Abstract
Background
Promoting medication adherence is a recognized challenge for prescribers. In this study we examine whether lower medication adherence is associated with adverse safety events in individuals with decreased estimated glomerular filtration rate (eGFR).
Study Design
Cross-sectional baseline analysis of prospective cohort.
Setting & Participants
Baseline analysis of the Safe Kidney Care (SKC) Cohort Study, a prospective study of individuals with an eGFR < 60 mL/min/1.73m2 intended to assess the incidence of disease-specific safety events. Kidney transplant recipients were excluded.
Predictor
Self-reported medication adherence based on responses to 3 questions ascertaining degree of medication regimen adherence.
Outcomes
Adverse safety events were self-reported at baseline (Class I events) such as hypoglycemia or fall thought related to a medication, or detected incidentally during the baseline visit (Class II events), for example hypotension or hyperkalemia. Potential drugrelated problems (DRPs) were determined by analyzing participants’ medications with respect to dosing guidelines based on their screening eGFR values at the time of medication reporting.
Measurements
Relationship between medication adherence and disease-specific patient safety events.
Results
Of 293 SKC participants, 154 (53%) were classified as having lower medication adherence. After multivariable adjustment, lower medication adherence was significantly associated with a Class I or II safety event (prevalence ratio [PR], 1.21; 95% CI, 1.04–1.41) and potential DRPs (PR, 1.29; 95% CI, 1.02–1.63). Lower medication adherence was also significantly associated with multiple (≥ 2) Class I events (PR, 1.71; 95% CI, 1.18–2.49), multiple Class I or II events (PR, 1.35; 95% CI, 1.04–1.76), and multiple potential DRPs (PR, 2.11; 95% CI, 1.08–2.69) compared to those with higher medication adherence.
Limitations
Use of self-reported medication adherence rather than pharmacy records. Clinical relevance of detected safety events is unclear.
Conclusions
Lower medication adherence is associated with adverse safety events in individuals with eGFR < 60 mL/min/1.73m2.
Keywords: reduced kidney function, chronic kidney disease (CKD), medication adherence, treatment compliance, polypharmacy, patient safety, drug-related problem (DRP), adverse safety event, Safe Kidney Care (SKC) Cohort Study
Medications remain an integral part of chronic disease management, yet promoting medication adherence among patients continues to be a challenge for prescribers.1–4 Complex medication regimens and lower socioeconomic status, both commonly observed within the chronic kidney disease (CKD) population, have been identified as key factors that contribute to poor medication adherence in the general population.5–8 Individuals with CKD often have numerous related comorbidities and resultant polypharmacy, and are at increased risk for adverse safety events related to their condition;9–12 however, the contribution of low medication adherence to a developing high-risk CKD safety phenotype is unclear. With poor medication adherence, patients may have a lessened therapeutic response such that providers increase dosing beyond safe thresholds, or patients may have highly variable dosing (missing a dose one day and doubling the next day). These dosing and management departures have the potential to lead to patient safety mishaps. Improving medication adherence has the potential of improving patient safety.
In this study we explore the relationship between self-reported medication adherence and adverse safety events among individuals enrolled in the Safe Kidney Care (SKC) Cohort Study, an ongoing cohort study of individuals with decreased estimated glomerular filtration rate (eGFR) structured to identify the frequency of adverse safety events related to kidney disease care. The SKC safety events of interest include potential drug-related problems (DRPs) based on the appropriateness of reported medications for each participant’s level of kidney function, along with patient-reported events (Class I) and those that are detected during an in-center study visit (Class II).
METHODS
Study Participants
The SKC Cohort Study (ClinicalTrials.gov study number NCT01407367) is approved by the University of Maryland School of Medicine Institutional Review Board and Veterans Affairs Maryland Health Care System Baltimore Research and Development committee. In April 2011, the study began enrollment to assess the frequency of adverse safety events in individuals with decreased eGFR.13 Study participants were recruited from disease-management nephrology clinics primarily caring for individuals with diabetes and hypertension as the etiology of their reduced kidney function at the University of Maryland School of Medicine and Baltimore Veterans Affairs Medical Center. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. To be eligible for the SKC cohort, participants had to be aged 21 years or older, with two assessments of kidney function with eGFR < 60 mL/min/1.73m2 at least 90 days apart and no more than 18 months prior to enrollment. Participants were excluded if they are expected to reach end-stage renal disease (ESRD) or die within one year from enrollment, or if they had previously received a kidney transplant. Measures of demographics including income and educational attainment were collected at baseline for all study participants, and the abbreviated Test of Functional Health Literacy Assessment (TOFHLA)14 was administered then as well. Details of SKC study procedures have been previously published.13,15
Medication Adherence
Participants were asked to answer “Yes” or “No” to three questions designed to ascertain different aspects of non-adherence to their medication regimen.16 Participants were asked the following questions starting with “During the past 30 days, did you…”: “forget to take a medicine?”; “not take a medicine on purpose?”, or “add an extra pill?” In order to sort participants based on the extent of non-adherence, responses to the medication adherence questionnaire were ordered based on the affirmative responses to each question. Purposefully not taking a medicine was thought to represent more significant non-adherence than adding an extra pill and was rated lowest among the intentional non-adherence questions. Unintentional non-adherence (forgetting to take a pill) was treated as the least significant form of non-adherence. To sort participants based on their overall adherence, they were first classified according to whether or not they reported purposefully not taking a medicine, with affirmative responses rated lowest. Next, affirmative or negative responses to the question regarding adding an extra pill were added to the sorting procedure. Finally, responses to the question regarding forgetting to take a medicine were added to create an unadjusted adherence scale. In order to account for the influence of pill burden on medication adherence, this adherence value was then divided by the number of reported daily pills to create a final adherence category adjusted for number of pills. Individuals who rated lower than or equal to the median adjusted value were classified as having relatively lower medication adherence. Conversely, those with a rating higher than the median were classified as having higher medication adherence relative to their counterparts.
