Abstract
Endurance exercise has emerged as a powerful intervention that promotes healthy aging by maintaining the functional capacity of critical organ systems. In addition, long-term exercise reduces the incidence of age-related diseases in humans and in model organisms. Despite these evident benefits, the genetic pathways required for exercise interventions to achieve these effects are still relatively poorly understood. Here, we compare gene expression changes during endurance training in Drosophila melanogaster to gene expression changes during selective breeding for longevity. Microarrays indicate that 65% of gene expression changes found in flies selectively bred for longevity are also found in flies subjected to three weeks of exercise training. We find that both selective breeding and endurance training increase endurance, cardiac performance, running speed, flying height, and levels of autophagy in adipose tissue. Both interventions generally upregulate stress defense, folate metabolism, and lipase activity, while downregulating carbohydrate metabolism and odorant receptor expression. Several members of the methuselah-like (mthl) gene family are downregulated by both interventions. Knockdown of mthl-3 was sufficient to provide extension of negative geotaxis behavior, endurance and cardiac stress resistance. These results provide support for endurance exercise as a broadly acting anti-aging intervention and confirm that exercise training acts in part by targeting longevity assurance pathways.
Keywords: endurance training, selective breeding, healthspan, cardiac performance, mobility
INTRODUCTION
Modern, sedentary lifestyles and highly nutritive diets have contributed to an increase in the incidence of obesity, cardiovascular disease, and diabetes [1]–[3]. These problems are particularly evident in the aging population, where these and other age-related diseases reduce independence and quality of life for the elderly [4], [5]. Endurance exercise has emerged as a powerful intervention to promote healthy aging [6]–[8]. Exercise training has been found to extend the healthy function of multiple organ systems, including heart [9], skeletal muscle [10], and brain [11], [12]. These functional effects are associated with substantial metabolic remodeling in humans [13]–[15] and in vertebrate models [16]–[18]. Less well understood are the changes to gene expression that are necessary for this remodeling to occur.
Although several important single genes and pathways have been identified using vertebrate models [19]–[22], the lack of an endurance exercise paradigm for an invertebrate species has impaired the use of large-scale genetic screens for exercise-induced factors. We have developed an endurance training paradigm for Drosophila, using a machine known as the Power Tower [23], that uses reiterated induction of the negative geotaxis instinct to allow controlled, daily training of fruit fly cohorts [23].
Following a three-week period of daily, ramped endurance exercise activity, wild-type flies display increased climbing speed [24], endurance [25], cardiac performance [24], [26], and mitochondrial enzyme activity [24]. Furthermore, endurance training has been demonstrated to increase mitochondrial number and reduce accumulated oxidative stress in fly cardiac muscle [27]. Exercise also induces consumption of accumulated fat stores in animals with excess lipid [26]. Taken together, these observations support the idea that endurance training in Drosophila induces similar effects as training in humans [28]–[30] and vertebrate models [15, 31–33].
These observations raise two important questions about this model: 1) what are the genes and pathways induced by exercise in the Drosophila model? 2) Which of these genes are associated with increased healthspan of the animal? One way to answer these questions would be to concurrently, but independently, induce longevity extension and exercise training in genetically isogenous fly cohorts, then compare the overlap in gene expression changes between the two interventions. The intersection of gene expression changes induced by both endurance training and longevity extension should be enriched for genes that act as intermediates to assure healthspan improve-ments in exercise trained animals.
To do this, we took advantage of a fly line generated by selective breeding for longevity, known as the La line [34]. This line exhibits extended longevity, particularly in the early, healthy portion of the survival curve [35]. This profile is characteristic of an intervention that genuinely extends healthspan, rather than simply extending the time between senescence and death. Longevity extension in La flies acts through mechanisms that are partially overlapping with those induced by dietary restriction [36] and curcumin feeding [37], and are also dependent, in part, on changes to mitochondrial efficiency [36], [38]. Dietary restriction, in turn, induces changes to wild type exercise capacity that are similar to those seen following exercise training [39].
We have previously shown that the La line climbs higher than wild-type in a rapid negative geotaxis assay [24], while its parental strain, known as Ra, behaves normally in negative geotaxis assays. Importantly, the Ra strain exhibits a robust exercise response, improving in speed following a three-week training program, while the La line shows much smaller improvement when trained [24], suggesting that La flies may already be receiving some of the benefits of exercise training through their selective breeding.
Here, we measure physiological changes to endurance and cardiac function induced by exercise training to Ra or La flies, then compare changes in gene expression induced in Ra flies by exercise to differences induced in Ra flies by selective breeding.
RESULTS
Lifespan/mortality
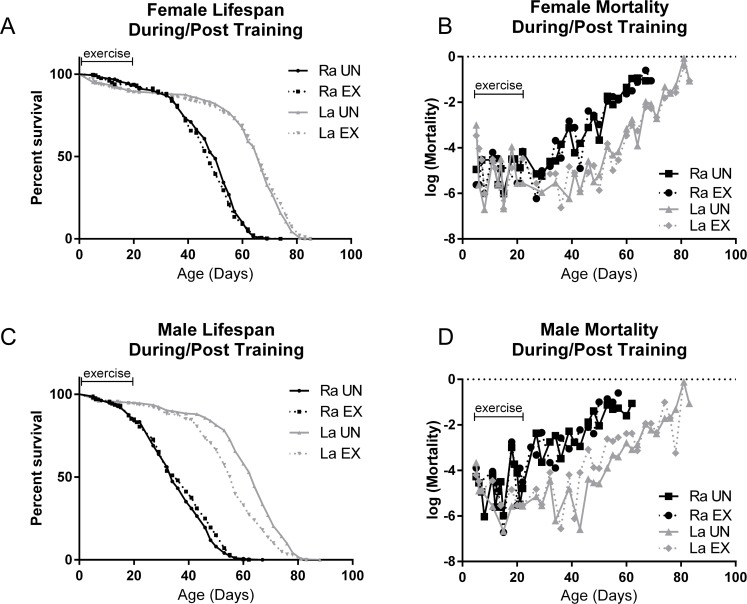
La flies are a product of selective breeding for longevity and are longer-lived than the parental Ra lines on standard laboratory diets [40]–[42]. We subjected La and Ra flies to three weeks of endurance exercise, using a ramped daily protocol [24]. Survival and age-specific mortality were measured during training and continued to be measured for the duration of the lifespan following the training period. Ra longevity was not significantly affected by three weeks of exercise training, when compared to control siblings that were placed on the machine for the same time, but restrained from exercising by a foam stopper pushed down into the vial. La longevity was not further extended by three weeks of exercise training, and La flies lived longer than Ra whether exercised or not (Figure 1A, B). In male flies only, La flies trended toward a slightly lower longevity following training, although this effect is small in comparison to the longevity difference between La and Ra males (Figure 1C).
Figure 1. Selectively bred La flies increase longevity whether exercised or not.
(A) Female La flies live longer than the control Ra females after three weeks of exposure to the Power Tower training machine (logrank; p <.0001). Training has little effect on longevity of either line. (B) La females have a delayed increase in mortality with age that is independent of exercise. Mortality during the training period is similar for all groups. (C) Male La flies live longer than control Ra males after three weeks of exposure to the training machine (logrank; p <.0001). Training does not increase longevity, and slightly decreases longevity in La males. (D) Mortality of La males is similar to Ra males during training, but is consistently lower than control Ra males after day 20. Exercised La males had somewhat increased mortality between days 40 and 65 compared to unexercised La males. n ≥ 250 for all survival and mortality experiments. Mortality and survival data are from same cohorts.
It is possible that the limitation of exercise to a three-week period may have limited the impact of training on longevity, as compared to lifelong exercise. However, in order to best visualize exercise-induced changes to gene expression, independent of changes induced late in life by aging, we stopped exercise training at three weeks for all experiments in this study and performed all assessments on age-matched animals immediately following training.
