Abstract
Objectives
Recently, increased rates of suicide in counties at higher altitudes have been noted. Due to the documented association between cocaine use and suicide, we hypothesized that there would be a correlation between incidence of cocaine use and altitude of residence.
Methods
Cocaine use data was obtained from the Substate Substance Abuse Estimates from the 1999-2001 National Surveys on Drug Use and Health. Data related to the percentages of people 12 years or older who used cocaine in the past year. Average elevation for United States counties was calculating using the Shuttle Radar Topography Mission elevation dataset and subject region elevation was calculated by averaging the weighted elevations of each region's relevant counties. The correlation between elevation of a substate region and cocaine use in that region was calculated using Pearson correlation coefficients.
Results
A significant correlation exists between mean altitude of a substate region and cocaine use in that region (r=0.34; p < 0.0001). Regression analysis controlling for age, gender, race, education level, income, unemployment, and population density was performed. Altitude remained a significant factor (p = 0.007), while male sex (p = 0.008) and possessing less than a college education (p < 0.0001) were also significant predictors of self-reported cocaine use in the past year. It is important to note that cocaine use was assessed in isolation of other drugs of abuse, an additional confounding variable.
Conclusions
This study demonstrates a significant correlation between altitude of substate region of residence and cocaine use. It is possible that stress response due to hypoxia is responsible, however, this requires further investigation. However, because other substance use was not assessed, specificity of this association is unknown Additionally, this correlation may help to explain the elevated rate of suicide in areas of higher elevation.
Cocaine use disorders are chronic and disabling conditions, typified by adverse life consequences and high rates of relapse. Despite decades of research, the pathophysiology of cocaine has not been fully elucidated (O'Brien, Childress et al. 1998; McLellan, Lewis et al. 2000; Lull, Freeman et al. 2008) and effective treatments are lacking (O'Brien 2005; Knapp, Soares et al. 2007; Xi and Gardner 2008; Vocci and Montoya 2009; Penberthy, Ait-Daoud et al. 2010). In the absence of evidence-based interventions, a large body of work has focused on identifying risk factors for human cocaine use and self-administration of cocaine in animals.
Approximately half the individual risk for cocaine dependence is genetic (Goldstein 2001; Nestler 2005), with the remainder consisting of environmental factors. The role of environmental stressors has long been recognized in self-administration of drugs of abuse (Piazza and Le Moal 1998). Clinical studies have suggested a role for environmental factors in the acquisition of, and relapse into, cocaine use in humans (Johanson and Fischman 1989; Gawin 1991). Preclinical findings (Hill and Powell 1976; Phillips, Howes et al. 1994; Phillips, Howes et al. 1994; Haney, Maccari et al. 1995; Tidey and Miczek 1997; Morgan, Grant et al. 2002) support the hypothesis that environmental stressors can facilitate the acquisition and maintenance of cocaine self-administration behaviors (LeSage, Stafford et al. 1999).
Suicidal behavior has also been shown to have a significant association with cocaine use (Vijayakumar, Kumar et al. 2011). In an urban emergency department setting, suicidal ideation (SI) is more common in patients with cocaine use disorder (40.5%) than those without cocaine use disorders (22.9%) (Garlow, Purselle et al. 2003). Two studies of cocaine dependent patients found that 39% and 43%, respectively, reported a previous suicide attempt (Renshaw, Parsegian et al. 2009; Roy 2009). Cocaine is associated with increased risk of attempt (Risk Ratio = 62; 95% Confidence Interval = 2.51–1528) but illicit use of cannabis, sedative-hypnotics and methamphetamine are not (Petronis, Samuels et al. 1990). In addition to suicidal ideation and attempts, cocaine also appears to be a risk factor for completed suicide. A study of all suicide deaths in New York City in a one-year period found positive cocaine toxicology tests in 21.8% of suicide decedents (Marzuk, Tardiff et al. 1992). Cocaine's association with suicidal ideation, suicide attempts and completed suicide is unexplained at present.