Participant Awareness of Kidney Disease
In order to better understand individual determinants that may be associated with medication adherence, such as limited insight of medical comorbidity, participants were asked at baseline “Have you ever been told you have kidney problems, weak kidneys, or kidney disease?” Possible answers include “Yes,” “No,” or “Don’t Know.” Those who answered “No” or “Don’t Know” to this question were categorized as having low disease awareness. Participants who answered “Yes” were asked further questions related to their perceived etiology of their kidney disease. Individuals with self-reported diabetes who answered “No” or “Don’t Know” to the question, “Have you ever been told that your kidney problem was caused by diabetes?” were categorized as having low disease awareness. Similarly, individuals with self-reported hypertension without a history of self-reported diabetes who answered “No” or “Don’t Know” to the question, “Have you ever been told that your kidney problem was caused by high blood pressure?” were classified as having low disease awareness. For those without self-reported hypertension or diabetes, a medication review for antihypertensive and diabetes-related medication was performed to confirm the accuracy of their negative responses.
Participant Perceptions of Provider Counseling
The extent to which participants understand their providers’ counseling was assessed through their responses to questions regarding common and expected disease-management discussions. Individuals with self-reported diabetes were asked, “Have you ever been instructed on how to avoid low sugars?” Possible responses include “Yes,” “No” or “Don’t Know.” All study participants were asked to indicate if a “kidney disease specialist or another healthcare provider” had “Told you to avoid anti-inflammatory drugs (e.g. NSAIDs) or other drugs that might harm your kidneys?”, “Told you to cut down on the amount of salt or high-sodium foods that you eat” or “Started you on a drug to raise your blood count (treat anemia)?” For the latter two questions, analysis of the responses was limited to those with self-reported hypertension and those with a documented prescription for an erythropoiesis-stimulating agent (ESA), respectively.
Perceived Barriers to Adherence
In order to examine additional factors that may relate to adherence, we asked participants several questions related to practices during times with and without health insurance. Individuals who answered “Yes” to the question, “Was there ever a time when you did not have health insurance?” were further prompted to answer “Yes” or “No” to a set of three follow-up questions: “Did the lack of health insurance cause you to put off seeing a healthcare provider because of the cost?”, “Did the lack of health insurance cause you to put off filling a prescription medicine because of the cost?” and “Did the lack of health insurance cause you to skip some of your doses or did you cut some of your pills in half to make them last longer?” All participants were asked, “During times when you did have health insurance, did you…”: “put off seeing a healthcare provider because of the cost?”, “put off filling a prescription medicine because of the cost?” or “skip some of your doses or cut some of your pills in half to make them last longer?” Possible answers included “Yes,” “No,” or “N/A [not applicable].”
Safety Event Assessment
Potential adverse patient safety events were classified as self-reported by a participant (Class I), noted during an in-center study visit (Class II), or potential DRP. Class I adverse safety incidents were ascertained from a baseline self-reported safety event questionnaire, which asks participants what incidents they had over the prior 12 months that they attributed to an administered medication or treatment. These potential incidents include the following: low blood sugar; high potassium blood level requiring a treatment or change in therapy or diet; falling or significant lightheadedness; bleeding; facial, tongue, and/or throat swelling (angioedema); confusion or inability to think clearly; nausea, vomiting and/or diarrhea; new or worsening ankle swelling, muscle weakness or muscle cramps; skin rash; and a final free-text category for any incident not listed. Class II adverse safety events were abnormalities in baseline laboratory and vital sign parameters with concomitant treatment with a drug from a class known to cause such a disturbance, and with the potential to normalize with withdrawal of the suspect agent (e.g., pulse less than 50 beats per minute in the setting of a β-blocker, hyperkalemia in the setting of a renin-angiotensin system blocker). Class I and II events were conditionally restricted to individuals who were on classes of medications that may conceivably be associated with their occurrence; for example, bleeding was a potential safety event only for individuals on platelet inhibitors or anticoagulants. Details of the conditional safety classifications have been previously reported.15 A detailed description of Class I and II safety events is provided in Item S1 (provided as online supplementary material).
Potential DRPs constitute nomenclature previously described by Strand et al and others17,18 and include any medication with: a) a dose that exceeds upper threshold of dosing for a given eGFR, b) a non-specific recommendation for dose reduction, c) a recommendation for avoidance in CKD, d) or general caution for use in CKD. Departures from recommendations for medication dosing were detected using a medication crosswalk of all medications relevant in kidney disease which has been previously described.9,10 Primary sources used to construct the tool include established general and CKD-specific texts on drug dosing.19–22 The renal drug crosswalk includes criteria for the appropriate frequency of administration and total daily dose of each drug dosage form based on a participant’s screening eGFR value as calculated by the IDMS-traceable 4-variable Modification of Diet in Renal Diseases (MDRD) Study equation, the most commonly used estimating equation for clinical prescribing purposes.23 At their baseline visit, SKC participants are asked to provide details regarding any prescription or over-the-counter medications they may have taken in the preceding 30 days, and medication bottles are brought in and recorded. Medications that were inappropriately dosed relative to a participant’s screening eGFR based on published dosing guidelines were flagged as needing adjustment and termed “Adjust” medications. Medications that were contraindicated for use per published guidelines based on a participant’s screening eGFR or any nonsteroidal anti-inflammatory medication (NSAID) were termed “Avoid” medications. For example, a participant with an eGFR of 30 mL/min/m2 prescribed colchicine 0.6 mg daily would receive an “Adjust” flag, while a participant with an eGFR of 20 mL/min/m2 prescribed any dose of colchicine would receive an “Avoid” flag.
