Abstract
The role of position L8′, located in transmembrane domain 1 of the neuronal nicotinic α3 subunit, was characterized by using two-electrode voltage clamp in Xenopus oocytes. Four amino acids (Ala, Ser, Phe, and Tyr) were inserted at this conserved position, and the mutant subunit was coexpressed with either wild-type β2 or β4 subunits. These substitutions led to significant alterations in the pharmacodynamic parameters of cholinergic agents, resulting in loss of function. Ala and Ser substitutions resulted in losses in agonist (ACh, nicotine, and DMPP) potency and intrinsic activity at both α3β2 and α3β4 receptors. Similarly, significant changes in antagonist potency were produced by the Ala and Ser substitutions. Phe and Tyr mutations did not alter the receptor's EC50 for ACh or nicotine but reduced the EC50 for DMPP at both receptors. The Phe mutation also reduced the intrinsic activity of all agonists tested at both receptors. The Tyr mutation, though, led to a decrease in intrinsic activity for all agonists at the α3β2 receptor, yet resulted in no changes for DMPP, a decrease for nicotine, and an increase for ACh at the α3β4 receptor. The most dramatic changes in the receptor's functional properties were produced by substitutions that introduced the largest changes in amino acid volume. Additional replacements (Gly, Thr, and Val) suggested an inverse correlation between amino acid volume at position α3L8′ and EC50 for α3β4 nAChRs; however, α3β2 nAChRs displayed a nonlinear correlation. These data demonstrate that structural alterations at position α3L8′ could propagate to the agonist-binding site.
Keywords: acetylcholine, nicotine, DMPP, neuronal nicotinic acetylcholine receptor, competitive antagonist, two-electrode voltage clamp
The muscle and neuronal nicotinic acetylcholine receptor (nAChR), as well as the γ-aminobutyric acid (GABA) and glycine receptors, belong to the superfamily of ligand-gated ion channels (LGIC). Neuronal and muscle nAChRs are allosteric integral membrane proteins assembled from five homologous polypeptide subunits. The five subunits are arranged pseudopentamerically around a central axis to form a cation-selective ion channel (Karlin, 2002). The muscle-type nAChR is composed of four different polypeptide subunits (α1, β1, γ or ε, and δ) with subunit stoichiometry of 2α:1β:1γ or ε:1δ, whereas neuronal nAChRs are composed of α and β subunits and in some cases five α subunits that form a functional homomeric receptor (e.g., α7). To date, 12 neuronal nAChR subunits have been described, α2–α10 and β2–β4 (Hogg et al., 2003). Each subunit is expressed throughout the nervous system, and the subunits can combine with each other to form nAChRs with a broad range of physiological and pharmacological profiles (Nai et al., 2003).
The subunits that form neuronal and muscle nAChRs share many structural features. Each subunit within a pentamer has a large, extracellular, amino terminal domain; four transmembrane domains (TMDs; TMD1–TMD4); a cytoplasmic loop between TMD3 and TMD4; and a small, extracellular, carboxy terminal (for review see Karlin, 2002). Of the four TMDs, TMD2 has been shown to form the receptor ion pore (Akabas and Karlin, 1995; Karlin and Akabas, 1995). TMD1 contributes to the ion pore in the open channel state (Akabas and Karlin, 1995). Alterations in the primary structure of these TMDs can affect the normal function of the channel (e.g., channel gating, desensitization). Mutations at TMD1 and TMD2 of muscle nAChRs have been found to alter the kinetics of nAChRs, leading to forms of congenital myasthenic syndrome (CMS; Engel et al., 2003). Furthermore, a group of mutations in the neuronal α4 and β2 subunits has been associated with autosomal dominant nocturnal frontal lobe epilepsy (ANFLE; Sutor and Zolles, 2001). Most of the mutations that cause either CMS or ANFLE occur at well-conserved positions in TMD1 of muscle-type nAChRs or TMD2 of neuronal nAChRs.
Considerable progress has been made in determining the role of the predominant receptor subtypes in the brain (α4β2 and α7 receptors) during physiological and pathophysiological conditions (Ochoa and Lasalde-Dominicci, 2007). However, the role of other neuronal nicotinic receptors found throughout the central nervous system is not well understood, and their expression in these regions may play a critical role. Such is the case for the neuronal α3 receptor subtype. The neuronal α3 receptor subtype—coexpressed with neuronal β2 and/or β4 subunit—is the predominant neuronal nicotinic receptor in the autonomic ganglia (Skok, 2002) and thus can play a critical role in the homeostatic function of every organ system in the body. Indeed, mice lacking α3 subunits either die early after birth or show defects that range from retarded growth and underweight to defects in the ocular and urinary systems (Xu et al., 1999). Furthermore, the phenotype produce by ablation of the α3 subunit gene resembles that of the human autosomal disease megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS; Anneren et al., 1991).
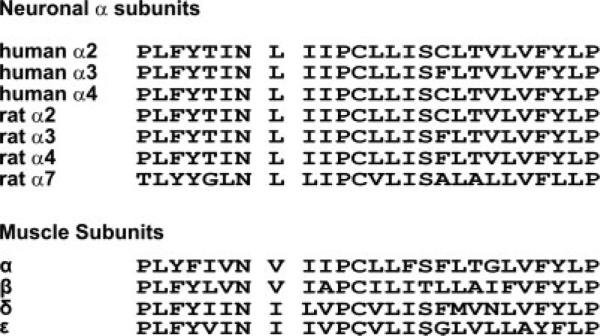
The aim of this study was to characterize the contribution of L8′ to the structure and function of neuronal α3 nAChR. We chose position L8′ of the neuronal α3 subunit, which is near the middle of TMD1, for two major reasons. First, this position is highly conserved in all neuronal α subunits of different vertebrate species and is substituted conservatively between TDM1 of other nicotinic subunits (Fig. 1). Second, a couple of naturally occurring mutations in the muscle-type AChR, shown to cause slow-channel syndrome (SCS), have been identified to occur at this position (βV229F; Navedo et al., 2006) or one position more N-terminal to it (αN217K; Wang et al., 1997). Albeit by different mechanisms, these two SCS mutations were shown to affect agonist binding, implying that this region may play an important role in coupling agonist binding to channel gating either allosterically or through the so-called pre-M1 region (Lee and Sine, 2005). The rationale for this study was to develop a profile of mutations that will mimic pathogenic mutations found in muscle nAChRs and also to assess their functional response in combination with two β subunits. The functional interactions between different neuronal nicotinic subunits are poorly understood. The “functional cross-talk” between the α3 nAChR subunit with different β subunits has barely been studied, and in the present study we used model pathogenic mutants to assess this differential “cross-talk.”
Fig. 1.
Primary structure of the nicotinic acetylcholine receptor TMD1. Amino acid sequence alignment of TMD1 of different neuronal α and β subunits and muscle α, β, δ, and ε subunits.