We recently reported a correlation between altitude and the rate of suicide in the U.S. (Kim, Mickelson et al. 2011). Altitude of residence was a greater risk factor for suicide than the regional rate of gun ownership or population density. This finding has been independently replicated, using mortality data covering two decades (1979-1998) and 2,584 U.S. counties (Betz, Valley et al. 2011; Brenner, Cheng et al. 2011). We additionally found an association between altitude and depression (DelMastro, Hellem et al. 2011). Comorbid depression and cocaine use disorder is a risk factor for relapse (Poling, Kosten et al. 2007; Stulz, Thase et al. 2011). In the U.S. National Comorbidity Survey, depressive psychopathology was found to precede, rather than follow, development of cocaine use disorders (Shaffer and Eber 2002). Pre-existing depressive symptomatology modulates both subjective and physiological responses to cocaine (Sofuoglu, Brown et al. 2001). A recent meta-analysis revealed that depression is consistently associated with cocaine use and impairment (Conner, Pinquart et al. 2008).
In consideration of the association of cocaine use with suicidal behaviors, and the association of altitude with suicide and depression, we hypothesized there would be a significant correlation between the rate of cocaine use and altitude of residence. For this study, we assessed the relationship of county and state altitude in the United States with the rate of self-reported cocaine use within the previous 12 months. In addition, using county-level data from the 2008 Area Resource File (U.S. Department of Health and Human Services 2009), we performed a multiple regression analysis on the rate of cocaine use with mean altitude, age, gender, race, education level, socioeconomic status and population density. In the context of these potentially confounding variables, we hypothesized that altitude would remain independently associated with cocaine use.
Methods
Data on cocaine use were obtained from the Substate Substance Abuse Estimates in the 1999-2001 National Survey on Drug Use and Health (NSDUH) (Substance Abuse and Mental Health Services Administration 2009). The data set included the percentage of persons aged 12 years and older that reported cocaine use in the past 12 months within the contiguous 48 states and the District of Columbia. The data were evaluated at a substate level for three age groups: 12-17 years, 18-25 years, and 26 years or older. These were combined to create a composite estimate for the rate of cocaine use in the population. Substate regions for each state are defined by the Substance Abuse and Mental Health Services Administration and include multiple counties or census tracts in each state. A total of 345 substate regions make up all contiguous 48 states and the District of Columbia (Substance Abuse and Mental Health Services Administration 2009).
The Shuttle Radar Topography Mission (SRTM) elevation dataset, a global digital topographic database of the earth, was used to calculate the average elevation for United States counties (National Geospatial-Intelligence Agency and National Aeronautics and Space Administration 2000). Alaska, Hawaii, one county in each Montana and Georgia, and two counties in Virginia are not included in the SRTM dataset and were therefore not included in this analysis. Zonal statistics in an ArcGIS/ArcInfo 9.3 (Esri, Redlands, CA) environment were used to calculate the average altitude of each of the remaining U.S. counties (n=3,108). County outlines from the National Atlas of the United States: County Boundaries of the United States (National Atlas of the United States 2001) were used to obtain the mean county elevation for each county based on the SRTM mean elevation calculations for each square kilometer. Mean altitude of each substate region was then calculated by averaging the weighted elevations of the counties for which data are available. Several counties were represented in multiple substate regions and, therefore, county elevation was weighted using its mapped area within a substate region. Notably, this method was utilized in our previous publications (DelMastro, Hellem et al. 2011; Kim, Mickelson et al. 2011) to calculate the mean altitude of U.S. county and substate regions.
Pearson correlation coefficients were calculated for the association between the mean substate altitude and cocaine use. Statistical significance was defined at an alpha level of 0.05 (two-tailed). R v2.10 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) was used for correlation analysis.
To increase our confidence in these results, we conducted an additional analysis that examined the relationship between cocaine use and altitude in the context of a number of possible covariates. With cocaine use as the dependent variable, we analyzed the following county-level independent variables: altitude; percentage of persons at or below the poverty line; unemployment rate; per capita income; percentage of persons 25 or older with less than 9 years of formal education; percentage of persons age 25 or older with at least a high school diploma; percentage of persons age 25 or older with 4 with a college education; population density; male population ratio; white male population ratio; white female population ratio; and divorced female population ratio. These data were taken from the Area Resource File 2008 (U.S. Department of Health and Human Services 2009) and analyzed using a normalized regression method. Regression calculations were performed in Stata 11 (StataCorp, College Station, TX).