Statistical Analysis
Binomial and categorical variables were expressed as number (percentage) with comparisons made using the Chi-square test statistic to assess differences in participants’ characteristics between those with lower and higher medication adherence, as dichotomized based on the adjusted adherence variable noted above. Mean, median, and standard deviation were reported for continuous variables. Modified Poisson regression was performed to identify participant characteristics independently associated with the occurrence of any Class I or II safety events (versus no safety events), or potential DRP (versus no DRP) using medication adherence as the primary predictor for adverse events, as well as to compute adjusted prevalence ratios (PRs) for the occurrence of multiple (≥ 2) Class I or II safety events, or multiple (≥2) potential DRPs (versus no DRP), comparing low medication adherence versus high medication adherence.25
RESULTS
Study Participants
Of 308 SKC participants with completed baseline visits, 293 were included in the analysis. Four SKC participants were not administered the medication adherence questionnaire and 11 participants did not complete the TOFHLA at baseline and were excluded. There were 174 participants (59%) who answered “Yes” to any of the 3 medication adherence questions; 61 individuals (21%) answered affirmatively to purposefully skipping a pill, 20 individuals (7%) answered affirmatively to taking an additional pill, and 135 individuals (46%) answered affirmatively to forgetting to take a pill. The median unadjusted adherence value was 80 (range, 1–181), with adjustment for number of participant pills resulting in a median value of 8 (range, 0.04–60). While the absolute value itself had no meaning per se, this approach to sorting allowed us to dichotomize study participants into those with lower and higher medication adherence based on how individual adherence values rated relative to the median value. There were 154 (53%) participants rated below the median and were classified as having lower medication adherence. Those with lower self-reported medication adherence were taking a median of 14 pills (range, 4–31), compared with 9 pills (range, 1–22) in participants with higher medication adherence. Table 1 shows the demographic characteristics of study participants classified by adherence group. Median age of participants was 66 (range, 21–91) years. Participants were predominantly male and African-American, without full-time employment, and with a high proportion reporting diabetes or hypertension. Approximately one third of participants had limited health literacy. Individuals with lower medication adherence were significantly more likely than those with higher medication adherence to have self-reported cardiovascular disease or diabetes.
TABLE 1.
CHARACTERISTICS OF SAFE KIDNEY CARE COHORT PARTICIPANTS BY MEDICATION ADHERENCE
| Characteristic | All (N=293) | Lower adherence (n = 154) |
Higher adherence (n = 139) |
P-value |
|---|---|---|---|---|
| Age | 0.9 | |||
| < 65 y | 125 (42.7) | 65 (42.2) | 60 (43.2) | |
| ≥ 65 y | 168 (57.3) | 89 (57.8) | 79 (56.8) | |
| Sex | 0.09 | |||
| Male | 214 (73.0) | 119 (77.3) | 95 (68.3) | |
| Female | 79 (27.0) | 35 (22.7) | 44 (31.7) | |
| Race/ethnicity | 0.7 | |||
| African-American | 199 (67.9) | 106 (68.8) | 93 (66.9) | |
| Non−African-American | 94 (32.1) | 48 (31.2) | 46 (33.1) | |
| Incomea | 0.4 | |||
| ≤ $20,000 | 86 (29.4) | 41 (26.6) | 45 (32.4) | |
| > $20,000 | 197 (67.2) | 109 (70.8) | 88 (63.3) | |
| Missing | 10 (3.4) | 4 (2.6) | 6 (4.3) | |
| Educational level | 0.8 | |||
| ≤ High school diploma | 144 (49.1) | 77 (50.0) | 67 (48.2) | |
| > High school diploma | 149 (50.9) | 77 (50.0) | 72 (51.8) | |
| Full-time employment | 0.3 | |||
| No | 253 (86.3) | 130 (84.4) | 123 (88.5) | |
| Yes | 40 (13.7) | 24 (15.6) | 16 (11.5) | |
| Health literacy | 0.3 | |||
| Limited: TOFHLA < 67 | 93 (31.7) | 53 (34.4) | 40 (28.8) | |
| Adequate: TOFHLA ≥ 67 | 200 (68.3) | 101 (65.6) | 99 (71.2) | |
| Baseline eGFR | 0.1 | |||
| < 45 mL/min/1.73 m2 | 170 (58.0) | 83 (53.9) | 87 (62.6) | |
| ≥ 45 mL/min/1.73 m2 | 123 (42.0) | 71 (46.1) | 52 (37.4) | |
| Cardiovascular disease | 0.01 | |||
| Yes | 164 (56.0) | 97 (63.0) | 67 (48.2) | |
| No | 129 (44.0) | 57 (37.0) | 72 (51.8) | |
| Diabetes/high blood sugar | < 0.001 | |||
| Yes | 185 (63.1) | 118 (76.6) | 67 (48.2) | |
| No | 108 (36.9) | 36 (23.4) | 72 (51.8) | |
| Hypertension | 0.3 | |||
| Yes | 283 (96.6) | 147 (95.5) | 136 (97.8) | |
| No | 10 (3.4) | 7 (4.5) | 3 (2.2) | |
| No. of pills | 12 (1–31) | 14 (4–31) | 9 (1–22) | < 0.001 |
| Skipped pill | <0.001 | |||
| Yes | 61 (20.8) | 60 (39.0) | 1 (0.7) | |
| No | 232 (79.2) | 94 (61.0) | 138 (99.3) | |
| Added extra pill | 0.01 | |||
| Yes | 20 (6.8) | 16 (10.4) | 4 (2.9) | |
| No | 273 (93.2) | 138 (89.6) | 135 (97.1) | |
| Forgot pill | <0.001 | |||
| Yes | 135 (46.1) | 110 (71.4) | 25 (18.0) | |
| No | 158 (53.9) | 44 (28.6) | 114 (82.0) |
Note: Unless otherwise indicated, values are given as number (percentage) or median (range).
