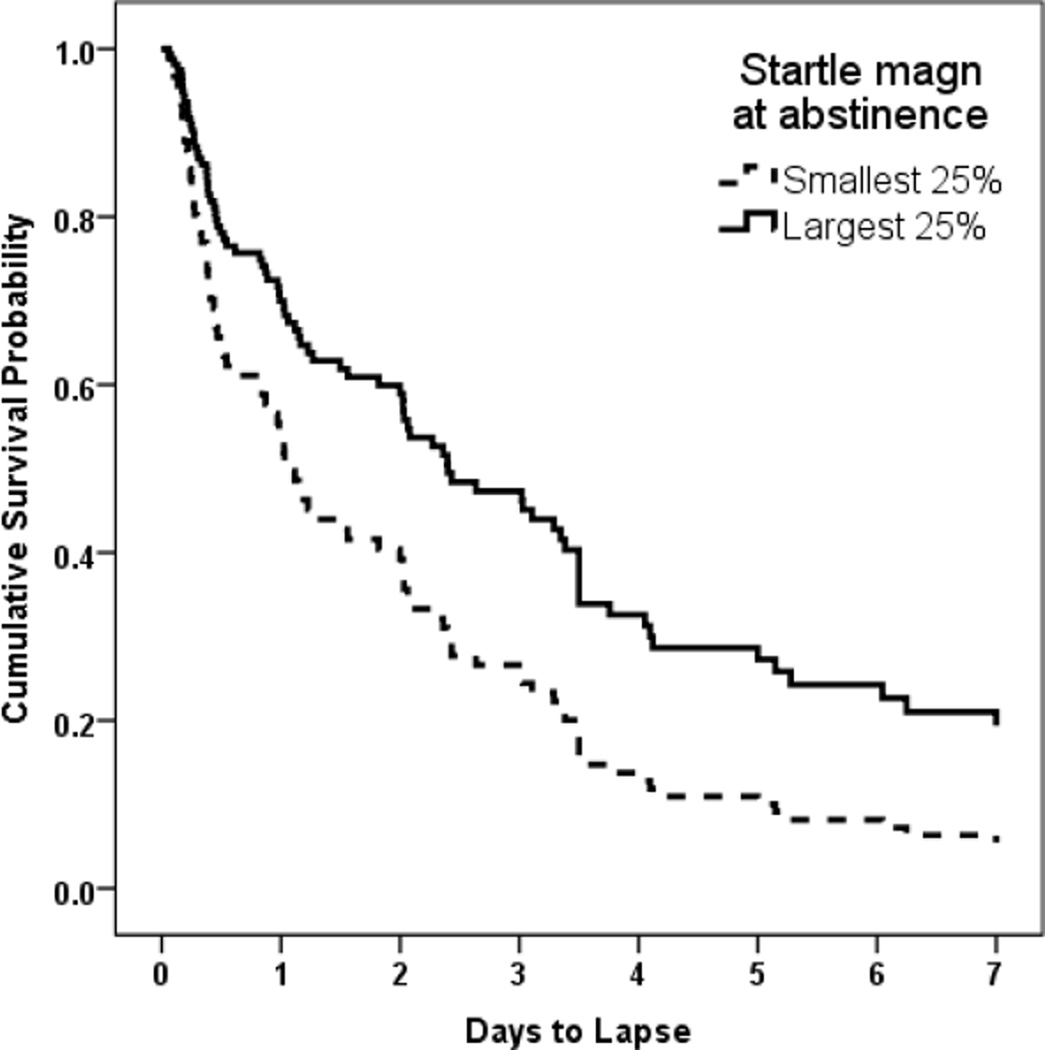
PTSD was associated with shorter time to lapse. After accounting for age, gender, and PTSD status, a larger startle magnitude during the smoking session marginally predicted a more successful smoking cessation attempt (e.g., longer time to smoking lapse), β = −4.57, p = .074. When startle magnitude during the abstinence session was added to the model, larger startle magnitude during abstinence significantly predicted a more successful cessation attempt, β = −7.93, p = .037 (HR = .0003, 95% CI = .0000 – 0.630), and startle magnitude during the smoking session was no longer a predictor, p = .912. Thus, after accounting for startle magnitude while participants were smoking, participants who maintained a larger startle magnitude when abstinent from smoking had a more successful quit attempt than those with smaller startle magnitude. To illustrate this effect, Figure 1 shows the probability of maintaining smoking cessation over the observation days for the participants in the top quartile and the bottom quartile of startle magnitude. As can be seen in the figure, about 20% of participants with the largest startle magnitude were still successfully abstinent after seven days, whereas less than 5% of participants with the smallest startle magnitude had not yet lapsed. The interactions between startle magnitude and PTSD status did not significantly predict time to smoking lapse when added to the model, both p >.80.