Results
Figure 1 depicts the positive correlation between mean altitude of a substate region of residence and cocaine use in the past year among persons aged > 12 years (r = 0.34; p < 0.0001).
Figure 1.
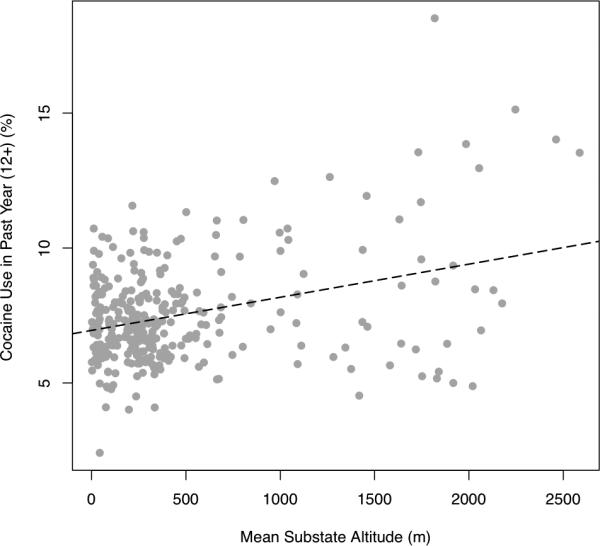
Rate of cocaine use vs. mean substate altitude
r = 0.34; df = 323, p<0.001
Multiple regression analysis was used to test if altitude significantly predicted cocaine use when potential confounding factors were included in the model (see Table 1). Additional predictor variables included: gender, race, educational attainment, per capita income, population density, and unemployment. The results of the regression indicated that three predictors explained 24% of the variance in self-reported cocaine use (r-squared = 0.24; df = 8; F(8,315) = 12.43; root MSE = 1.6714; p < 0.0001). Altitude remained a significant factor (β = 0.345; p = 0.007). Male sex (β = 0.263; p = 0.008) and possessing less than a college education (β = 0.269; p < 0.0001) were also significant predictors of endorsing cocaine use in the past 12 months. Age, race, per capita income, and population density were not significant predictors.
Table 1.
Demographic Predictors of Self-Reported Cocaine Use in U.S. Substate Regions
| Measure | Coefficient | t | p value |
|---|---|---|---|
| Altitude (meters) | 0.0006516 | 2.72 | 0.007 |
| Sex | 30.56331 | 2.68 | 0.008 |
| Years of Education | 11.0684 | 4.05 | < 0.0001 |
| White | −2.509071 | −0.64 | 0.523 |
| Black / African-American | −3.914166 | −0.99 | 0.323 |
| American Indian / Native American | −4.18068 | −0.86 | 0.391 |
| Income | −0.0000519 | −1.60 | 0.110 |
| County Population Density | −2.61e-06 | −0.55 | 0.580 |
| Unemployment | −6.197669 | −0.81 | 0.419 |
Data Sources: National Survey on Drug Use and Health (1999-2001); Area Resource File (2008); National Atlas of the United States: County Boundaries of the United States (2001)
To further evaluate the relationship between cocaine use, elevation and suicide, we completed two additional multiple regression analyses. When considered together with elevation, cocaine use was still significantly associated with suicide (see Table 2). Additionally, when considered together with elevation, population density, per capita income, and unemployment rate, cocaine use was still significantly associated with suicide rate (see Table 3). All data was assessed at a substate level using the same regions that were defined by the data obtained from Substate Substance Abuse Estimates from the 1999-2001 National Surveys on Drug Use and Health. Age-adjusted suicide data was obtained from the CDC WONDER database (data collected from 1979-1998) as described in Kim et al. (2010), elevation data was obtained from the Shuttle Radar Topography Mission elevation dataset as described abose, and all other data was obtained from the Area Resource File (2008).
Table 2.
Predictors of Suicide Rate in U.S. Substate Regions
| Measure | Coefficient | t | p value |
|---|---|---|---|
| Cocaine Use | .3998457 | 3.81 | 0.000 |
| Elevation (m) | .0026235 | 7.00 | 0.000 |
Data Sources: National Survey on Drug Use and Health (1999-2001); National Atlas of the United States: County Boundaries of the United States (2001)
Table 3.
