Abstract
Physical dependence on alcohol and anesthetics stems from neuroadaptive changes that act to counter the effects of sedation in the brain. In Drosophila, exposure to either alcohol or solvent anesthetics have been shown to induce changes in expression of the BK-type Ca2+- activated K+ channel gene slo. An increase in slo expression produces an adaptive modulation of neural activity that generates resistance to sedation and promotes drug tolerance and dependence. Increased BK channel activity counteracts the sedative effects of these drugs by reducing the neuronal refractory period and enhancing the capacity of neurons for repetitive firing. However, the brain regions or neuronal populations capable of producing inducible resistance or tolerance remain unknown. Here we map the neuronal substrates relevant for the slo-dependent modulation of drug sensitivity. Using spatially-controlled induction of slo expression we identify the mushroom bodies, the ellipsoid body and a subset of the circadian clock neurons as pivotal regions for the control of recovery from sedation.
Keywords: Drug tolerance, Ellipsoid body, Mushroom bodies, BK channels, Pigment dispersing factor, Drosophila
Introduction
Repeated exposure to sedative drugs, such as alcohol and solvent anesthetics, is known to induce compensatory changes in the physiology of an organism that ameliorate the effects of the drug. These adaptive changes underlie functional drug tolerance—manifested as a decrease in a drug effect upon re-exposure to the drug (Young and Goudie 1995). Drug tolerance has been mechanistically linked to withdrawal symptoms (indicative of physiological dependence) in that the cellular changes that cause tolerance are thought to persist and cause withdrawal symptoms (Koob and Bloom 1988; Littleton 1998; Ghezzi and Atkinson 2011). Drug tolerance promotes increased drug consumption, as ever-increasing doses are required to achieve the same initial pleasurable effects (Schuckit 1994).
In Drosophila, sedation with solvent anesthetics such as benzyl alcohol or ethanol induces tolerance to later sedation by these drugs. Recent genetic evidence has shown that tolerance to the sedative effects of these drugs is mediated, at least in part, by a drug-induced increase in expression of the BK-type Ca2+- activated K+ channel gene slo (Ghezzi et al. 2004; Cowmeadow et al. 2006). Exposure to a sedating dose of either ethanol or benzyl alcohol has been shown to induce neural expression of the slo gene. Blocking neural expression from the slo gene blocks the acquisition of tolerance to both drugs. Meanwhile, transgenic over-expression of slo, induced globally throughout the fly, causes resistance to both drugs in drug-naive animals. The drug induced enhancement of the Ca2+-activated K+ channel activity causes resistance to sedation by increasing neural excitability. In Drosophila, sedative exposure to alcohols and volatile anesthetics have been shown to inhibit neural excitability, at least in part, by increasing the neuronal refractory period and thus reducing the capacity for repetitive firing (Lin and Nash 1996; Ghezzi et al. 2010). Meanwhile, increased slo expression was shown to decrease the neuronal refractory period and enhance repetitive firing, thereby opposing the acute effects of acute drug sedation (Ghezzi et al. 2010). After drug clearance however, the slo-dependent increase in neural excitability persists and results in an increase in seizure susceptibility—a symptom typical of severe alcohol withdrawal (Ghezzi et al. 2010; Ghezzi et al. 2012).
In mammals, BK channel activity has also been shown to play a pivotal role in the development of tolerance to ethanol (Treistman and Martin 2009). For example, microRNA regulation of slo-encoded BK channel expression in rat magnocellular neurons has been shown to underlie a form of functional ethanol tolerance (Pietrzykowski et al. 2008). Furthermore, as in flies, increased BK channel activity has also been linked to enhanced neuronal firing frequency in rats (Gu et al. 2007) and to contribute to the development of temporal lobe seizures in mice (Brenner et al. 2005). In humans, increased BK channel activity also promotes neural excitability. A genetic predisposition for coexistent generalized epilepsy and paroxysmal dyskinesia has been linked to a gain-of-function mutation in the slo gene (Du et al. 2005). The function of slo in flies and mammals, both with respect to drug responses and with respect to modulating overall neural excitability appears to be highly conserved.
Because a global induction of slo has been linked to the acquisition of drug tolerance we thought that it was important to determine where in the brain slo induction would produce benzyl alcohol resistance and phenocopy drug tolerance (Ghezzi et al. 2004; Cowmeadow et al. 2006). To do this we use the versatile Gal4/UAS system (Brand and Perrimon 1993) to spatially restrict slo over-expression to distinct brain regions in order to determine the effects of localized slo over-expression on drug resistance.
Results
To achieve the desired localized over-expression of the slo gene, the Gal4/UAS system combines two independent transgenic constructs—a Gal4 driver and a UAS responder. Through the collective endeavors of the Drosophila community, hundreds of Gal4 driver lines with unique expression patterns have been produced. These lines are readily available for use from the Drosophila community and stock centers. In addition, for this study we have generated a transgenic line that carries a slo cDNA under the regulation of the UAS promoter (Figure 1A and methods). By mating a collection of Gal4 lines with the UAS-slo line, we can produce a variety of animals that express UAS-slo in discrete parts of the brain.
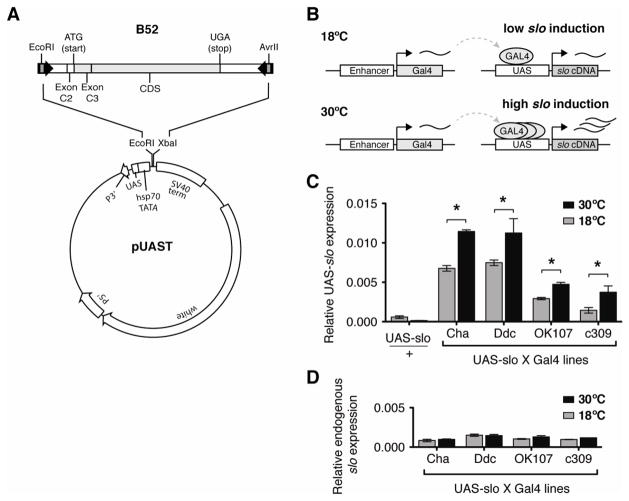
Figure 1.
