Abstract
IMPORTANCE
Centralization of specialized health care services such as organ transplantation and bariatric surgery is advocated to improve quality, increase efficiency, and reduce cost. The effect of increased travel on access and outcomes from these services is not fully understood.
OBJECTIVE
To evaluate the association between distance from a Veterans Affairs (VA) transplant center (VATC) and access to being waitlisted for liver transplantation, actually having a liver transplant, and mortality.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective study of veterans meeting liver transplantation eligibility criteria from January 1, 2003, until December 31, 2010, using data from the Veterans Health Administration’s integrated, national, electronic medical record linked to Organ Procurement and Transplantation Network data.
MAIN OUTCOMES AND MEASURES
The primary outcome was being waitlisted for transplantation at a VATC. Secondary outcomes included being waitlisted at any transplant center, undergoing a transplantation, and survival.
RESULTS
From 2003–2010, 50 637 veterans were classified as potentially eligible for transplant; 2895 (6%) were waitlisted and 1418 of those were waitlisted (49%) at 1 of the 5 VATCs. Of 3417 veterans receiving care at a VA hospital located within 100 miles from a VATC, 244 (7.1%) were waitlisted at a VATC and 372 (10.9%) at any transplant center (VATC and non-VATCs). Of 47 219 veterans receiving care at a VA hospital located more than 100 miles from a VATC, 1174 (2.5%) were waitlisted at a VATC and 2523 (5.3%) at any transplant center (VATC and non-VATCs). In multivariable models, increasing distance to closest VATC was associated with significantly lower odds of being waitlisted at a VATC (odds ratio [OR], 0.91 [95% CI, 0.89–0.93] for each doubling in distance) or any transplant center (OR, 0.94 [95% CI, 0.92–0.96] for each doubling in distance). For example, a veteran living 25 miles from a VATC would have a 7.4% (95% CI, 6.6%–8.1%) adjusted probability of being waitlisted, whereas a veteran 100 miles from a VATC would have a 6.2% (95% CI, 5.7%–6.6%) adjusted probability. In adjusted models, increasing distance from a VATC was associated with significantly lower transplantation rates (subhazard ratio, 0.97; 95% CI, 0.95–0.98 for each doubling in distance). There was significantly increased mortality among waitlisted veterans from the time of first hepatic decompensation event in multivariable survival models (hazard ratio, 1.03; 95% CI, 1.01–1.04 for each doubling in distance). For example, a waitlisted veteran living 25 miles from a VATC would have a 62.9% (95% CI, 59.1%–66.1%) 5-year adjusted probability of survival from first hepatic decompensation event compared with a 59.8% (95% CI, 56.3%–63.1%) 5-year adjusted probability of survival for a veteran living 100 miles from a VATC.
CONCLUSIONS AND RELEVANCE
Among VA patients meeting eligibility criteria for liver transplantation, greater distance from a VATC or any transplant center was associated with lower likelihood of being waitlisted, receiving a liver transplant, and greater likelihood of death. The relationship between these findings and centralizing specialized care deserves further investigation.
Centralization of specialized health care services is used to control costs, concentrate expertise, and minimize regional differences in quality of care. Such efforts are common in national health systems. In the United States, insurers regionalize care by contracting with centers of excellence for services like bariatric surgery, cardiac interventions, and treatment for some cancers.1–3 Although efficient, centralization may offset any gains in care delivery by increasing the distance between patients and hospitals.2,4–9 Prior studies relating geography to health care access found less access for rural patients and for those patients living far away from hospitals delivering specialized services.2,5,10–12 Few studies have examined specialized care restricted to a limited number of centers. Previous studies of access to care were limited by not knowing the total population in need of care.2,12,13
Organ transplantation is a highly specialized service requiring concentrated medical and surgical expertise, resulting in de facto centralization in metropolitan regions.14 Veterans with Veterans Health Administration (VHA) benefits receive care at 1 of 128 Veterans Affairs (VA) hospitals or associated community-based clinics. Within the VA, liver transplantation is offered at only 5 VA transplant centers (VATCs) located in Houston, Texas (since 2008); Nashville, Tennessee; Pittsburgh, Pennsylvania; Portland, Oregon; and Richmond, Virginia. Veterans with secondary insurance (ie, Medicare) may obtain care at either a VATC or non-VATC. Patients at the VA lacking other health insurance generally receive care at a VATC except in rare emergencies (ie, fulminant hepatic failure).
Liver transplantation in the VA system serves as a model to study the association between distance and access to centralized medical resources. We tested the hypothesis that increasing distance between a patient and a liver transplant center (ie, VATC) is associated with a lower likelihood of being waitlisted for transplantation, a lower likelihood of getting a liver transplant, and an increased risk for mortality.
Methods
We evaluated liver transplantation in the VA between January 1, 2003, and September 20, 2012. January 1, 2003, was selected as the start date because it was about 1 year after the implementation of the current model for end-stage liver disease (MELD) allocation system. MELD shifted liver transplantation priority away from wait time to illness severity.15–17
The study was approved by the institutional review boards at the Philadelphia VA Medical Center and the University of Pennsylvania, which included a waiver of informed consent.