Additional Predictors of Suicide Rate in U.S. Substate Regions
| Measure | Coefficient | t | p value |
|---|---|---|---|
| Cocaine Use | .429386 | 4.12 | 0.000 |
| Elevation (m) | .0021825 | 5.81 | 0.000 |
| Population Density (pop/sq mile) | −.0000351 | −3.90 | 0.000 |
| Per Capita Income (USD) | −2.64e-06 | −0.08 | 0.933 |
| Unemployment Rate | 39.93691 | 3.59 | 0.000 |
Data Sources: National Survey on Drug Use and Health (1999-2001); Area Resource File (2008); National Atlas of the United States: County Boundaries of the United States (2001)
Discussion
This study reports a significant correlation between mean altitude of a substate region of residence and cocaine use. At an altitude of 2,000 meters and above, we found that cocaine use in the previous year is 34.5% greater than the rate at sea level. Kim et al. reported that the suicide rate per 100,000 population increases 68.4% from sea level to 2,000 meters (Kim, Mickelson et al. 2011). Multiple lines of evidence indicate that cocaine use is associated with suicidal behavior (Petronis, Samuels et al. 1990; Marzuk, Tardiff et al. 1992; Garlow, Purselle et al. 2003; Roy 2009; Vijayakumar, Kumar et al. 2011), and this relationship may contribute to the correlation between altitude and suicide demonstrated in Haws et al. (Haws, Gray et al. 2009) and Kim et al. (Kim, Mickelson et al. 2011). It is important to note that several of these studies exploring suicidality and cocaine use, including those by Garlow and Roy, are based on cocaine dependent persons in treatment whereas our data is based on cocaine users in the community. However, the etiology of increased cocaine use at higher altitudes remains unclear.
The predisposition towards relapse in addiction may reflect a behavioral sensitization phenomenon (Robinson and Berridge 1993; Kalivas, Pierce et al. 1998). In addition to challenge doses of certain drugs, cross-sensitization effects are also observed following exposure to environmental stressors (Sorg 1992; Shaham, Erb et al. 2000) including non-contingent footshock (Goeders and Guerin 1994), food deprivation (Carroll, France et al. 1979; Papasava and Singer 1985), and physical relocation (Badiani, Browman et al. 1995). Environmental variables such as rearing history, conditioned stimuli, social defeat and access to food also affect cocaine self-administration (LeSage, Stafford et al. 1999). Psychogenic and environmental stressors are thought to converge on common stress-responsive brain structures, including the paraventricular nucleus (Pacak, Palkovits et al. 1995; Sawchenko, Brown et al. 1996; Herman, Flak et al. 2008). In fact, activation of the hypothalamic-pituitary-adrenal (HPA) axis, and in particular corticotropin releasing factor (CRF) (Corominas, Roncero et al. 2010), is implicated in behavioral sensitization to cocaine (Motheral, Cox et al. 2002).
Hypoxia is a form of environmental stress employed in preclinical animal models (Majmundar, Wong et al. 2010; Miyata, Takizawa et al. 2011). The systemic stress of increased altitude and reduced fraction of inspired oxygen (FiO2) is simulated with hypobaric hypoxia (HH). Laboratory experiments have shown that HH induces changes in dopaminergic tone (Heien, Khan et al. 2005; Sulzer 2011), which is associated with cocaine use. HH also increases CRF (Chen, Du et al. 2004), although it has not been shown that this occurs at simulated altitudes comparable to 2,000 meters of altitude. Cocaine is also associated with altered mitochondrial function (Dietrich, Poirier et al. 2004). This observation may be related to cocaine's association with bipolar disorder (Swann 2010), in which mitochondrial dysfunction is implicated (Stork and Renshaw 2005; Young 2007; Kato 2008; Quiroz, Gray et al. 2008; Cataldo, McPhie et al. 2010). We now briefly review the evidence for hypoxia's effect on dopaminergic tone, CRF and mitochondrial function.