Because we often observe age-independent deaths due to damage during the training of any fly line, we wondered whether a selection effect for the fittest flies might contribute to the improved physiology of trained flies. To address this question, we measured age-specific mortality during the training period. If exercise acts by selecting flies that are resistant to training stress, then exercised Ra flies should have higher mortality during training than unexercised Ra flies. Furthermore, the La flies, previously established as long-lived and stress-resistant, should have lower mortality during training than Ra flies.
Age-specific mortality measures reveal that La flies have a delayed induction of age-related mortality increase rather than a decrease in the slope of age-related mortality increase (Figure 1B, D), in agreement with previous observations [43]. Mortality slope of both lines during the training period was sporadic and showed no consistent difference between exercised and unexercised (Figure 1B, D).
Negative geotaxis performance of exercised and long-lived Flies
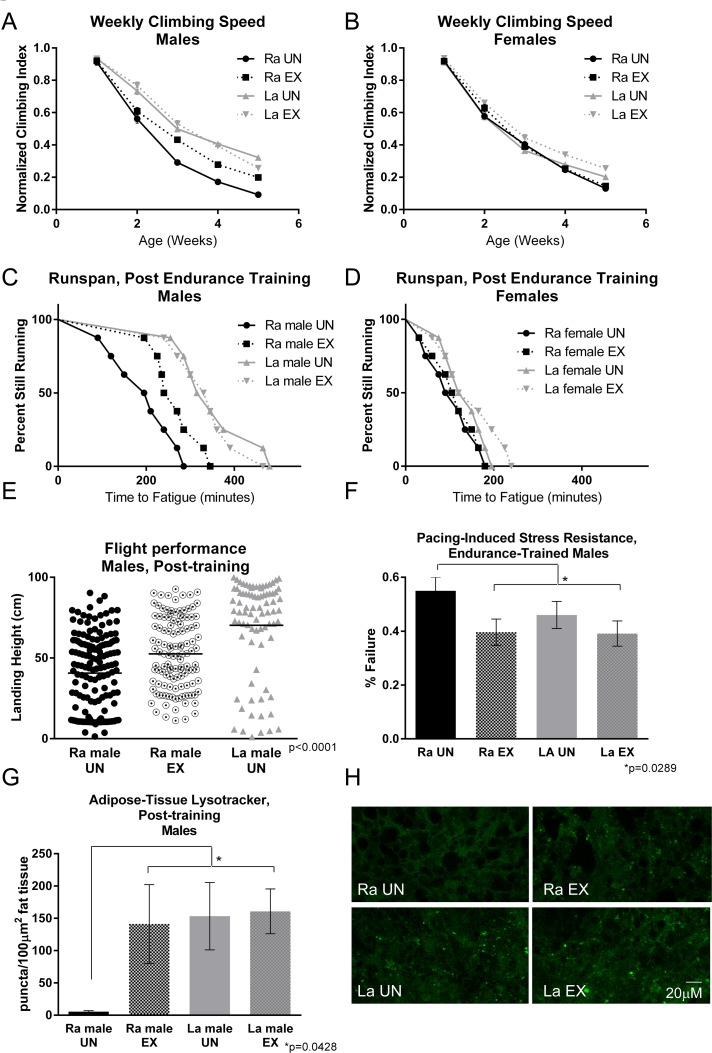
La flies have a higher climbing index than Ra flies in an acute test of climbing speed measured longitudinally across five weeks (Figure 2A,B), as previously observed [24]. However, when exercise trained, male Ra flies improve their speed significantly (Figure 2A). Age-matched La males, by contrast, derive no further benefit from training. Female flies of either genotype do not significantly benefit from training (Figure 2B).
Figure 2. Exercise training increases performance of male control flies, but not long‐lived La flies.
(A) Exercised Ra males are protected against declining negative geotaxis speed with age compared to unexercised controls (two‐way ANOVA, p <.0001, n ≥ 100). La males have higher negative geotaxis speed than Ra males across ages and receive no further benefit from exercise. (B) Female control flies were unaffected by training in negative geotaxis speed across five weeks of age, while female La flies showed a slight trend toward increased speed. (C) Ra males improved endurance significantly following training (log‐rank; p <.0001, n ≥ 160). La males showed higher endurance than Ra (log‐rank; p <.0001), but received no further benefit from exercise. (D) Neither Ra nor La females receives a benefit to endurance following training, although La showed a trend toward an increase (log‐rank; p = .2290, n ≥ 160). (E) Ra males improved flight ability following training. La males display higher landing height than both untrained and trained Ra flies. (ANOVA, p <.0001, n ≥ 160). (F) Unexercised La males had significantly less cardiac failure in response to electrical pacing stress than did unexercised Ra (error bars indicate ±SD, t‐test; p = .0289, n ≥ 100). Following training, Ra males had statistically similar failure rates to exercised or unexercised La. (G) Unexercised La males showed significantly higher Lysotracker staining in dissected abdominal adipose tissue than unexercised Ra (error bars indicate ±SD, t‐test; p =.0428, n ≥ 10). Following training, Ra flies showed statistically similar Lysotracker staining to exercised or unexercised La.
In order to examine endurance directly, we developed a fatigue tolerance assay [23], in which flies are placed on the exercise training device in vials of 20 and allowed to run to exhaustion. Vials are removed from the machine when less than five flies are still running and considered “fatigued”. Vial removal is analyzed as a time-to-event curve with each removal treated as an event, equivalent to a death in a survival curve. We refer to the resulting curve as a “runspan”.
Male La flies showed clear improvements in endurance compared to age-matched, three-week old Ra flies (Figure 2C). Exercise training caused a dramatic increase in the endurance of three-week old male Ra flies compared to unexercised sibs (Figure 2C), although they still did not equal the endurance of La males. La males do not gain further benefit from training. Three-week old female flies of either genotype showed less endurance than male sibs, and neither responded significantly to exercise training, although La females showed a slight trend toward improvement (Figure 2D).
Flight performance
Untrained three-week old La flies have significantly higher flight index than age-matched, untrained Ra flies (Figure 2E). Three-week old male Ra flies improve their score on a flight index significantly following exercise training, indicating that running induces a systemic response that improves other functional assays as well (Figure 2E). This result is consistent with a genuine endurance training response, not simply a behavioral improvement in negative geotaxis.
Cardiac performance
We have previously established that exercise training reduces cardiac failure in response to external electrical pacing [24]. External pacing is a cardiac stress assay that paces hearts to twice their normal heartbeat, then measures the percentage of flies that undergo arrest following treatment [44]. The percentage of hearts that undergo arrest following pacing is highly age-dependent [45] and acts as a marker for overall cardiac health. Three-week old La flies have a lower failure rate than age-matched unexercised Ra flies (Figure 2F) and do not receive further benefit from training (Figure 2F). However, after three weeks of exercise training, Three-week old Ra males had a similar failure rate to that of age-matched La males, indicating that exercise training in males can mimic the cardioprotective effect seen in La flies. Three-week old female Ra flies did not respond to training with significant improvement.
Exercise training increases Lysotracker staining in adipose tissue, but not heart, of wild type male flies [26]. Lysotracker staining is also high in adipose tissue of three-week old La flies in both males and females, consistent with a genetic increase in fat-specific autophagy levels in the La line (Figure 2G, H).
In both the Ra and La lines, males showed a stronger physiological response to exercise training than females, a phenomenon that we have previously observed across genotypes. This difference is due to a dimorphic behavioral response to the exercise stimulus which will be described in a separate manuscript. Therefore, we concentrated on males for microarray analysis.