Temperature-dependent Gal4 induction of UAS-slo expression. A) Map of the UAS-slo construct. Black arrows indicate primers used for PCR amplification from the previously described B52 construct. White boxes indicate untranslated regions, gray boxes indicate protein coding regions. B) Schematic representation of the two-part Gal4/UAS system. A cell- or tissue-specific enhancer drives expression of the Gal4 transcription factor gene. Once translated, GAL4 binds to its DNA binding target UAS to induce expression from the adjacent slo cDNA. GAL4 binding is enhanced at higher temperatures, resulting in higher transcription rates from the UAS-slo cDNA. C) Expression from the UAS-slo transgene in an uninduced state (lacking a Gal4 driver, but otherwise identical) and induced by four independent Gal4 drivers after incubation of female adult flies at 18°C or 30°C for 3 consecutive days. Gal4 drivers were: the cholinergic Cha-Gal4 driver, the dopaminergic and serotonergic Ddc-Gal4 driver, and the OK107-Gal4 and c309-gal4 mushroom body drivers. In all 4 Gal4 lines, induction of UAS-slo was significantly higher in flies incubated at 30°C than those of flies incubated at 18°C. D) Expression of the endogenous neural slo transcript in the four independent UAS-slo/Gal4 lines after incubation at 18°C or 30°C for 3 consecutive days. In all 4 lines, endogenous slo expression was unaffected by the temperature treatment. Error bars show SEM, n=3, * indicates p<0.05 by Student’s t-test.
The Gal4/UAS system not only allows for spatial regulation of gene expression, but the levels of transcript expression can also be manipulated. The interaction between the GAL4 transcription factor and the UAS DNA element is temperature dependent. GAL4 binds more effectively to the UAS at higher temperatures than at lower ones (Jarrett 2000; Duffy 2002). Therefore, the level of expression from the Gal4/UAS system is positively correlated with the incubation temperature. By manipulating the incubation temperature, one can achieve distinct expression levels in given set of cells (Figure 1B).
Temperature-dependent Gal4 induction of UAS-slo expression
To test the efficacy of the temperature control of Gal4-induced expression we first crossed the UAS-slo line to four different Gal4 driver lines. The Gal4 lines used were: Cha-Gal4, Ddc-Gal4, OK107-Gal4 and c309-Gal4. The expression patterns of these reporters have been described in detail (for references, see Table I). The Cha-Gal4 construct consists of the promoter for the Choline acetyltransferase gene Cha and therefore has an expression pattern restricted to cholinergic neurons. The Ddc-Gal4 construct consists of the promoter for the Dopa decarboxylase gene Ddc, and has an expression pattern restricted to dopaminergic and serotonergic neurons. The OK107-Gal4 and c309-Gal4 constructs are enhancer trap insertions that drive expression primarily within mushroom body neurons. The progeny of these crosses, carrying both the Gal4 driver transgene and the UAS-slo transgene, were raised at room temperature throughout the initial developmental stages. Two days after eclosion from the pupal stage, adult flies were collected and separated into two groups. One group was incubated at 18°C and the other at 30°C for three consecutive days. After incubation, total RNA was extracted and slo expression from the UAS transgene was measured by real-time quantitative RT-PCR. The primers that we used reported the expression pattern only from the UAS-slo transgene. Figure 1C shows expression from the UAS-slo transgene in flies lacking a Gal4 Driver, or in combination with the four different Gal4 drivers after incubation at 18°C and 30°C. While expression from the transgene was minimal in the UAS-slo / + control, strong expression was evident across all four Gal4/UAS-slo lines. Most importantly, an increase in slo expression was evident after incubation at 30°C when compared to animals incubated at 18°C. On the contrary, expression from the endogenous slo gene was relatively low and was unaffected by the temperature induction protocol in the Gal4/UAS-slo stocks (Figure 1D).
Table I.
Gal4 driver lines used in this study
| Gal4 Lines | Expression patterna | References |
|---|---|---|
| Cha | All cholinergic neurons; mostly optic lobes and antennal lobes; faint expression in central complex | Salvaterra and Kitamoto 2001 |
| Ddc | Dopaminergic and serotenergic neurons; multiple tracts | Li et al. 2000; Sitaraman et al. 2008 |
| 10y | Multiple tracts scattered throughout the brain | www.fly-trap.org |
| 71y | Multiple extrinsic neurons innervating the fan shaped body | Young and Armstrong 2010; www.fly-trap.org |
| 103y | Most Kenyon cells of Mushroom bodies projecting to the α/β lobes, except core of each cluster; fan shaped bodies and pars intercerebralis | Tettamanti et al. 1997; Gatti et al. 2000; www.fly-trap.org |
| c758 | Mushroom body α/β lobes; some optic and antennal lobe neurons | Pitman et al. 2006; www.fly-trap.org |
| 11y | Mushroom bodies, restricted to α/β lobes | www.fly-trap.org |
| 201y | Mushroom bodies Kenyon cells projecting to core elements of the α/β lobes and to a subset of fibres within the γ lobe; pars intercerebralis | O’Dell et al. 1995; Connolly et al. 1996; www.fly-trap.org |
| 43y | Mushroom body α/β lobes and some output neurons from γ lobe | Armstrong et al. 1998; www.fly-trap.org |
| 238y | Mushroom bodies (α/β, α′/β′ and γ lobes); pars intercerebralis; other extrinsic tracts | Connolly et al. 1996; Aso et al. 2009; www.fly-trap.org |
| OK107 b | Mushroom bodies neurons from α/β, α′/β′ and γ lobes; some antennal lobe peripheral neurons | Lee et al. 1999; Kitamoto 2002 |
| c309 b | Mushroom bodies (α/β, α′/β′ and γ lobes); pars intercerebralis; optic lobes; antennal lobe; subesophageal ganglion neurons | Connolly et al. 1996; Kitamoto 2002; www.fly-trap.org |
| 106y b | Mushroom bodies (α/β, α′/β′ and γ lobes); ellipsoid body ring neurons (R1-4); pars intercerebralis; optic lobes; retina; antennal lobe; subesophageal ganglion neurons | www.fly-trap.org |
| c041 b | Ellipsoid body Ring neurons R1 and R4 projecting from the Lateral triangle | www.fly-trap.org |
| c232 | Cell bodies and the neuropil of the ellipsoid body, specifically R3 and R4d ring neurons projecting to the Lateral triangle | Connolly et al. 1996; Renn et al. 1999; www.fly-trap.org |
| c819 | R2 and R4m Ring neurons of the ellipsoid body | Renn et al. 1999; www.fly-trap.org |
| 16y b | Antennal and optic lobe neurons as well as lateral neurons | Bhandari et al. 2006; www.fly-trap.org |
| tim b | Dorsal and ventral lateral neurons | Kaneko and Hall 2000 |
| Pdf b | Ventral lateral neurons | Renn et al. 1999; Park et al. 2000 |
| 167Y | Larval central brain and ventral ganglion; unknown adult expression | Manseau et al. 1997; www.fly-trap.org |
Expression pattern descriptions are approximate. Detailed expression patterns can be found in the adjacent references.