eGFR, estimated glomerular filtration rate; TOFHLA, Test of Functional Health Literacy Assessment;
11 participants declined to report their income
Factors Associated With Medication Adherence
While the overwhelming majority of participants were aware of their CKD diagnosis, over half showed evidence of limited disease awareness. The majority of participants reported receiving provider counseling on CKD-related content and those with lower medication adherence were nominally more likely to endorse receiving counseling from their provider regarding salt consumption if they had hypertension than those with higher medication adherence; however, this finding did not reach statistical significance. Those with lower medication adherence were more likely than participants with higher medication adherence to report putting off seeing a provider and to report putting off filling a medication during times when they did not have health insurance, although the latter did not reach statistical significance. Similarly, participants with lower medication adherence were more likely to put off filling a prescription during times when they had active health insurance compared with participants with higher medication adherence. (Table 2)
TABLE 2.
PARTICIPANT FACTORS ASSOCIATED WITH MEDICATION ADHERENCE
| Characteristic | All (N=293) |
Lower adherence (n = 154) |
Higher adherence (n = 139) |
P-value |
|---|---|---|---|---|
| Patient awareness of kidney disease and comorbidity | ||||
| Been told to have: | ||||
| Kidney disease | 282 (96.2) | 149 (96.8) | 133 (95.7) | 0.6 |
| Kidney disease due to diabetesa | 99 (55.9) | 62 (54.9) | 37 (57.8) | 0.7 |
| Kidney disease due to hypertensionb | 35 (34.0) | 14 (41.2) | 21 (30.4) | 0.4 |
| Disease awareness | 0.3 | |||
| Limited | 154 (52.6) | 76 (49.4) | 78 (56.1) | |
| Adequate | 139 (47.4) | 78 (50.6) | 61 (43.9) | |
| Patient perceptions of provider counseling | ||||
| Been told: | ||||
| How to avoid low sugarsa | 152 (82.2) | 99 (83.9) | 53 (79.1) | 0.4 |
| To reduce salt consumptionc | 264 (93.3) | 141 (96.0) | 123 (90.4) | 0.07 |
| To avoid NSAIDs | 195 (66.6) | 99 (64.3) | 96 (69.1) | 0.4 |
| Aware of purpose of ESAd | 12 (100.0) | 8 (100.0) | 4 (100.0) | |
| Perceived barriers to engaged care | ||||
| Ever had no health insurance | 89 (30.4) | 46 (29.9) | 43 (30.9) | 0.8 |
| Put off seeing provider | 41 (46.1) | 27 (58.7) | 14 (32.6) | 0.01 |
| Put off filling medication | 36 (40.9) | 23 (50.0) | 13 (31.0) | 0.07 |
| Cut pills in half or skip doses | 21 (23.9) | 13 (28.3) | 8 (19.0) | 0.3 |
| During times with health insurance | ||||
| Put off seeing provider | 26 (8.9) | 18 (11.7) | 8 (5.8) | 0.08 |
| Put off filling medication | 31 (10.6) | 23 (14.9) | 8 (5.8) | 0.01 |
| Cut pills in half or skip doses | 18 (6.1) | 11 (7.1) | 7 (5.0) | 0.5 |
ESA, erythropoiesis-stimulating agent; NSAID, nonsteroidal anti-inflammatory drug
Limited to those with self-reported diabetes (n = 185).
Limited to those with self-reported hypertension with no diabetes (All, n = 103; Lower medication adherence, n=34; Higher medication adherence, n=69).
Limited to those with self-reported hypertension (n = 283).
Limited to those who are on ESAs (n =12).