Gene expression profiles of exercised and long-lived flies
Whole-fly transcript levels from untreated three-week old male Ra flies were compared to whole-fly transcript levels from untreated three-week old male La flies, and separately to whole-fly transcript levels from male Ra flies that were subjected to exercise training for the first three weeks of life. Three weeks was selected as a timepoint because it is a time before log-phase of mortality begins in either line, allowing a look at transcript changes occurring during the healthy phase of life, rather than at changes in response to advanced age and increasing pathology. Three weeks of training is sufficient to generate training-induced improvements to mobility and cardiac function in Ra flies (Figure 2B, D-F).
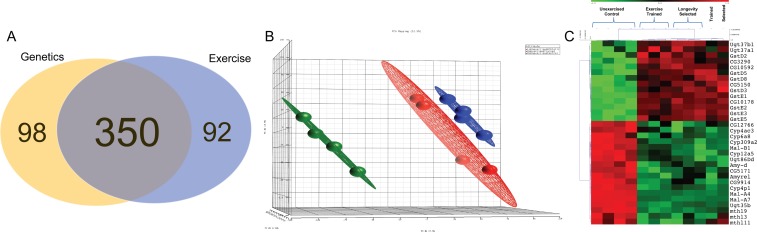
448 genes had altered expression following selective breeding from Ra to the La line (Supplementary Table S1). 442 genes had altered expression following exercise training in Ra flies in comparison to isogenic sibs (Supplementary Table S2). In order to highlight genes that may be involved in the effects of training on healthspan, we looked for the intersection of genes whose transcription was altered similarly by both selective breeding for longevity and by exercise training. 350 genes, or 65% of genes with transcription altered by breeding alone, were altered by both interventions in the same direction (Figure 3A). Orthogonal linear transformations of the input data were performed in order to assess portions of the variability associated with each principal component. The result of this Principal Component Analysis was consistent with the idea that selective breeding and exercise produce a similar pattern of variability in gene expression, represented graphically by the colored clouds in Figure 3B. Members of four KEGG pathways were significantly upregulated by both interventions, including Xenobiotic/Drug metabolism, Glutathione Metabolism and Folate biosynthesis (Table 1, Figure 3C). Members of four KEGG pathways were significantly downregulated by both interventions, including several aspects of carbohydrate metabolism (Table 1, Figure 3C).
Figure 3. Transcriptional changes induced by exercise training and by selective breeding for longevity are highly overlapping.
(A) Venn diagram showing transcripts altered by breeding from Ra to La (beige), transcripts altered by exercise training Ra males (blue), and the transcripts commonly altered by both interventions (overlap). (B) Plot of variance in gene expression explained by each treatment, when each is treated as the principal component (PCA). Training creates an expression pattern in Ra that is substantially similar to La. Ra (green), exercise trained Ra (blue) and selectively bred La (red). (C) Heat map of genes contained in KEGG pathways that are significantly altered by both interventions.
Table 1. KEGG pathway transcripts altered in the same direction by both endurance exercise and longevity selection.
| Pathway | Hits | KEGG ID | |
|---|---|---|---|
| Upregulated KEGG pathway genes | metabolism of xenobiotics drug metabolism | 10 | Ugt37b1, Ugt37a1, [GstD2, GstD5, GstD8, GstD3, GstE1, GstE2, GstE3, GstE5] |
| [glutathione metabolism] | 8 | ||
| folate biosynthesis | 3 | CG3290, CG5150, CG10592 | |
| Downregulated KEGG pathway genes | starch/sucrose metabolism• | 8 | CG12766•, CG9914•, CG5171•, Mal-A4•‡, Mal-B1•‡, Amy-d•‡, Amyrel•‡, Mal-A7•*, CG10178*, Ugt35b*, Ug86Dd* |
| galactose metabolism‡ | 4 | ||
| pentose/glucuronate interconversions* | 4 | ||
| limonene/pinene degradation | 5 | Cyp4ac3, Cyp6a8, Cyp309a2, Cyp12a5, Cyp4p1 |
Brackets indicate genes that are members of both the glutathione metabolism and the metabolism of xenobiotics KEGG pathways. Gray upregulated genes are part of the KEGG folate biosynthesis pathway. Dots, crosses and stars indicate membership in downregulated KEGG pathways. Some genes are members of multiple pathways. Gray downregulated genes are members of the KEGG limonene/pinene degradation pathway.
In addition to families identified by KEGG pathway analysis, we also noted several cases where multiple genes involved in similar functions were altered by both interventions. We chose two such functional groups, sensory receptors and methuselah-like genes, and confirmed the expression changes of individual genes from each group by qRT-PCR in order to validate the microarray results (Supplementary Figure 1A-H). These were chosen because they had previously been associated with longevity and movement behaviors, but not specifically linked to exercise capacity. Each is discussed separately below.
Sensory genes
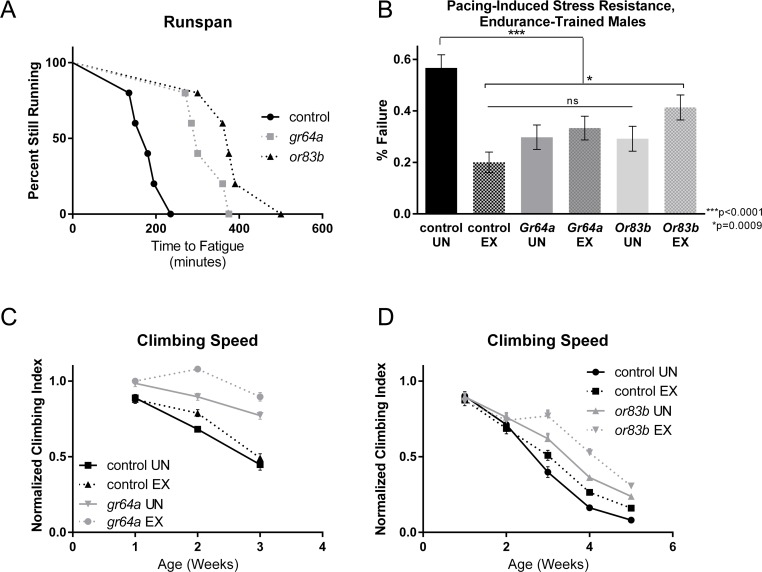
We noted several changes to the expression of odorant or gustatory receptors in exercised flies, including Or59a, Or59b, Gr98a and Gr32a, leading us to wonder if alterations to nutrient sensing could improve exercise capacity. Since alteration of several receptors was detected, we reasoned that a general reduction in odorant sensing might be induced by exercise. Therefore, we tested a null mutation in Or83b, a co-receptor with heteromeric G protein-coupled receptors, including odorant receptors. This mutation is thought to generally reduce detection of a wide variety of odorants [46], [47] and Or83b mutants have extended longevity [48]. Or83b mutants showed significant improvement over background controls in runspan of one-week old flies (Figure 4A), and in cardiac stress resistance of three-week old flies (Figure 4B). As previously observed [49], Or83b mutants also have a preserved climbing index at older ages (Figure 4C).
Figure 4. Sensory receptor mutations increase exercise capacity.
(A) Endurance of both gr64a and or83b mutants is significantly higher than a w1118 background control (log-rank; p <.0001 for each, n ≥ 100). (B) Exercised w1118 background control males had significantly less cardiac failure in response to electrical pacing stress than did unexercised cohorts (ANOVA with Tukey post-hoc comparison, p <.0001). Both Gr64a and Or83b flies had significantly less cardiac failure than unexercised w1118 controls whether exercised or not (ANOVA with Tukey post-hoc comparison, p <.0001). Exercised Or83b flies displayed a higher failure rate than exercised background controls (t-test, p=.0009) while maintaining a failure rate significantly lower than unexercised w1118 flies (t-test, p <.0001). (C) Unexercised gr64a males have a slower decline in negative geotaxis speed than control males (two-way ANOVA, p <.0001). Nevertheless, gr64a males still display significant improvement in negative geotaxis following training (two-way ANOVA, p <.0001, n ≥ 100). Exercised controls have higher climbing index across ages compared to unexercised controls (two-way ANOVA, p <.0001, n ≥ 100). (D) Unexercised or83b males have a slower decline in negative geotaxis speed than control males (two-way ANOVA, p <.0001, n ≥ 100). Nevertheless, or83b males still display significant improvement in negative geotaxis following training (two-way ANOVA, p <.0001, n ≥ 100). Exercised controls have higher climbing index across ages compared to unexercised controls (two-way ANOVA, p <.0001, n ≥ 100).