Four different amino acids (Ala, Ser, Phe, and Tyr) were substituted into position L8′ of the neuronal α3 nAChR. We generated a phenylalanine replacement, homologous to mutation βV229F in the muscle type, to assess the effect of amino acid atomic volume. Tyrosine was selected to increase amino acid atomic volume and to confer a more polar environment. Alanine was selected to conserve hydrophobicity while reducing amino acid volume, and serine was selected to decrease the volume and change the polar character of the position. Our results suggested that side chain volume at position L8′ affected ion channel function and the pharmacological profile of neuronal α3 nAChR subtypes. Furthermore, the functional analysis of the present mutations indicated that volume at position L8′ differentially affected receptor properties of α3 nAChR subtypes when coexpressed with β2 or β4 subunits.
MATERIALS AND METHODS
Generation of α3 Subunit Mutations
This study employed only neuronal α and β subunits from Rattus norvegicus; α3, β2, and β4 clones (GeneBank Nos. L31621, L31622, and U42976, respectively) were kindly provided by Dr. Jim Patrick (Baylor College of Medicine). The coding region of the α3 subunit of rat neuronal nAChR was subcloned into the EcoRI/Hind III site of the pcDNA3 vector under the T7 promoter (Promega, Madison, WI). Amino acid side chain substitutions at position L8′ (α3–L8′A, L8′S, L8′F, L8′Y, L8′V, L8′T, and L8′G) were prepared using the Quik-Change Site Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Oligonucleotide primers containing the desired mutation were generated (Invitrogene), and sample reactions were prepared as instructed by the manufacturer. The successful inclusion of mutations was confirmed by DNA sequence analysis performed at the DNA Sequencing Facility in the section of Evolution and Ecology, University of California, Davis. All pcDNA3 plasmids containing the coding region of the desired neuronal subunit were linearized using the EcoRI site, and cRNA was produced using the T7 mMessage mMachine Kit (Ambion, Austin, TX). The integrity and quantity of each cRNA were verified by gel electrophoresis, weight markers, and spectrophotometry.
nAChR Expression in Xenopus Oocytes
Stage V–VI oocytes were extracted from Xenopus laevis in accordance with the guidelines of the University of Puerto Rico Institutional Animal Care and Use Committee. The oocytes were incubated in collagenase type IA (Sigma-Aldrich, St. Louis, MO) to remove the follicles. The enzymatic treatment was followed by manual defolliculation to remove any remaining follicles. Single oocytes were microinjected within 1 hr of manual defolliculation with α3 wild-type (WT) or mutant cRNA and β2 or β4 WT cRNAs in a subunit stoichiometry of 2α:3β (40 ng total cRNA/oocyte). The cRNA mixtures were pressure injected using a positive displacement injector (Drummond Instruments, Broomhall, PA). The injected oocytes were incubated at 19°C in 0.5× Leibovitz's L-15 medium (Invitrogene) supplemented with 400 μg/ml bovine serum albumin and 10 mg/liter of antibiotic/antimycotic (Invitrogene). The incubation medium was changed daily. Electrophysiology experiments were carried out 3 days after cRNA injection.
Electrophysiological Recordings
The Gene Clamp 500 amplifier (Axon Instruments, Foster City, CA) in its two-electrode voltage clamp configuration was used to record agonist-induced currents. The electrodes were pulled with a vertical pipette puller (model PP-830; Narishige, Japan). Voltage electrodes with a resistance of <10 MΩ were used to record current traces. Current electrodes had a resistance of <2 MΩ. Electrodes were filled with 3 M KCl. Impaled oocytes in the recording chamber were perfused at a rate of 10.0 ml/min with MOR-2 buffer [115 mM NaCl, 2.5 mM KCl, 5 mM MgCl2, 1 mM Na2HPO4, 5 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), and 0.2 mM CaCl2 (pH 7.4)]. Membrane potential was held at –70 mV, unless otherwise indicated. Membrane currents were digitalized at 2 kHz by an Axon Digidata 1200 interface (Axon Instruments) and recorded using the Whole Cell Program 2.3 (kindly provided by Dr. J. Demspter).
Concentration-response data were collected from peak currents at seven agonist concentrations (in μM: 0.1, 1, 3, 10, 30, 100, and 300). Concentrations ranging from 30 μM to 1 mM were used to determine the EC50 for loss-of-function mutants. We employed three different agonists: ACh, nicotine, and DMPP. GraphPad Prism (GraphPad Software, San Diego, CA) was used to perform a nonlinear regression fit with a sigmoid concentration-response variable slope equation (Ortiz-Acevedo et al., 2004):
where I is the macroscopic current at a given agonist concentration; Imax and Imin are the maximum and minimum responses recorded, respectively; EC50 is the agonist concentration required to achieve half-maximal response; [Agonist] is the concentration of agonist; and nH is the Hill slope.
Inhibition curves were generated by coapplication of 100 μM ACh and increasing concentrations (in μM: 0.001, 0.01, 0.1, 1, 3, 10, 30, and 300) of the proper antagonist. We used DHβE to determine the effect of mutations on the α3β2 receptor subtype and MLA for the α3β4 receptor subtype.
Inhibition curves were fitted with the equation (Everhart et al., 2003):
where I is the current at a given antagonist concentration; Imax is the current in the absence of antagonist; IC50 is the concentration of antagonist needed to cause 50% inhibition of the agonist response; and [Antagonist] is the concentration of antagonist. The Hill slope (nH) was fixed to nH = 1. A system-independent estimate of the apparent antagonist potency (apparent KB) was performed from an estimate of the IC50 corrected for the level of agonism (100 μM ACh) using a modified Cheng-Prusoff equation (Leff and Dougall, 1993):
where KB is the apparent antagonist potency; IC50 is the concentration of antagonist needed to cause 50% inhibition of the agonist response; EC50 is the agonist concentration required to achieve a half-maximal response; [Agonist] is the concentration of agonist; and nH is the Hill slope.
Correlation Between Amino Acid Volume and Channel Function
To determine whether there was a correlation between amino acid volume and the receptor's functional properties, the log of the EC50 for ACh activation of each mutant was plotted as a function of amino acid volume. Additional amino acids (Gly, Thr, and Val) were inserted at position L8′ to confirm or discard such a correlation. These new mutants were characterized with ACh as described above.
Chemicals
All chemicals, including agonists and antagonists, were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis
EC50, Hill slope, and IC50 estimated errors are provided as 95% confidence intervals. Two-sample comparisons were made using Student's t-test or a Wilcoxon signed-rank test. For more than two groups, an ANOVA with a Dunnett's post-test analysis was used. P < 0.05 was considered significant.
