Abstract
The suprachiasmatic nucleus (SCN) drives and synchronizes daily rhythms at the cellular level via transcriptional-translational feedback loops comprised of clock genes such as Bmal1 and Period (Per). Glycogen synthase kinase 3 (GSK3), a serine/threonine kinase, phosphorylates at least five core clock proteins and shows diurnal variation in phosphorylation state (inactivation) of the GSK3β isoform. Whether phosphorylation of the other primary isoform (GSK3α) varies across the subjective day-night cycle is unknown. The purpose of this study was to determine if the endogenous rhythm of GSK3 (α and β) phosphorylation is critical for rhythmic BMAL1 expression and normal amplitude and periodicity of the molecular clock in the SCN. Significant circadian rhythmicity of phosphorylated GSK3 (α and β) was observed in the SCN from wild-type mice housed in constant darkness for two weeks. Importantly, chronic activation of both GSK3 isoforms impaired rhythmicity of the GSK3 target BMAL1. Furthermore, chronic pharmacological inhibition of GSK3 with 20 μM CHIR-99021 enhanced the amplitude and shortened the period of PER2::luciferase rhythms in organotypic SCN slice cultures. These results support the model that GSK3 activity status is regulated by the circadian clock and that GSK3 feeds back to regulate the molecular clock amplitude in the SCN.
At the cellular level, 24-hour timing is maintained by transcriptional-translational feedback loops resulting in rhythmic expression of core “clock genes,” in which the activators (CLOCK and BMAL1) are expressed in anti-phase to the suppressors (PERs and CRYs; (Partch et al., 2014 for review). A secondary feedback loop consists of CLOCK-BMAL1 activation of a nuclear orphan receptor REV-ERBα which feeds back to transcriptionally repress Bmal1. The length of the molecular clock cycle can be regulated by post-translational events including phosphorylation by kinases such as casein kinase I enzymes (Partch et al., 2014). We know much less about how other kinases, such as glycogen synthase kinase 3 (GSK3), regulate the period and/or amplitude of the molecular clock, especially in neurons of the central circadian pacemaker, the suprachiasmatic nucleus (SCN) of the hypothalamus. Given that dysregulated GSK3 signaling has been implicated in psychiatric and neurological disorders (Hooper et al., 2008; Li and Jope, 2010; Duncan, M J, 2013), and many therapeutic drugs target GSK3 (e.g., lithium, valproate, risperidone, etc; Li and Jope, 2010), important questions to address are how GSK3 activity varies over the course of the day and how the molecular clock is affected by these rhythms.
A key feature of GSK3 is that it is active in its default state and that its two isoforms are inactivated by phosphorylation (at Ser-21 for GSK3α [S21-GSK3α] and Ser-9 for GSK3β [S9-GSK3β]). This critically important phosphorylation state balance of S9-GSK3β changes across the light-dark cycle in the SCN (Iwahana et al., 2004; Iitaka et al., 2005). In some brain regions, the two distinct GSK3 isoforms can have functional differences, such as differences in substrate recognition, axonal growth, regulation of synaptic plasticity, and formation of senile plaques in an Alzheimer's mouse model (Castaño et al., 2010; Soutar et al., 2010; Hurtado et al., 2012; Shahab et al., 2014). Therefore, it is important to determine whether phosphorylation of S21-GSKα also varies across the subjective day-night cycle in the SCN. Because rhythmic phosphorylation of S9-GSK3β is dependent upon the molecular clock in peripheral tissues such as the heart (Durgan and Young, 2010), we hypothesized that rhythmic phosphorylation of GSK3α and GSK3β persists in constant darkness in the SCN.