Because we find that genes involved in carbohydrate metabolism are downregulated by exercise, and because several gustatory receptors showed altered expression in both interventions, we wondered whether reduction in sugar tasting might potentiate exercise capacity. Flies mutant for Gr64a, a receptor for sweet taste involved in sensing of carbohydrate moieties [50], [51], showed improvement to runspan in one-week old flies (Figure 4A), to cardiac stress tolerance in three-week old flies (Figure 4B), and to climbing index across five weeks of age (Figure 4C). This result is also consistent with a link between nutrient sensing and exercise behavior.
Methuselah-like genes
Three members of the G-protein coupled receptor-encoding methuselah-like gene family, mthl-3, mthl-9 and mthl-11 were downregulated by both interventions. As members of this gene family have variously been associated with changes to negative geotaxis behavior and longevity [52]–[54], we tested these genes individually for their ability to extend lifespan or performance. Although wild-type females do not respond to exercise training with physiological improvements, this lack of response is due to their failure to complete the training program, not necessarily due to dimorphic responses to the training (see Discussion). Therefore, we measured the effects of altering mthl-3 gene expression in both females and males.
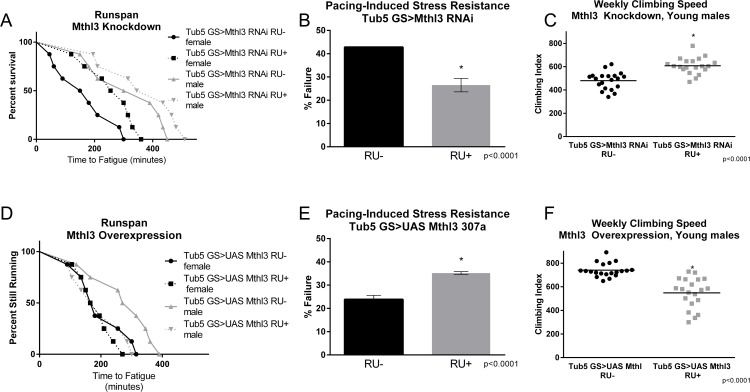
Knockdown of either mthl-9 or mthl-11 by ubiquitous expression of an RNAi construct did not improve climbing index or runspan (data not shown). However, ubiquitous knockdown of mthl-3 alone in adult flies with an RU-inducible tub5-Gal4 significantly improved runspan (Figure 5A), cardiac stress resistance (Figure 5B) and climbing index (Figure 5C) compared to RU- controls, despite only inducing a partial knockdown (Supplementary Figure S1I). Conversely, ubiquitous overexpression in adult flies (tub5-Gal4 >UAS-mthl-3) was sufficient to reduce runspan (Figure 5D), cardiac stress resistance (Figure 5E) and climbing index (Figure 5F) compared to RU- controls. By contrast, overexpression of two different UAS-mthl-3 lines with tub5-Gal4 had no significant effect on longevity (Supplementary Figure S2A, C) or flight performance (Supplementary Figure S2B, D). However, RNAi against mthl-3 somewhat reduced longevity (Figure 6A), while extending three-week old flight performance in females only (Figure 6B).
Figure 5. methuselah-like 3 knockdown mimics healthspan benefits of exercise training.
(A) Ubiquitous expression of RNAi against mthl3 using an RU-inducible driver increases the endurance of both males (log-rank; p = .0452) and females (log-rank; p <.0001) compared to RU- controls. n ≥ 160 for all endurance assays. (B) mthl3 knockdown reduces cardiac failure in response to pacing stress compared to RU- controls (t-test; p <.0001, n ≥ 100). (C) mthl3 knockdown increases negative geotaxis in males compared to RU- controls (2-way ANOVA; p <.0001, n ≥ 100). (D) Ubiquitous overexpression of mthl3 reduces the endurance of males compared to RU- controls (log-rank; p =.0005, n ≥ 160). (E) Ubiquitous overexpression of mthl3 increases cardiac failure in response to pacing (error bars indicate ±SD, t-test; p <.0001, n ≥ 100). (F) Ubiquitous overexpression of mthl3 decreases negative geotaxis in males compared to RU- controls (2-way ANOVA; p <.0001, n ≥ 100).
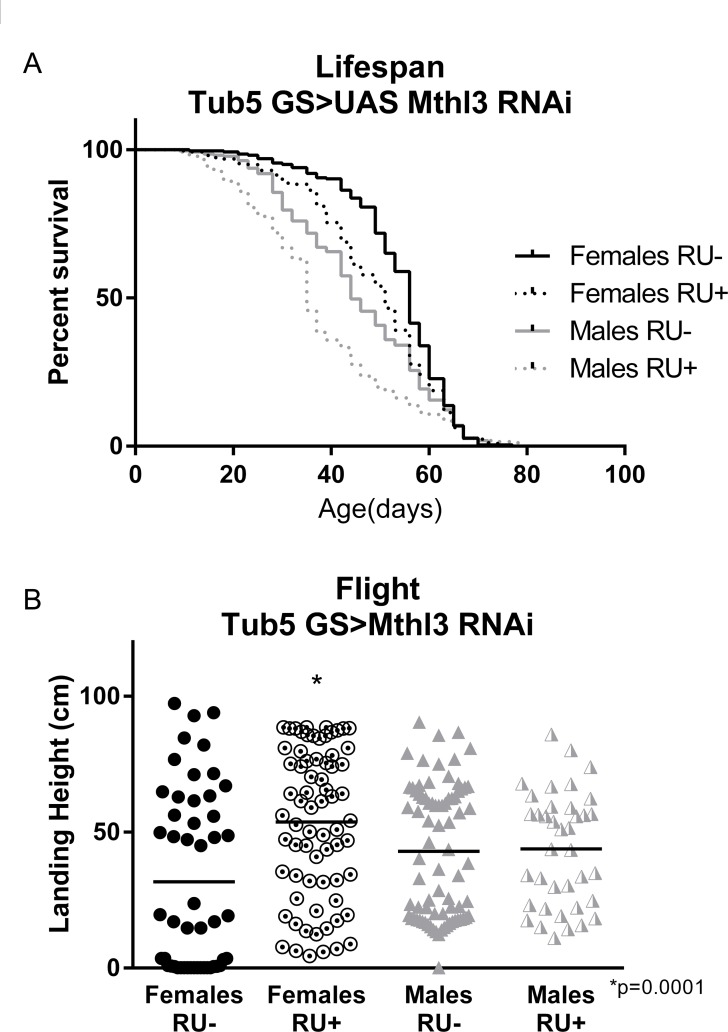
Figure 6. methuselah-like 3 knockdown fails to extend longevity.
(A) Ubiquitous knockdown of mthl3 does not extend lifespan, and in fact reduces longevity of both males and females (log-rank; p <.0001 for each, n ≥ 260). (B) Ubiquitous knockdown of mthl3 improves flight ability of females compared to RU- control (t-test, p = .0001, n ≥ 160), but not males.
DISCUSSION
Exercise and longevity
These results demonstrate that a three-week training program is sufficient to induce a change in gene expression that confers a gene expression profile resembling that of long-lived flies. This change is associated with a boost to healthspan, as measured by several indices of performance that persist after training, although age-related decline proceeds at a normal rate once exercise is discontinued [24]. These results further support the use of the Drosophila model to probe mechanisms of healthspan extension.