RESULTS
Effect of Amino Acid Side Chain Substitution on the Pharmacological Responses to ACh
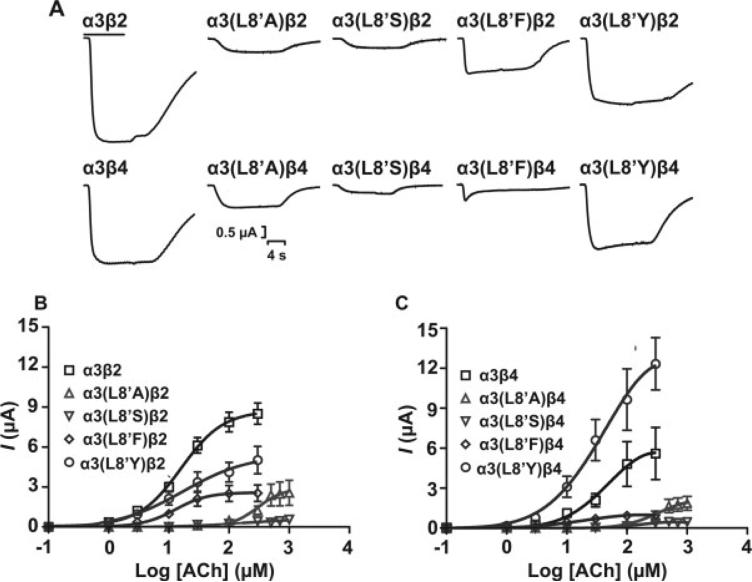
Functional expression of the receptors was evaluated with a two-electrode voltage clamp. Representative current traces from oocytes expressing either WT or mutant α3β2 or α3β4 receptors are shown for ACh (Fig. 2A), nicotine (Fig. 3A), and DMPP (Fig. 4A). Application of 300 μM ACh to oocytes expressing the mutant constructs resulted in loss of function as revealed by the effects on the macroscopic response. A detailed pharmacological analysis of the agonist pharmacodynamic profile (potency and intrinsic activity) was therefore undertaken. Peak currents at different ACh concentrations (see Materials and Methods) were used to generate a concentration-response (CR) curve for each WT and mutant receptor. The EC50 for ACh activation for α3(L8′A)β2 and α3(L8′S)β2 increased 22.9- and 6.1-fold, respectively, compared with WT (P < 0.05). A similar increase in EC50 was observed for α3(L8′A)β4 and α3(L8′S)β4, indicating a decrease in ACh's potency accompanying the apparent loss-of-function for these mutant receptors. These results were consistent with the reduction in macroscopic response observed in Figure 2A and its intrinsic activity (see Table II). However, an effect of the mutations on receptor expression could not be ruled out. Nevertheless, because EC50 is a functional property of the receptor that is independent of expression levels, the significant increase in EC50 (P < 0.05) produced by Ala and Ser substitutions suggested an effect of the mutations on channel function. Although the L8′A and L8′S mutations produced an apparent decrease in the receptor's functional properties, the replacement of Tyr or Phe for Leu at this position did not cause significant alterations (P > 0.05) in the potency estimates for ACh when coexpressed with the β2 subunit (Fig. 2B, Table I). The CR curve for the α3(L8′F)β4 mutant receptor shows a slight reduction in EC50 compared with WT, although this difference was not statistically significant (P > 0.05; Fig. 2B,C, Table I).
Fig. 2.
Functional consequences of amino acid substitution at position α3L8′. A: Representative ACh-induced peak current traces for wild-type and mutant α3 nAChR subtypes in response to 300 μM ACh. Agonist application (6 sec) is indicated by the bar on top of trace. Concentration-response data for α3β2 (B) and α3β4 (C) WT and mutant receptors (n = 5–9 individually tested oocytes). Additional agonist concentrations of 30, 100, 300, 500, 700, and 1,000 μM were used to obtain concentration-response data for L8′A and L8′S mutants. Error estimates for EC50 values are presented as 95% CI. Results are summarized in Table I.
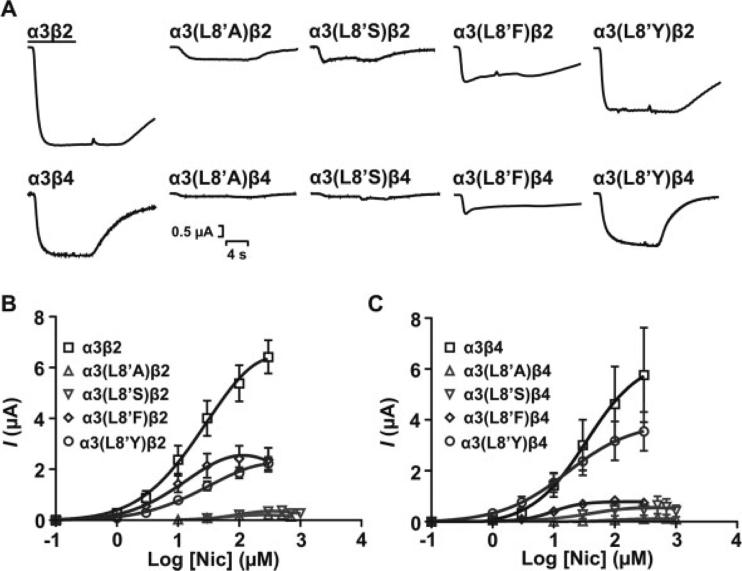
Fig. 3.
Functional characterization of WT and mutant α3 receptor subtypes upon activation by nicotine. A: Representative peak current traces of wild-type and mutant α3 nAChR subtypes in response to 300 μM nicotine. Bar indicates agonist application (6 sec). Niotine concentration-response curves for wild-type and mutant α3β2 (B) and α3β4 (C) receptor subtypes. Each concentration-response curve was obtained as for Figure 2. Additional agonist concentrations of 30, 100, 300, 500, 700, and 1,000 μM were used to obtain concentration-response data for L8′A and L8′S mutants. Error estimates for EC50 values are given as 95% CI. Results are summarized in Table I.
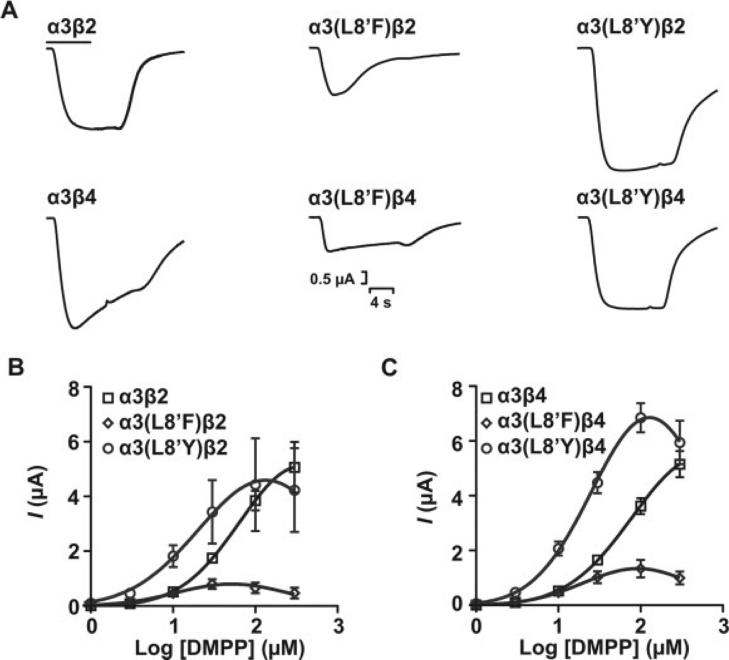
Fig. 4.