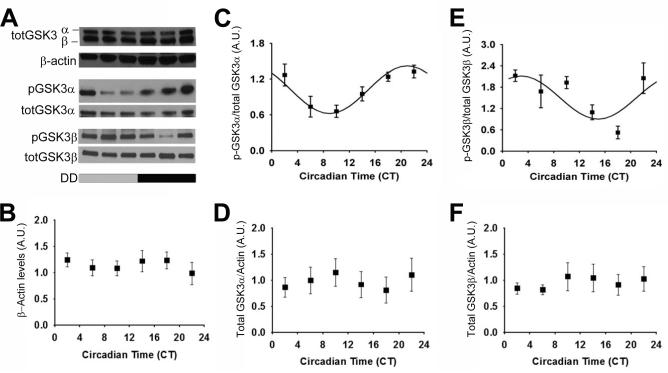
Using Western blot analysis (as in Paul et al., 2012), we investigated p-GSK3α and p-GSK3β levels in mice housed in constant darkness (DD) for 14-20 days. Both p-GSK3α and p-GSK3β (as a percentage of total GSK3α or GSK3β, respectively) displayed a statistically significant rhythm (n = 27/time course; p < 0.05; Figure 1A, C, E) during DD conditions. Interestingly, p-GSK3α peaked during the late subjective night (circadian time or CT phase, 20.94 ± 0.47, where CT 12 refers to activity onset; mesor, 1.02 ± 0.51; amplitude, 0.41 ± 0.08). In contrast, p-GSK3β peaked during the early subjective day (circadian time or CT phase, 2.93 ± 1.45; mesor, 1.51 ± 0.16; amplitude, 0.61 ± 0.22). There were no significant differences in total GSK3α or GSK3β expression with respect to time (cosinor non-linear regression, p > 0.05; Figure 1A, D, F), consistent with previous reports in the SCN and liver from mice housed in LD (Iitaka et al., 2005). The persistence of rhythmic GSK3α/β inactivation in the absence of light cues provides evidence for intrinsic clock regulation of GSK3 inactivation state.
Figure 1. Rhythmic phosphorylation of GSK3 in the SCN persists in constant darkness.
(A) Representative western blots of total GSK3α/β re-blotted for beta-actin (top); p-GSK3α re-blotted for GSK3α (middle); p-GSK3β re-blotted for GSK3β (bottom). Quantification (mean ± SEM per CT bin) of (B) beta-actin levels, (C) p-GSK3α to total GSK3α ratio, (D) total GSK3α (normalized to beta-actin), (E) p-GSK3β to total GSK3β ratio, and (F) total GSK3β (normalized to beta-actin) when sampled at various times across DD. Cosinor non-linear regression: (C) R2 = 0.53, F(2, 24) = 13.73, p < 0.05, N = 27/time course; (D) R2 = 0.25, F(2, 24) = 3.96, p < 0.05; N = 27/time course; (E) R2 = 0.21, F(2, 22) = 2.91, p > 0.05; N = 26/time course.
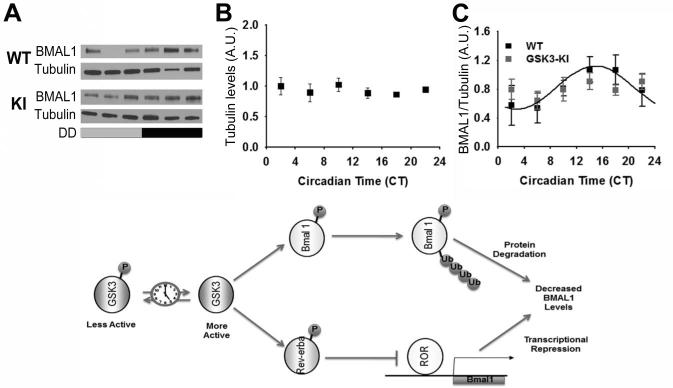
Previous evidence suggests that GSK3 directly phosphorylates at least five core clock proteins: PER2, CRY2, CLOCK, BMAL1, and REVERBα) (Kaladchibachi et al., 2007; Spengler et al., 2009; Kurabayashi et al., 2010; Sahar et al., 2010). Given that phosphorylation of BMAL1 by GSK3β impacts Bmal1 translation and protein stability in vitro (Yin et al., 2006; Sahar et al., 2010; Valnegri et al., 2011), we tested the initial hypothesis that the time-dependent balance of phosphorylated to de-phosphorylated GSK3 is critical for BMAL1 expression rhythms in the SCN. Specifically, p-GSK3α and p-GSK3β rhythms were eliminated using a double transgenic mouse model of chronic GSK3 activity (GSK3-KI mice) in which two serine-alanine mutations (GSK3αS21A/S21A and GSK3βS9A/S9A) render both isoforms of GSK3 constitutively active (but at endogenous levels; McManus et al., 2005; Paul et al., 2012). Wheel running rhythms of these mice have decreased rhythmic amplitude, lengthened alpha (active period), increased activity bouts per day, and increased SCN excitability at night compared to wild-type (WT) controls (Paul et al., 2012). This circadian phenotype was not observed in mice bearing single KI mutations (GSK3αS21A/S21A or GSK3βS9A/S9A), likely due to functional redundancy between the GSK3 isoforms, and therefore, only mice with both α and β isoform mutations were investigated in the current studies. Using Western blot analysis of isolated SCN from individual animals, we quantified BMAL1 expression (as a percentage of α-Tubulin expression) over a 24-h period in isolated SCN after GSK3-KI or WT control mice were housed in DD for at least 2 weeks.