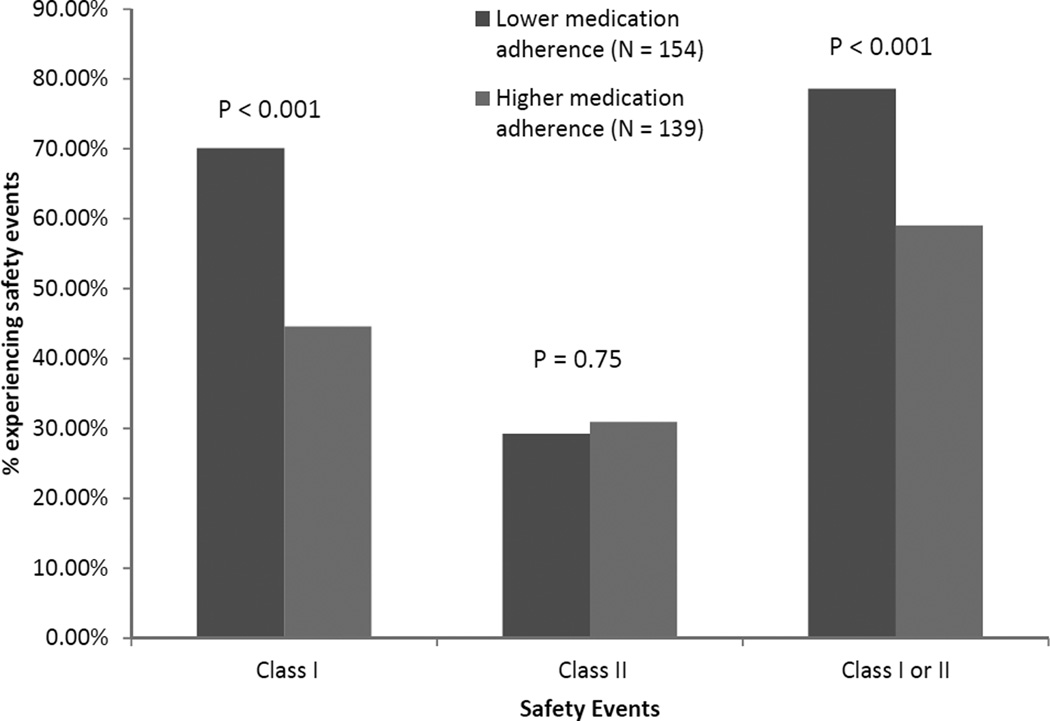
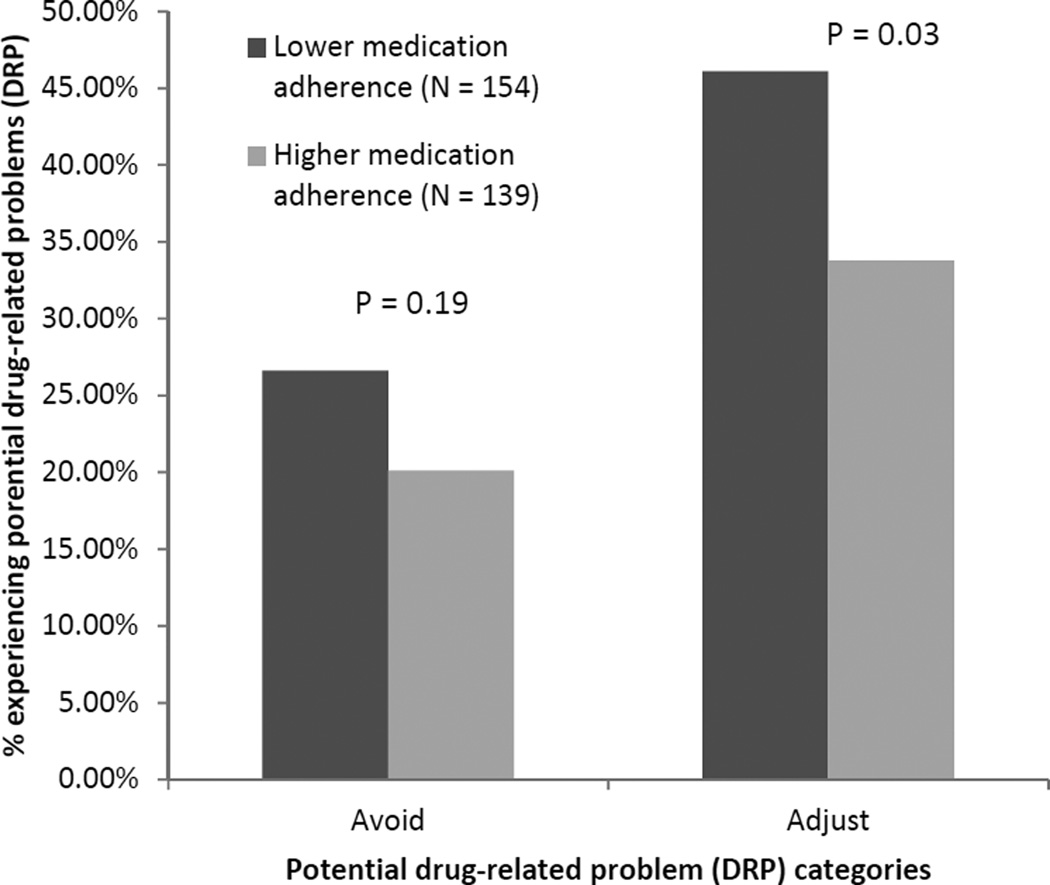
Adverse Safety Events
Participants found to have lower medication adherence had higher frequencies of Class I events and composite Class I or II events than those with higher medication adherence (Figure 1). Those with lower medication adherence were also more likely to have medications with a DRP in the “Adjust” category than those with higher medication adherence (Figure 2). After multivariable adjustment, individuals with lower medication adherence were more likely to have a Class I safety event than those with higher medication adherence (PR, 1.39; 95% confidence interval [CI], 1.12–1.72), 20% more likely to have a Class I or II safety event than those with higher medication adherence, and almost 30% as likely to have a potential DRP than those with higher medication adherence (Table 3). Lower medication adherence was significantly associated with having multiple (≥ 2) Class I safety events (PR, 1.71; 95% CI, 1.18–2.49), multiple (≥ 2) composite Class I or II safety events (PR, 1.35; 95% CI, 1.04–1.76), and multiple (≥ 2) potential DRPs (PR, 2.11; 95% CI, 1.08–2.69).