Veterans Eligible for Waitlisting at a VATC
Any veteran with VHA health benefits who used the VA health system was eligible for inclusion. We queried the VHA’s Corporate Data Warehouse18 to identify transplant-eligible veterans meeting the following minimal waitlisting criteria established by the American Association for the Study of Liver Diseases: cirrhosis with a complication of liver disease(ascites, variceal bleeding, or hepatic encephalopathy) or hepatocellular carcinoma.19,20 Transplant-eligible veterans were identified using a validated International Classification of Diseases, Ninth Revision, coding algorithm.21,22 We excluded veterans aged 70 years or older (only 4 veterans aged ≥70 years were waitlisted at a VATC from 2003–2010) with malignancies precluding transplantation or having the human immunodeficiency virus (eTable 1 in Supplement).20 We only included veterans with incident decompensated cirrhosis from January 1, 2003, until December 31, 2010, to ensure sufficient follow-up for outcomes assessment. Veterans Affairs physicians may not directly refer veterans who have secondary insurance to non-VA transplant facilities. They may, however, inform patients of their ability to refer themselves for non-VA health care. The VA does not reimburse veterans for co-pays or deductibles related to non-VA care.
We restricted our cohort to veterans who were active users of VA outpatient care to ensure the ability to be referred for liver transplantation in the VA system. We defined active users as patients who were seen in VA outpatient clinics for at least 2 physician or clinician outpatient visits in the 365 days following the first decompensation event or hepatocellular carcinoma event (including the index visit if outpatient). Two visits were required based on previous studies evaluating use of VA care,23,24 and the assumption that to complete testing prior to referral to a VATC, a veteran must have at least 2 outpatient visits. Veterans were assigned to a local VA medical hospital using Corporate Data Warehouse data, which identified the VA medical hospital where a patient received his or her medical care. Patients receiving care at more than 1 VA facility were assigned to the first hospital where he or she met the coding algorithm criteria for having decompensated cirrhosis, hepatocellular carcinoma, or both.
Identification of Waitlisted Veterans
We cross-referenced Social Security numbers of all waitlisted liver transplant candidates from 2003–2012 using the Organ Procurement and Transplantation Network (OPTN) database25 linked with the VA Corporate Data Warehouse. Among the 110 US liver transplant centers, the waitlists of only 5 (the VATCs) were solely composed of patients with VA insurance, and these transplant centers could be discriminated based on the distribution of zip codes of the waitlisted patients at each center.
Statistical Analysis
Access to Waitlisting
In our primary analysis, we evaluated the relationship between a transplant-eligible veteran’s distance from the local VA hospital to a VATC and being placed on the waitlist for a liver transplant at a VATC. Secondarily, we evaluated the association between distance to a VATC and being placed on the waitlist at any transplant center (VATC and non-VATC) to determine whether access to a local non-VATC mitigates this relationship between distance and waitlisting. We chose a binary waitlisting outcome because access to transplantation once waitlisted is based on severity of illness not waiting time unlike kidney transplantation.
Distance was modeled as a continuous variable. The relationship between distance and waitlisting was not linear so distance was linearized by log transformation in the log 2 base scale.26 In a secondary analysis, distance was modeled as a categorical variable with 5 categories. To our knowledge, no prior regionalization study has modeled the effect of distance with the conditions we studied. Thus, we created 5 distance categories having broad ranges to prevent identification of individual hospitals (ie, no hospital was 100 miles from a VATC, but hospitals were 90 or 110 miles, thus 100 miles was a cutoff not associated with a specific VA hospital) that were based on the observed relationships between certain distance and waitlisting outcomes upon initial evaluation of the data (to convert miles to kilometers, multiply by 1.6). Because these categories were defined after examination of the data, these analyses should be considered post hoc.
We assumed that veterans receiving care at a VA within 100 miles of a VATC would live at home after discharge from the transplant hospitalization given that travel times for these veterans would be less than 90 minutes. Thus, the first distance cut point was selected to be 100 miles. Distances longer than 100 miles were categorized relative to travel times or mode of transportation to a VATC (ie, necessity to travel by plane for those living >500 miles from a VATC). Privacy regulations precluded our access to a veteran’s home address. Consequently, the shortest distance in miles was measured between the VA medical hospital where the patient received routine care and the closest VATC or non-VA transplant facility.
Regression analyses were performed using generalized estimating equation models with a logit link, an exchangeable correlation structure, and a robust variance estimator to account for patient clustering within VA hospitals27 using Stata version 13.0 (StataCorp). Models were adjusted for age at the time of hepatic decompensation without inclusion of other patient-level covariates. We did not have access to other patient-level covariates because the VA data use agreement only authorized identification of date and age at the time of hepatic decompensation. The following data were captured for all patients with hepatitis C at a given VA hospital and were adjusted to account for hospital characteristics that may be associated with waitlisting independently of distance: (1) age (median); (2) socioeconomic status estimated by the proportion of patients who are below the federal poverty level; (3) race/ethnicity (proportion self-reported as white); and (4) mental illness (proportion with anxiety, bipolar disorder, depression, posttraumatic stress disorder, and/or schizophrenia). Hospital-level measurements of these covariates were obtained from the VA Clinical Case Registry: Hepatitis C, which is anational VA registry of all patients with hepatitis C because such measurements are not available among other data for the entire VA population.28–30 We assumed the distribution of these covariates mimicked the broader chronic liver population at each VA medical hospital.
