Abstract
Objectives
To describe the results of a Phase I dose escalation trial for newly diagnosed glioblastoma multiforme (GBM) using a hypofractionated concurrent intensity-modulated radiotherapy (IMRT) boost.
Methods
Twenty-one patients were enrolled between April 1999 and August 2003. Radiotherapy consisted of daily fractions of 1.8 Gy with a concurrent boost of 0.7 Gy (total 2.5 Gy daily) to a total dose of 70, 75, or 80 Gy. Concurrent chemotherapy was not permitted. Seven patients were enrolled at each dose and dose limiting toxicities were defined as irreversible Grade 3 or any Grade 4–5 acute neurotoxicity attributable to radiotherapy.
Results
All patients experienced Grade 1 or 2 acute toxicities. Acutely, 8 patients experienced Grade 3 and 1 patient experienced Grade 3 and 4 toxicities. Of these, only two reversible cases of otitis media were attributable to radiotherapy. No dose-limiting toxicities were encountered. Only 2 patients experienced Grade 3 delayed toxicity and there was no delayed Grade 4 toxicity. Eleven patients requiring repeat resection or biopsy were found to have viable tumor and radiation changes with no cases of radionecrosis alone. Median overall and progression-free survival for this cohort were 13.6 and 6.5 months, respectively. One- and 2-year survival rates were 57% and 19%. At recurrence, 15 patients received chemotherapy, 9 underwent resection, and 5 received radiotherapy.
Conclusions
Using a hypofractionated concurrent IMRT boost, we were able to safely treat patients to 80 Gy without any dose-limiting toxicity. Given that local failure still remains the predominant pattern for GBM patients, a trial of dose escalation with IMRT and temozolomide is warranted.
Keywords: Glioblastoma Multiforme, Radiotherapy, Dose escalation, IMRT, Temozolomide
INTRODUCTION
Glioblastoma multiforme (GBM) remains the most common and most aggressive primary brain malignancy in adults. Historically, the mainstay of treatment for GBM was maximal safe surgical resection (MSR) followed by adjuvant radiotherapy. In recent years, the addition of the oral alkylating agent temozolomide concurrent and adjuvant to postoperative radiotherapy has demonstrated a survival benefit and become the accepted standard of care (1). However, outcomes remain poor with disease-free survival (DFS) measured in months, and only approximately 20% of patients surviving beyond 2 years.
Extracranial metastases are rare, and relapses up to several centimeters away from the tumor have been documented. However, mortality is primarily from local tumor recurrence or progression in or adjacent to the resection cavity (2–5). This suggests that improving local control may improve overall survival, a concept affirmed by the survival advantage conferred by radiotherapy.
One means to improve local control may be radiotherapy dose escalation. Dose response for malignant gliomas has been established up to 60 Gy (6, 7). Attempts at dose escalation for GBM above 60 Gy have been met with limited success (8–11), although some studies have suggested benefit (12–15). A theoretical benefit to dose escalation may have been offset by increased neurotoxicity in trials using older treatment techniques and therapeutic targeting, such as whole-brain radiotherapy.
Novel approaches for dose escalation in GBM are being explored. Intensity-modulated radiotherapy (IMRT) is now used in the treatment of a number of different cancers for its ability to improve dose conformity and spare critical neighboring or adjoining normal tissues when compared with conventional radiotherapy. In the present study, we conducted a Phase I dose escalation study of IMRT with field-in-field boost using magnetic resonance imaging (MRI)-based treatment planning for newly diagnosed glioblastoma multiforme. The purpose of this study is to report treatment tolerability and toxicity for IMRT to a maximum dose of 80 Gy in which the central tumor volume received a hypofractionated daily dose of 2.5 Gy.
METHODS AND MATERIALS
Patient eligibility
This study was approved by our institutional review board. Study enrollment was offered to all eligible patients presenting at our institution. Eligible patients were 18 years of age or older, had a Karnofsky performance score ≤70, with histologically confirmed initial presentation of supratentorial GBM. Additionally, a preoperative contrast enhanced MRI of the brain was required and only those patients with T1 enhancing tumors ≥5 cm diameter after biopsy or a preoperative T1 enhancing tumor of ≥8 cm before resection were included. Patients with any previous history of brain irradiation or radiotherapy within 6 months of entrance into study were excluded. Furthermore, patients with recurrent or multifocal glioblastoma multiforme as well as tumors centered in the pons, medulla, cerebellum, or optic pathway were excluded. Patients receiving investigational agents or concurrent chemotherapy were also deemed ineligible.