Figure 1.
Frequency of patient safety events by medication adherence status
Figure 2.
Frequency of potential drug-related problem classification by medication adherence status
TABLE 3.
ADJUSTED PREVALENCE RATIOS OF SAFETY EVENTS IN SAFE KIDNEY CARE PARTICIPANTS)a
| Characteristic | Class I or II safety event | Potential drug-related problem |
||
|---|---|---|---|---|
| PR (95% CI) | P-value | PR (95% CI) | P-value | |
| Medication adherence | ||||
| Lower | 1.21 (1.04–1.41) | 0.02 | 1.29 (1.02–1.63) | 0.03 |
| Higher | 1.00 (reference) | --- | 1.00 (reference) | --- |
| Age | ||||
| ≥ 65 y | 0.94 (0.91–1.10) | 0.5 | 0.86 (0.69–1.08) | 0.2 |
| < 65 y | 1.00 (reference) | --- | 1.00 (reference) | --- |
| Sex | ||||
| Male | 0.87 (0.73–1.03) | 0.1 | 0.83 (0.65–1.05) | 0.1 |
| Female | 1.00 (reference) | --- | 1.00 (reference) | --- |
| Race/ethnicity | ||||
| African-American | 0.90 (0.76–1.06) | 0.2 | 0.73 (0.58–0.92) | 0.01 |
| Non−African-American | 1.00 (reference) | --- | 1.00 (reference) | --- |
| Income | ||||
| ≤ $20,000 | 1.00 (0.85–1.18) | 0.9 | 0.96 (0.75–1.23) | 0.7 |
| > $20,000 | 1.00 (reference) | --- | 1.00 (reference) | --- |
| Missing | 1.08 (0.71–1.66) | 0.7 | 1.23 (0.76–2.00) | 0.4 |
| Educational level | ||||
| ≤ high school diploma | 1.00 (0.86–1.16) | 0.9 | 1.07 (0.85–1.35) | 0.6 |
| > high school diploma | 1 (ref) | --- | 1 (ref) | --- |
| Full-time employment | ||||
| No | 0.86 (0.67–1.12) | 0.3 | 0.86 (0.60–1.21) | 0.4 |
| Yes | 1 (ref) | --- | 1 (ref) | --- |
| Health literacy | ||||
| Limited: TOFHLA < 67 | 0.89 (0.75–1.06) | 0.2 | 0.99 (0.77–1.26) | 0.9 |
| Adequate: TOFHLA ≥ 67 | 1 (ref) | --- | 1 (ref) | --- |
| Disease awareness | ||||
| Limited | 0.87 (0.75–1.01) | 0.07 | 1.16 (0.93–1.44) | 0.2 |
| Adequate | 1 (ref) | --- | 1 (ref) | --- |
| Baseline eGFR | ||||
| < 45 mL/min/1.73 m2 | 1.09 (0.94–1.26) | 0.3 | 1.21 (0.96–1.52) | 0.1 |
| ≥ 45 mL/min/1.73 m2 | 1 (ref) | --- | 1 (ref) | --- |
| Cardiovascular disease | ||||
| Yes | 1.41 (1.18–1.67) | 0.001 | 1.30 (1.02–1.65) | 0.03 |
| No | 1 (ref) | --- | 1 (ref) | --- |
| Diabetes/high blood sugar | ||||
| Yes | 1.35 (1.12–1.64) | 0.002 | 1.19 (0.94–1.52) | 0.2 |
| No | 1 (ref) | --- | 1 (ref) | --- |
| Hypertension | ||||
| Yes | 1.07 (0.76–1.49) | 0.7 | 0.87 (0.52–1.46) | 0.6 |
| No | 1 (ref) | --- | 1 (ref) | --- |
Note: N=293.
CI, confidence interval; eGFR, estimated glomerular filtration rate; PR, prevalence ratio; TOFHLA, Test of Functional Health Literacy Assessment
Note: N=293. Adjustment variables: medication adherence, age, gender, race/ethnicity, income, education level), employment, health literacy, disease awareness, baseline eGFR, cardiovascular disease, diabetes, hypertension
DISCUSSION
In this study of individuals with decreased eGFR, lower self-reported medication adherence was associated with more self-reported comorbidity and higher medication burden, with individuals with lower medication adherence taking over 50% more medications than individuals with higher medication adherence. This might be partially explained by diabetic status, with over three quarters of individuals with lower medication adherence reporting diabetes compared to roughly half of those with higher medication adherence. Despite no difference in the frequency of being uninsured, people with lower medication adherence were more likely to put off seeing a provider while uninsured and to put off filling a medication while insured than those with higher medication adherence, suggesting influences beyond health insurance status might contribute to non-adherence. Individuals with lower medication adherence were more likely to have one or multiple adverse safety events or DRPs than people with higher medication adherence, which supports the notion of a potentially classifiable high risk group of individuals with decreased eGFR.