Cosinor non-linear regression revealed a significant BMAL1 expression rhythm in WT mice (n = 27/time course; cosinor nonlinear regression analysis; p < 0.05 for SCN) with peak BMAL1 expression in the subjective night (mesor, 0.82 ± 0.08; amplitude, - 0.30 ± 0.11; phase, 14.85 ± 10.61; Figure 2). In GSK3-KI mice, however, BMAL1 expression did not exhibit a significant 24-h rhythm (n = 25/time course; as determined by cosinor nonlinear regression analysis, p = 0.91; Figure 2). There were no significant differences in α-Tubulin expression levels with respect to time or genotype (p > 0.05). These results indicate that constitutive GSK3 activation disrupts circadian expression of BMAL1 in the central pacemaker. Based on phosphorylation status, we predict that GSK3β activity is highest in WT SCN neurons at approximately CT16, when BMAL1 protein levels peak. GSK3α activity likely peaks around CT9, a time at which BMAL1 protein levels are increasing. These observations are consistent with the conceptual model that GSK3-mediated phosphorylation is an early event, which primes BMAL1 for subsequent degradation via ubiquitin/proteosomal degradation (Figure 2; Sahar et al., 2010). The possibility also remains that chronic GSK3 activation influences BMAL1 protein levels indirectly, through phosphorylation of other clock components (e.g., REV-ERBα, which in turn represses Bmal1 transcription).
Figure 2. Rhythmic BMAL1 expression in mice with chronic GSK3 activation.
(A) Representative western blots of BMAL1 re-blotted for alpha-tubulin. Quantification (mean ± SEM per CT bin) of (B) alpha-tubulin levels and (C) BMAL1 levels (normalized to alpha-tubulin) when sampled across DD for WT (black; N = 27/time course) and GSK3-KI (gray; N = 25/time course) mice. Cosinor non-linear regression: WT, R2 = 0.23, F(2, 24) = 3.50, p < 0.05; GSK3-KI, R2 = 0.01, F(2, 22) = 0.57, p = 0.91. (Bottom) Schematic representing hypothetical model for the interrelationship between GSK3 and the circadian clock. Circadian clock dependent regulation of GSK3 results in time-ofday-dependent oscillations in target proteins, including BMAL1 and REV-ERBα. Phosphorylation of BMAL1 primes this clock component for ubiquitination and subsequent degradation, while phosphorylation of REV-ERBα promotes nuclear translocation and inhibition of ROR-mediated Bmal1 transcription. Increased BMAL1 protein degradation coupled with diminished Bmal1 transcription will result in diminished BMAL1 protein levels and therefore attenuated circadian clock output.