Pretreatment evaluation and treatment design
A comprehensive history and physical and baseline laboratory tests including serum chemistries and hematologic profile evaluations were conducted within 1 week of administration of protocol therapy. Scans were conducted 4 weeks before the start of therapy with all patients beginning postoperative radiotherapy no earlier than 28 days and no later than 35 days after biopsy or resection.
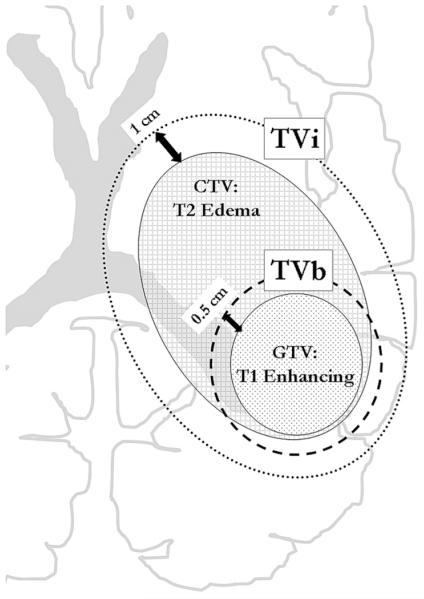
For the purposes of treatment planning, all patients underwent computed tomography simulation in the supine treatment position with appropriate immobilization using a custom Aquaplast face mask and head holder. Computed tomography slices for each patient were 2.5 mm or 5 mm thick, and all treatment plans were generated by inverse planning using Pinnacle software. Target volumes were defined by fusing the treatment planning computed tomography with the patient’s postoperative MRI scan and treatments consisted of a combination of coplanar and noncoplanar treatment fields. Gross tumor volume (GTV) was defined as the contrast-enhancing tumor visible on T1-weighted MRI images as well as the entire resection cavity. The clinical target volume (CTV), representing the area of subclinical (microscopic) tumor involvement, was defined as the edema visible on T2-weighted MRI images. The initial target volume (TVi) consisted of (GTV + CTV) + 1.0 cm to the planning target volume (PTV), and the boost target volume (TVb) consisted of the GTV + 0.5 cm margin to PTV with the entire TVb encompassed by the TVi (Figure 1). IMRT plans required that 95% of the prescribed dose cover 95% of the target volume for both TVi and TVb. Margins of as little as 0.5 cm were used on TVi or TVb to spare critical normal structures when necessary.
Fig. 1.
Radiotherapy Target Volumes. The initial planning target volume (TVi - dotted line) consisted of a 1 cm geometric margin around the gross tumor volume (defined as the T1-weighted MRI enhancing tumor + the resection cavity) and the clinical target volume (defined as edema visible on T2-weighted MRI). The boost planning target volume (TVb - dashed line) consisted of a 0.5 cm geometric margin on the gross tumor volume.
Critical tissues, including the brainstem, spinal cord, temporal lobes, optic chiasm, globes, lenses, and cochlea were delineated and dose constraints were applied in treatment planning. A dose of 54 Gy to the entire optic chiasm or brainstem or 60 Gy to any part of the structures was tolerated. The globes were limited to 45 Gy to the whole structure and 50 Gy to any part of the structure for at least one globe. The spinal cord dose was limited to 45 Gy to any part of the structure. When possible, the contralateral temporal lobe and cochlea were limited to 30 Gy. Dose–volume histograms were generated for TVi, TVb, optic chiasm, pons, medulla, midbrain, pituitary gland, optic nerves, globes, and normal brain tissue surrounding the TVi and TVb.