Persistent induction of negative geotaxis involves repetitive falling and loud noise, conditions which are likely to induce some degree of stress in experimental flies. Indeed, some degree of early mortality was observed in all training cohorts. This mortality was sporadic and not consistently different between genotypes, or between sibs that trained and sibs that were placed on the training device but prevented from running. This strongly suggests that increased mortality in flies of all genotypes during training is likely to be caused by acute damage (e.g. damage to a leg causing flies to get stuck in food), and not by a systemic stress response. Importantly, this result strongly excludes the possibility that the Power Tower training device has a “selection” effect that removes the weakest flies from the cohort. Nevertheless, all “unexercised” groups throughout the study, including the flies used for the arrays, were placed on the machine at the same time as “exercised” groups, but prevented from running by a foam stopper. This controlled for any potential exercise-independent effects of the training regimen.
Individual genes
Or83b mutant phenotypes are similar to those seen in dietary restricted flies, and are thought to act through similar mechanisms [48]. Dietary restriction also increases exercise capacity acutely, both in negative geotaxis assays and in runspan [39]. Furthermore, longevity extension of La flies acts through mechanisms that overlap with dietary restriction [36]. These results are consistent with a link between the sensing of food odors and exercise behavior. In cases where the sensory perception of food is reduced, flies increase movement behavior, exercise capacity and longevity.
Several genes encoding odorant binding proteins are altered specifically by selective breeding, but not exercise, including Obp99b. Interestingly, Obp99b has recently been associated with extended longevity and proposed to act as a humoral signaling factor [55]. A possible role for Obp99b in mediating longevity in La flies warrants further study.
These results implicate mthl-3 as a potentially important mediator of a subset of exercise-induced improvements to healthspan. mthl-3 is a member of the methuselah family of Secretin-like G-protein coupled receptors. Mutation of the founding member of this family, methuselah, has been associated with increased longevity [56] and extended sensorimotor function [57]. mthl-3 has previously been identified as a gene whose transcription is increased during aging [58], but mthl-3 has not previously been examined for longevity or motor phenotypes.
Here, we observe that mthl-3 is downregulated by exercise training in Ra flies and reduced in La flies. Endurance, negative geotaxis and cardiac performance were significantly improved by knockdown of mthl-3. Unlike the original methuselah or its ligand stunted [59], knockdown of mthl-3 did not significantly extend lifespan, and instead somewhat reduced it. While this result may be surprising in this context, it is important to note that two other mthl genes were similarly downregulated by both exercise and selective breeding. It may be that the combined effects of several mthl knockdowns would have a different effect on longevity and/or healthspan. It is also possible that mthl-3 would extend longevity under different temperature conditions or different activity levels, as the effects of methuselah are conditional [60], [61]. Alternately, mthl-3 may simply contribute to healthy physiology without extending longevity.
In addition to the individually tested genes above, we also noted several other interesting trends in the array data that would be useful to follow up further in the future. We discuss these separately below.
Lipases
Although microarrays were performed on whole flies, one gene classification that was highly upregulated in both interventions was lipases. Several of these are normally enriched in gut, where they contribute to breakdown of food components, including magro, an important gene for homeostasis of both triglycerides and cholesterol [62]. Declining function of the fly gut has recently been described as a highly relevant biomarker for aging and an efficient predictor of time of death [63]. Lipase expression in enterocytes has also been proposed to be important in maintenance of gut stem cell function in flies, with JNK and Foxo activity as important intermediates [64], [65]. Interestingly, the exercise-associated PGC-1αhomolog, spargel, has been shown to extend lifespan when activated in the gut [63], suggesting that the gut may be a highly relevant target tissue for exercise-induced changes in gene expression.
Folate metabolism
Folate biosynthesis is a significantly upregulated KEGG pathway in both interventions. This is intriguing in light of other recent results identifying folate metabolism as upregulated in the rapid response to dietary restriction in flies [66]. In C. elegans, knockdown of SAMe, a folate cycle inhibitor, increases lifespan by a mechanism that is not additive with dietary restriction [67], [68] In addition, the mechanism by which metformin extends lifespan in C. elegans is dependent on modifications to folate metabolism in the worms' E. coli food source [69]. Folate is an essential precursor to methionine, and thus necessary for methylation [70]. Hypomethylation has been proposed to be a general feature in metazoan aging [71]. Taken together, these results may point to availability of methylation precursors as a common target in multiple anti-aging mechanisms, including dietary restriction, endurance exercise, selective breeding for longevity, and metformin treatment.
Stress response
La flies have previously been observed to be resistant to multiple forms of stress [40, 72–75], and this is reflected in the upregulation of genes in KEGG pathways that promote stress resistance. Here we observe that endurance training also upregulates a set of genes in common with La that are associated with stress resistance pathways. These include Xenobiotic metabolism, drug metabolism, and glutathione metabolism genes (Table 1). Glutathione metabolism is particularly intriguing here, since endurance exercise has been proposed to have a hormetic effect on oxidative stress resistance in vertebrates [76]–[78].
Autophagy
It is perhaps surprising that we did not identify upregulation of pathways associated with autophagy in exercised flies. We have observed upregulation of lysosomal activity in adipose tissue of both La flies and exercised Ra flies (Figure 2G, H), and we have previously observed similar upregulation in wild-type exercised animals [26].
Upregulation of autophagy in muscle has been proposed to be an important mediator of the benefits of exercise in vertebrate muscle [19], [22]. Fly cardiac muscle mitochondria show evidence of less accumulation of oxidative damage in chronically exercised flies [27], consistent with increased mitophagy in cardiac muscle with exercise. In mutant flies with increased intra-myocellular lipid stores, cardiac muscle displays increased autophagy [26], but exercise training reverses lipid accumulation and lowers autophagy correspondingly in this model.
These results are consistent with a model in which autophagy in muscle is only upregulated in a transitory fashion by exercise, and autophagy genes are not chronically upregulated in muscle by training. Although upregulation in adipose tissue appears to be continuous during exercise training, this tissue alone may not be sufficient to provide statistically significant upregulation in a whole-animal gene expression analysis.
Sexual dimorphism
Ra flies show a strong sexual dimorphism in response to exercise (Figure 2A-D), as we have previously observed for several other wild-type genetic backgrounds. Males respond to training with consistent improvement in several functional assays, while females typically show a reduced response or no response. The lack of female response is not due to a reduced initial capacity, because initial negative geotaxis speed is nearly identical between males and females. Instead, we have observed sex-specific differences in the behavioral response to repeated negative geotaxis stimuli, with females frequently pausing or failing to respond. This is consistent with exercise experiments in other model systems that also show substantial effects of sex, age, and diet on both the amount of voluntary exercise and on the resulting adaptations, e.g. [79], [80]. Interestingly, knockdown of mthl3 improves runspan of both sexes, suggesting that females could benefit from training if they successfully executed the program long enough to induce the gene expression changes observed in males.
Conserved effects of exercise/selective breeding
The large degree of overlap between genes induced by exercise and those induced by breeding for longevity is highly suggestive of common genetic mechanisms. This is consistent with work from a rodent model in which rats were selectively bred for oxidative capacity based on performance on a treadmill test. High capacity running (HCR) rats also gained an extension of longevity in the process [81]. Thus, breeding for longevity confers exercise capacity and vice versa.
HCR rats also demonstrated improved cardiac performance and delayed cardiac senescence [81], as did La flies. Furthermore, improvements to aerobic efficiency were also observed in both models [38], [82]. Aging mitochondria in vivo are also protected from accumulated oxidation exposure by exercise training in both mouse skeletal muscle and fly cardiac muscle [27], indicating that the relationship between longevity, mitochondrial function and exercise capacity is highly conserved.