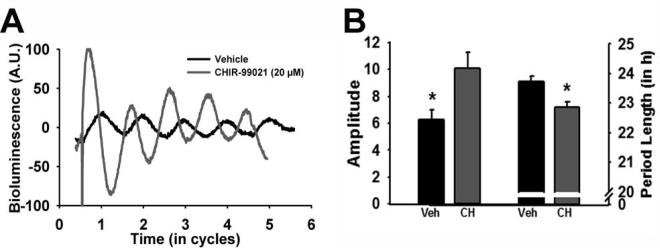
While it is appreciated that Bmal1 is a necessary molecular clock component for behavioral rhythmicity (Bunger et al., 2000), whether or not BMAL1 protein level rhythmicity is necessary for a functional pacemaker is unclear (Shi et al., 2010). In order to test the idea that GSK3 activation rhythms regulate molecular clock amplitude and periodicity, we used the Period2Luciferase (Per2Luc) mouse model as a read-out of real-time molecular clock function (Yoo et al., 2004). Specifically, a selective GSK3 inhibitor (20 μM CHIR-99021) was chronically applied to the media of organotypic tissue cultures of SCN from mPer2Luc+/− reporter mice (Yoo et al., 2004; Besing et al., 2012). This compound inhibits both GSK3 isoforms by competing with ATP for the ATP-binding site of the kinase (Cline et al., 2002; Ring et al., 2003). Application of this small molecule GSK3 inhibitor nearly doubled the amplitude of the chi-squared periodogram analysis and significantly shortened the period length of SCN explant PER2::LUC rhythms (n = 9/group; t-tests, p < 0.05; Figure 3A,B), suggesting that chronic GSK3 inhibition enhances the amplitude and speeds up the periodicity of the molecular clock. These results are consistent with previous in vitro studies that show that molecular clock amplitude is enhanced and period is shortened by pharmacological GSK3 inhibition in fibroblasts and mammalian cell lines (Hirota et al., 2008; Vougogiannopoulou et al., 2008; Li et al., 2012). On the other hand, molecular clock amplitude is reduced by GSK3 over-expression (Sahar et al., 2010; Li et al., 2012). Although it is well documented that the GSK3 inhibitor lithium lengthens the period of circadian rhythms (Mason and Biello, 1992; Abe et al., 2000; Hirota et al., 2008; Mohawk et al., 2009; Osland et al., 2011), our results with a more selective GSK3 inhibitor raise the possibility that lithium-induced effects may be due to nonspecific targets, perhaps via inositol phosphatase inhibition (Quiroz et al., 2004).
Figure 3. GSK3 inhibition modulates the molecular clock.
(A) Representative bioluminescent traces from PER2::LUC organotypic SCN cultures treated with vehicle (black) or CHIR-99021 (20 μM; gray). (B) Bar graph (mean ± SEM) of amplitude (left; t(16) = 2.68; *p < 0.05) and period length (right; t(16) = −3.83, *p < 0.05).
The present study shows that both GSK3 isoforms are rhythmically inactivated in the SCN in constant conditions consistent with previous evidence in LD (Iwahana et al., 2004; Iitaka et al., 2005). These results support the model that GSK3 activity is clock-controlled (Durgan and Young, 2010) potentially regulated by rhythmic phosphorylation of AKT (Panda et al., 2002; Durgan and Young, 2010), a primary upstream kinase of GSK3 (Hur and Zhou, 2010). In addition, our data suggests that GSK3 feeds back to impact molecular clock function in SCN neurons. Specifically, when both GSK3 isoforms were constitutively activated, BMAL1 rhythms within the SCN became arrhythmic. It is interesting that BMAL1 levels do not fall below baseline levels in mice with chronic GSK3 activity. Future studies should investigate whether there is a pool of BMAL1 that is resistant to degradation via the GSK3-E3-ubiquitin pathway and whether chronic GSK3 activation affects transcriptional clock gene rhythms and/or cellular compartmentalization of clock proteins. Our study also found that disruption of GSK3 activation rhythms with chronic pharmacological inhibition nearly doubled PER2::LUC rhythms in SCN organotypic cultures. Investigation of the effects of phase-specific inhibition of GSK3 on the molecular clock will be important to address in the future. Taken together, our results support the conceptual model that disruption of GSK3 activity rhythms impacts the molecular clock such that chronic activation impairs rhythmicity of some molecular clock components (such as BMAL1; Figure 2), while chronic inhibition enhances rhythmicity of the molecular clock (as indicated by real-time PER2::LUC rhythms).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R00GM086683 and R01NS082413 to K.L.G, F31NS086282 to J.R.P, F31NS084683 to LMH, R01HL123574 to M.E.Y. and T32NS061788 – Training Program in the Neurobiology of Cognition and Cognitive Disorders. We thank Rita M. Cowell for technical assistance.