Seven patients were enrolled in each of three dose levels (70 Gy, 75 Gy, and 80 Gy) with a constraint on enrollment of no more than 4 patients from RTOG GBM Recursive Partitioning Analysis (RPA) Classes III or IV/V entering each level to balance the RPA status of patients at each dose level. All patients were treated using mega-voltage linear accelerators, with a 100 cm source to axis distance isocentric setup and photon energies >6 MV. The prescribed dose was 50.4 Gy to the TVi and 70 Gy to the TVb. For the first 28 fractions, the TVi received 1.8 Gy/fraction with an additional 0.7/Gy fraction concurrent boost to the TVb for a total of 2.5 Gy/fraction to the TVb. TVb dose escalation higher than 70 Gy was achieved with the addition of supplemental fractions of 2.5 Gy/fraction at the end of the treatment, extending the total length of treatment beyond 28 days (Table 1).
Table 1.
Dose escalation schema
| Total dose (Gy)/No. of fractions (treatment days 1–28) |
Total dose (Gy)/No. of fractions (treatment days ≥29) |
Total dose TVb/fraction |
2 Gy fraction BED10 to TVb |
|||
|---|---|---|---|---|---|---|
| Dose level | TVi | TVb | TVi | TVb | ||
| 1 | 50.4/28 | 70/28 | 0/0 | 0/0 | 70/28 | 73 |
| 2 | 50.4/28 | 70/28 | 0/0 | 5/2 | 75/30 | 78 |
| 3 | 50.4/28 | 70/28 | 0/0 | 10/4 | 80/32 | 83 |
Abbreviations: TVb = boost target volume; TVi = initial target volume.
Treatment quality assurance was maintained through the comparison of weekly port films of all fields to simulation films or digitally reconstructed computed radiographs of the appropriate fields. Planning information was reviewed on a biweekly basis by one of the principal investigators.
Toxicity and dose escalation
Toxicities were defined as acute if they occurred during or within 3 calendar months of the last day of treatment and delayed if they occurred more than 3 months after treatment completion. Acute neurotoxicity was graded by the Radiation Therapy Oncology Group (RTOG) Acute CNS Toxicity Criteria. Delayed neurotoxicity was graded according to the Late Effects on Normal Tissues toxicity tables. Nonneurologic toxicities were graded according to Common Toxicity Criteria.
Dose-limiting toxicities (DLTs) were defined as irreversible Grade 3 or any Grade 4–5 acute neurotoxicity attributable to radiotherapy per RTOG Acute CNS Toxicity Criteria. Briefly, these would include toxicities requiring inpatient management for progressive neurologic symptoms or signs attributable to radiotherapy and refractory to steroid management (i.e., irreversible), life-threatening neurologic symptoms or signs including status epilepticus, coma, or nonfatal herniation attributable to radiotherapy, or neurologic death attributable to radiotherapy. Seven patients were enrolled to each dose level of radiotherapy with dose escalation dependent on the observed toxicities of the previously administered dose group. Doses were escalated to the next dose level for the subsequent group of 7 patients enrolled in the study provided that ≥2 patients experienced DLTs that were determined to be possibly, probably, or definitely related to protocol treatment. If at any dose level, 3 or more patients experienced DLTs, then the prior dose level would be considered the maximum tolerated dose. After completing treatment for the last patient at a given dose, 90 days of observation for acute DLTs was required before enrolling patients to the next dose level.
Patient follow-up, response criteria, and data analysis
Study participants were evaluated with a complete history and physical and medication review 1 month after completion of radiation, every 3 months for four visits followed by every 6 months for the subsequent two visits, and then annually, unless more frequent follow-up visits were clinically indicated. An MRI with contrast and spectroscopy were obtained midway through treatment and at all follow-up visits. All adverse effects/toxicities were recorded and a description of the type, acuity (acute versus delayed); grade and assessment of attribution were assigned.
Progression and radionecrosis are often indistinguishable from one another by MRI. Accordingly, suspected progression or radionecrosis was validated by pathology or MRI spectroscopy. In the presence of progressive disease on two consecutive imaging studies with progressive neurologic symptoms despite the use of steroids, progression was identified by MRI alone. Progression was determined to be in-field if it was deemed to be within the radiotherapy target volume. As such, although the main end point of the study was to determine the maximum tolerated dose for radiotherapy in this setting, survival end points including overall survival (OS) and progression-free survival (PFS) were also measured from the date of protocol enrollment and described via Kaplan-Meier methods. Log–rank tests were used to assess dose group differences in OS and PFS.