Functional characterization of WT and mutant α3 receptor subtypes upon activation by DMPP. A: Representative peak current traces for wild-type and mutant α3 nAChR subtypes in response to 300 μM DMPP. The bar indicates agonist application (6 sec). DMPP concentration-response curves for wild-type and mutant α3β2 (B) and α3β4 (C) receptor subtypes. Application of DMPP to L8′A and L8′S constructs failed to produce detectable currents. Error estimates for EC50 values are given as 95% CI. Results are summarized in Table I.
TABLE II.
Summary of Imax and Intrinsic Activity Estimates Using ACh as Full Agonist†
| ACh response |
Nicotine response |
DMPP response |
||||
|---|---|---|---|---|---|---|
| nAChR | Imax | Alpha | Imax | Alpha | Imax | Alpha |
| α3β2 | 8730 ± 577 | 1 | 6982 ± 1130 | 0.80 | 5602 ± 440 | 0.64 |
| α3(L8’A)β2 | 1679 ± 1153★ | 0.19 | 436 ± 52★ | 0.06 | — | — |
| α3(L8'S)β2 | 588 ± 239★ | 0.07 | 318 ± 30★ | 0.05 | — | — |
| α3(L8’F)β2 | 2578 ± 345★ | 0.30 | 2438 ± 272★ | 0.35 | 639 ± 52★ | 0.07 |
| α3(L8’Y)β2 | 5445 ± 1401 | 0.62 | 2472 ± 380★ | 0.35 | 4408 ± 676 | 0.50 |
| α3β4 | 6151 ± 2110 | 1 | 6429 ± 2688 | 1.05 | 6429 ± 477 | 1.05 |
| α3(L8’A)β4 | 2236 ± 977★ | 0.36 | 240 ± 18★ | 0.03 | — | — |
| α3(L8'S)β4 | 441 ± 65★ | 0.07 | 273 ± 149★ | 0.04 | — | — |
| α3(L8’F)β4 | 993 ± 95★ | 0.16 | 2376 ± 49★ | 0.40 | 1165 ± 144★ | 0.19 |
| α3(L8’Y)β4 | 12317 ± 1959★ | 2.00 | 2234 ± 1051 | 0.36 | 6489 ± 307 | 1.05 |
Alpha (intrinisic activity) is the ratio between the maximum current elicited by a 300 μM agonist concentration for a particular receptor and the maximum current elicited by a 300 μM ACh concentration for the corresponding wild-type receptor.
Error estimates are expressed as the mean ± SEM of 5–10 oocytes.
P < 0.05 vs. response in WT receptor.
TABLE I.
Funtional Characterization of Mutant α3 Receptor Subtypes†
| ACh response |
Nicotine response |
DMPP response |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| nAChR | EC50 (μM) | nH | n | EC50 (μM) | nH | n | EC50 (μM) | nH | n |
| α3β2 | 17 (13–22) | 1.2 (0.7–1.3) | 7 | 21.3 (10–35) | 1.1 (0.8–1.3) | 6 | 55 (54–76) | 1.3 (1.1–1.4) | 6 |
| α3(L8’A)β2 | 389 (197–767) | 1.8 (0.6–3.0) | 5 | 123 (48–276) | 1.7 (0.7–1.8) | 3 | ND | ND | >10 |
| α3(L8'S)β2 | 103 (51–205) | 1.7 (0.0–3.5) | 9 | 98 (90–131) | 1.8 (1.5–2.5) | 10 | ND | ND | >10 |
| α3(L8’F)β2 | 13 (11–18) | 1.2 (1.0–1.0) | 7 | 8 (7–12) | 1.4 (1.0–1.7) | 5 | 7 (11–14) | 2.1 (1.5–2.3) | 5 |
| α3(L8’Y)β2 | 17 (10–25) | 1.0 (0.5–1.0) | 6 | 24 (17–23) | 1.0 (1.0–1.2) | 7 | 13 (12–24) | 2.2 (0.8–2.3) | 8 |
| α3β4 | 44 (14–102) | 1.3 (0.8–1.4) | 9 | 30 (19–46) | 1.1 (0.6–1.3) | 5 | 58 (40–87) | 1.5 (1.0–1.6) | 6 |
| α3(L8’A)β4 | 367★ (156–865) | 1.6 (0.4–2.8) | 6 | 104 (98–183) | 1.5 (1.0–2.5) | 5 | ND | ND | >10 |
| α(L8'S)β4 | 130★ (61–276) | 2.6 (0.0–7.1) | 5 | 90 (58–139) | 1.4 (0.8–2.3) | 6 | ND | ND | >10 |
| α3(L8’F)β4 | 13 (9–23) | 1.4 (0.9–1.4) | 8 | 10 (9–11) | 1.8 (1.5–2.2) | 6 | 15 (9–15) | 2.0 (1.0–2.0) | 5 |
| α3(L8’Y)β4 | 36 (11–80) | 1.3 (0.7–1.3) | 7 | 29 (15–30) | 1.2 (0.5–1.6) | 5 | 16 (14–20) | 2.0 (1.0–2.0) | 6 |
n = number of individually tested oocyte.
Error estimates are expressed as the 95% confidence interval.
P < 0.05 compared with response in WT receptor.
Effect of Amino Acid Side Chain Substitution on the Potency of Nicotine and DMPP
The reduction in the EC50 for ACh observed for L8′A and L8′S mutants raised two questions: Do these mutations affect the potency of other agonists, and is there a correlation between amino acid side chain and agonist potency? We characterized the activation of α3 WT and mutant receptors by nicotine and DMPP. Similarly to ACh, application of 300 μM nicotine to oocytes expressing the mutant constructs resulted in loss of function as revealed by the effects on the macroscopic response (Fig. 3A). Nicotine-elicited macroscopic currents were used to establish CR curves, from which EC50 values were estimated. Compared with α3β2 and α3β4 WT receptors, L8′A and L8′S mutations significantly increased (P < 0.05) the EC50 for nicotine activation (Fig. 3B,C, Table I). The L8′F mutant receptor displayed a slight reduction in the EC50 for nicotine activation, whereas the L8′Y mutation did not produce a significant change (P > 0.05) in nicotine's EC50 (Table I). Nicotine-elicited responses for α3(L8′A)β2, α3(L8′S)β2, α3(L8′F)β2, and α3(L8′Y)β2 mutant receptors were smaller than the WT α3β2 response, which is consistent with its decrease in the agonists’ elicited Imax responses (Fig. 3B, Table II). Similar results were obtained for α3β4 receptor subtypes.