Gal4 lines shown to affect recovery from sedation by benzyl alcohol in this study.
Effects of localized slo-induction on behavioral recovery from benzyl alcohol sedation
We utilized the temperature-induction protocol described above to test the effects of spatially-restricted UAS-slo induction on the behavioral recovery from benzyl alcohol sedation. For this assay, flies were raised at room temperature throughout the initial developmental stages. Two days after eclosion from the pupal stage, adult flies were collected and separated into two groups. One group was incubated at 18°C and the other at 30°C for three consecutive days. After incubation, flies in both groups were sedated with benzyl alcohol in glass vials. Once sedated (which took about 15 minutes) flies were transferred to benzyl alcohol-free vials and recovery from sedation was monitored (Al-Hasan et al. 2011). Because flies recovering from benzyl alcohol immediately begin to climb the walls of the vial, recovered flies can be easily scored using automated image processing software (Ramazani et al. 2007). To circumvent behavioral differences associated with genetic background, comparisons are only made within the same line of flies. For every drug resistance assay performed, we compared the recovery time from drug sedation between animals that were incubated at 30°C and animals of the same genotype that were incubated at 18°C.
To determine whether the manipulation of temperature has a non-specific effect on behavioral drug resistance, we first tested non-transgenic wild-type flies for an effect of the temperature shift protocol on drug sensitivity. Two groups of age-matched adult female flies from the Canton S wild-type strain (CS) were incubated for three consecutive days at either 18°C or 30°C and then subjected to a sedation recovery assay. We observed that the recovery rate from benzyl alcohol sedation of flies incubated at 18°C was statistically indistinguishable from that of CS flies incubated at 30°C (Figure 2A). Similarly, in flies carrying the transgenic UAS-slo construct in the absence of a Gal4 driver, recovery from sedation was unaffected by the temperature induction protocol (Figure 2B). These control flies were also heterozygous for the slo4 mutation, as all other transgenic stocks tested in this study. The slo4 heterozygous background was chosen in an attempt to avoid high basal expression of slo. The heterozygous slo4 background however does not affect the capacity of flies to acquire tolerance to benzyl alcohol as compared to wild type-flies (Figure 2C–D). Both CS wild-type flies and UAS-slo flies heterozygous for slo4 recover faster from their second benzyl alcohol exposure than from their first (Figure 2C–D).
Figure 2.
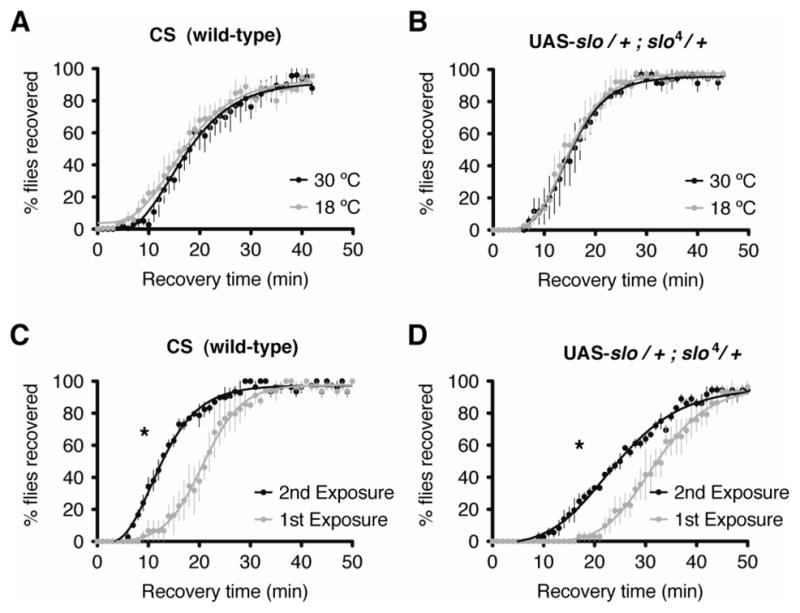
The temperature induction protocol does not affect recovery from sedation in flies lacking a Gal4 driver. Shown are recovery curves from benzyl alcohol sedation of wild-type CS flies and the UAS-slo line generated for this study. Plotted are averages of the percentage of flies that resumed climbing the walls of the vial over time. A–B) The gray curve represents the recovery of a population incubated at 18°C for 3 consecutive days prior to the benzyl alcohol sedation treatment. The black curve represents the recovery of a population incubated at 30°C for 3 consecutive days prior to the benzyl alcohol sedation treatment. Both, wild-type CS (A) and the UAS-slo transgenic flies lacking a Gal4 driver (B) show no statistical difference between the recovery rates of the groups incubated at the different temperatures. C–D) Capacity to develop tolerance to benzyl alcohol is also unaffected by the presence of the UAS-slo transgene. The gray curve represents the recovery of a population recovering from its 1st exposure to benzyl alcohol. The black curve represents the recovery of a population recovering from its 2nd exposure to benzyl alcohol. Both, wild-type CS (C) and the UAS-slo transgenic flies (D) develop tolerance to benzyl alcohol after a single exposure, as shown by an the more rapid recovery from sedation from their second exposure than from the first. Error bars represent SEM (* indicates p<0.05 by the Log-rank test, n=6).
In order to identify the parts of the fly brain in which slo induction can generate benzyl alcohol-resistance, we tested the effects of spatially-restricted increased slo expression on the behavioral recovery from drug sedation. For this experiment, we targeted transgenic expression of slo using twenty different Gal4 driver lines, each with a distinct neuronal expression pattern, encompassing different types of neurons and brain structures. A list of the different Gal4 lines used, along with a description of the expression pattern, is presented in Table I.