REFERENCES
- Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11:3261–3264. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- Besing RC, Hablitz LM, Paul JR, Johnson RL, Prosser RA, Gamble KL. Neuropeptide Y–Induced Phase Shifts of PER2::LUC Rhythms Are Mediated by Long-Term Suppression of Neuronal Excitability in a Phase-Specific Manner. Chronobiol Int. 2012;29:91–102. doi: 10.3109/07420528.2011.649382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA, Hughes H. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño Z, Gordon-Weeks PR, Kypta RM. The neuron-specific isoform of glycogen synthase kinase-3beta is required for axon growth. J Neurochem. 2010;113:117–130. doi: 10.1111/j.1471-4159.2010.06581.x. [DOI] [PubMed] [Google Scholar]
- Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L, Damico C, Shulman GI. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes. 2002;51:2903–2910. doi: 10.2337/diabetes.51.10.2903. [DOI] [PubMed] [Google Scholar]
- Duncan MJZP. Watching the clock and hitting the snooze button: introduction to the special issue on circadian rhythms and sleep in neurological disorders. Exp Neurol. 2013:1–3. doi: 10.1016/j.expneurol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lewis WG, Liu AC, Wook J, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3β. 2008;105:1–6. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E-M, Zhou F-Q. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado DE, Molina-Porcel L, Carroll JC, Macdonald C, Aboagye AK, Trojanowski JQ, Lee VM-Y. Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer's disease. J Neurosci. 2012;32:7392–7402. doi: 10.1523/JNEUROSCI.0889-12.2012. Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Akiyama M, Miyakawa ÃK, Uchida A, Kasahara J, Fukunaga K. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. 2004;19 doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Kaladchibachi S a, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms. 2007;5:3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabayashi N, Hirota T, Sakai M, Sanada K, Fukada Y. DYRK1A and glycogen synthase kinase 3beta, a dual-kinase mechanism directing proteasomal degradation of CRY2 for circadian timekeeping. Mol Cell Biol. 2010;30:1757–1768. doi: 10.1128/MCB.01047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lu W, Beesley S, Loudon ASI, Meng Q-J. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 2012;7:e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R, Biello SM. A neurophysiological study of a lithium-sensitive phosphoinositide system in the hamster suprachiasmatic (SCN) biological clock in vitro. Neurosci Lett. 1992;144:135–138. doi: 10.1016/0304-3940(92)90734-o. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Miranda-Anaya M, Tataroglu O, Menaker M. Lithium and genetic inhibition of GSK3beta enhance the effect of methamphetamine on circadian rhythms in the mouse. Behav Pharmacol. 2009;20:174–183. doi: 10.1097/FBP.0b013e32832a8f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osland TM, Fernø J, Håvik B, Heuch I, Ruoff P, Lærum OD, Steen VM. Lithium differentially affects clock gene expression in serum-shocked NIH-3T3 cells. J Psychopharmacol. 2011;25:924–933. doi: 10.1177/0269881110379508. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul JR, Johnson RL, Jope RS, Gamble KL. Disruption of circadian rhythmicity and suprachiasmatic action potential frequency in a mouse model with constitutive activation of glycogen synthase kinase 3. Neuroscience. 2012;226:1–9. doi: 10.1016/j.neuroscience.2012.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- Ring DB, Johnson KW, Henriksen EJ, Nuss JM, Goff D, Kinnick TR, Ma ST, Reeder JW, Samuels I, Slabiak T, Wagman AS, Hammond M-EW, Harrison SD. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-corsi P. Regulation of BMAL1 Protein Stability and Circadian Function by GSK3 b -Mediated Phosphorylation. 2010;5 doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Plattner F, Irvine EE, Cummings DM, Edwards F a. Dynamic range of GSK3α not GSK3β is essential for bidirectional synaptic plasticity at hippocampal CA3-CA1 synapses. Hippocampus. 2014;4:1–4. doi: 10.1002/hipo.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Curr Biol. 2010;20:316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutar MPM, Kim W-Y, Williamson R, Peggie M, Hastie CJ, McLauchlan H, Snider WD, Gordon-Weeks PR, Sutherland C. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. J Neurochem. 2010;115:974–983. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Schumer M, Antoch MP. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle. 2009;8:4138–4146. doi: 10.4161/cc.8.24.10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnegri P, Khelfaoui M, Dorseuil O, Bassani S, Lagneaux C, Gianfelice A, Benfante R, Chelly J, Billuart P, Sala C, Passafaro M. A circadian clock in hippocampus is regulated by interaction between oligophrenin-1 and Rev-erbα. Nat Neurosci. 2011;14:1293–1301. doi: 10.1038/nn.2911. [DOI] [PubMed] [Google Scholar]
- Vougogiannopoulou K, Ferandin Y, Bettayeb K, Myrianthopoulos V, Lozach O, Fan Y, Johnson CH, Magiatis P, Skaltsounis A-L, Mikros E, Meijer L. Soluble 3’,6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase -3 alter circadian period. J Med Chem. 2008;51:6421–6431. doi: 10.1021/jm800648y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar M a. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.