DMPP did not produce a significant activation of L8′A and L8′S mutant receptors at any concentration tested; therefore, we were unable to estimate the EC50 for DMPP activation for these mutations. In contrast, L8′F and L8′Y mutations reduced the EC50 for DMPP activation significantly compared with WT (P < 0.05; Fig. 4, Table I). Although L8′F and L8′Y mutations reduced the EC50 for DMPP activation, they did not change the EC50 for ACh or nicotine activation. These results suggested differences among the interactions governing α3 nAChR subtype activation by DMPP, ACh, and nicotine.
Effect of Amino Acid Side Chain Substitution on the Intrinsic Activity of Cholinergic Agonists (ACh, Nicotine, and DMPP)
Inspection of the nonnormalized CR curves for ACh reveals that dramatic changes in the intrinsic activity of ACh are produced by the mutant receptors. ACh as an endogenous full agonist at the WT receptors is assigned an intrinsic activity value of 1 (corresponding to its Imax value of 8,730 ± 577 nA and 6,151 ± 2,110 nA, for α3β2 and α3β4, respectively; Table II). The L8′S, L8′A, and L8′F mutations resulted in a loss of intrinsic activity for ACh and nicotine at both receptors. At the two L8′S mutant receptors, ACh's intrinsic activity is 0.07. This mutation also affects nicotine's intrinsic activity, lowering its value to 0.05 and 0.04 at the α3β2 and α3β4 receptors, respectively (Table II). The second largest change in agonist intrinsic activity for both ACh and nicotine at the α3β2 receptors is observed with the L8′A mutation. The L8′F mutation also diminishes ACh's and nicotine's intrinsic activity at both the α3β2 and the α3β4 receptors. The L8′Y mutation, though, led to a decrease in intrinsic activity for ACh and nicotine at the α3β2 receptor yet resulted in a decrease for nicotine and an increased value for ACh at the α3β4 receptor.
In the case of DMPP, these mutations also reduce its intrinsic activity, with the largest change corresponding also to the L8′S and L8′A mutations, insofar as DMPP was unable to elicit activation of these mutant receptors (Table II). A decrease in DMPP's intrinsic activity is also seen with the L8′F mutation, being comparable to the levels attained with the L8′S mutation for ACh and nicotine. The L8′Y mutation, though, led to a decrease in intrinsic activity for DMPP at the α3β2 receptor yet resulted in no changes at the α3β4 receptor. Therefore, mutations at the L8′ position led to a loss of function reflected by these alterations in cholinergic agonists’ intrinsic activities. In particular, the L8′S, L8′A, and L8′F mutations produce the largest losses for all cholinergic agonists (ACh, nicotine, and DMPP) at both the α3β2 and the α3β4 cholinergic receptors.
Effect of Mutations at α3L8′ Position on Competitive Antagonist Sensitivity
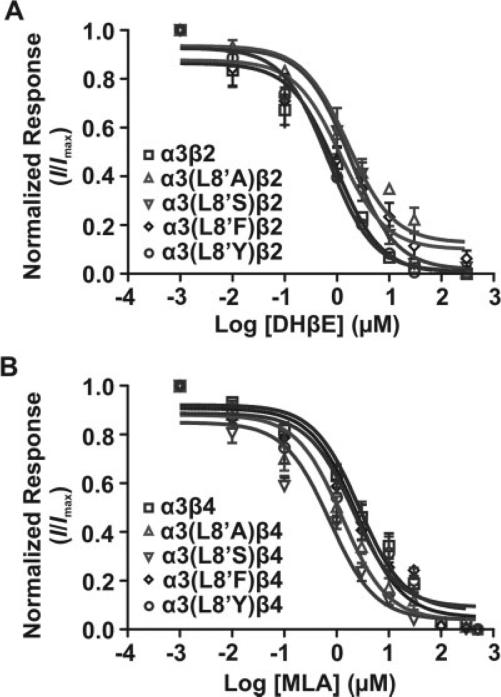
To examine the contribution of position L8′ to competitive antagonist sensitivity, we characterized the effect of amino acid substitutions on the relative potency of two competitive antagonists: dihydro-β-erythroidine (DHβE) and methyllycaconitine (MLA). DHβE is an alkaloid derived from Erythrina seeds that has submicromolar affinity for neuronal α3β2 receptors (Harvey and Luetje, 1996; Chavez-Noriega et al., 1997). Although DHβE has a high sensitivity for α3β2 receptors, it has a relatively low sensitivity for α3β4 receptors (IC50 = 23.1 μM; Harvey et al., 1996; Chavez-Noriega et al., 1997). MLA is a tertiary diterpenoid alkaloid produced by Delphinium brownie with high selectivity for α7 nAChRs (Ward et al., 1990). MLA has been found to display a moderate affinity (Ki 5 1.3 μM) for α3β4 ganglionic nAChRs (Free et al., 2002) and to block α3β4-mediated bovine adrenal catecholamine secretion with an IC50 value of 2.6 μM (Bryant et al., 2002). We used DHβE to determine the effect of mutations on the α3β2 receptor subtype and MLA for the α3β4 receptor subtype.
Inhibition curves were obtained by coapplication of 100 μM ACh with increasing concentrations of the appropriate antagonist. A concentration of 100 μM ACh was used to produce significant activation of the mutant receptors. The apparent potency of DHβE was significantly decreased (P < 0.05) by 67.0- and 26.0-fold for L8′A and L8′S mutants, respectively, in comparison with the α3β2 WT receptor (Table III). No evident changes in antagonist potency were produced by L8′F and L8′Y mutants relative to the α3β2 WT receptor (Fig. 5A, Table III). In the case of the apparent potency of the MLA antagonist for the α3β4 receptor, data analysis revealed a significant (P < 0.05) increase in the apparent potency for L8′A and L8′S mutant receptors of 1.4- and 3.6-fold, respectively. The L8′F and L8′Y substitutions did not produce a significant effect on the apparent potency of the antagonist (Fig. 5B, Table III). Similar to the case of the agonists pharmacodynamic parameters (potency and intrinsic activity), the most significant changes in antagonist potency were observed with the L8′A and L8′S mutants. Interestingly, these results showed a differential effect on antagonist potency on the L8′A and L8′S mutants. This was revealed as a decreased antagonist potency at α3β2 and increased antagonist potency at the α3β4 receptors.
TABLE III.