Dopamine
Multiple reports indicate that HH significantly increases dopamine levels in the striatum of rats (Akiyama, Koshimura et al. 1991; Parrot, Cottet-Emard et al. 2003; Ray, Dutta et al. 2011). Even exposure to mild hypoxia (15% FiO2 rather than the 21% found at sea level) increases extracellular dopamine in rat striatum by 76% (Broderick and Gibson 1989). In addition, application of the DAT inhibitor nomifensine prior to hypoxia inhibits the efflux of extracellular dopamine (Orset, Parrot et al. 2005). Perhaps as a compensatory and homeostatic mechanism, in mice hypoxia induces a 21% increase in dopamine uptake sites (Arregui, Hollingsworth et al. 1994). Therefore, decreased FiO2 not only increases ambient dopamine levels but also appears to increase the loci for cocaine's mechanism of action. Finally, work with humans demonstrates that hypoxia alters the plasma concentration of dopamine (Gamboa, Gamboa et al. 2006).
CRF
The role of CRF in cocaine addiction is well-established (Corominas, Roncero et al. 2010). CRF binding protein and the type 2 CRF receptor play key roles in cocaine-seeking behavior (Wang, You et al. 2007). Furthermore, CRF receptor antagonists reduce cocaine self-administration and attenuate drug-seeking (Gurkovskaya, Palamarchouk et al. 2005), and blockade of CRF receptors has been proposed as a treatment strategy for cocaine use disorders (Lodge and Grace 2005). Simulated altitude of 5,000 meters increases CRF levels in rats (Chen, Du et al. 2004), raising the question of whether the response to environmental hypoxia could potentially be related to cocaine use. In addition to CRF levels, gene expression of CRF type 1 and 2 receptors is modulated by HH (Wang, Chen et al. 2004). As noted above, however, it has not been determined whether mild-to-moderate hypoxia has the same physiological effects on the CRF system. These small animal studies are often completed at simulated altitudes that are much higher than those in the continental United States. It is presently unclear whether or not the results from these small animal studies can be extrapolated to the moderate altitudes we have in the United States. However, Brenner et al. (2011), have shown that suicide rates in the U.S. begin to increase at as little as 2000 ft of elevation.
Mitochondrial Function
Genetic expression studies demonstrate that cocaine affects mitochondrial proteins (Lull, Freeman et al. 2008). Human cocaine abusers experience both acute (London, Stapleton et al. 1996) and long-term (Volkow, Hitzemann et al. 1992) declines in frontal lobe energy metabolism. Cocaine exerts cytotoxic effects on the electron transport chain, activating the mitochondrial apoptosis pathway in rodent neurons (Cunha-Oliveira, Rego et al. 2006). Finally, it was recently reported that inhibition of the mitochondrial enzyme aldehyde dehydrogenase 2 suppresses cocaine self-administration, and prevents cue-induced relapse in a rodent model (Mague, Pliakas et al. 2003).
This report is limited by its analysis of cocaine use in isolation, due to a lack of substate region data at our disposal on concomitant drugs of abuse such as opiates, methamphetamine, or alcohol. Thus, it is possible that the observed associations with cocaine use are a manifestation of a general association between altitude and psychoactive substance use or are confounded by associations with other specific substance use. In the future, we hope to assess the confound of other drugs of abuse. One review concluded that there are few high-quality studies focused on cocaine use disorders and suicide (Wilcox, Conner et al. 2004), suggesting an additional avenue by which this relationship could be explored. Recognizing the cross-sensitization between cocaine and other substances (Bonate, Swann et al. 1997; Desai and Terry 2003; Panlilio, Solinas et al. 2007), it would also be of interest to examine the relationship between cocaine use and altitude in light of individual drug use histories, and the geographic availability of cocaine within the U.S. As with all retrospective surveys, the data used for this analysis are subject to participant recall bias.
Genetic epidemiology has shown that environmental factors unique to individual patients determine whether predisposed persons will abuse one class of psychoactive substances versus another (Kendler, Jacobson et al. 2003). We propose that altitude, and the associated reduction in FiO2, may eventually be confirmed as one such environmental factor.