For each sedation recovery test, homozygous Gal4 driver lines were crossed to the homozygous UAS-slo line. The benzyl alcohol recovery analysis was carried out as described above upon age-matched female progeny from each Gal4 X UAS-slo cross. Flies were divided into two groups 2 to 3 days after eclosion. For one group, slo expression was induced with a 3 day incubation period at 30°C (slo-induced), whereas for the other group, slo expression was minimized by incubating flies at 18°C for 3 days (slo-uninduced). Of the twenty lines tested with benzyl alcohol, only four lines showed a significant (P<0.05) slo-dependent increase in resistance to sedation. These are: 106y, c041, c309 and OK107 (Figure 3). On the other hand, another three Gal4 drivers produced a slo-dependent decrease in benzyl alcohol resistance upon localized slo overexpression. These are: tim-Gal4, Pdf-Gal4, and 16y-Gal4 (Figure 4). None of these lines showed a change in sedation recovery in the absence of the UAS-slo transgene (Figures 3 and 4). However, we noticed that there was high degree of variability in the sedation recovery time amongst the different lines. From our replicate experiments, we suspect that a significant portion of this variability is due to day-to-day variations in environmental factors, however, genetic background can also affect behavioral recovery from sedation.
Figure 3.
Induction of slo in the mushroom and ellipsoid bodies causes resistance to sedation. Shown are recovery curves from benzyl alcohol sedation of four different Gal4 lines driving expression of the transgenic slo cDNA and the respective Gal4 lines lacking the UAS-slo transgene as a control. Plotted are averages of the percentage of flies that resumed climbing the walls of the vial over time. The black curve represents the recovery of a population incubated at 30°C for 3 consecutive days prior to the benzyl alcohol sedation treatment (slo-induced). The gray curve represents the recovery of a population incubated at 18°C for 3 consecutive days prior to the benzyl alcohol sedation treatment (slo-uninduced). Temperature-dependent induction of the UAS-slo transgene with 106y-Gal4 (A), c041-Gal4 (C), c309-Gal4 (E), and OK107 (G) enhanced the rate of recovery from benzyl alcohol sedation, while the respective controls (B, D, F and H) did not. Error bars represent SEM (* indicates p<0.05 by the Log-rank test, n=6).
Figure 4.
Induction of slo in the lateral ventral neurons causes sensitization to sedation. Shown are recovery curves from benzyl alcohol sedation of three different Gal4 lines driving expression of a transgenic slo cDNA and the respective Gal4 lines lacking the the UAS-slo transgene as a control. Plotted are averages of the percentage of flies that resumed climbing the walls of the vial over time. The black curve represents the recovery of a population incubated at 30°C for 3 consecutive days prior to the benzyl alcohol sedation treatment (slo-induced). The gray curve represents the recovery of a population incubated at 18°C for 3 consecutive days prior to the benzyl alcohol sedation treatment (slo-uninduced). Temperature-dependent induction of the UAS-slo transgene with tim-Gal4 (A), Pdf-Gal4 (C), and 16y-Gal4 (E) causes sensitization to sedation by benzyl alcohol as indicated by the slower recovery rate. Sedation recovery of the respective controls (B, D, and F) remained unaffected by the temperature induction protocol. Error bars represent SEM (* indicates p<0.05 by the Log-rank test, n=6).
For these reason, when evaluating whether a line is capable of producing inducible slo-dependent benzyl alcohol resistance, we compared the recovery from sedation profile of a stock when it is incubated at 30°C (induced transgenic slo expression) to the same stock when it is incubated at 18°C. Because we are comparing a stock to itself, we avoid almost all variability in response that arises from differences in genetic background from interfering with our ability to determine whether or not slo induction phenocopies tolerance (produces inducible resistance). Furthermore, because the resistance test for a stock are performed at the same time, the day-today variability in benzyl alcohol sensitivity cannot confound our interpretation. Finally, the temperature shift produces changes in behavior in the Gal4/UAS lines but not in the parental lines indicating that this effect is not due to the Gal4 insertion or UAS-slo alone.
The four lines that caused a slo-dependent increase in resistance to sedation direct expression to either the mushroom bodies, the ellipsoid body, or to both structures. Lines c309-Gal4 and OK107-Gal4 predominantly drive expression in the mushroom body lobes but also elicit low level expression in foci scattered throughout the brain (Connolly et al. 1996; Kitamoto 2002). Expression of the c041-Gal4 line is limited to the ellipsoid body and to a series of clustered cell bodies around the central complex, while in line 106y-Gal4, expression is evident in both the mushroom bodies and the ellipsoid body (fly-trap.org).
The ellipsoid body is an almost circular structure of neuropil within the central complex of the fly brain that lays anterior to the fan-shaped body. It is mainly composed of ring-like neuronal projections that arise from the lateral protocerebrum. There are five known types of ellipsoid body ring neurons; R1, R2, R3, R4m, and R4d each of which occupy different concentric rings of the ellipsoid body (Renn et al. 1999). From the twenty enhancer-trap lines tested, four lines express in different combinations of ellipsoid body R1–R4 neurons (Table I). Line 106y expresses in all ring neurons; line c041 expresses in R1 and R4 neurons; line c232 expresses in R3 and R4d; and line c819 expresses in R2 and R4m neurons. However, when crossed to UAS-slo, we found that only lines 106y and c041 caused resistance to sedation after slo induction, indicating that overexpression of slo in the 106y- and c041-positive ring neurons (but not those in the other c232- and c819-positive ring neurons) can produce the drug-resistance phenotype (Figure 5). Therefore, we infer that within the ellipsoid body, the R1 neurons are the critical element in slo-induced resistance to sedation.
Figure 5.

Increased expression of slo in distinct neuronal subgroups of the mushroom bodies, ellipsoid bodies and Lateral Neurons differentially affect resistance to sedation. A) Chart shows the change in resistance to sedation after temperature-dependent slo induction in the twenty different Gal4-lines tested. The UAS-slo/+ line lacking a Gal4 Driver is displayed as a reference. Change in resistance was determined by calculating the relative difference in the 50% recovery time between slo-induced and slo-uninduced flies. Error bars are SEM, * denotes a significant difference between the recovery curves of the slo-induced and slo-uninduced flies as calculated by Log-rank test. Three lines expressing slo in the mushroom bodies (Ok107, c309, and 106y) and two lines expressing in the ellipsoid body (106y and c041) induce a significant increase in resistance to sedation. Three lines expressing slo in the lateral neurons (16y, tim and Pdf) induce a significant decrease in resistance to sedation. B) Expression patterns within distinct neuronal subgroups of the mushroom bodies, ellipsoid body and Lateral Neurons (LN) are indicated for each Gal4 line (+). Except for line 238y (gray rectangle), all mushroom body lines expressing in the α′/β′ lobes (Ok107, c309, and 106y) induce a significant increase in resistance to sedation (light-red rectangle). For the ellipsoid body, only those lines expressing in the R1 ring neurons (106y and c041) induce a significant increase in resistance to sedation (dark-red rectangle). All lines expressing in the LNV neurons (16y, tim and Pdf) induce a significant decrease in resistance to sedation (green rectangle).