Figure 1.
Cumulative probability of maintaining abstinence for participants in the upper and lower quartile of startle response magnitude during the abstinence session.
Prepulse inhibition (PPI) of startle magnitude
Greater PPI of startle magnitude during the smoking session predicted a more successful smoking cessation attempt, β = −.004, p = .023 (HR = .996, 95% CI = .993–.99950). PPI during the abstinence session did not add significantly to the model, β = −.002, p = .467. The interaction between PTSD status and PPI during the smoking session was marginally significant, β = .008, p = .087. Since one of the goals of the study was to investigate whether startle response would differentially predict smoking lapses in people with and without PTSD, the predictive models were examined separately in the PTSD and the control group. For the PTSD group, greater PPI during the smoking session predicted a more successful cessation attempt, β = −.007, p = .013 (HR = .993, 95% CI = .987–.998), whereas this relationship was not found in the control group, β = −.001, p = .512.
Combined model
Finally, a combined model was conducted with age, gender, PTSD status, and the variables that were significant in the previous models; that is, startle magnitude during the abstinence session and PPI during the smoking session. In this model both startle magnitude during abstinence β = −.003, p = .041 (HR = .001, 95% CI = .000–.498) and PPI during smoking β = −6.67, p = .029 (HR = .997, 95% CI = .993–.99986) were significant, suggesting that the two variables were explaining non-overlapping percentages of the variance in time to lapse. Further, the model containing these two variables was a significant improvement in predicting a successful cessation attempt over the model including only age, gender, and PTSD status, χ2 = 12.04, p = .002.
Discussion
The current study found that startle response and prepulse inhibition of startle response, measures of arousal and early attentional processes, predicted the length of a smoking quit attempt. These findings are important both in providing evidence that regulation of arousal and attention by nicotine are important factors in smoking cessation success, and in providing objective, neurophysiological measures of these factors. Although participants were followed for only a week following a quit attempt, this is a crucial period to study given that most smokers lapse during their first week of a quit attempt, and 90% of those who lapse will fully relapse [Kenford et al., 1994]. As described in earlier reports on the electronic diary recordings and gender and racial differences in this dataset [Beckham et al., 2013, Wilson et al., 2014], the majority of participants (88%) lapsed within the first week following a quit attempt and PTSD was significantly related to time to lapse. Participants who had a larger startle magnitude when smoking tended to be more successful in their quit attempt. As can be seen in Figure 1, when controlling for the magnitude of the startle response when smoking, participants who exhibited a larger magnitude startle on the first day of smoking abstinence had a significantly more successful quit attempt, with 20% of the top quartile of largest startle responders remaining abstinent after seven days compared to less than 5% of the quartile with the smallest startle responses. It may be that a larger initial startle response, and then the ability to preserve that response when not smoking, identifies smokers who are less dependent on nicotine to maintain arousal and attention, and so have more success in continued smoking abstinence after a quit attempt.
The association between a larger magnitude startle response during the smoking session and a more successful quit attempt is consistent with Postma et al.’s [Postma et al., 2001] finding in a much smaller sample. Postma et al. [Postma et al., 2001] found, in a small sample (N=18) of mostly female participants, that smokers who remained abstinent for a month had larger startle magnitudes during the smoking assessment compared to those who relapsed. The present study also found that, after controlling for startle magnitude while smoking, maintaining a large startle response during the abstinence session predicted a more successful quit attempt. Larger startle magnitude typically indexes greater arousal/attention or higher levels of negative affect [Witvliet and Vrana, 1995, Panayiotou et al., 2011]. If abstinence-induced negative affect was making smoking cessation more difficult, then a larger startle magnitude during abstinence would have predicted a less successful quit attempt. However, we found the opposite, suggesting that a successful quit attempt was more likely among people who were able to maintain their arousal and alertness in the absence of nicotine.