MATERIALS AND METHODS
Fly stocks and maintenance
All fly lines were reared and aged at 25°C; 50% humidity with a 12 hour light-dark cycle and provided with a standard 10% yeast/10% sucrose diet. Brewer's Yeast was obtained from MP Biomedicals (Solon, OH). Or83b and Gr64a lines were provided by Scott Pletcher and John Carlson, respectively [48], [50] and backcrossed into a w1118background. UAS-mthl3(307a) and UAS-mthl3(365) were provided by Mark Vanberkum. UAS-mthl3 RNAi (Bloomington Stock #36822), Tub5 GS gal-4 and y1w1 flies were obtained from the Bloomington Drosophila Stock Center. All UAS and Gal4 containing lines were backcrossed into the y1w1 background for ten generations before analysis.
Microarray analysis
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) for each genotype. RNA was then column purified using the RNeasy Plus Mini Kit (QIAGEN, Redwood City, CA, USA). At least three independent RNA extractions were prepared for each sample. Total RNA was analyzed by the Wayne State University School of Medicine Karmanos Cancer Institute using Drosophila GeneChip (Affymetrix) assay. The Affymetrix expression image files were uploaded to Partek® software, version 6.6 Copyright © 1993–2012 Partek Inc., St. Louis, MO, USA. Data was normalized using the quantile normalization method and the RMA background subtraction was applied. Differentially expressed genes were identified using a 1-way ANOVA. Genes were filtered according to differential p-value with FDR correction < 0.05 [83] as well as fold-change +/− 1.5. Heatmaps were generated using TMeV [84] software with Pearson Correlation distance metric.
qRT-PCR
Total RNA was prepared from 20 whole, age-matched flies of indicated genotype and treatment using Trizol (Invitrogen, Carlsbad, USA). At least three independent RNA extractions were prepared for each sample. Relative message abundance was determined by amplification and staining with SYBR Green I using an ABI 7000 SDS (Applied Biosystems). Expression of Rp49 was used for normalization. Differences between genotypes were assessed by t-test or nested ANOVA. Primer sequences are listed below.
Mthl3:
Forward: 5′-GATCCCCGCCCATTTGACAG-3′
Reverse: 5′-GGCTCGCCACCTTCTCCTTC-3′
Mthl9:
Forward: 5′-TACGCCCACACGGTCAACAT-3′
Reverse: 5′-GCCCGGTACTCCACTCCATC-3′
Mthl11:
Forward: 5′-GCAAGCGGTGGGTTTTCTGT-3′
Reverse 5′-TTCTACGTCGGCCATTTTCTCA-3′
Gr64a:
Forward: 5′-ACGGCGGCGGACATCAAT-3′
Reverse: 5′-CTCCACCTCGACGCACCAG-3′
Gr98a:
Forward: 5′-CATGCGGCGACTGATGAAGTGT-3′
Reverse: 5′-CGAAGCTGAAGCGCCAGTAGC-3′
Gr32a:
Forward: 5′-TCGCATCGGCTTTGCTCAGG-3′
Reverse: 5′-CGCCTCGCTCGTGCTCCAC-3′
Or59b:
Forward: 5′-GCCGGGCGAGTTCCTTACCT-3′
Reverse: 5′-CGTTCGCCAGCCTCTTGTCC-3′
Or83b:
Forward: 5′-CCAGAAAGAACAGCTTCCTCATCT-3′
Reverse: 5′-CGAGTCGGATGCTCGTTACC-3′
Rp49:
Forward: 5′-ACTCAATGGATACTGCCCAAGAA-3′
Reverse: 5′-CAAGGTGTCCCACTAATGCATAAT-3′
Survival analysis
Prior to all experiments, fly cultures were maintained at a constant density for at least two generations. 15 virgin females and 5 males were mated in 300 mL bottles with 50 mL standard 10% sucrose 10% yeast, with or without mifepristone or vehicle as described in text/figures [85]. Adult progeny were synchronized by collecting within 2 hours of eclosion, over a 24 hour time period. During and after three weeks of exercise training, groups of 20 age and gender-matched exercised or control unexercised flies were transferred into narrow polypropylene vials containing 5mL of appropriate food medium. Food vials were changed every second day, at which time dead flies were removed and counted. Flies were housed in a 25°C incubator on a 12:12 light:dark cycle at 50% relative humidity. Differences in survival were analyzed by log-rank. n > 280 for all longevity experiments.
Endurance exercise
Exercise was performed as previously described [23], [86]. Cohorts of at least 320 flies were collected under light CO2 anesthesia within 2 hours of eclosion and separated into vials of 20. Flies were then further separated into 2 large cohorts of at least 160 flies divided into exercised and unexercised groups. The unexercised groups were placed on the exercise training device, but were prevented from running by the placement of a foam stopper low in the vial in order to control for exercise-independent environmental factors. Exercised flies were placed in identical vials with normal cotton stoppers. The exercise device drops the vials of flies every 15 seconds, inducing a repetitive, innate negative geotaxis response. Exercised flies are free to run to the top of the vial. A ramped program of gradually increasing daily exercise time was previously established to generate significant alterations in mobility and cardiac performance [24].
Negative geotaxis behavior
Adult flies were collected with light CO2 anesthesia within 2 hours of eclosion and housed in appropriate fresh food vials. Negative geotaxis was assessed in Rapid Negative Geotaxis (RING) assays in groups of 100–120 flies as described [87]. Flies were transferred to individual polypropylene vials in a RING apparatus and allowed to equilibrate for 1 minute. Negative geotaxis was elicited by sharply rapping the RING apparatus four times in rapid succession. The positions of the flies were captured in digital images taken 2s after eliciting the behavior. Images were analyzed using ImageJ. The relative distance climbed by each fly was converted into quadrants using Microsoft Excel. The performance of 20 flies was calculated as the average of four consecutive trials to generate a single datum. Flies were tested 5 times per week for 5 weeks to assess decline in negative geotaxis speed with age. Between assessments, flies were returned to food vials and housed until the following RING test. Negative geotaxis results were analyzed using two-way ANOVA analysis with post hoc Tukey multiple comparison tests in GraphPad Prism (San Diego, CA, USA).
Endurance
Climbing endurance was measured using the fatigue assay described previously [23]. Eight vials of flies from each cohort were subjected to the fatigue assay at two time points: once in the first week of life, and once in the fourth week of life. For each assessment, the flies were placed on the Power Tower exercise machine and made to climb until they were fatigued, or no longer responded to the negative geotaxis stimulus. Monitored at 15 min intervals, a vial of flies was visually determined to be “fatigued” when five or fewer flies could climb higher than 1 cm after four consecutive drops. The time from the start of the assay to the time of fatigue was recorded for each vial, and the data analyzed using log-rank analysis in Prism.
Flight performance
Flight was analyzed as described in Babcock et al. [88]. Cohorts of at least 160 flies were aged and/or exercise trained in narrow vials housing groups of 20 age-matched siblings. Acrylic sheeting with paintable adhesive was placed in the flight tube, and fly cohorts were ejected into the apparatus to record flight performance and subsequent landing height after release. Fly cohorts were introduced to the flight tester one vial at a time using a gravity-dependent drop tube in order to reduce variability [88]. After a full cohort of flies was captured on the adhesive, the sheeting was removed to a white surface in order to digitally record the landing height of each fly. Images were analyzed using ImageJ. Landing height was averaged and compared in Prism using a student t-test.
Electrical pacing
Once per week a minimum of 100 males and 100 females were removed from the cohort and subjected to electrical pacing as described [44]. The percentage of fly hearts that responded to pacing with either fibrillation or arrest was recorded as “% failure”. Percent failure is a marker for stress sensitivity [44]. Data were analyzed in Prism using student's t-test.