The second structure implicated as having a role in slo-dependent resistance to sedation is the mushroom body. Each mushroom body (one per hemisphere) is made of approximately 2500 Kenyon cells arising from a dense cluster of cell bodies located dorsal and posterior to each brain hemisphere (Technau and Heisenberg 1982; Aso et al. 2009). Kenyon cells project their axons to the front of the brain through a densely packed stalk-like structure called the peduncle. From the peduncle axons branch into 5 distinct lobes. The α and α′ lobes project Kenyon cell axons vertically, whereas the β, β′ and γ lobes project medially. Three major classes of Kenyon cells have been identified to innervate distinct subsets of mushroom body lobes. One class project to the α and β lobes, another to the α′ and β′ lobes, and the third projects to the γ lobes (Crittenden et al. 1998; Lee et al. 1999; Tanaka et al. 2008). In this study, nine of the Gal4 lines used express at least partially in the mushroom bodies. Of these, lines 103y, 11y, 201y, and c758 restrict expression to subsets of neurons within the α/β lobes, the γ lobes, or both, but notably lack expression in the α′/β′ lobes (Table I). Lines 238y, OK107, c309, and 106y, on the other hand, express robustly in the α′/β′ lobe. When crossed to the UAS-slo transgenic line, none of the lines lacking expression in the α′/β′ lobe induced resistance to sedation after induction (Figure 5). On the contrary, 3 out of 4 lines that direct expression to all lobes, including the α′/β′ lobes, result in a significant increase in resistance to sedation after slo induction (Figure 5). The sole exception was line 238y, which expresses in all 5 lobes but failed to induce drug resistance upon slo induction (Figure 5). Although these results provide support for a potential involvement of mushroom body neurons in slo-dependent resistance to sedation, putatively through the involvement of the α′/β′ neurons, the contradictory pattern presented in line 238y suggests perhaps a more intricate neuroanatomical involvement of the mushroom bodies. In fact, recent studies revealed that α′/β′ and α/β lobes encompass further neuronal subtypes, each with differentiated gene expression profiles, neurotransmitter systems, connectivity to extrinsic neurons, and behavioral functions (Yang et al. 1995; Strausfeld et al. 2003; Keene and Waddell 2007). A conclusive assessment of the role of the sub-regions within the mushroom body responsible for this phenotype will require further genetic dissection.
In contrast to the ellipsoid and mushroom body lines that produce a slo-dependent increase in resistance to sedation, induction of slo by tim-Gal4, Pdf-Gal4, and 16y-Gal4, caused animals to become more sensitive to benzyl alcohol sedation (Figure 5). One commonality between the expression patterns of these drivers is that all three lines express in cells of the central circadian pacemaker but lack expression in the mushroom or ellipsoid body neurons. The tim-Gal4 driver has a well defined expression pattern within the Drosophila circadian clock neurons that includes the dorsal and ventral lateral neurons (LND and LNV) and the dorsal neurons (DN1 and DN3). Pdf-Gal4 is the most restrictive line tested in this study. It exclusively expresses within the LNV circadian clock neurons. Finally, the 16y-Gal4 line displays a broader expression pattern that includes antennal and optic lobe neurons, but also induces expression in the LNV neurons. These results suggest that slo over-expression within the Drosophila clock neurons, specifically the LNV neurons, exerts an unexpected negative effect on the recovery from sedation (Figure 5).
Discussion
Serial sedation with benzyl alcohol induces slo gene expression and this induction has been shown to produce functional tolerance (inducible resistance) to subsequent drug sedation (Ghezzi et al. 2004). While pan-neural induction of slo from a transgene has been shown to phenocopy tolerance (Ghezzi et al. 2004), drug sedation may generate tolerance because of induction in only a part of the brain. Because slo expression is difficult to visualize by in situ hybridization (Becker et al. 1995) it has not been possible to directly determine where in the brain induction occurs. Instead, we used Gal4 drivers to increase slo expression in different structures in the brain and asked if increased expression resulted in behavioral drug resistance. In this manner we can determine which structures are capable of producing a drug resistance phenotype in response to increased slo expression. While the use of temperature to alter Gal4 expression is not the predominant use of Gal4 in Drosophila research, this is mostly because most studies benefit from a scenario in which Gal4 expression is either turned completely ON or OFF. Because in our study we wished to study the effects of slightly increasing expression from a low level to a higher level, the temperature paradigm better suits our needs.
Using this method, we have identified three neural structures as having a central role in slo-mediated resistance to sedation by the organic solvent anesthetic benzyl alcohol. We found that increased expression of slo only within the mushroom bodies or the ellipsoid bodies caused the animals to recover more rapidly from benzyl alcohol sedation (resistance to drug sedation) phenocopying functional drug tolerance. On the other hand, we found that increased expression of slo in the Pdf-expressing LNV neurons caused the animals to recover more slowly from benzyl alcohol sedation (drug sensitization).