Greater PPI of startle magnitude while smoking was significantly associated with longer time to lapse. In addition, when the PTSD and control groups were examined separately, increased magnitude PPI was associated with longer time to lapse only in those with PTSD. PPI has been identified with sensorimotor gating of attention [Graham, 1975], and is larger to the extent that the prepulse stimulus is more fully processed [Franklin et al., 2011]. Nicotine enhances attention, and increases PPI, in smokers and nonsmokers alike [Postma et al., 2006, Ashare and Hawk, 2012, Vrana et al., 2013]. These results suggest that smokers with better early attentional processing capabilities as indexed by PPI are likely to be more successful in quitting smoking. The model predicting smoking lapse found that greater PPI in the smoking session predicted a more successful quit attempt, and PPI during the abstinence session did not add to this prediction. However, correlations between PPI and time to lapse were the same (r = .17) during both the smoking and abstinence session, suggesting that it is a person’s overall early attentional capabilities, and not maintenance of attention with nicotine, that is associated with a successful cessation attempt.
Greater PPI during the smoking session predicted a longer time before smoking lapse in the full sample; however, when the groups were looked at separately greater PPI predicted cessation success only for the participants diagnosed with PTSD. The separate analysis of the two groups occurred after a marginally significant Session × Group interaction, and so the findings should be considered tentative. Nevertheless, the results are consistent with findings of attention and concentration problems among people with PTSD. It may be that the people with more acute attention and concentration problems as indexed by PPI may find it particularly difficult to successfully navigate a week without smoking, and that people with PTSD are particularly susceptible to the attention-enhancing effects of nicotine. It should be noted that within this study the PTSD group did not exhibit less PPI than the control group overall, and in fact PPI generally does not discriminate between samples with and without a PTSD diagnosis [Pole, 2007]. It may be that making a quit attempt is especially difficult for people who must also cope with the twin stresses of PTSD and poor attentional regulation. Indeed, fMRI evidence suggests that difficulty maintaining smoking abstinence is linked to reduced brain activity in the dorsal anterior cingulate cortex and dorsal lateral prefrontal cortex, areas associated with top-down control over interoceptive awareness and self-regulation [Janes et al., 2010]. One implication is that individual differences in early attentional processes are especially important to take into account in designing smoking cessation programs for people with PTSD. Another potential implication is that the same logic may need to be applied to people with schizophrenia, who smoke at a high rate [De Leon and Diaz, 2005], and have smaller PPI than control groups [Braff and Geyer, 1990]—individual difference in attentional abilities within this group may need to be taken into account when understanding their smoking cessation process.
This study was limited by the relatively brief time to follow-up. Although a large majority of smokers who lapse during the first week following a quit attempt subsequently relapse [Kenford et al., 1994], a longer assessment period would allow assessment of relapse (i.e., a return to sustained smoking at baseline levels). Study results are also limited by the lack of a comparison group with non-PTSD psychiatric disorders. Thus, it is unclear whether differences were due to the effects of PTSD in particular or the presence of psychopathology more generally. Participants were paid a small incentive ($25) for maintaining abstinence. A direction for future research is to examine the robustness of these findings when smokers are provided a more salient incentive to achieve and maintain abstinence. We recently documented that as many as 80% of smokers with PTSD could achieve and maintain abstinence for one month when given a larger financial incentive and 55% remained abstinent at three months even after all financial incentives were removed [Hertzberg et al., 2013].
Despite its limitations, the current study suggests that startle magnitude and PPI are significantly associated with time to smoking lapse among smokers with PTSD. Results suggest that inclusion of smoking cessation intervention components that address PTSD symptoms and deficits in attentional processes may be needed to maximize quit rates in this population.
Acknowledgments
This work was supported by the National Institutes of Health [Grant numbers 2R01CA081595, 2K24DA016388]; and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Science Research and Development. The views expressed in this presentation are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health. We would like to thank the participants who volunteered to participate in this study. The views expressed in this presentation are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Scott R. Vrana, Email: srvrana@vcu.edu.
Patrick S. Calhoun, Email: patrick.calhoun2@va.gov.
Michelle F. Dennis, Email: michelle.dennis@va.gov.
Angela C. Kirby, Email: angela.kirby5@va.gov.