RESULTS
Between April 1999 and August 2003, 21 patients with histologically proven GBM ages 37–76 years (mean age, 55) with normal organ and marrow function, and Karnofsky performance status ≤70 were enrolled in this Phase I trial. Sixteen patients were male and 5 were female. Nine patients were RPA Class III and 12 were RPA Class IV. Eight patients underwent gross total resection, 9 patients underwent subtotal resection, and 4 patients underwent biopsy only.
Toxicity
Acutely, all patients had Grade 1 or 2 toxicity, 8 patients had Grade 3 toxicity, and 1 patient had a Grade 3 and 4 toxicity (Table 2). The most common acute toxicities were alopecia, fatigue, weakness, dermatitis, loss of appetite, nausea, and thrush. Headache, memory difficulties or confusion, word finding difficulties, aphasia, or changes in vision were also seen in some patients. Severe acute toxicities included 2 patients with Grade 3 deep venous thrombosis, 1 patient with Grade 3 gastrointestinal toxicity, 2 patients with Grade 3 otitis media, and 4 patients with Grade 3 neurotoxicity of whom 1 also had Grade 4 hyperglycemia. Most toxicities were not attributable to protocol treatment but rather to the disease process itself or to steroid therapy. Only the two cases of otitis media were directly attributable to radiotherapy and these were reversible. Of 11 patients requiring repeat resection or biopsy, all were found to have viable tumor and radiation changes. No patients required surgery for radionecrosis alone. No dose-limiting toxicities were encountered. Delayed toxicities were much less pronounced with no Grade 4 toxicity and only two Grade 3 toxicities (one deep venous thrombosis and one neurotoxicity). Delayed toxicities were mostly limited to Grade 1 and 2 fatigue, weakness, and headache with some patients experiencing Grade 1 neurocognitive deficits. No differences in toxicity were observed among the different dose-level groups. Dose escalation above 80 Gy was initially planned; however, the trial was stopped early because of new evidence indicating the efficacy of concomitant Temozolomide in the management of GBM (1).
Table 2.
Acute and delayed toxicity
| Acute toxicity ≤90 days from completion of therapy |
Delayed toxicity >90 days from completion of therapy |
|||||
|---|---|---|---|---|---|---|
| Dose | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 |
| 70 Gy | 7 | 3 | 0 | 5 | 2 | 0 |
| 75 Gy | 7 | 2 | 0 | 5 | 0 | 0 |
| 80 Gy | 7 | 4 | 1 | 3 | 0 | 0 |
Outcomes and OS
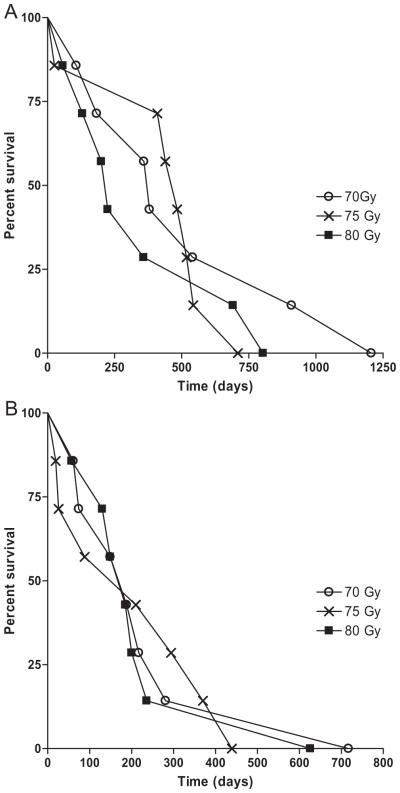
The median OS and PFS for this cohort were 13.6 months (range, 0.9–40.2 months) and 6.5 months (range, 0.9–40.2 months) respectively (Figure 2). Fifty-seven percent of patients were alive at 1 year and 19% of patients were alive at 2 years. There were no differences in OS (p = 0.57) or PFS (p = 0.91) detected among dose groups (Figure 3) and this trial was not powered to detect such a difference. All of the patients enrolled in this study have died at last follow-up. The site of first progression was in field for 17 patients, out of field for 1 patient, and 3 patients never had radiographic or pathologic evidence of disease progression. All 3 patients without evidence of failure were in the 80 Gy dose level group. These 3 patients survived 57, 130, and 200 days and likely died of disease sequelae before they could be confirmed to have disease progression.