Transplantation
The distance to a transplant center may affect the likelihood of receiving a liver transplant. For example, patients living closer to a transplant center might have increased access to transplantation because they can reach the center in the narrow time window of an organ offer, or by virtue of proximity, serve more readily as a backup recipient. To evaluate this, we analyzed all waitlisted veterans, and modeled deaths while waitlisted as identified by OPTN coding or within 90 days of being removed from the list. Deaths were identified from the Social Security Death Master File found within OPTN. Pre-transplant deaths were modeled as competing risks31,32 because death while on the waitlist serves as a competing risk to transplantation.
We fit competing risk Cox regression models with transplantation as the outcome and all other waitlist removals (ie, condition improved) other than death (modeled as the competing risk) as censoring events.31,32 The exposure was distance from a patient’s home VA hospital to a VATC. Covariates included sex, race/ethnicity, age, laboratory MELD score,16,17 and albumin measured when waitlisted, diagnosis, and hepatocellular carcinoma (binary yes or no as to whether a patient was receiving additional waitlist priority for hepatocellular carcinoma33). We tested for interactions between distance and being waitlisted at a VATC to determine if the probability of being waitlisted is directly influenced by distance. We used a robust standard error estimator to adjust for the clustering of veterans within VA hospitals.34,35
Survival
The relationship between mortality and distance to a VATC among all waitlisted veterans was modeled with Cox regression. Time from the first hepatic decompensation event to death ora censoring event (eg, condition improved)was modeled with the exposure variable being distance from the patient’s home hospital to a VATC. Follow-up began at the date of first hepatic decompensation event to account for the time a patient first became eligible for transplant, which may have been associated with delays in being waitlisted as a function of distance. We adjusted the model for covariates available in OPTN (sex, race/ethnicity, age, laboratory MELD score,16,17 albumin level measured when waitlisted, diagnosis, and hepatocellular carcinoma) and insurance status at the time of waitlisting. Residential-level poverty was adjusted for using OPTN zip code data.36 Death dates were ascertained as specified above. We used a robust variance estimator to adjust for clustering within VA hospitals.34 The proportional hazard assumption was tested for using Schoenfeld residuals.
Sensitivity Analyses
Although veterans with decompensated cirrhosis met minimal clinical criteria for being waitlisted, a MELD score of 15 or greater may better determine eligibility.37 In a preplanned sensitivity analysis, we restricted our cohort to veterans having MELD scores of 15 or higher following the diagnosis of decompensated cirrhosis, hepatocellular carcinoma, or both. The influence of a patient’s base hospital having advanced liver care available (defined by being located within 20 miles of any transplant center, being affiliated with an academic liver transplant center, and having a clinician specialized in hepatology) was modeled by a distance × advanced liver care interaction analysis. Availability of secondary insurance status (defined as none, Medicaid, secondary non-Medicaid, or Medicaidplus secondary non-Medicaid) was modeled as a covariate for the 45 792 (90.4%) of the cohort who had this information available in the VA Corporate Data Ware house (10% had missing data or insurance status reported as unknown).
Statistical significance was defined as P < .05 using 2-sided tests. The final multivariable models also include variables with biological plausibility for the association with the outcome, even if the P value was above the prespecified P value threshold (ie, diagnosis). All analyses used Stata version 13.0 (StataCorp), including the xtgee module.
Results
Among all veterans in the United States having VHA health benefits and using VHA medical care, 79 899 had incident decompensated cirrhosis or hepatocellular carcinoma (of any stage) and used VA outpatient services from 2003–2010. Although hepatocellular carcinoma stage could not be ascertained, which affects transplant eligibility,38 results were unchanged when patients with hepatocellular carcinoma were excluded. Of the 79 899 veterans, 29 262 were excluded (18 041 were aged ≥70 years and 11 221 were <70 years, but had a malignancy precluding transplantation). This left a total analytic cohort of 50 637. Overall, 2895 (5.7%) veterans meeting our predefined criteria of using VA outpatient care were waitlisted (1418 [49.0%] at a VATC and 1477 [51.0%] at a non-VATC). Waitlisted veterans had significantly more VA clinician visits than veterans who were not waitlisted, but there were no differences based on distance to a VATC. Demographic characteristics are listed in Table 1 (additional clinical data in eTable 2 in Supplement).
Table 1.