Jean C. Beckham, Email: jean.beckham@va.gov.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Ashare RL, Hawk LW., Jr Effects of Smoking Abstinence on Impulsive Behavior among Smokers High and Low in Adhd-Like Symptoms. Psychopharmacology. 2012;219:537–547. doi: 10.1007/s00213-011-2324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA. Predictors of Lapse in First Week of Smoking Abstinence in Ptsd and Non-Ptsd Smokers. Nicotine & Tobacco Research. 2013;15:1122–1129. doi: 10.1093/ntr/nts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Kirby AC, Feldman ME, Hertzberg MA, Moore SD, Crawford AL, et al. Prevalence and Correlates of Heavy Smoking in Vietnam Veterans with Chronic Posttraumatic Stress Disorder. Addictive Behaviors. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Roodman AA, Shipley RH, Hertzberg MA, Cunha GH, Kudler HS, et al. Smoking in Vietnam Combat Veterans with Posttraumatic Stress Disorder. Journal of Traumatic Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FS, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The Development of a Clinician-Administered Ptsd Scale. Journal of Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor Gating and Schizophrenia: Human and Animal Model Studies. Archives of General Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow N. Human Studies of Prepulse Inhibition of Startle: Normal Subjects, Patient Groups, and Pharmacological Studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Burns DM, Anderson C, Johnson M, Major JM, Biener L, Vaughn J, et al. Cessation and Cessation Measures among Adult Daily Smokers: National and State-Specific Data. Population-Based Smoking Cessation: What Works Smoking and Tobacco Control Monograph No. 2000;12:25–97. [Google Scholar]
- Bushnell PJ, Levin ED, Marrocco RT, Sarter MF, Strupp BJ, Warburton DM. Attention as a Target of Intoxication: Insights and Methods from Studies of Drug Abuse. Neurotoxicology and Teratology. 2000;22:487–502. doi: 10.1016/s0892-0362(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Healthy Aging: Helping People to Live Long and Productive Lives and Enjoy a Good Quality of Life at a Glance. 2011 [Google Scholar]
- De Leon J, Diaz FJ. A Meta-Analysis of Worldwide Studies Demonstrates an Association between Schizophrenia and Tobacco Smoking Behaviors. Schizophrenia Research. 2005;76:1351–1357. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to Smoke During the First Month of Abstinence: Relationship to Relapse and Predictors. Psychopharmacology. 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, et al. Effects of Smoking on Acoustic Startle and Prepulse Inhibition in Humans. Psychopharmacology. 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I Dsm-Iv Disorders. 2nd ed. New York, NY: Biometrics Research Department; 1994. [Google Scholar]
- Franklin JC, Murray CT, Lane ST, Lee KM, Shorkey SP. A Test of the Protection Function of Prepulse Inhibition. Psychophysiology. 2011;48:S115–S115. [Google Scholar]
- George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, et al. A Preliminary Study of the Effects of Cigarette Smoking on Prepulse Inhibition in Schizophrenia: Involvement of Nicotinic Receptor Mechanisms. Schizophrenia Research. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Graham FK. The More or Less Startling Effects of Weak Prestimuli. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A Review of the Modulation of the Startle Reflex by Affective States and Its Application in Psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of Experimental Context and Explicit Threat Cues on Acoustic Startle in Vietnam Veterans with Posttraumatic Stress Disorder. Psychiatry Research. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What Aspects of Human Performance Are Truly Enhanced by Nicotine? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and Cognitive Effects of Smoking: Relationship to Nicotine Addiction. Nicotine & Tobacco Research. 1999;(1) suppl 2:S143–S147. doi: 10.1080/14622299050011971. [DOI] [PubMed] [Google Scholar]
- Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Mobile Contingency Management as an Adjunctive Smoking Cessation Treatment for Smokers with Posttraumatic Stress Disorder. Nicotine & Tobacco Research. 2013;15:1934–1938. doi: 10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Lindgren PG, Connett JE, Nides MA. Smoking Reduction in the Lung Health Study. Nicotine & Tobacco Research. 2004;6:275–280. doi: 10.1080/14622200410001676297. [DOI] [PubMed] [Google Scholar]
- Hutchinson KE, Niaura R, Swift R. The Effects of Smoking High Nicotine Cigarettes on Prepulse Inhibition, Startle Latency, and Subjective Responses. Psychopharmacology. 2000;150:244–252. doi: 10.1007/s002130000399. [DOI] [PubMed] [Google Scholar]