Conclusions
The authors report what is, to the best of our knowledge, the first report of a significant correlation between cocaine use in the past year and altitude of substate region of residence. When potential confounding demographic factors are controlled for, altitude remains a significant predictor of self-reported cocaine use; male gender and having less than a college education were also associated with cocaine use. Age, race, poverty, unemployment, and county population density do not predict cocaine use. The association of altitude with cocaine use may be related to increased rates of suicide and depression at altitudes > 2,000 meters that have recently been reported. Increased cocaine use at altitude may involve a stress response to hypoxia, which animal studies show involves neuroadaptation in dopamine neurotransmission and the HPA axis. However, additional research is needed to replicate this finding and clarify the mechanism of the association. This limitation is applicable to the link between the findings reported here, and increased suicidal ideation (Garlow, Purselle et al. 2003) and attempts (Roy 2009) in cocaine use disorders. Additionally, because other substance use was not assessed, the specificity of this association is unknown. Future studies should examine the role played by other drugs such as nicotine, alcohol and opiates in the relationship between altitude, suicidal behavior and cocaine use.
Acknowledgements
We would like to thank NIH Grants DA015116 and DA031247, VISN 19 MIRECC, and the Utah Science Technology and Research (USTAR) initiative for supporting this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Area Resource File (ARF) H. R. a. S. A. US Department of Health and Human Services, Bureau of Health Professions; Rockville, MD.: 2008. [Google Scholar]
- Akiyama Y, Koshimura K, et al. Effects of hypoxia on the activity of the dopaminergic neuron system in the rat striatum as studied by in vivo brain microdialysis. J Neurochem. 1991;57(3):997–1002. doi: 10.1111/j.1471-4159.1991.tb08249.x. [DOI] [PubMed] [Google Scholar]
- Arregui A, Hollingsworth Z, et al. Autoradiographic evidence for increased dopamine uptake sites in striatum of hypoxic mice. Neurosci Lett. 1994;167(1-2):195–197. doi: 10.1016/0304-3940(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Browman KE, et al. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674(2):291–298. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- Betz M, Valley M, et al. Elevated Suicide Rates at High Altitude: Sociodemographic and Health Issues May Be to Blame. Suicide Life Threat Behav. 2011;41(5):562–573. doi: 10.1111/j.1943-278X.2011.00054.x. [DOI] [PubMed] [Google Scholar]
- Bonate PL, Swann A, et al. Context-dependent cross-sensitization between cocaine and amphetamine. Life Sci. 1997;60(1):PL1–7. doi: 10.1016/s0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- Brenner B, Cheng D, et al. Positive association between altitude and suicide in 2584 U.S. counties. High Alt Med Biol. 2011;12(1):31–35. doi: 10.1089/ham.2010.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick PA, Gibson GE. Dopamine and serotonin in rat striatum during in vivo hypoxic-hypoxia. Metab Brain Dis. 1989;4(2):143–153. doi: 10.1007/BF00999391. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, et al. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205(4403):319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, McPhie DL, et al. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol. 2010;177(2):575–585. doi: 10.2353/ajpath.2010.081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-Q, Du J-Z, et al. Regulation of hypoxia-induced release of corticotropin- releasing factor in the rat hypothalamus by norepinephrine. Regul Pep. 2004;119:221–228. doi: 10.1016/j.regpep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, et al. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98(1-2):13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas M, Roncero C, et al. Corticotropin releasing factor and neuroplasticity in cocaine addiction. Life Sci. 2010;86(1-2):1–9. doi: 10.1016/j.lfs.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, et al. Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res. 2006;1089(1):44–54. doi: 10.1016/j.brainres.2006.03.061. [DOI] [PubMed] [Google Scholar]
- DelMastro K, Hellem T, et al. Incidence of major depressive episode correlates with elevation of substate region of residence. J Affect Disord. 2011;129(1-3):376–379. doi: 10.1016/j.jad.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Terry P. Evidence of cross-tolerance between behavioural effects of nicotine and cocaine in mice. Psychopharmacology (Berl) 2003;166(2):111–119. doi: 10.1007/s00213-002-1319-4. [DOI] [PubMed] [Google Scholar]