Effect of Mutation at Position α3(L8’) on Competitive Antagonist sensitivity†
| DHβE |
MLA |
|||||||
|---|---|---|---|---|---|---|---|---|
| nAChR | IC50 (μM) | Delta (x) | Apparent KB (μM) | Delta (x) | IC50 (μM) | Delta (x) | Apparent KB (μM) | Delta (x) |
| α3β2 | 0.6 (0.6–1.6) | — | 0.1 | — | — | — | — | — |
| α3(L8’A)β2 | 3.4★ (1.0–2.7) | 5.7 | 6.7 | 67.0 | — | — | — | — |
| α3(L8'S)β2 | 2.3★ (0.9–3.4) | 3.8 | 2.6 | 26.0 | — | — | — | — |
| α3(L8’F)β2 | 0.6 (0.6–2.3) | 1.0 | 0.1 | 1.0 | — | — | — | — |
| α3(L8’Y)β2 | 0.6 (0.5–0.9) | 1.0 | 0.1 | 1.0 | — | — | — | — |
| α3β4 | — | — | — | — | 4.4 (1.8–3.9) | — | 1.8 | — |
| α3(L8’A)β4 | — | — | — | — | 0.8★ (0.9–1.8) | 0.2 | 1.3 | 0.7 |
| α3(L8'S)β4 | — | — | — | — | 0.2★ (0.4–1.3) | 0.05 | 0.5 | 0.3 |
| α3(L8’F)β4 | — | — | — | — | 2.8 (1.6–3.7) | 0.1 | 0.4 | 0.2 |
| α3(L8’Y)β4 | — | — | — | — | 3.1 (1.1–4.0) | 0.7 | 1.1 | 0.6 |
Dose-response curves were obtained after coapplication of 100 μM ACh and increasing concentrations of the appropriate competitive antagonist. Errors for IC50 values are given as the 95% confidence interval of 6–12 individually tested oocytes. Apparent KB values were calculated using a modified Cheng-Prusoff equation (Leff and Dougall, 1993).
P < 0.05 compared with response in WT receptor.
Fig. 5.
Effect of mutations at position α3L8′ on competitive antagonist sensitivity. Effects of α3L8′ mutations on the competitive antagonist sensitivity of WT and mutant α3β2 (A) and α3β4 (B) receptor subtypes. Inhibition curves were generated by coapplication of increasing concentrations of the appropriate antagonist and a fixed concentration of ACh (100 μM). Each concentration-response curve was normalized to maximal ACh-induced current for each oocyte tested in the absence of the competitive antagonist. Results are summarized in Table III.
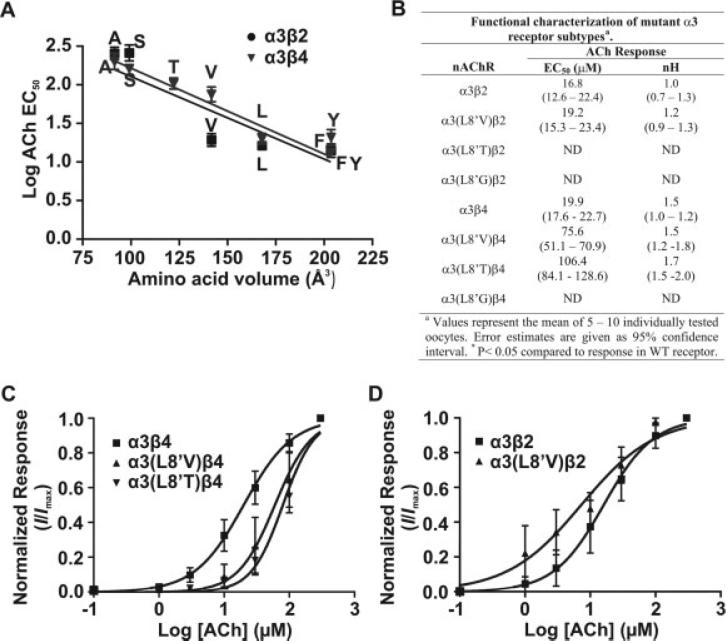
Side Chain Volume and Receptor Function
Amino acid side chain volume seemed to be an important structural element of position L8′, in that the most significant changes in the receptor's functional properties were observed with amino acid substitutions that introduce the largest changes in amino acid volume. The substitution of Ala or Ser in L8′ decreases amino acid volume by approximately 44% and 40%, respectively. In contrast, the substitution of Phe and Tyr produces an increase in amino acid volume of approximately 20%. Amino acid atomic volumes were estimated by using data produced by Chothia (1975). To test this hypothesis further, we mutated position L8′ to Val and Thr, with atomic volumes that stand between those of Ser and Leu. We also mutated this position to Gly in order to explore the effect of the smallest amino acid on channel function. We determined the EC50 for ACh activation for these mutant receptors as described previously (see Materials and Methods).
Expression of the mutant receptor α3(L8′G) did not produce measurable responses when coexpressed with β2 or β4 WT subunits. Analysis of the linear regression of log EC50 as a function of amino acid volume showed an inverse correlation between amino acid volume and the agonist EC50 for α3β4 receptor subtypes (Fig. 6A). A correlation between amino acid volume and function was difficult to establish for α3β2. For the α3β2 subtype, a Val substitution produced a remarkable deviation from a linear correlation, and the Thr mutation led to a nonfunctional nAChR (Fig. 6A,B).
Fig. 6.
Correlation of amino acid volume of substituted residues at position α3L8′ with EC50 values for ACh activation. A: The log of the EC50 value for ACh activation for individual WT and mutant α3 receptor subtypes was plotted against the volume of the corresponding amino acid residue inserted at position L8′. B: Summary of the mutant α3(L8′V), α3(L8′T), and α3(L8′G) receptor subtype functional properties in response to ACh. C,D: Concentration-response curves for α3(L8′V), α3(L8′T), and α3(L8′G) mutant receptors. Each concentration-response curve was obtained as for Figure 2. Concentration-response curves were normalized to maximal current for each oocyte tested. Error estimates for EC50 values are given as 95% CI.
DISCUSSION
We characterized the contribution of position α3L8′, a conserved residue in TMD1, to the functional properties of α3β2 and α3β4 neuronal nicotinic receptor subtypes. L8′ is homologous to position V229 in TMD1 of the muscle β subunit, in which a natural substitution of Phe for the WT Val causes CMS (Vohra et al., 2004; Navedo et al., 2006). The high degree of amino acid conservation and the disruption of receptor function caused by the spontaneous mutation in the homologous position in muscle-type receptors suggested a role of L8′ in ion channel function. We provide evidence that supports a contribution of position L8′ to the functionality of α3 nAChR subtypes. The two most important findings of the present study are 1) that substitution of L8′ in α3TMD1 by Ala and Ser alters agonist potency and intrinsic activity and antagonist potency and 2) that amino acid side chain volume at this position can dramatically affect the receptor's functional properties.
Substitution of Ala or Ser for L8′ produced a receptor loss of function characterized by a reduced macroscopic response (and reduced intrinsic activity) and an increased EC50 (decreased potency) for all cholinergic agonists tested. Furthermore, these mutant receptors did not show significant activation in response to DMPP. Loss of function was observed whether the mutant α subunit was coexpressed with β2 or with β4 WT subunits. Although we could not rule out a decrease in channel expression, the EC50 data for L8′A and L8′S mutants suggested an effect of the mutations on channel functionality, since EC50 is a channel functional property independent of receptor expression. Conversely, the substitution of Phe or Tyr for L8′ did not produce a significant effect on the agonist potency of ACh and nicotine. Both of these mutations decreased the EC50 for DMPP activation significantly.