- Janes A, Pizzagalli D, Richardt S, Deb Frederick B, Chuzi S, Pachas G, et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biological Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, Stress, and Negative Affect: Correlation, Causation, and Context across Stages of Smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting Smoking Cessation: Who Will Quit with and without the Nicotine Patch. Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, et al. Development and Preliminary Validation of a Brief Broad-Spectrum Measure of Trauma Exposure: The Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley W, Gray JA. Effect of Cigarette Smoking on Prepulse Inhibition of the Acoustic Startle Reflex in Healthy Male Smokers. Psychopharmacology. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA, Gray JA. Effect of Acute Subcutaneous Nicotine on Prepulse Inhibition of the Acoustic Startle Inhibition of the Acoustic Startle Reflex in Heathy Male Non-Smokers. Psychopharmacology. 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking Withdrawl, Nicotine Dependence, and Prepulse Inhibition of the Acoustic Startle Reflex. Psychopharmacology. 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: The self medication hypotheses. Neuroscience and Biobehavioral Reviews. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of Cigarette Smoking on Prepulse Inhibition of the Acoustic Startle Response in Schizophrenia. Human Psychopharmacology: Clinical and Experimental. 2001;16:321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Lando HA, Mccovern PG, Barrios FX. Comparative Evaluation of American Cancer Society and American Lung Association Smoking Cessation Clinics. American Journal of Public Health. 1990;80:554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, Mccormick D, Bor DH. Smoking and Mental Illness: A Population-Based Prevalence Study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Monso E, Campbell J, Tonnsen P, Gustavson G, Morera J. Sociodemographic Predictors of Success in Smoking Intervention. Tobacco Control. 2001;10:165–169. doi: 10.1136/tc.10.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotou G, Witvliet CVO, Robinson JD, Vrana SR. A Startling Absence of Emotion Effects: Active Attention to the Startle Probe as a Motor Task Cue Appears to Eliminate Modulation of the Startle Reflex by Valence and Arousal. Biological psychology. 2011;87:226–233. doi: 10.1016/j.biopsycho.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, Race, and Education Differences in Abstinence Rates among Participants in Two Randomized Smoking Cessation Trials. Nicotine & Tobacco Research. 2010;12:647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N. The Psychophysiology of Posttraumatic Stress Disorder: A Meta-Analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, et al. A Behavioural and Functional Neuroimaging Investigation into the Effects of Nicotine on Sensorimotor Gating in Healthy Subjects and Persons with Schizophrenia. Psychopharmacology. 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Postma P, Kumari V, Sharma T, Hines M, Gray JA. Startle Response During Smoking and 24 Hours after Withdrawal Predicts Successful Smoking Cessation. Psychopharmacology. 2001;156:360–367. doi: 10.1007/s002130100829. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to Smoking During Unaided Cessation: Clinical, Cognitive and Motivational Predictors. Psychopharmacology. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Rissling AJ, Dawson ME, Schell AM, Nuechterlein KH. Effects of Cigarette Smoking on Prepulse Inhibition, Its Attentional Modulation, and Vigilance Performance. Psychophysiology. 2007;44:627–634. doi: 10.1111/j.1469-8986.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the Rewarding Value of Smoke Cues: Pharmacologic and Behavioral Treatments. Nicotine & Tobacco Research. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Shiffman S. A Cluster-Analytic Classification of Smoking Relapse Episodes. Addictive Behaviors. 1986;11:295–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Paty JA, Engberg J, Gwaltney CJ, Liu K. Dynamic Effects of Self-Efficacy on Smoking Lapse and Relapse. Health Psychology. 2000;19:315–323. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards T. Progression from a Smoking Lapse to Relapse: Predictions from Abstinence Violation Effects and Nicotine Dependence. Journal of Consulting & Clinical Psychology. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. A Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2014. The Health Consequences of Smoking 50 Years of Progress. [Google Scholar]
- Vrana SR, Calhoun PS, Mcclernon FJ, Dennis MF, Lee ST, Beckham JC. Effects of Smoking on the Acoustic Startle Reponse and Prepulse Inhibitions in Smokers with and without Posttraumatic Stress Disorder. Psychopharmacology. 2013;230:477–485. doi: 10.1007/s00213-013-3181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The Startle Probe Response: A New Measure of Emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Dedert EA, Dennis PA, Dennis MF, Calhoun PS, Kirby AC, et al. Do Ethnicity and Gender Moderate the Influence of Posttraumatic Stress Disorder on Time to Smoking Lapse? Addictive Behaviors. 2014;39:1163–1167. doi: 10.1016/j.addbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvliet C, Vrana S. Psychophysiological Responses as Indices of Affective Dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Woznica AA, Sacco KA, George TP. Prepulse Inhibition Deficits in Schizophrenia Are Modified by Smoking Status. Schizophrenia Research. 2009;112:86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]