All three neural structures identified here have been previously shown to regulate some of the higher order processes of the insect brain, and are implicated in arousal, the regulation of wake-sleep cycles, locomotor activity, and learning and memory. In particular, arousal and the regulation of wake-sleep cycles are processes that are likely to be relevant to understanding the regulation of the rate of recovery from anesthesia (van Swinderen and Andretic 2003; van Swinderen 2006). In Drosophila and other insects the mushroom bodies mediate complex behaviors such as place and associative memory (Mizunami et al. 1998b; Rybak and Menzel 1998), context dependent sensory filtering (Liu et al. 1999), motor control and the regulation of sleep (Mizunami et al. 1998a; Joiner et al. 2006; Pitman et al. 2006). The mushroom bodies receive neuronal inputs from several sensory modalities, including visual, olfactory, and somatosensory and are thought to be the insect homologue to the mammalian forebrain (Kurusu et al. 2000). The ellipsoid body is part of the central complex of the Drosophila brain and is tightly associated with the control of locomotor, geotactic and flight behaviors (Martin et al. 1999; Ilius et al. 2007; Rezaval et al. 2007). The ellipsoid body has also been implicated in the formation of visual and spatial memories (Neuser et al. 2008; Pan et al. 2009). Finally, the ventral lateral neurons (LNV) regulate circadian rhythmicity and wake/sleep cycles in flies by coupling autonomously oscillating cells. The LNV neurons release the neuropeptide pigment dispersing factor (Pdf) that synchronizes the autonomous clocks within neurons to produce a single coherent biological rhythm throughout the animal (Kaneko et al. 1997; Nitabach et al. 2006). Recent studies have shown that the ellipsoid body expresses the Pdf receptor, thus the ellipsoid body is presumed to be one of the innervating targets of the LNV neurons and responsive to Pdf release (Parisky et al. 2008).
A global increase in slo expression has been shown to enhance neural excitability by reducing the neural refractory period, thereby increasing the capacity of CNS neurons to fire repeatedly and as a side-effect increasing the probability of seizure (Ghezzi et al. 2010). Thus, we associate increased slo expression, benzyl alcohol resistance and tolerance, with increased neural activity. However, we do not know that increased slo expression is a neural excitant in all of the structures tested. Here we show that in some parts of the brain increased slo expression generates benzyl alcohol resistance while in others it generates benzyl alcohol sensitivity. It may be that increased slo expression has opposing effects on firing rates in the mushroom bodies, ellipsoid bodies, and LNV neurons. These are not contradictory hypotheses. If the LNV neurons innervate the ellipsoid bodies, in a sense, the ellipsoid bodies could act epistatically to the LNV neurons and therefore when slo is over-expressed in both neural structures only the consequence of over-expression of slo in the ellipsoid body is manifested. In this circumstance, the behavioral output might only report the change associated with increased ellipsoid body expression. It is a limitation of this study that we can only observe the behavioral output of the change in expression to determine which structures are capable of producing the drug resistance phenotype.
The ellipsoid bodies have been previously implicated in the production of drug induced behaviors. Urizar et al. (2007) have shown that mutations in the homer gene interfere with the acquisition of tolerance to the onset of ethanol sedation. Homer was shown to be expressed in the ellipsoid body but not in the mushroom bodies and transgenic expression of homer in the ellipsoid bodies was shown to complement homer mutations with respect to their ethanol phenotypes. Furthermore, Kong et al. (2010) found that expression of the Drosophila dopamine D1 receptor ortholog, DopR, exclusively within the ellipsoid body is required for the ethanol-stimulated increase in locomotor activity upon acute exposure to ethanol. However, in both these cases, the R2 and R4 neurons of the ellipsoid body were implicated in the behavioral responses under study, but not the R1 neurons as seen here. This discrepancy suggests that different arousal phenotypes may be under control of distinct neuronal substrates within the ellipsoid body.
A previous study has identified independent systems for the control of distinct states of arousal in Drosophila (Lebestky et al. 2009). A mechanistic distinction has been made between the exogenous control of arousal in response to environmental stimulus, such as the mechanically induced startle response, and the endogenous control of arousal, such as the circadian regulation of sleep-wake transitions. Lebestky and colleagues showed that DopR function in the ellipsoid body inhibits exogenous arousal but when expressed in the LNV neurons it stimulates endogenous arousal. In our survey, we also show that the ellipsoid body and the LNV neurons have opposing effects on an arousal phenotype, supporting the notion that these neural substrates complement each other to finely tune the emergent arousal state.
Based on these results and on the published neuroanatomical and physiological profiles of the structures identified in this study, we propose a hypothesis for the regulation of drug resistance (Figure 6). In this model, the ellipsoid body is the central controller of arousal, whereas the mushroom bodies and ventral lateral clock neurons modulate the activity of the ellipsoid body in opposing ways in response to slo induction. Increased slo expression in the mushroom bodies promotes ellipsoid body-mediated arousal generating benzyl alcohol resistance, while increased slo expression in the ventral lateral clock neurons suppresses this function of the ellipsoid body, leading to a reduction in arousal and sensitization to benzyl alcohol. While currently there is no neuroanatomical evidence of a direct connection between the PDF-positive LNVs and the ellipsoid body neurons, robust expression of PDF receptors in ellipsoid bodies suggests this possibility (Parisky et al. 2008; Shang et al. 2008; Lear et al. 2009). However, this evidence is not conclusive, and connectivity is merely hypothetical. More work needs to be done to test this model, however, the model summarizes our observations in this initial investigative foray to identify the neural origins of functional drug tolerance in Drosophila.
Figure 6.

Hypothetical model for neuronal control of tolerance to sedation. A) Schematic representation of the fly brain highlighting the structures shown to influence drug resistance in a slo-dependent manner. Induction of slo within the ellipsoid body R1 ring neurons (ebR1/dark red) and mushroom bodies α′/β′ lobes (light red) results in drug resistance, while slo induction in the large and small ventral lateral neurons (lLNV and sLNV) causes drug sensitization (green). While there is no neuroanatomical evidence of a direct interaction between the PDF-positive LNVs and the ellipsoid body, strong PDF-receptor expression in ellipsoid body neurons suggests this possibility. This hypothetical scenario is marked with a “?”. B) A working model for the regulation of drug resistance is that the ellipsoid body is the central controller of arousal and that the mushroom bodies and ventral lateral clock neurons modulate the activity of the ellipsoid body in opposite ways in response to slo induction. Increased slo expression in the mushroom bodies promotes ellipsoid body-mediated arousal generating benzyl alcohol resistance while increased slo expression in the ventral lateral clock neurons suppress this function of the ellipsoid body leading to a reduction in arousal and sensitization to benzyl alcohol.