Resembling the reported potency alterations elicited by the L8′S mutations were the dramatic changes in the intrinsic activity of ACh, which could bear important consequences for patients that may eventually be discovered to carry these mutations. For instance, irrespective of the potency values for ACh, in a patient expressing the (L8′S) α3β2 and (L8′S) α3β4 mutant receptors, ACh will elicit only minute responses at concentrations of up to 1 mM, with even minimal responses attained at lower physiological concentrations of ACh. The impact of the L8′S mutations on these two neuronal AChR subtypes seems to apply to all agonists (Figs. 2–4). Meanwhile, ACh will behave as a “partial agonist,” compared with WT receptors, in patients exhibiting (L8′S), (L8′A; L8′F), and (L8′Y) α3β2 mutations and (L8′A), (L8′S), and (L8′F) α3β4 mutations. In the case of the (L8′Y) α3β4 mutant receptors, ACh is predicted to conserve or even increase its endogenous full agonist property. Indeed, the lesser impact on agonist intrinsic activity is produced by the L8′Y substitution mutation at either receptor for DMPP. These considerations are of crucial significance in the assessment and development of cholinergic agents for central neurodegenerative disorders such as Alzheimer's disease and may help in explaining and understanding the refractoriness exhibited by certain patients in diverse therapeutic regimes.
DHβE and MLA block ACh's responses, maintaining their behavior as antagonists, whereas the receptor mutations clearly introduce documented shifts in their potency (as determined by their apparent KB values) compared with WT. Even more, once again the most dramatic changes in antagonist potency are produced by the L8′S and L8′A mutations for DHβE at the α3β2 receptor and for MLA at the α3β4 receptors, suggesting that these mutations are producing an alteration of the agonist binding site. Meanwhile, the lesser impact on antagonist potency is again produced for both selective antagonists by the L8′Y mutation of both receptor subtypes. The results clearly demonstrate that these specific mutations of the two neuronal nicotinic receptor subtypes affect the pharmacodynamic parameters of both the agonists (potency and intrinsic activity) and the antagonists (potency). The primary conclusions drawn from these results bear strong clinical and translational importance in the development and implementation of therapeutic regimes with central nicotinic agonists and antagonists used in an ample array of neurological conditions.
There was a difference between the effects of different substitutions at L8′ on the potency of DHβE as an α3β2 inhibitor and MLA as an inhibitor of α3β4 receptors. For the α3β4 receptor, Ala and Ser substitution decreased the MLA IC50, whereas, for the α3β2 receptor, the same amino acid substitution increased the IC50 for DHβE. Previous reports have suggested that regions in the β subunit are responsible for the differential sensitivity of α3β2 and α3β4 neuronal receptor subtypes to inhibition by substance P (Stafford et al., 1998). Thus, the differential effect of Ala and Ser substitution at L8′ on DHβE and MLA antagonism in α3β2 and α3β4 receptors, respectively, might have been due to structural and/or conformational differences between the association of α and β subunits in the two receptor subtypes.
The present study could lead to the development of mice with targeted altered function of neuronal nicotinic receptors. A remarkable study by the group of Henry Lester engineered mice hypersensitive to nicotine by the introduction of the M2 α4L9′→A mutation (Tapper et al., 2004). This study demonstrated that nicotine activation of the α4 subunit nAChRs was sufficient for reward, tolerance, and sensitization. Along these lines, the pronounced partial agonism for nicotine shown for the α3(L8′A) mutant in the present study could be used to develop a mutant mice line with reduced sensitivity to nicotine.
A remarkable observation in this study was the production of the most significant changes in EC50 by the amino acid substitutions that decreased amino acid volume. For example, the insertion of Ala or Ser at position L8′ reduced the volume occupied by the natural amino acid (Leu) by 45% and 40%, respectively. These substitutions produced up to a 23-fold increase in the EC50 for ACh activation. A Gly substitution did not produce a functional nAChR when coexpressed with β2 and β4 WT subunits. The Val substitution produced functional α3β4 and α3β2 nAChRs; however, the Thr substitution produced only functional α3β4 nAChR. When coexpressed with the β4 WT subunit, the L8′V mutation produced a 3.8-fold increase in the EC50 for ACh activation, and reduced amino acid volume by only 15%. A Thr substitution, which reduces side chain volume by about 27%, produced a 5.3-fold increase in the EC50 for ACh activation for α3β4 nAChRs,. In contrast, Phe and Tyr mutations, which increase amino acid volume by only 20%, had minor effects on ion channel function, suggesting that amino acid volume at position 8′ was an important physical property for proper ion channel function. These results suggested that decreasing amino acid side chain volume at position L8′ was more critical than increasing volume to the same extent. It is noteworthy that Gly and Thr are α-helix breakers; the lack of functional response from these two mutants could be due to altered folding of the α3 subunit. The lack of functional response from α3(L8′T)β2 nAChR could also suggest that oligomerization of the α3 subunit with the β2 subunit had more structural constraints than the β4 subunit at the L8′ position. The remarkable functional differences observed for Val and Thr mutations suggest that interactions of β2 and β4 subunits with the L8′ position of the α3 subunit have very specific structural requirements.
Reduction in amino acid side chain volume could hinder helix–helix interactions that are key to the function of the channel. According to the effects of Ala and Ser mutations on agonist potency and on the sensitivity of the receptor to competitive inhibition, it is likely that the changes introduced by the insertion of Ala or Ser at position L8′ allosterically propagated to the agonist binding site.
Previous reports have shown that mutations at the amino terminal portion of muscle α and β subunits’ TMD1 allosterically affect agonist binding (Wang et al., 1997; Navedo et al., 2006), although effects on channel gating are also possible. These spontaneous mutations occurred at the same position studied in this work (βV229F) or one position pre-N-terminal to it (αN217K). These reports suggested that changes in the primary structure of the TMD1 could modify the agonist's binding site through disruption of allosteric interactions that affect the proper propagation of agonist binding to ion channel gating.
The results of mutating position L8′ in the α3 nAChR contrast quite dramatically with those obtained for the SCS mutation βV229F, for which this study was, in part, designed (Navedo et al., 2006). Although the βV229F mutation produced a 10-fold decrease in ACh EC50, the α3 mutant receptors showed higher or unaffected EC50s. Our results suggest that this disparity may be explained based on differences in the local environment of the studied position between α and β subunits (Akabas and Karlin, 1995) and in subunit composition between neuronal and muscle nAChRs. Accordingly, specific interactions, within and between subunits, are major factors regulating proper function of nAChR and other ion channels (Quiram et al., 1999; Skok, 2002; Raike et al., 2007). Future studies should investigate the role of position L8′ in different nAChR subunits to assess its role in agonist binding and channel gating.