Lysotracker
Lysotracker staining of adult fat bodies was performed as in Sujkowski et al. [26] Adult flies separated by age, genotype, and or treatment were dissected, ventral side up, in room temperature PBS. Having exposed fat bodies, partially dissected flies were rinsed 1X in fresh PBS. Lysotracker green (Molecular Probes, Eugene, OR) was diluted to 0.01 μM in PBS and applied to dissected preps for 30 seconds. Samples were washed 3 times in fresh PBS. Stained fat bodies were subsequently removed and mounted in Vectashield reagent (Vector Laboratories, Burlingame, CA, USA). Confocal work was done at the Microscopy, Imaging and Cytometry Resources Core at Wayne State University, School of Medicine on a Zeiss Laser Scanning LSM 780 (Jena, Germany) using a 100X oil immersion objective. Images were analyzed using ImageJ. A minimum of 10 samples were analyzed for each sample. Data were subjected to student t-test following quantification.
SUPPLEMENTARY INFORMATION FIGURES AND TABLES
Acknowledgments
We thank Joy Alcedo, Monica Driscoll, Zhen Yan, Donald DeGracia and Jun-Hee Lee for helpful discussions, John Carlson, Scott Pletcher and Mark Van Berkum for stocks, Karmanos Cancer Institute Microarray Core Facility and Microscope and Imaging Core Facility for assistance with analysis and imaging.
Footnotes
Funding
We acknowledge funding from the Wayne State University School of Medicine Department of Physiology, the Karmanos Cancer Institute Core Facility Pilot Grant Program and the Malaysian Palm Oil Board.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur J. A comprehensive review on metabolic syndrome. Cardiology research and practice. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 4.Fontana L. Modulating human aging and age-associated diseases. Biochimica et biophysica acta. 2009;1790:1133–1138. doi: 10.1016/j.bbagen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strasser B. Physical activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadore EL, Pinto RS, Bottaro M, Izquierdo M. Strength and endurance training prescription in healthy and frail elderly. Aging and disease. 2014;5:183–195. doi: 10.14336/AD.2014.0500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rengo G, Parisi V, Femminella GD, Pagano G, de Lucia C, Cannavo A, Liccardo D, Giallauria F, Scala O, Zincarelli C, Perrone Filardi P, Ferrara N, Leosco D. Molecular aspects of the cardioprotective effect of exercise in the elderly. Aging clinical and experimental research. 2013;25:487–497. doi: 10.1007/s40520-013-0117-7. [DOI] [PubMed] [Google Scholar]
- 8.Topp R, Fahlman M, Boardley D. Healthy aging: health promotion and disease prevention. The Nursing clinics of North America. 2004;39:411–422. doi: 10.1016/j.cnur.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Kemi OJ, Wisloff U. Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta physiologica. 2010;199:425–439. doi: 10.1111/j.1748-1716.2010.02132.x. [DOI] [PubMed] [Google Scholar]
- 10.Bishop-Bailey D. Mechanisms governing the health and performance benefits of exercise. British journal of pharmacology. 2013;170:1153–1166. doi: 10.1111/bph.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radak Z, Hart N, Sarga L, Koltai E, Atalay M, Ohno H, Boldogh I. Exercise plays a preventive role against Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 12.Raichlen DA, Polk JD. Linking brains and brawn: exercise and the evolution of human neurobiology. Proceedings Biological sciences / The Royal Society. 2013;280:20122250. doi: 10.1098/rspb.2012.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell metabolism. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. American journal of physiology Endocrinology and metabolism. 2004;286:E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 15.Fluck M. Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. The Journal of experimental biology. 2006;209:2239–2248. doi: 10.1242/jeb.02149. [DOI] [PubMed] [Google Scholar]
- 16.Cameron I, Alam MA, Wang J, Brown L. Endurance exercise in a rat model of metabolic syndrome. Canadian journal of physiology and pharmacology. 2012;90:1490–1497. doi: 10.1139/y2012-097. [DOI] [PubMed] [Google Scholar]
- 17.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annual review of biochemistry. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 18.Bo H, Zhang Y, Ji LL. Redefining the role of mitochondria in exercise: a dynamic remodeling. Annals of the New York Academy of Sciences. 2010;1201:121–128. doi: 10.1111/j.1749-6632.2010.05618.x. [DOI] [PubMed] [Google Scholar]
- 19.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1alpha. Experimental gerontology. 2013;48:1343–1350. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmoller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:3211–3224. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 22.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tinkerhess MJ, Ginzberg S, Piazza N, Wessells RJ. Endurance training protocol and longitudinal performance assays for Drosophila melanogaster. Journal of visualized experiments : JoVE. 2012;61 doi: 10.3791/3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PloS one. 2009;4:e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinkerhess MJ, Healy L, Morgan M, Sujkowski A, Matthys E, Zheng L, Wessells RJ. The Drosophila PGC-1alpha homolog spargel modulates the physiological effects of endurance exercise. PloS one. 2012;7:e31633. doi: 10.1371/journal.pone.0031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sujkowski A, Saunders S, Tinkerhess M, Piazza N, Jennens J, Healy L, Zheng L, Wessells R. dFatp regulates nutrient distribution and long-term physiology in Drosophila. Aging cell. 2012;11:921–932. doi: 10.1111/j.1474-9726.2012.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laker RC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, Adler PN, Wessells RJ, Saucerman JJ, et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. The Journal of biological chemistry. 2014;289:12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira ML, Sherrington C, Smith K, Carswell P, Bell R, Bell M, Nascimento DP, Maximo Pereira LS, Vardon P. Physical activity improves strength, balance and endurance in adults aged 40–65 years: a systematic review. Journal of physiotherapy. 2012;58:145–156. doi: 10.1016/S1836-9553(12)70105-4. [DOI] [PubMed] [Google Scholar]
- 29.Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports medicine. 2013;43:121–133. doi: 10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers SK, Sollanek KJ, Wiggs MP, Demirel HA, Smuder AJ. Exercise-induced improvements in myocardial antioxidant capacity: the antioxidant players and cardioprotection. Free radical research. 2014;48:43–51. doi: 10.3109/10715762.2013.825371. [DOI] [PubMed] [Google Scholar]
- 31.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circulation research. 2012;110:1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolaidis MG, Mougios V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports medicine. 2004;34:1051–1076. doi: 10.2165/00007256-200434150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. Journal of applied physiology. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arking R. Successful selection for increased longevity in Drosophila: analysis of the survival data and presentation of a hypothesis on the genetic regulation of longevity. Experimental gerontology. 1987;22:199–220. doi: 10.1016/0531-5565(87)90040-4. [DOI] [PubMed] [Google Scholar]
- 35.Arking R, Buck S, Hwangbo DS, Lane M. Metabolic alterations and shifts in energy allocations are corequisites for the expression of extended longevity genes in Drosophila. Annals of the New York Academy of Sciences. 2002;959:251–262. doi: 10.1111/j.1749-6632.2002.tb02097.x. discussion 463–255. [DOI] [PubMed] [Google Scholar]
- 36.Soh JW, Hotic S, Arking R. Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mechanisms of ageing and development. 2007;128:581–593. doi: 10.1016/j.mad.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Soh JW, Marowsky N, Nichols TJ, Rahman AM, Miah T, Sarao P, Khasawneh R, Unnikrishnan A, Heydari AR, Silver RB, Arking R. Curcumin is an early-acting stage-specific inducer of extended functional longevity in Drosophila. Experimental gerontology. 2013;48:229–239. doi: 10.1016/j.exger.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Ross RE. Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. Journal of insect physiology. 2000;46:1477–1480. doi: 10.1016/s0022-1910(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 39.Bazzell B, Ginzberg S, Healy L, Wessells RJ. Dietary composition regulates Drosophila mobility and cardiac physiology. The Journal of experimental biology. 2013;216:859–868. doi: 10.1242/jeb.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arking R. Gene expression and regulation in the extended longevity phenotypes of Drosophila. Annals of the New York Academy of Sciences. 2001;928:157–167. doi: 10.1111/j.1749-6632.2001.tb05645.x. [DOI] [PubMed] [Google Scholar]