At a systems level, the circuitry in the Drosophila brain differs greatly from the circuitry in a mammalian brain. Thus, we would not expect that the identification of the structure in the Drosophila brain that underlies functional tolerance will, by itself, have direct relevance to the understanding of how the mammalian brain produces functional tolerance. However, we do expect that the cellular responses that underlie functional tolerance will be sufficiently conserved to allow one to identify genes and signaling pathways involved in the process. We expect that the identification of the neural structures might identify relevant and conserved cellular functions. For instance, the LNV neurons are integral components of the central circadian pacemaker of Drosophila. In mammals it has been long recognized that drug sensitivity oscillates with circadian time and that mutations in some mammalian circadian genes influence drug responses (Falcon and McClung 2009). In both mammals and flies the slo gene is expressed in the central pacemakers and null mutations in slo render the animals arrhythmic (Ceriani et al. 2002; Meredith et al. 2006). Thus, even though the circuitry may differ, cells involved in circadian rhythms and a gene that they express, slo, are important modulators of drug effects. Although, we cannot yet sensibly interpret how the central pacemaker neurons are involved in solvent tolerance, it appears that both these cells and slo are important for drug responses in flies and mammals.
Methods
Generation of transgenic UAS-slo flies
The UAS-slo transgene was constructed by cloning the B52 neuronal slo cDNA in the pUAST Drosophila transformation vector. The insert was amplified by PCR from the B52 neuronal slo cDNA construct described previously (Atkinson et al. 1998; Ghezzi et al. 2004). The forward primer used for amplification contained an EcoRI restriction site sequence, whereas the Reverse primer contained an AvrII restriction site sequence. The sequence of each primer is: EcoRI/forward: 5′-GCAT/GAATTC/GGATCTTTGGTGGATCAT-3′ and AvrII/Reverse: 5′-GTCCAGT/CCTAGG/ACCATCAGTTAAG-3′. The amplified product spans the entire B52 slo cDNA coding sequence and includes the 5′ UTR located between the vector and the ATG start site and the 3′UTR located between the TGA stop site and the other end of the vector. The PCR product was digested with EcoRI and AvrII restriction enzymes to create compatible sticky ends for ligation into the pPUASt vector. The pUAST vector was digested with EcoRI and XbaI restriction enzymes and ligated to the digested PCR insert to generate the final transformation construct. The construct was sent for microinjection into w1118 embryos to Rainbow Transgenics Flies Inc. (Camarillo, CA). The transgene insertion site maps to the Drosophila X chromosome. The UAS-slo lines used in these study were crossed to a homozygous slo4 null mutant background.
Fly stocks
Drosophila stocks used were Canton S (wild type), UAS-slo (w1118, UAS-slo; + ; slo4), and a collection of enhancer trap Gal4 lines (Table I) obtained from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, IN) and from J. Douglas Armstrong (fly-trap.org). Stocks were raised on standard cornmeal agar medium in a 12/12 h light/dark cycle. For all sedation recovery assays, newly eclosed flies were collected over a two day interval and studied five days after collection. Homozygous UAS-slo; slo4 flies, were crossed to the distinct homozygous Gal4 drivers flies. Age-matched female offspring from each cross, heterozygous for UAS-slo, slo4, and the Gal4 driver were collected and sorted into groups of 10 animals per vial under light CO2 anesthesia and allowed to age for 2 days at 18°C. After the second day, one group of vials was transferred to 30°C for 3 days to promote GAL4 induction, whereas another group was left at 18°C for the same period of time. After the induction period, both groups of flies were subjected to behavioral analysis of resistance to sedation. For the UAS-slo control lines, homozygous UAS-slo; slo4 were crossed to wild-type CS flies, yielding offspring heterozygous for UAS-slo, slo4, but lacking a Gal4 driver. For the Gal4-only control lines, homozygous Gal4 driver lines were crossed to homozygous slo4, yielding offspring heterozygous for the Gal4 driver and for slo4, but lacking the UAS-slo transgene.
Quantification of UAS-slo transgene induction
Total RNA was extracted from groups of 20 flies six hours after the start of the induction protocol using a single-step RNA isolation protocol (Ausubel 1994). Residual genomic DNA was digested by incubating the RNA samples at 37°C for 30 minute with RNase free DNase I (Ambion Inc., Austin, TX) and further purified by acid phenol/chloroform extraction (Ambion Inc., Austin, TX) an ethanol precipitation. RNA quality was determined by agarose gel electrophoresis and quantified using a NanoDrop Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). Quantification of induction of the slo cDNA under the control of the UAS promoter was performed using a quantitative real-time RT-PCR SYBR green assay (Applied Biosystems, Carlsbad, CA). The slo mRNA was quantified relative to the Cyclophilin gene (Cyp1) mRNA. For UAS-slo, the reverse primer used was 5′-ATCAGTTGGCAGGTTGGAACGATG-3′ and was designed to overlap the SV40 polyadenylation site found in the transgene cDNA whereas the forward primer was 5′-GATTACGACCATAACTTGCGTGCC-3′, specific to a region within the slo coding sequence included in the same cDNA. For endogenous slo transcripts, the reverse primer was 5′-GATAGTTGTTCGTTCTTTTGAATTTGA-3′ and the forward primer was 5′-AAACAAAGCTAAATAAGTTGTGAAAGGA-3′. For Cyp1, the reverse primer was 5′-TGCTTCAGCTCGAAGTTCTCATC-3′ and the forward primer was 5′-ACCAACCACAACGGCACTG-3′. First-strand cDNA was synthesized from 50 ng of total RNA, primed with 200 nM each of the gene-specific reverse primers for the UAS-slo and Cyp1 transcripts using Superscript II reverse transcriptase (Invitrogen, Grand Island, NY). The cDNAs were amplified by real-time PCR using the Power SYBR® Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) in an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Carlsbad, CA). UAS-slo mRNA abundance was quantified relative to Cyp1 mRNA abundance, using the standard curve method. Each RT-PCR reaction was performed in triplicate from independent RNA samples, and the yields thereof were expressed as an average. Statistical significance was calculated using the Student’s t-test.′
Behavioral analysis of resistance to sedation
Age-matched female flies were exposed to benzyl alcohol as described previously (Al-Hasan et al. 2011). Briefly, groups of ten age matched female flies were placed in benzyl alcohol coated vials for fifteen minutes. Flies were then transferred to clean, benzyl alcohol free glass vials and allowed to recover as a camera took images of the recovery vials every 10 seconds. Because flies are normally negatively geotactic; climbing flies were recorded as recovered from sedation. Automated image processing software was used to detect when the flies recover from sedation and return to climbing the walls of their vials (Ramazani et al. 2007). The software subtracts images of each vial from the image where all flies are sedated. The resulting subtracted image is void of background and contains only recovered flies. The number of non-black pixels was calculated as a measure of the number of recovered flies. For each vial, the pixel-count value at each time point is normalized to the complete recovery value, and expressed as a percent recovery. The percent recovery of each vial within a population was averaged and plotted as a function of time, with error bars describing the standard error of the mean (SEM) of six vials for each group. A left-ward shift in the recovery graph indicates resistance to the sedative affects of benzyl alcohol. Statistically significant difference between the two curves was determined using the Log-rank test. The 50% recovery time (and the associated SEM) for each treatment group was calculated by performing a Richard’s five parameter non-linear regression curve fit on the respective recovery curves using GraphPad Prism software. The relative change in resistance between the slo-induced and slo-uninduced groups for each line tested was determined by calculating the difference in the 50% recovery time between the groups relative to the slo-uninduced group.