Demographics of Veterans Receiving Outpatient Veterans Affairs (VA) Medical Care Prior to Being Waitlisted for Liver Transplantation
| No. (%) of Veterans Waitlisted at Transplant Centera | P Valueb | ||
|---|---|---|---|
| VA Center (n = 1418) | Other Center (n = 1477) | ||
| Age, median (IQR), y | 56 (52–60) | 57 (53–60) | .08 |
| Male sex | 1378 (97.2) | 1402 (94.9) | .002 |
| Race/ethnicity | |||
| White | 1101 (77.6) | 1093 (74.0) | .09 |
| Black | 152 (10.7) | 171 (11.6) | |
| Hispanic | 138 (9.7) | 180 (12.2) | |
| Asian | 6 (0.4) | 13 (0.9) | |
| Otherc | 20 (1.4) | 21 (1.5) | |
| Primary etiology | |||
| Hepatitis C virus | 909 (64.1) | 879 (59.5) | .003 |
| Alcohol | 222 (15.7) | 209 (14.2) | |
| Hepatitis B virus | 19 (1.3) | 27 (2.8) | |
| NASH or cryptogenic | 112 (7.9) | 128 (8.7) | |
| Cholestatic | 25 (1.8) | 49 (3.3) | |
| Autoimmune | 24 (1.7) | 28 (1.9) | |
| Otherd | 107 (7.6) | 157 (10.6) | |
| Poverty category, %e | |||
| 0–4.9 | 87 (6.1) | 116 (7.9) | .04 |
| 5–9.9 | 267 (18.8) | 325 (22.0) | |
| 10–14.9 | 308 (21.7) | 333 (22.6) | |
| 15–19.9 | 250 (17.6) | 251 (17.0) | |
| 20–24.9 | 178 (12.6) | 160 (10.8) | |
| 25–29.9 | 110 (7.8) | 91 (6.2) | |
| ≥30.0 | 176 (12.4) | 149 (10.1) | |
| Missing | 42 (3.0) | 52 (3.5) | |
Abbreviations: IQR, interquartile range; NASH, nonalcoholic steatohepatitis.
Unless otherwise indicated.
Derived from the χ2 test for categorical variables and the Wilcoxon rank sum test for the continuous variables.
Other race/ethnicity included multiracial, Pacific Islander, and individuals who responded as other.
Included metabolic liver diseases, acute liver failure, polycystic liver disease, and all other diagnoses.
Defined as the proportion of people residing in the zip code who are living below the federal poverty level. Patient-level zip code data were only available for the 2895 waitlisted veterans registered with the Organ Procurement and Transplantation Network.
Validation of Distance
Our method of measuring distance was validated by analyzing the cohort of veterans waitlisted at the Pittsburgh VATC (eTable 3 in Supplement). Because the home zip codes of waitlisted veterans is provided in OPTN data, the distance from the centroid of a respective veteran’s home zip code to the Pittsburgh VATC was compared with the measured distance from that veteran’s local VA hospital to the Pittsburgh VATC. The median distance between these 2 measured distances was 18.7 miles (interquartile 100 miles based on distance from a local VA hospital remaining in that category when using home zip code as the measure (eTable 3 in Supplement).
Multivariable Regression Results
In multivariable models, increasing distance to a VATC was associated with significantly lower odds of being waitlisted either at a VATC or any transplant center (Table 2). The odds ratio (OR) in the multivariable generalized estimating equation model evaluating distance and waitlisting at a VATC was 0.91 (95% CI, 0.89–0.93, P<.001; Table 2). For example, a veteran living 25 miles from a VATC would have a 7.4% (95% CI, 6.6%–8.1%) adjusted probability of being waitlisted, whereas a veteran 100 miles from a VATC would have a 6.2%(95% CI, 5.7%–6.6%) adjusted probability. The OR signifies a 9% lower odds of being waitlisted at a VATC between 2 populations whose distance from a local VA hospital to a VATC differs by a multiplicative factor of 2. Veterans Affairs hospital academic affiliation or an advanced liver care center was neither a significant covariate nor an effect modifier. Similar results were obtained when we excluded veterans with hepatocellular carcinoma or those with a MELD score of less than 15. Even though veterans with secondary non-Medicaid insurance were significantly more likely to be waitlisted at a VATC (OR, 1.60; 95% CI, 1.43–1.81) or any transplant center (OR, 2.22; 95% CI, 2.04–2.41), secondary insurance status did not confound the relationship between distance and waitlisting with unchanged ORs for distance with inclusion of this insurance variable. Increasing distance from a local VA hospital to the closest transplant center (VA or non-VA) was also associated with a lower odds of being waitlisted overall (OR, 0.94 [95% CI, 0.92–0.96] for log 2 base distance variable in multivariable generalized estimated equation model, P = .004; Table 3). Similar results were seen when distance was modeled as a categorical variable (eTables 4 and 5 in Supplement).
Table 2.
Association Between Distance From Veterans Affairs (VA) Center to VA Transplant Center (VATC) and Being Waitlisted for Transplantation Among Veterans With Decompensated Cirrhosis or Hepatocellular Carcinoma
| Being Waitlisted at a VATC | Being Waitlisted at Any Transplant Center | |||
|---|---|---|---|---|
| Multivariable OR (95% CI)a | P Value | Multivariable OR (95% CI)a | P Value | |
| Distanceb | 0.91 (0.89–0.93) | <.001 | 0.94 (0.92–0.96) | .004 |
| Age at first hepatic decompensation event per 1-year increments | 0.97 (0.96–0.97) | <.001 | 0.97 (0.96–0.97) | <.001 |
| Racial/ethnic composition of VA centerc | ||||
| 76%–100% White (n = 3461) | 1 [Reference] | .14d | 1 [Reference] | .76d |
| 51%–75% White (n = 22 026) | 0.97 (0.71–1.32) | 1.12 (0.87–1.43) | ||
| 26%–50% White (n = 21 107) | 0.80 (0.58–1.09) | 1.02 (0.80–1.31) | ||
| 0%–25% White (n = 4403) | 1.03 (0.68–1.57) | 1.04 (0.74–1.46) | ||
| Median center agee | 1.10 (0.98–1.57) | .08 | 1.13 (1.03–1.23) | .006 |
Center-specific covariates of proportion of veterans with mental illness and percentage of veterans with a low socioeconomic status excluded from final model for listing at VATC because they were not significant in univariable or multivariable models (P>.50) and were not confounders of the relationship between distance and waitlisting. None of the variables were collinear and the models were not overfit due to a large number of outcomes relative to the number of covariates examined.