- Dietrich JB, Poirier R, et al. Cocaine downregulates the expression of the mitochondrial genome in rat brain. Ann N Y Acad Sci. 2004;1025:345–350. doi: 10.1196/annals.1316.042. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Gamboa J, et al. Plasma catecholamines and blood volume in native Andeans during hypoxia and normoxia. Clin Auton Res. 2006;16(1):40–45. doi: 10.1007/s10286-006-0305-z. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Purselle D, et al. Cocaine use disorders and suicidal ideation. Drug Alcohol Depend. 2003;70:101–104. doi: 10.1016/s0376-8716(02)00337-x. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251(5001):1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114(1):63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Addiction: From Biology to Drug Policy. Oxford University Press USA; New York: 2001. [Google Scholar]
- Gurkovskaya OV, Palamarchouk V, et al. Effects of corticotropin-releasing hormone receptor antagonists on cocaine-induced dopamine overflow in the medial prefrontal cortex and nucleus accumbens of rats. Synapse. 2005;57(4):202–212. doi: 10.1002/syn.20172. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, et al. Social stress increases the acquisition of cocaine self- administration in male and female rats. Brain Res. 1995;698(1-2):46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Haws CA, Gray DB, et al. The possible effect of altitude on regional variation in suicide rates. Med Hypotheses. 2009;73:587–590. doi: 10.1016/j.mehy.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102(29):10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Flak J, et al. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Powell BJ. Cocaine and morphine self-administration: effects of differential rearing. Pharmacol Biochem Behav. 1976;5(6):701–704. doi: 10.1016/0091-3057(76)90315-4. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41(1):3–52. [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, et al. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12(1):49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Kato T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder. Cell Calcium. 2008;44(1):92–102. doi: 10.1016/j.ceca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kim N, Mickelson JB, et al. Altitude, gun ownership, rural areas, and suicide. Am J Psychiatry. 2011;168(1):49–54. doi: 10.1176/appi.ajp.2010.10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp WP, Soares BG, et al. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007;(3):CD003023. doi: 10.1002/14651858.CD003023.pub2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, et al. Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev. 1999;23(5):717–741. doi: 10.1016/s0149-7634(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314(1):201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- London ED, Stapleton JM, et al. PET studies of cerebral glucose metabolism: acute effects of cocaine and long-term deficits in brains of drug abusers. NIDA Res Monogr. 1996;163:146–158. [PubMed] [Google Scholar]
- Lull ME, Freeman WM, et al. Correlating human and animal studies of cocaine abuse and gene expression. Ann N Y Acad Sci. 2008;1141:58–75. doi: 10.1196/annals.1441.013. [DOI] [PubMed] [Google Scholar]
- Mague S, Pliakas A, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305(1):323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, et al. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuk PM, Tardiff K, et al. Prevalence of Cocaine Use Among Residents of New York City Who Committed Suicide During a One-Year Period. Am J Psychiatry. 1992;149:371–375. doi: 10.1176/ajp.149.3.371. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, et al. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Miyata T, Takizawa S, et al. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. Am J Physiol Cell Physiol. 2011;300(2):C226–231. doi: 10.1152/ajpcell.00430.2010. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, et al. Social dominance in monkeys: dopamine D 2 receptors and cocaine self-administration. Nat Neurosci. 2002;5(2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Motheral B, Cox E, et al. Prescription Drug Atlas, Express Scripts. 2002 [Google Scholar]
- National Atlas of the United States County Boundaries of the United States. 2001 Sep 17; 2009 from http://www.nationalatlas.gov/atlasftp.html.