Fig. 2.
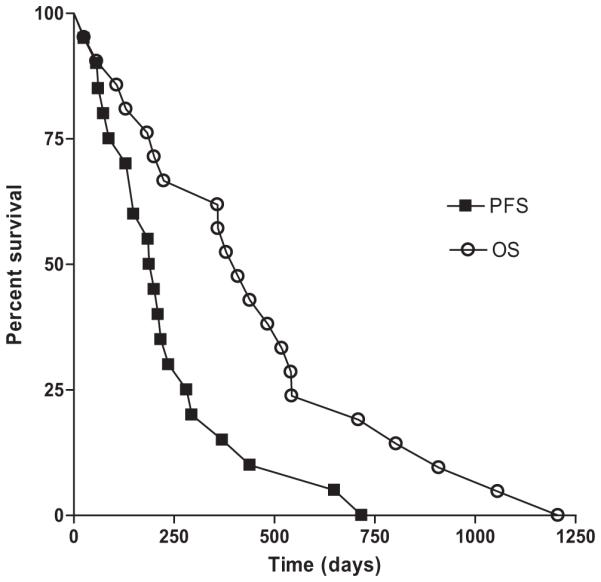
Overall and progression-free survival. Outcomes for all 21 patients enrolled in the trial are depicted. Median overall survival was 13.6 months and median progression-free survival was 6.5 months.
Fig. 3.
Outcomes by radiotherapy dose. Overall (A) and progression-free survival (B) curves for patients by radiotherapy dose are depicted. No significant difference is found in outcomes based on dose group.
DISCUSSION
In the present study, we describe the long-term results of a prospective Phase I trial of dose escalation with radiotherapy alone for newly diagnosed GBM using a concurrent hypofractionated boost delivered by IMRT field-in-field technique. The results of the study indicate that a total dose of 80 Gy may be safely administered to patients with newly diagnosed glioblastoma without DLTs or increased radionecrosis.
The theoretical benefit of IMRT in the treatment of glioblastoma lies in the possible decrease in toxicity to structures including brain stem and optic pathways that otherwise may be above radiation tolerance doses using conventional planning techniques. However, some theoretical disadvantages of IMRT also exist including the potential for greater dose heterogeneity in the radiation plan and increased integral dose, both of which may ultimately affect the radionecrosis rate. In 2006, Narayana et al. reported a retrospective analysis of 58 patients with World Health Organization Grade III and IV gliomas treated with IMRT to doses of 59.4–60 Gy (16). Neither PFS nor OS were significantly changed as compared with historical controls with the use of IMRT. Fuller et al. also reported a series of 42 patients treated with IMRT alone (72%) or as a boost (28%) after three-dimensional conformal radiotherapy with no observed survival benefit or decrement when compared with historical controls (17). Because these series used conventional doses of radiotherapy, they would not be expected to significantly improve tumor control rates. However, IMRT does provide the potential for escalation of doses beyond conventional doses, an advantage that our trial has attempted to exploit.
Early randomized trials conducted in the 1970s demonstrated a dose response to radiotherapy for the treatment of GBM (6, 7). Attempts at dose escalation above 60 Gy have had equivocal results (6, 8–15) and several randomized trials have been unable to show a survival benefit to further dose escalation. The multiarmed RTOG 7401 trial found no benefit to the addition of a 10-Gy boost to standard 60 Gy whole brain radiotherapy (8). Other RTOG trials investigating hyperfractionation (18) or stereotactic radiosurgical boost (19) also found no benefit to dose escalation. More recent studies have likewise shown no added benefit to dose escalation (9–11). The University of Michigan reported 35/36 recurrences within the high-dose central region in patients with malignant astrocytoma who were treated with computed tomography–guided radiotherapy to a dose of 70–80 Gy (9). Further dose escalation to 90 Gy at the University of Michigan demonstrated similar results with 18/23 recurrences within the 95% isodose line and 21/23 recurrences within the 80% isodose line (11). Despite the lack of efficacy, these trials do demonstrate the relative safety of dose escalation using modern fields and techniques.