The odds ratio (OR) for distance corresponds to the difference in the odds of being waitlisted between 2 populations whose distance from a local VA center to a VATC differs by a multiplicative factor of 2.
The number within each racial/ethnic category represents the total number of transplant-eligible veterans receiving care at a VA center with that specific racial/ethnic composition. The waitlisting rate at a VATC is 2.6% (89/3461) for 76%–100% white, 3.0% (670/22 026) for 51%–75% white, 2.6% (539/21 107) for 26%–50% white, and 3.0% (120/1043) for 0%–25% white. The waitlisting rate at any transplant center was 4.7% (163/3461) for 76%–100% white, 5.9% (1296/22 026) for 51%–75% white, 5.8% (1213/21 107) for 26%–50% white, and 5.5% (223/1043) for 0%–25% white.
Omnibus P value for the overall category.
The median center age was based on center-level data from the VA Hepatitis C Clinical Case Registry, and for each VA center, there is an age in years that is the median center age. The OR thus signifies the increase in the odds of waitlisting for every increase in 1 year of the median center age when comparing 2 centers.
Table 3.
Hospital-Level Percentages of Veterans With Decompensated Cirrhosis Receiving Outpatient Care at a Veterans Affairs (VA) Center Who Were Waitlisted for Transplantation From 2003–2010
| Distance in Miles From VA Center to Closest VA Transplant Centera | P Valueb | |||||
|---|---|---|---|---|---|---|
| 0–100 (n = 3417) | 101–200 (n = 5122) | 201–300 (n = 7906) | 301–500 (n = 9528) | >500 (n = 24 664) | ||
| No. of VA centers within zone | 8 | 14 | 26 | 28 | 61 | |
| Veterans waitlisted, No. (%) | ||||||
| For a transplant at any center | 372 (10.9) | 279 (5.5) | 424 (5.4) | 550 (5.8) | 1270 (5.2) | <.001 |
| For a transplant at a VATC | 244 (7.1) | 142 (2.8) | 184 (2.3) | 245 (2.6) | 603 (2.4) | <.001 |
| Per center % waitlisted, median (IQR)c | ||||||
| At any transplant center | 7.9 (5.0–12.5) | 6.0 (4.4–6.8) | 5.1 (3.3–6.6) | 5.7 (2.8–7.2) | 4.9 (3.5–6.1) | .18 |
| At a VATCd | 5.0 (3.5–7.4) | 2.7 (1.9–4.0) | 2.1 (1.3–2.9) | 2.7 (1.6–4.1) | 1.9 (1.1–3.4) | .007 |
| Per center % waitlisted veterans waitlisted at a VATC, median (IQR)e | 82.4 (37.5–93.1) | 56.7 (42.8–68.8) | 42.9 (23.1–66.7) | 56.5 (35.0–71.4) | 50.0 (32.6–64.6) | .09 |
Abbreviations: IQR, interquartile range; VATC, VA transplant center.
Data presented per VA medical center and distance category. The 5 distance categories reflect the distribution of the data and cut points in the relationship between distance and waitlisting. Only veterans receiving care at a VA center within 100 miles of a VATC would be expected to have the opportunity to live at home after discharge from the transplant hospitalization.
Derived from χ2 tests for the proportion of veterans waitlisted (yes or no) within each distance category or the Kruskall-Wallis test when comparing median and ranges between centers across distance categories.
The median values for percentages listed at a VATC vs any transplant center do not add upbecausea different VA medical center may represent the median for different variables.
From January 1, 2003, through December 31, 2007, the distance from a VA medical center to the closest VATC was measured from the Nashville VA for the 10 centers for which the Houston VATC is the closest because only 1 liver transplant was performed at the Houston VATC priortoJanuary1, 2008.
For each VA center, this value represents the proportion of veterans eligible for inclusion in the study who were waitlisted at a VATC among eligible veterans waitlisted overall (ie, 20% if a specific center has 50 veterans waitlisted, of whom 10 are waitlisted at a VATC).