The association of lower self-reported medication adherence to adverse safety events is not suggestive of a causal relationship between medication adherence and patient safety, but rather contributes to a developing phenotype of potentially identifiable individuals with decreased eGFR who are at high risk of adverse events. Individuals with CKD are at risk of CKD-pertinent adverse safety events such as hypoglycemia and hyperkalemia,9,12,26,27 and while medication non-adherence has been shown to be associated with uncontrolled hypertension in this population,28 the overall influence of adherence on patient safety in CKD to our knowledge has not been previously evaluated. Our findings offer further insight into associated determinants that may be the target of future interventions designed to engage and educate this high-risk group. For example, the association of lower medication adherence to higher perceived insight regarding appropriate provider counseling corroborates prior research suggesting that patient satisfaction with provider counseling in CKD is more common in individuals with more limited understanding of their illness.29 Better understanding of this paradox may help inform educational strategies designed to raise measurable, rather than perceived, disease awareness. Interestingly, lower medication adherence was also associated with higher odds of potential DRPs; this suggests that providers are prescribing medications to individuals whose lower adherence may have dampened the anticipated therapeutic response, resulting in dose titrations above recommended guidelines and a greater potential for adverse events. It is also possible that providers have prioritized other comorbidities such as diabetes and cardiovascular disease more highly than CKD, which may be appropriate at the time based on the individual’s needs, but should certainly not occur at the expense of patient safety. Innovative and collaborative strategies such as the routine inclusion of pharmacists in the CKD care model or the use of health information technology have the potential not only to improve medication safety and adherence in this at-risk population, but to also promote patient-centered engaged care in CKD.30,31
Previous qualitative studies of medication adherence in the CKD population have noted medication regimen complexity and polypharmacy as two driving factors for nonadherence to prescribed therapies,32–34 which is consistent with the current findings. Rifkin et al. interviewed 20 community-dwelling older adults with CKD stages 3–5D and noted that polypharmacy led many to prioritize their medications and not adhere to medications that they deemed less important.32 Interestingly, Rifkin’s study also revealed that many individuals disagreed with conventional medical opinion about their medications, and reported a lack of discussion with their providers regarding their medication prioritization, beliefs, and adherence. This finding corroborates other studies suggesting a dissatisfaction among patients with provider communication and patient involvement in the medical decision making process,29,35 and suggests patient engagement is a critical component of adherence to any therapy, medication or otherwise.
Our study has limitations that should be addressed. First, our study uses self-reported medication adherence rather than medication possession ratios which have been used in other studies across several disciplines. However, self-report has been used as a standard adherence tool as significant heterogeneity in medication adherence measurement has yet to result in a gold standard tool for capturing adherence.16,36 Secondly, our evaluation of medication adherence uses both intentional non-adherence (skipped a pill, took an extra pill) and unintentional non-adherence (forgot a pill) in our adherence variable; we acknowledge that interventions targeted to promote adherence in the CKD population may employ varying strategies based on whether non-adherence to medication was intentional or not, which may limit the generalizability of our findings.37 In addition, while medication dosing recommendations were extracted from published pharmaceutical sources, routine clinical practice may purposefully contradict dosing recommendations when individualized benefit is perceived to outweigh the risk suggested by pharmaceutical guidelines (for example, the use of aspirin in individuals with cardiovascular disease). Further, self-reported hypertension rather than medication review was used when assessing patients’ perceptions of provider counseling, and in those variables based on medication review (i.e. use of an ESA), it remains possible that participants did not account for all medications taken or prescribed. Our categorization of disease awareness may also have limitations, as despite recruitment from a nephrology clinic caring predominantly for individuals with diabetic or hypertensive kidney disease, it remains possible that the etiology of decreased kidney function might have differed from these two conditions. Finally, the impact of noted safety events and potential DRPs on clinical outcomes has yet to be determined in the CKD population.
Individuals with eGFR < 60 mL/min/1.73m2 who reported lower medication adherence were found to be at higher risk of adverse safety events. Further studies are needed to explore innovative strategies to measure and attenuate the impact of medication non-adherence in an evolving CKD-risk phenotype.
Supplementary Material
Acknowledgements
Support: The work included in this paper was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-DK084017; J.S.G., M.Z., C.W., J.C.F.) and in part by the National Institute on Aging Short-Term Training Program on Aging Grant (T35AG036679; K.H.) to the University of Maryland School of Medicine and the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center. The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; nor the decision to submit the report for publication.
Dr Fink has received prior research funding from Amgen Inc and honoraria from Sandoz Inc and Amgen Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design CJD, KLH, JCF, JSG, MY, MZ, WF; data acquisition JSG, MY, WF, CMW; data analysis KLH, JSG, MZ, CMW; supervision or mentorship JCF, CJD. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CJD takes responsibility that this study has been reported honestly, accurately and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Item S1: Detailed description of class I and II safety events.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Supplementary Table S1 (PDF)
Detailed description of class I and II safety events.