Acknowledgments
This work was supported by National Institute of Health grant R01 AA018037 to NSA.
References
- Al-Hasan YM, Krishnan HR, Ghezzi A, Prado F, Jr, Robles RB, Atkinson NS. Tolerance to anesthesia depends on synaptic proteins. Behav Genet. 2011;41(5):734–745. doi: 10.1007/s10519-011-9451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Grubel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23(1–2):156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Atkinson NS, Brenner R, Bohm RA, Yu JY, Wilbur JL. Behavioral and electrophysiological analysis of Ca-activated K-channel transgenes in Drosophila. Ann N Y Acad Sci. 1998;860:296–305. doi: 10.1111/j.1749-6632.1998.tb09057.x. [DOI] [PubMed] [Google Scholar]
- Ausubel FM. Current Protocols in Molecular Biology. John Wiley & Sons, Inc; USA: 1994. [Google Scholar]
- Becker MN, Brenner R, Atkinson NS. Tissue-specific expression of a Drosophila calcium-activated potassium channel. J Neurosci. 1995;15(9):6250–6259. doi: 10.1523/JNEUROSCI.15-09-06250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8(12):1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22(21):9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, Tully T, O’Kane CJ. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274(5295):2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Ghezzi A, Al’Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30(5):745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5(1–2):38–51. [PMC free article] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, et al. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37(7):733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34(1–2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Sup 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc Natl Acad Sci U S A. 2004;101(49):17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Atkinson NS. Homeostatic control of neural activity: a Drosophila model for drug tolerance and dependence. Int Rev Neurobiol. 2011;99:23–50. doi: 10.1016/B978-0-12-387003-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Krishnan HR, Atkinson NS. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Pohl JB, Wang Y, Atkinson NS. BK channels play a counter-adaptive role in drug tolerance and dependence. Proc Natl Acad Sci U S A. 2010;107(37):16360–16365. doi: 10.1073/pnas.1005439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580(Pt.3):859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilius M, Wolf R, Heisenberg M. The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid body open. J Neurogenet. 2007;21(4):321–338. doi: 10.1080/01677060701693503. [DOI] [PubMed] [Google Scholar]
- Jarrett HW. Temperature dependence of DNA affinity chromatography of transcription factors. Anal Biochem. 2000;279(2):209–217. doi: 10.1006/abio.2000.4489. [DOI] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441(7094):757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Helfrich-Forster C, Hall JC. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: newly identified pacemaker candidates and novel features of clock gene product cycling. J Neurosci. 1997;17(17):6745–6760. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8(5):341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional disruption of synaptic transmission induces male-male courtship behavior in Drosophila. Proc Natl Acad Sci U S A. 2002;99(20):13232–13237. doi: 10.1073/pnas.202489099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE. 2010;5(4):e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Nagao T, Walldorf U, Flister S, Gehring WJ, Furukubo-Tokunaga K. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and Dachshund genes. Proc Natl Acad Sci U S A. 2000;97(5):2140–2144. doi: 10.1073/pnas.040564497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;7(7):e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64(4):522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126(18):4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Lin M, Nash HA. Influence of general anesthetics on a specific neural pathway in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1996;93(19):10446–10451. doi: 10.1073/pnas.93.19.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World. 1998;22(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400(6746):753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol A. 1999;185(3):277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9(8):1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunami M, Okada R, Li Y, Strausfeld NJ. Mushroom bodies of the cockroach: activity and identities of neurons recorded in freely moving animals. J Comp Neurol. 1998a;402(4):501–519. [PubMed] [Google Scholar]
- Mizunami M, Weibrecht JM, Strausfeld NJ. Mushroom bodies of the cockroach: their participation in place memory. J Comp Neurol. 1998b;402(4):520–537. [PubMed] [Google Scholar]
- Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453(7199):1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26(2):479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem. 2009;16(5):289–295. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60(4):672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441(7094):753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- Ramazani RB, Krishnan HR, Bergeson SE, Atkinson NS. Computer automated movement detection for the analysis of behavior. J Neurosci Methods. 2007;162(1–2):171–179. doi: 10.1016/j.jneumeth.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, Taghert PH. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol. 1999;41(2):189–207. [PubMed] [Google Scholar]
- Rezaval C, Werbajh S, Ceriani MF. Neuronal death in Drosophila triggered by GAL4 accumulation. Eur J Neurosci. 2007;25(3):683–694. doi: 10.1111/j.1460-9568.2007.05317.x. [DOI] [PubMed] [Google Scholar]
- Rybak J, Menzel R. Integrative properties of the Pe1 neuron, a unique mushroom body output neuron. Learn Mem. 1998;5(1–2):133–145. [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008;105(50):19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech. 2003;62(2):151–169. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508(5):711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Technau G, Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature. 1982;295(5848):405–407. doi: 10.1038/295405a0. [DOI] [PubMed] [Google Scholar]
- Treistman SN, Martin GE. BK Channels: mediators and models for alcohol tolerance. Trends Neurosci. 2009;32(12):629–637. doi: 10.1016/j.tins.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27(17):4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen B. A succession of anesthetic endpoints in the Drosophila brain. J Neurobiol. 2006;66(11):1195–1211. doi: 10.1002/neu.20300. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Andretic R. Arousal in Drosophila. Behav Processes. 2003;64(2):133–144. doi: 10.1016/s0376-6357(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15(1):45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Young AM, Goudie AJ. Adaptive processes regulating tolerance to behavioral effects of drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: Fourth Generation of Progress. Raven Press; New York, NY: 1995. pp. 733–742. [Google Scholar]