- National Geospatial-Intelligence Agency and National Aeronautics and Space Administration . Shutte Radar Topography Mission (STRM) Dataset. United States Geological Survey; Souix Falls, SD.: 2000. [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3(1):4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162(8):1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, et al. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Orset C, Parrot S, et al. Dopamine transporters are involved in the onset of hypoxia- induced dopamine efflux in striatum as revealed by in vivo microdialysis. Neurochem Int. 2005;46(8):623–633. doi: 10.1016/j.neuint.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, et al. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol. 1995;16(2):89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, et al. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32(3):646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Papasava M, Singer G. Self-administration of low-dose cocaine by rats at reduced and recovered body weight. Psychopharmacology (Berl) 1985;85(4):419–425. doi: 10.1007/BF00429657. [DOI] [PubMed] [Google Scholar]
- Parrot S, Cottet-Emard JM, et al. Effects of acute hypoxic conditions on extracellular excitatory amino acids and dopamine in the striatum of freely-moving rats. Adv Exp Med Biol. 2003;536:433–444. doi: 10.1007/978-1-4419-9280-2_55. [DOI] [PubMed] [Google Scholar]
- Penberthy JK, Ait-Daoud N, et al. Review of treatment for cocaine dependence. Curr Drug Abuse Rev. 2010;3(1):49–62. doi: 10.2174/1874473711003010049. [DOI] [PubMed] [Google Scholar]
- Petronis KR, Samuels JF, et al. An epidemiologic investigation of potential risk factors for suicide attempts. Soc Psychiatry Psychiatr Epidemiol. 1990;25(4):193–199. doi: 10.1007/BF00782961. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, et al. Isolation rearing impairs the reinforcing efficacy of intravenous cocaine or intra-accumbens d-amphetamine: impaired response to intra-accumbens D1 and D2/D3 dopamine receptor antagonists. Psychopharmacology (Berl) 1994;115(3):419–429. doi: 10.1007/BF02245085. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, et al. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berl) 1994;115(3):407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, et al. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Gray NA, et al. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33(11):2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- Ray K, Dutta A, et al. Hypobaric hypoxia modulates brain biogenic amines and disturbs sleep architecture. Neurochem Int. 2011;58(1):112–118. doi: 10.1016/j.neuint.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Renshaw P, Parsegian A, et al. Lovastatin potentiates the antidepressant efficacy of fluoxetine in rats. Pharmacol Biochem Behav. 2009;92(1):88–92. doi: 10.1016/j.pbb.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive- sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roy A. Characteristics of cocaine dependent patients who attempt suicide. Arch Suicide Res. 2009;13(1):46–51. doi: 10.1080/13811110802572130. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, et al. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–222. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Eber GB. Temporal progression of cocaine dependence symptoms in the US National Comorbidity Survey. Addiction. 2002;97(5):543–554. doi: 10.1046/j.1360-0443.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, et al. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Brown S, et al. Depressive symptoms modulate the subjective and physiological response to cocaine in humans. Drug Alcohol Depend. 2001;63(2):131–137. doi: 10.1016/s0376-8716(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Sorg BA. Mesocorticolimbic dopamine systems: cross-sensitization between stress and cocaine. Ann N Y Acad Sci. 1992;654:136–144. doi: 10.1111/j.1749-6632.1992.tb25962.x. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10(10):900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Stulz N, Thase ME, et al. Psychosocial treatments for cocaine dependence: the role of depressive symptoms. Drug Alcohol Depend. 2011;114(1):41–48. doi: 10.1016/j.drugalcdep.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, O. o. A. S. [May 24, 2011];Substate Substance Abuse Estimates from the 1999-2001 NSDUH. 2009 Jan 15; 2009, from http://www.oas.samhsa.gov/subStateTABS/AgeTabs.htm#tab6.
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69(4):628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC. The strong relationship between bipolar and substance-use disorder. Ann N Y Acad Sci. 2010;1187:276–293. doi: 10.1111/j.1749-6632.2009.05146.x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130(3):203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, U. S. H. R. a. S. A., Bureau of Health Professions . Area Resource File (ARF) 2008. U. S. D. o. H. a. H. Services; U.S. Department of Health and Human Services; Rockville, MD: 2009. [Google Scholar]
- Vijayakumar L, Kumar MS, et al. Substance abuse and suicide. Arch Suicide Res. 2011;24(3):197–202. doi: 10.1097/YCO.0b013e3283459242. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263–268. doi: 10.1097/YCO.0b013e32832a3b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, et al. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193(2):283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang TY, Chen XQ, et al. Corticotropin-releasing factor receptor type 1 and 2 mRNA expression in the rat anterior pituitary is modulated by intermittent hypoxia, cold and restraint. Neuroscience. 2004;128(1):111–119. doi: 10.1016/j.neuroscience.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Wilcox HC, Conner KR, et al. Association of alcohol and drug use disorders and completed suicide: an empirical review of cohort studies. Drug Alcohol Depend. 2004;76(Suppl):S11–19. doi: 10.1016/j.drugalcdep.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1(3):303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LT. Is bipolar disorder a mitochondrial disease? J Psychiatry Neurosci. 2007;32(3):160–161. [PMC free article] [PubMed] [Google Scholar]