However, several series do suggest that there may be a dose sufficient to change the pattern of failure from within the high-dose region. Fitzek et al. showed an increase in median survival secondary to improved central local control in patients receiving 90 cobalt Gray equivalent (CGE) when compared to controls (15). With dose escalation to 90 CGE using conformal protons and photons in accelerated fractionation, tumor progression occurred most commonly in areas that received doses of 60–70 CGE or less, whereas recurrent tumor in an area treated with 90 CGE was found in only one case. In addition, they found that among the treated patients demonstrating radionecrosis, there was a statistically significant increase in survival for patients with radionecrosis alone than that those with recurrent tumor in the face of radionecrosis (29 months vs. 16 months, p = 0.01). Mehta et al. (20) treated patients with glioblastoma with conventional fractionated radiotherapy followed by a radiosurgical boost. With median doses of 54 Gy using external beam radiotherapy and an 18.6 Gy boost, they found 79% of failures to be in the region just peripheral to the radiosurgical boost region, and the remaining failures to be distant. Conversely, as mentioned previously, the University of Michigan’s data with dose escalation to 90 Gy showed a predominantly local failure pattern (11). The small sample size and the fact that a larger proportion of the patients in the Michigan study had gross residual disease after surgery may be the reason that this study was unable to show the local control benefit seen in the Massachusetts General Hospital (Boston, MA) and Wisconsin series. An alternative explanation could lie in the fact that the Massachusetts General Hospital series used proton beam radiotherapy, which theoretically has slightly higher relative biologic effectiveness than conventional photons, and the Wisconsin series used a single fraction boost, which may also increase the biologic effect. This hypothesis supports the rationale for a hypofractionated boost as used in this study.
In our current study, a total hypofractionated dose of up to 80 Gy was safely administered to GBM patients without DLTs, suggesting that further dose escalation may be possible. It has been suggested that the incidence of radionecrosis rises with increased fraction size and increased total dose. Emami established the TD5/5 for radionecrosis of partial brain as 60 Gy, but more recently the Quantitative Analyses of Normal Tissue Effects in the Clinic has increased this to 72 Gy (21). Our study differs from many past dose escalation studies in that an IMRT field-in-field technique was used for dose escalation and the daily dose to the central tumor volume was hypofractionated at 2.5 Gy per fraction. The toxicities seen in our series were no greater than what could reasonably be expected for patients receiving standard dose cranial radiotherapy despite the larger daily and total doses. Severe toxicities included 2 patients with acute Grade 3 deep venous thrombosis, 2 patients with acute Grade 3 otitis media, 4 patients with Grade 3 neurotoxicity, 1 patient with Grade 3 diarrhea, and 1 patient with Grade 4 hyperglycemia. Only the two cases of otitis media were directly attributable to radiotherapy. No patients required surgery for radionecrosis alone but 11 patients underwent repeat resection or biopsy and were found to have viable tumor and radiation changes.
Although the primary end point of this study was toxicity, the effects of IMRT dose escalation on survival end points were evaluated. The median OS and PFS for this cohort were 13.6 months and 6.5 months, respectively, with 57% of patients alive at 1 year and 19% alive at 2 years. The survival of this cohort treated with IMRT alone compared favorably with the historical 12 month median survival for conventional radiotherapy alone, and more importantly, with the current standard of care as reported by Stupp et al. (1) There was no significant difference in OS (p = 0.57) or PFS (p = 0.91) detected among the dose groups, although this trial was not powered for these end points.