Categorical Analysis
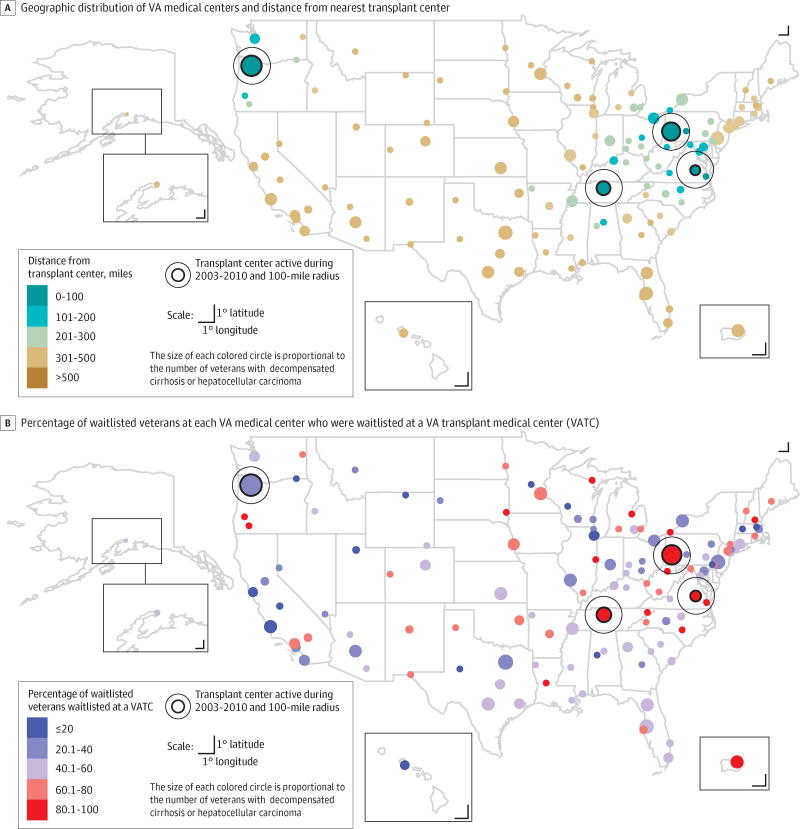
The proportion of transplant-eligible veterans waitlisted for transplantation at any transplant center differed significantly by distance from a VATC (≤100 miles, 372/3417 [10.9%; 95% CI, 9.9%–12.0%]; 101–200 miles, 279/5122 [5.5%; 95% CI, 4.8%–6.1%]; 201–300 miles, 424/7906 [5.4%; 95% CI, 4.9%–5.9%]; 301–500 miles, 550/9528 [5.8%; 95% CI, 5.3%–6.3%]; >500 miles, 1270/24 664 [5.2%; 95% CI, 4.9%–5.4%]; P < .001; Table 3). Of 47 219 veterans receiving care at a VA hospital located more than100 miles from a VATC, 1174 (2.5%) were waitlisted at a VATC and 2523 (5.3%) at any transplant center (VATC and non-VATCs). The proportion specifically waitlisted at a VATC was also significantly varied by distance to a VATC (≤100 miles, 244/3417 [7.1%; 95% CI, 6.3%–8.1%]; 101–200 miles, 142/5122 [2.8%; 95% CI, 2.3%–3.3%]; 201–300 miles, 184/7906 [2.3%; 95% CI, 2.0%–2.7%]; 301–500 miles, 245/9528 [2.6%; 95% CI, 2.3%–2.9%]; >500 miles, 603/24 664 [2.4%; 95% CI, 2.3%–2.6%]; P < .001; Table 3). Among all veterans who were waitlisted, the proportion specifically waitlisted at a VATC varied by distance. There was a broad range specifically waitlisted at a VATC across VA locations within each distance category (Table 3); however, when aggregated by distance, 66% of waitlisted veterans from the 8 VA hospitals within 100 miles of a VATC were waitlisted at a VATC compared with less than 51% across the other distance categories (Figure).
Figure. Geographic Distribution of VA Medical Centers (n=128), Including 4 Transplant Centers, and Variation in Rates of Waitlisting for Liver Transplantation.
The median proportion of veterans waitlisted at a VA transplant center (VATC) was 2.3% (interquartile range [IQR], 1.4%–3.7%), and waitlisted at any transplant center was 5.5% (IQR, 3.5%–6.7%). The median center-specific percentage of veterans waitlisted at a VATC relative to overall waitlistings was 54.3% (IQR, 35.1%–66.7%).
Access to Transplantation
Waitlisted veterans who received care more than 100 miles from a VATC were significantly less likely to receive a transplant once waitlisted at a VATC or at any transplant center (eTable 6 in Supplement). Among veterans waitlisted at a VATC, the proportion who received transplants at a VATC differed by distance from a VATC (≤100 miles, 156/244 [63.9%]; 101–200 miles, 76/142 [53.5%]; 201–300 miles, 103/184 [56.0%]; 301–500 miles, 125/245 [51.0%]; and >500 miles, 326/604 [54.1%]; P = .045). Among all waitlisted veterans, the proportion who received transplants at any transplant center varied significantly by distance from a VATC (≤100 miles, 262/372 [70.4%]; 101–200 miles, 164/279 [58.8%]; 201–300 miles, 243/424 [57.3%]; 301–500 miles, 294/550 [53.5%]; and >500 miles, 700/1270 [55.1%]; P < .001). In multivariable models of all waitlisted veterans, increasing distance from a local VA hospital to a VATC was associated with a 3% lower odds of transplantation at any transplant center between 2 populations of waitlisted veterans whose distance from a local VA hospital to a VATC differs by a multiplicative factor of 2 (subhazard ratio, 0.97 [95% CI, 0.95–0.98] for log 2 base distance variable; P < .001; Table 4).
Table 4.