According to our current understanding of the nAChR activation mechanism, upon agonist binding, a series of conformational changes begins at the binding region and propagates to the protein subunits in a concerted fashion in order to open the ion channel pore (Chakrapani et al., 2004; Mitra et al., 2004). The present data suggest that a position downstream from this pathway of concerted allosteric transitions could affect agonist binding. In summary, our results suggest that side chain volume at position L8′ affected ion channel function and the pharmacological profile of neuronal α3 nAChR subtypes.
ACKNOWLEDGMENTS
M.N.-C. was sponsored by the Research Initiative for Scientific Enhancement (RISE) Program (NIH grant-1-R25-GM61151-01A1). D.C. was sponsored by the Graduate Assistance in Areas of National Need (GAANN) Program (U.S. Department of Education grant P200A030197-04) and the Research Initiative for Scientific Enhancement (RISE) Program (NIH grant 2 R25 GM061151).
Contract grant sponsor: National Institutes of Health; Contract grant numbers: SNRP U54NS0430311, 2RO1GM56371-10 and 2RO1-N33202; Contract grant sponsor: UPR Institutional Funds for Research (to J.A.L.-D.).
REFERENCES
- Akabas MH, Karlin A. Identification of acetylcholine receptor channel-lining residues in the M1 segment of the alpha-subunit. Biochemistry. 1995;34:12496–12500. doi: 10.1021/bi00039a002. [DOI] [PubMed] [Google Scholar]
- Anneren G, Meurling S, Olsen L. Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS), an autosomal recessive disorder: clinical reports and review of the literature. Am J Med Genet. 1991;41:251–254. doi: 10.1002/ajmg.1320410224. [DOI] [PubMed] [Google Scholar]
- Bryant DL, Free RB, Thomasy SM, Lapinsky DJ, Ismail KA, Arason KM, Bergmeier SC, McKay DB. Effects of methyllycaconitine and related analogues on bovine adrenal alpha3beta4* nicotinic acetylcholine receptors. Ann N Y Acad Sci. 2002;971:139–141. doi: 10.1111/j.1749-6632.2002.tb04448.x. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Bailey TD, Auerbach A. Gating dynamics of the acetylcholine receptor extracellular domain. J Gen Physiol. 2004;123:341–356. doi: 10.1085/jgp.200309004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975;254:304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Shen XM, Sine SM. Congenital myasthenic syndromes: multiple molecular targets at the neuromuscular junction. Ann N Y Acad Sci. 2003;998:138–160. doi: 10.1196/annals.1254.016. [DOI] [PubMed] [Google Scholar]
- Everhart D, Reiller E, Mirzoian A, McIntosh JM, Malhotra A, Luetje CW. Identification of residues that confer alpha-conotoxin-PnIA sensitivity on the alpha 3 subunit of neuronal nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;306:664–670. doi: 10.1124/jpet.103.051656. [DOI] [PubMed] [Google Scholar]
- Free RB, McKay SB, Boyd RT, McKay DB. Evidence for constitutive expression of bovine adrenal alpha3beta4* nicotinic acetylcholine receptors. Ann N Y Acad Sci. 2002;971:145–147. doi: 10.1111/j.1749-6632.2002.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Maselli R, Staub J, Day JW, Cens T, Wollman RL, Char-net PC. Novel beta and delta subunit acetylcholine receptor-mutations in the slow-channel syndrome demonstrate phenotypic variability. 1998 [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of dihydro-beta-erythroidine sensitivity on rat neuronal nicotinic receptor alpha subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Karlin A, Akabas MH. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15:1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Leff P, Dougall IG. Further concerns over Cheng-Prusoff analysis. Trends Pharmacol Sci. 1993;14:110–112. doi: 10.1016/0165-6147(93)90080-4. [DOI] [PubMed] [Google Scholar]
- Mitra A, Bailey TD, Auerbach AL. Structural dynamics of the M4 transmembrane segment during acetylcholine receptor gating. Structure. 2004;12:1909–1918. doi: 10.1016/j.str.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nai Q, McIntosh JM, Margiotta JF. Relating neuronal nicotinic acetylcholine receptor subtypes defined by subunit composition and channel function. Mol Pharmacol. 2003;63:311–324. doi: 10.1124/mol.63.2.311. [DOI] [PubMed] [Google Scholar]
- Navedo MF, Lasalde-ominicci JA, Baez-Pagan CA, Diaz-Perez L, Rojas LV, Maselli RA, Staub J, Schott K, Zayas R, Gomez CM. Novel beta subunit mutation causes a slow-channel syndrome by enhancing activation and decreasing the rate of agonist dissociation. Mol Cell Neurosci. 2006;32:82–90. doi: 10.1016/j.mcn.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa EL, Lasalde-Dominicci J. Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol Neurobiol. 2007;27:609–639. doi: 10.1007/s10571-007-9149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Acevedo A, Melendez M, Asseo AM, Biaggi N, Rojas LV, Lasalde-Dominicci JA. Tryptophan scanning mutagenesis of the gammaM4 transmembrane domain of the acetylcholine receptor from Torpedo californica. J Biol Chem. 2004;279:42250–42257. doi: 10.1074/jbc.M405132200. [DOI] [PubMed] [Google Scholar]
- Quiram PA, Ohno K, Milone M, Patterson MC, Pruitt NJ, Brengman JM, Sine SM, Engel AG. Mutation causing congenital myasthenia reveals acetylcholine receptor beta/delta subunit interaction essential for assembly. J Clin Invest. 1999;104:1403–1410. doi: 10.1172/JCI8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raike RS, Kordasiewicz HB, Thompson RM, Gomez CM. Dominant-negative suppression of Cav2.1 currents by alpha(1)2.1 truncations requires the conserved interaction domain for beta subunits. Mol Cell Neurosci. 2007;34:168–177. doi: 10.1016/j.mcn.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- Stafford GA, Oswald RE, Figl A, Cohen BN, Weiland GA. Two domains of the beta subunit of neuronal nicotinic acetylcholine receptors contribute to the affinity of substance P. J Pharmacol Exp Ther. 1998;286:619–626. [PubMed] [Google Scholar]
- Sutor B, Zolles G. Neuronal nicotinic acetylcholine receptors and autosomal dominant nocturnal frontal lobe epilepsy: a critical review. Pflugers Arch. 2001;442:642–651. doi: 10.1007/s004240100614. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Groshong JS, Maselli RA, Verity MA, Wollmann RL, Gomez CM. Focal caspase activation underlies the endplate myopathy in slow-channel syndrome. Ann Neurol. 2004;55:347–352. doi: 10.1002/ana.10823. [DOI] [PubMed] [Google Scholar]
- Wang HL, Auerbach A, Bren N, Ohno K, Engel AG, Sine SM. Mutation in the M1 domain of the acetylcholine receptor alpha subunit decreases the rate of agonist dissociation. J Gen Physiol. 1997;109:757–766. doi: 10.1085/jgp.109.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: a selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]