After recurrence, 15 patients received chemotherapy (8 TMZ),9 had further resection, and5 received further radiotherapy. In an unplanned analysis, patients receiving TMZ at recurrence had improved median (20.7 vs. 12 months) and 2 year OS (38% vs. 0%, p = 0.02). Given the retrospective nature there was likely a bias in which patients were perceived healthy enough to receive TMZ and which patients survived long enough to receive TMZ at recurrence. An important point is that our study and previous dose escalation trials were all undertaken before the establishment of adjuvant and concurrent chemotherapy with TMZ as standard of care. Recently, a Phase I dose escalation study (ISIDE-BT-1) was published by Morganti et al., in which 19 patients were treated with accelerated hypofractionated irradiation with IMRT and standard concurrent and adjuvant TMZ for 1 year (22). PTV1 dose escalation at dose levels of 60 Gy, 62.5 Gy, and a maximum dose of 65 Gy were administered with no DLTs experienced among the cohort. However, even with the addition of TMZ, an agent shown to improve median survival, the authors found a high rate of in-field failures, with an observed 78.9% relapse rate. The data suggest that even in the presence of concurrent TMZ, local control is not well achieved and may benefit from further dose escalation. It is unclear whether aggressive dose escalation in the context of concurrent alkylators such as TMZ would increase the toxicity seen in our series.
In conclusion, using an IMRT field-in-field concurrent boost, we were able to safely treat patients with GBM to 80 Gy with hypofractionated daily doses to the central tumor without any DLT. Given that local failure still remains the predominant pattern for GBM patients (even those treated with TMZ), we plan to undertake a trial of aggressive dose escalation with field-in-field hypofractionated concurrent boost IMRT + TMZ.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.Oppitz U, Maessen D, Zunterer H, Richter S, Flentje M. 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol. 1999;53:53–57. doi: 10.1016/s0167-8140(99)00117-6. [DOI] [PubMed] [Google Scholar]
- 4.Liang BC, Thornton AF, Jr., Sandler HM, Greenberg HS. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75:559–563. doi: 10.3171/jns.1991.75.4.0559. [DOI] [PubMed] [Google Scholar]
- 5.Garden AS, Maor MH, Yung WK, et al. Outcome and patterns of failure following limited-volume irradiation for malignant astrocytomas. Radiother Oncol. 1991;20:99–110. doi: 10.1016/0167-8140(91)90143-5. [DOI] [PubMed] [Google Scholar]
- 6.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 7.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson DF, ener-West M, Horton J, Chang CH, Schoenfeld D, Nelson JS. Combined modality approach to treatment of malignant gliomas—re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;6:279–284. [PubMed] [Google Scholar]
- 9.Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 10.Douglas JG, Stelzer KJ, Mankoff DA, et al. [F-18]-fluorodeoxyglucose positron emission tomography for targeting radiation dose escalation for patients with glioblastoma multiforme: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2006;64:886–891. doi: 10.1016/j.ijrobp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 12.Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyper-fractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83-02. Cancer. 1996;77:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Nwokedi EC, DiBiase SJ, Jabbour S, Herman J, Amin P, Chin LS. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50:41–46. doi: 10.1097/00006123-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa K, Aoki Y, Fujimaki T, et al. High-dose conformal radiotherapy influenced the pattern of failure but did not improve survival in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:1141–1149. doi: 10.1016/s0360-3016(97)00911-5. [DOI] [PubMed] [Google Scholar]
- 15.Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: Results of a phase II prospective trial. J Neurosurg. 1999;91:251–260. doi: 10.3171/jns.1999.91.2.0251. [DOI] [PubMed] [Google Scholar]
- 16.Narayana A, Yamada J, Berry S, et al. Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;64:892–897. doi: 10.1016/j.ijrobp.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 17.Fuller CD, Choi M, Forthuber B, et al. Standard fractionation intensity modulated radiation therapy (IMRT) of primary and recurrent glioblastoma multiforme. Radiat Oncol. 2007;2:26. doi: 10.1186/1748-717X-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coughlin C, Scott C, Langer C, et al. Phase II, two-arm RTOG trial (94-11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (> 20 or < or = 20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–1358. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 19.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Mehta MP, Masciopinto J, Rozental J, et al. Stereotactic radio-surgery for glioblastoma multiforme: Report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys. 1994;30:541–549. doi: 10.1016/0360-3016(92)90939-f. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morganti AG, Balducci M, Salvati M, et al. A Phase I Dose-Escalation Study (ISIDE-BT-1) of Accelerated IMRT with Temozolomide in Patients with Glioblastoma. Int J Radiat Oncol Biol Phys. 2010;77:92–97. doi: 10.1016/j.ijrobp.2009.04.064. [DOI] [PubMed] [Google Scholar]