Competing Risk Model Evaluating Transplantation Among Veterans Waitlisted for Liver Transplantation (n=2895)
| Total No. | Proportion Received Transplant Within Each Category, No. (%) | Multivariable Subhazard Ratio (95% CI)a | P Valueb | |
| Distance | 0.97 (0.95–0.98)c | <.001 | ||
| Age at listing | 0.95 (0.88–1.03)d | .21 | ||
| Male sex | 1.15 (0.97–1.38) | .12 | ||
| Race/ethnicity | ||||
| White | 2194 | 1253 (57.1) | 1 [Reference] | <.001 |
| Black | 323 | 189 (58.5) | 0.88 (0.65–1.19) | |
| Hispanic | 318 | 182 (57.2) | 0.88 (0.75–1.03) | |
| Asian | 19 | 13 (68.4) | 1.86 (1.13–3.07) | |
| Other | 41 | 26 (63.4) | 1.40 (0.95–2.07) | |
| Primary etiology | ||||
| Hepatitis C | 1788 | 1055 (59.0) | 1 [Reference] | <.001 |
| Alcohol | 431 | 214 (49.7) | 0.93 (0.77–1.13) | |
| Hepatitis B | 46 | 22 (47.8) | 0.52 (0.26–1.04) | |
| NASH or cryptogenic | 240 | 127 (52.9) | 0.99 (0.82–1.20) | |
| Cholestatic | 74 | 45 (60.8) | 1.07 (0.70–1.63) | |
| Autoimmune | 52 | 35 (67.3) | 1.51 (1.09–2.08) | |
| Other | 264 | 165 (62.5) | 1.03 (0.85–1.25) | |
| Blood type | ||||
| O | 1309 | 718 (54.9) | 1 [Reference] | |
| A | 1123 | 630 (56.1) | 1.06 (0.95–1.20) | <.001 |
| B | 337 | 214 (63.5) | 1.25 (1.04–1.51) | |
| AB | 126 | 101 (80.2) | 2.64 (2.28–3.05) | |
| Hepatocellular carcinoma | 4.04 (3.64–4.49) | <.001 | ||
| Measured at time of waitlisting | ||||
| Laboratory MELD scoree | 1.06 (1.05–1.08) | <.001 | ||
| Serum albumin levelf | 0.91 (0.82–1.01) | .07 |
Abbreviations: MELD, model for end-stage liver disease; NASH, nonalcoholic steatophepatitis.
Competing risk model of all waitlisted veterans (Veterans Affairs [VA] transplant center [VATC] or non-VATC) with the outcome of transplant and the competing risk of death on the waitlist or within 90 days of waitlist removal. Outcomes reported as subhazard ratios because of the competing risk model. The distance × waitlisting at a VA interaction term was not included in the final multivariable model because it was not significant (P = .22), although waitlisting at a VATC was included in the model even though it was not significant (P = .60). Primary insurance type was also not significant (P = .72). Residential-level poverty was neither independently associated with mortality nor was it a confounder.
The P value for the individual distance variables represents the pairwise comparison in the fully adjusted multivariable model, with 0 to 100 miles as the reference, whereas the P value for racial/ethnic composition, diagnosis, and blood type is the omnibus P value for the overall category.
The subhazard ratio for distance corresponds to the difference in the hazard of transplantation between 2 populations whose distance from a local VA center to a VATC differs by a multiplicative factor of 2.
The subhazard ratio for every 10-year increase in age at time of waitlisting.
Unit of comparison is per increase in 1 unit of MELD score.
Unit of comparison is per 1-mg/dL increase in albumin.
Survival
The overall survival rate of waitlisted veterans from the time of hepatic decompensation event differed by distance from a local VA hospital to a VATC (Table 5). Although the 1-year survival rates were similar, they dispersed over time. In multi-variable survival models of all waitlisted veterans with high health care use, increasing distance from a local VA hospital to a VATC was associated with a significantly increased risk of mortality after hepatic decompensation event, with a 3% increased risk of mortality between 2 populations for every doubling of distance from a local VA hospital to a VATC (HR, 1.03 [95% CI, 1.01–1.04]; P = .001). For example, a waitlisted veteran living 25 miles from a VATC would have a 62.9% (95% CI, 59.1%–66.1%) 5-year adjusted probability of survival from first hepatic decompensation event compared with a 59.8% (95% CI, 56.3%–63.1%) 5-year adjusted probability of survival for a veteran living 100 miles from a VATC.
Table 5.
Unadjusted 1-, 3-, and 5-Year Survival Rates Among Waitlisted Veterans From the Time of Initial Hepa Decompensation Event (n=2895)
| Survival Rate From Date of First Hepatic Decompensation Event (95% CI) | |||
|---|---|---|---|
| 1y | 3y | 5y | |
| Distance category of local VA center to VATC, miles | |||
| ≤100 | 90.5 (86.9–93.1) | 72.4 (67.0–77.1) | 57.5 (51.3–63.3) |
| 101–200 | 92.5 (88.7–95.1) | 64.0 (57.3–69.9) | 50.2 (42.6–57.3) |
| 201–300 | 89.4 (76.0–92.0) | 67.1 (61.8–71.8) | 51.6 (45.6–57.4) |
| 301–500 | 90.7 (87.9–92.9) | 66.1 (61.6–71.8) | 41.9 (36.7–47.1) |
| >500 | 91.2 (89.4–92.6) | 66.8 (63.9–69.6) | 45.4 (42.0–48.9) |
Discussion
Greater distance between a patient’s local VA hospital and a transplant center was associated with a lower likelihood of being placed on a transplant list when liver transplant was indicated. Once waitlisted, longer distances were also associated with a lower likelihood of receiving a transplant and increased mortality. These findings may be explained by (1) living remotely from a transplant center reducing the likelihood of getting evaluated for transplantation because of long travel times; or (2) reduced ability to proceed with transplantation because of the need for a patient or his or her family members to relocate. When analyzed as a continuous variable, distance had a dose-response relationship with increasing distance resulting in decreased likelihood of being put on a waitlist, receiving a transplant, and having a higher mortality. When analyzed as a categorical variable, distance appeared to have a threshold effect, whereby veterans living more than 100 miles from a VATC had a decreased likelihood of transplantation compared with patients who had their base hospital located within 100 miles of a liver transplant center.