REFERENCES
- 1.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 3.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Cummings DM, Letter AJ, Howard G, et al. Medication adherence and stroke/TIA risk in treated hypertensives: results from the REGARDS study. J Am Soc Hypertens. 2013;7(5):363–369. doi: 10.1016/j.jash.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990;150(7):1509–1510. [PubMed] [Google Scholar]
- 6.Fujii J, Seki A. Compliance and compliance-improving strategies in hypertension: the Japanese experience. J Hypertens Suppl. 1985;3(1):S19–S22. [PubMed] [Google Scholar]
- 7.Swanson MA, Palmeri D, Vossler ED, Bartus SA, Hull D, Schweizer RT. Noncompliance in organ transplant recipients. Pharmacotherapy. 1991;11(6):173S–174S. [PubMed] [Google Scholar]
- 8.Takemoto S, Terasaki PI. A comparison of kidney transplant survival in white and black recipients. Transplant Proc. 1989;21(6):3865–3868. [PubMed] [Google Scholar]
- 9.Diamantidis CJ, Seliger SL, Zhan M, et al. A varying patient safety profile between black and nonblack adults with decreased estimated GFR. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(1):47–53. doi: 10.1053/j.ajkd.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Chapin E, Zhan M, Hsu VD, Seliger SL, Walker LD, Fink JC. Adverse safety events in chronic kidney disease: the frequency of "multiple hits". Clin J Am Soc Nephrol. 2010;5(1):95–101. doi: 10.2215/CJN.06210909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink JC, Brown J, Hsu VD, Seliger SL, Walker L, Zhan M. CKD as an underrecognized threat to patient safety. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53(4):681–688. doi: 10.1053/j.ajkd.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(6):1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamantidis CJ, Fink W, Yang S, et al. Directed use of the internet for health information by patients with chronic kidney disease: prospective cohort study. Journal of medical Internet research. 2013;15(11):e251. doi: 10.2196/jmir.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Education and Counseling. 1999;38(1):33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg JS, Zhan M, Diamantidis CJ, Woods C, Chen J, Fink JC. Patient-reported and actionable safety events in CKD. Journal of the American Society of Nephrology: JASN. 2014;25(7):1564–1573. doi: 10.1681/ASN.2013090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Strand LM, Cipolle RJ, Morley PC. Documenting the clinical pharmacist's activities: back to basics. Drug Intell Clin Pharm. 1988;22(1):63–67. doi: 10.1177/106002808802200116. [DOI] [PubMed] [Google Scholar]
- 18.Cardone KE, Bacchus S, Assimon MM, Pai AB, Manley HJ. Medication-related problems in CKD. Adv Chronic Kidney Dis. 17(5):404–412. doi: 10.1053/j.ackd.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.G S. Seyffart's Directory of Drug Dosage in Kidney Disease. Orlando, FL: Dustri-Verlag Dr. Karl Feistle GmbH & Co. KG; 2011. [Google Scholar]
- 20.Lexicomp Online TM. [Accessed October 8, 2012];2012 http://online.lexi.com/lco/action/home/switch.
- 21.Micromedex Solutions. [Accessed October 8, 2013]; http://www.micromedexsolutions.com/home/dispatch.
- 22.Aronoff G, Berns JS, Brier ME, Golper TA, Morrison G, Singer I, Sawn SK, Bennett WM. Drug Prescribing in Renal Failure: Dosing Guidelines for Adults. 4th ed. Philadelphia: American College of Physicians; 1999. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Einhorn LM, Zhan M, Hsu VD, et al. The Frequency of Hyperkalemia and Its Significance in Chronic Kidney Disease. Arch Intern Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg JS, Zhan M, Diamantidis CJ, Woods C, Chen J, Fink JC. Patient-Reported and Actionable Safety Events in CKD. J Am Soc Nephrol. 2014 Jul;25(7):1564–1573. doi: 10.1681/ASN.2013090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muntner P, Judd SE, Krousel-Wood M, McClellan WM, Safford MM. Low medication adherence and hypertension control among adults with CKD: data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2010;56(3):447–457. doi: 10.1053/j.ajkd.2010.02.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright Nunes JA, Wallston KA, Eden SK, Shintani AK, Ikizler TA, Cavanaugh KL. Associations among perceived and objective disease knowledge and satisfaction with physician communication in patients with chronic kidney disease. Kidney Int. 2011;80(12):1344–1351. doi: 10.1038/ki.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St Peter WL, Wazny LD, Patel UD. New models of chronic kidney disease care including pharmacists: improving medication reconciliation and medication management. Curr Opin Nephrol Hypertens. 2013;22(6):656–662. doi: 10.1097/MNH.0b013e328365b364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamantidis CJ, Zuckerman M, Fink W, Aggarwal S, Prakash D, Fink JC. Usability testing and acceptance of an electronic medication inquiry system for CKD patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(4):644–646. doi: 10.1053/j.ajkd.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Rifkin DE, Laws MB, Rao M, Balakrishnan VS, Sarnak MJ, Wilson IB. Medication adherence behavior and priorities among older adults with CKD: a semistructured interview study. Am J Kidney Dis. 2010;56(3):439–446. doi: 10.1053/j.ajkd.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKillop G, Joy J. Patients' experience and perceptions of polypharmacy in chronic kidney disease and its impact on adherent behaviour. J Ren Care. 2013;39(4):200–207. doi: 10.1111/j.1755-6686.2013.12037.x. [DOI] [PubMed] [Google Scholar]
- 34.Magacho EJ, Ribeiro LC, Chaoubah A, Bastos MG. Adherence to drug therapy in kidney disease. Braz J Med Biol Res. 2011;44(3):258–262. doi: 10.1590/s0100-879x2011007500013. [DOI] [PubMed] [Google Scholar]
- 35.Tuot DS, Plantinga LC, Hsu CY, et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clifford S, Perez-Nieves M, Skalicky AM, Reaney M, Coyne KS. A systematic literature review of methodologies used to assess medication adherence in patients with diabetes. Curr Med Res Opin. 2014;30(6):1071–1085. doi: 10.1185/03007995.2014.884491. [DOI] [PubMed] [Google Scholar]
- 37.Vaughn-Cooke M. Development of an integrated model for predicting patient compliance. Paper presented at: Proceedings of the 53rd Human Factors and Ergonomics Society Annual Meeting; 2009; San Antonio, TX. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.