- 41.Arking R, Buck S, Novoseltev VN, Hwangbo DS, Lane M. Genomic plasticity, energy allocations, and the extended longevity phenotypes of Drosophila. Ageing research reviews. 2002;1:209–228. doi: 10.1016/s1568-1637(01)00010-1. [DOI] [PubMed] [Google Scholar]
- 42.Arking R, Force AG, Dudas SP, Buck S, Baker GT. Factors contributing to the plasticity of the extended longevity phenotypes of Drosophila. Experimental gerontology. 1996;31:623–643. doi: 10.1016/s0531-5565(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 43.Arking R. Multiple longevity phenotypes and the transition from health to senescence. Annals of the New York Academy of Sciences. 2005;1057:16–27. doi: 10.1196/annals.1356.001. [DOI] [PubMed] [Google Scholar]
- 44.Wessells RJ, Bodmer R. Screening assays for heart function mutants in Drosophila. BioTechniques. 2004;37:58–60. doi: 10.2144/04371ST01. [DOI] [PubMed] [Google Scholar]
- 45.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nature genetics. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 46.Elmore T, Ignell R, Carlson JR, Smith DP. Targeted mutation of a Drosophila odor receptor defines receptor requirement in a novel class of sensillum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, Heijne G, Nilsson I. Membrane topology of the Drosophila OR83b odorant receptor. FEBS letters. 2007;581:5601–5604. doi: 10.1016/j.febslet.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 49.Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Experimental gerontology. 2008;43:739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gimenez LE, Ghildyal P, Fischer KE, Hu H, Ja WW, Eaton BA, Wu Y, Austad SN, Ranjan R. Modulation of methuselah expression targeted to Drosophila insulin-producing cells extends life and enhances oxidative stress resistance. Aging cell. 2013;12:121–129. doi: 10.1111/acel.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ja WW, Carvalho GB, Madrigal M, Roberts RW, Benzer S. The Drosophila G protein-coupled receptor, Methuselah, exhibits a promiscuous response to peptides. Protein science : a publication of the Protein Society. 2009;18:2203–2208. doi: 10.1002/pro.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrosyan A, Goncalves OF, Hsieh IH, Saberi K. Improved functional abilities of the life-extended Drosophila mutant Methuselah are reversed at old age to below control levels. Age. 2014;36:213–221. doi: 10.1007/s11357-013-9568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alic N, Giannakou ME, Papatheodorou I, Hoddinott MP, Andrews TD, Bolukbasi E, Partridge L. Interplay of dFOXO and two ETS-family transcription factors determines lifespan in Drosophila melanogaster. PLoS genetics. 2014;10:e1004619. doi: 10.1371/journal.pgen.1004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 57.Petrosyan A, Hsieh IH, Saberi K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- 58.Lai CQ, Leips J, Zou W, Roberts JF, Wollenberg KR, Parnell LD, Zeng ZB, Ordovas JM, Mackay TF. Speed-mapping quantitative trait loci using microarrays. Nat Methods. 2007;4:839–841. doi: 10.1038/nmeth1084. [DOI] [PubMed] [Google Scholar]
- 59.Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat Cell Biol. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- 60.Baldal EA, Baktawar W, Brakefield PM, Zwaan BJ. Methuselah life history in a variety of conditions, implications for the use of mutants in longevity research. Experimental gerontology. 2006;41:1126–1135. doi: 10.1016/j.exger.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Experimental gerontology. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Sieber MH, Thummel CS. Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell metabolism. 2012;15:122–127. doi: 10.1016/j.cmet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell metabolism. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karpac J, Biteau B, Jasper H. Misregulation of an adaptive metabolic response contributes to the age-related disruption of lipid homeostasis in Drosophila. Cell reports. 2013;4:1250–1261. doi: 10.1016/j.celrep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 66.Whitaker R, Gil MP, Ding F, Tatar M, Helfand SL, Neretti N. Dietary switch reveals fast coordinated gene expression changes in Drosophila melanogaster. Aging. 2014;6:355–368. doi: 10.18632/aging.100662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ching TT, Paal AB, Mehta A, Zhong L, Hsu AL. drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging cell. 2010;9:545–557. doi: 10.1111/j.1474-9726.2010.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS genetics. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon YK, Lu W, Melamud E, Khanam N, Bognar A, Rabinowitz JD. A domino effect in antifolate drug action in Escherichia coli. Nature chemical biology. 2008;4:602–608. doi: 10.1038/nchembio.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim KC, Friso S, Choi SW. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. The Journal of nutritional biochemistry. 2009;20:917–926. doi: 10.1016/j.jnutbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barnes VL, Bhat A, Unnikrishnan A, Heydari AR, Arking R, Pile LA. SIN3 is critical for stress resistance and modulates adult lifespan. Aging. 2014;6:645–660. doi: 10.18632/aging.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arking R, Burde V, Graves K, Hari R, Feldman E, Zeevi A, Soliman S, Saraiya A, Buck S, Vettraino J, Sathrasala K. Identical longevity phenotypes are characterized by different patterns of gene expression and oxidative damage. Experimental gerontology. 2000;35:353–373. doi: 10.1016/s0531-5565(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 74.Arking R, Burde V, Graves K, Hari R, Feldman E, Zeevi A, Soliman S, Saraiya A, Buck S, Vettraino J, Sathrasala K, Wehr N, Levine RL. Forward and reverse selection for longevity in Drosophila is characterized by alteration of antioxidant gene expression and oxidative damage patterns. Experimental gerontology. 2000;35:167–185. doi: 10.1016/s0531-5565(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 75.Vettraino J, Buck S, Arking R. Direct selection for paraquat resistance in Drosophila results in a different extended longevity phenotype. The journals of gerontology Series, A, Biological sciences and medical sciences. 2001;56:B415–425. doi: 10.1093/gerona/56.10.b415. [DOI] [PubMed] [Google Scholar]
- 76.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. Journal of applied physiology. 2008;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 77.Goto S, Naito H, Kaneko T, Chung HY, Radak Z. Hormetic effects of regular exercise in aging: correlation with oxidative stress. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007;32:948–953. doi: 10.1139/H07-092. [DOI] [PubMed] [Google Scholar]
- 78.Kang C L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Annals of the New York Academy of Sciences. 2012;1271:110–117. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konhilas JP, Chen H, Luczak E, McKee LA, Regan J, Watson PA, Stauffer BL, Khalpey ZI, McKinsey TA, Horn T, LaFleur B, Leinw LA. Diet and sex modify exercise and cardiac adaptation in the mouse. American journal of physiology Heart and circulatory physiology. 2015;308:H135–145. doi: 10.1152/ajpheart.00532.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neuroscience and biobehavioral reviews. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circulation research. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends in cardiovascular medicine. 2012;22:29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 84.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 85.Roman G, Davis RL. Conditional expression of UAS-transgenes in the adult eye with a new gene-switch vector system. Genesis. 2002;34:127–131. doi: 10.1002/gene.10133. [DOI] [PubMed] [Google Scholar]
- 86.Piazza N, Hayes M, Martin I, Duttaroy A, Grotewiel M, Wessells R. Multiple measures of functionality exhibit progressive decline in a parallel, stochastic fashion in Drosophila Sod2 null mutants. Biogerontology. 2009;10:637–648. doi: 10.1007/s10522-008-9210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Babcock DT, Ganetzky B. An improved method for accurate and rapid measurement of flight performance in Drosophila. Journal of visualized experiments : JoVE. 2014:e51223. doi: 10.3791/51223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.