Our study has the advantage of a large sample of patients eligible for a lifesaving health care service. Our findings are consistent with other studies examining the relationship between distance and access to transplant services.4,5,7,10 One study did show the opposite effect; an examination of US dialysis patients found a greater likelihood of being waitlisted for renal transplant for patients living farther from a transplant center.13 The investigators hypothesized that rural residents treated with dialysis were a highly selected, motivated group to even initiate dialysis given the likely longer distances needed to travel for this service, that physicians in rural areas were aware of the challenges of having rural patients waitlisted due to difficulties in access distant transplant centers, thus expediting transplant referrals, or both reasons.13 Our cohort met inclusion criteria simply by having a disease warranting a transplant, thereby avoiding the selection bias that could have influenced that study, which required both the presence of a condition (end-stage renal disease) as well as receiving routine continuous therapy for that disease (dialysis).
Because we could access the medical records for all VA patients in the United States, we could directly estimate the denominator of patients eligible for waitlisting. Prior studies relied on estimates of hypothetical cohorts of patients who might be at risk for receiving a transplant based on census information.10,11 Most prior studies assessed care offered at many centers, with travel times of 15 minutes to 2 hours. Few prior studies evaluated services offered at only a very limited number of transplant centers. Patients in our study who were far away from a VATC did not necessarily reside in rural areas (ie, the Bronx VA Medical Center is >300 miles from the Pittsburgh VATC), resulting in our study being more of an examination of distance rather than urban vs rural. Our results were insensitive to adjusting for VA hospital academic affiliation, suggesting that our findings were related to distance rather than access to advanced liver care services.
Our findings suggest a need to improve access to liver transplantation in the VA. Increasing the number of VATCs is one solution, and the VA National Transplant Program has approved the opening of 2 VATCs: one in Madison, Wisconsin, and the other in Miami, Florida. However, this will not eliminate problems related to distance from a VATC for many veterans. Other solutions might include (1) streamlining referral to VATCs; (2) using telehealth or allowing local clinician teams to perform initial waitlisting evaluations; (3) active monitoring of liver disease burden at all VA hospitals with assessment of hospitals with low transplant referral rates; and (4) lowering financial disincentives for access to local transplant services through VA-purchased care (ie, payment of medical services delivered outside of the VHA health system for VHA beneficiaries). Such measures would require significant investment to enact.
Broader Implications
This issue of distance and access to care is critical given the focus on accountable care organizations that create large networks of physicians and hospitals. As complex, expensive medical technology evolves, certain services may only be offered at a limited number of sites (eg, proton beam therapy). Although our findings are consistent with prior studies evaluating the association of distance to care, our study is the first, to our knowledge, to demonstrate the adverse consequences of centralization of specialized care at a limited number of sites.8
For example, since 2006, hospitals performing bariatric surgeries on Medicare beneficiaries are required to be a designated as centers of excellence.39 A subsequent single-center study demonstrated that this initiative was associated with reduced access to bariatric surgery based on distance (a subset of patients had to travel distances of >800 miles)1 despite similar bariatric surgical outcomes at non–centers of excellence vs centers of excellence.35 However, such an analysis in a national sample of bariatric surgery candidates is practically in-feasible due to an inability to nationally define potential candidates based on body mass index data. Similarly, Blue Cross and Blue Shield restricts referrals for complex and rare cancers to centers receiving Blue Distinction.2 By demonstrating that increasing distance is associated with decreased access to care in a national sample of patients, our analysis may serve as a model of the national association of centralized care on services offered at selected centers. Future work must evaluate whether a causal relationship exists.
Limitations
As with any observational study, there may be unmeasured confounding, including that veterans living closer to a VATC have more severe liver disease. However, we specifically identified veterans with decompensated cirrhosis or hepatocellular carcinoma, thus warranting a transplant evaluation. Second, we identified our cohort using International Classification of Diseases, Ninth Revision, codes, not chart review. Even though a subset may be ineligible due to comorbid conditions or psychosocial contraindications (ie, alcohol use or homelessness), this proportion should not differ by hospital or distance. Also, the proportion of veterans waitlisted at a VATC track with those of a single VA hospital study,36 and a study of all patients hospitalized in Pennsylvania for liver-related conditions.37 Third, our results may have been related to factors beyond distance (ie, VATC preference for waitlisting patients from their hospital), yet the potential dose-response relationship seen with the continuous distance variable may suggest otherwise. Fourth, distance was measured from the VA hospital. Nonetheless, hospital assignment is based on geographic proximity to a hospital, thus hospital-level distances are representative of the distance a veteran would need to travel. Fifth, categorical analyses were based on distance grouping that was determined after examination of the data; therefore, these analyses should be considered post hoc and the categorical findings exploratory. Sixth, we could not determine hepatocellular carcinoma stage to determine transplant eligibility criteria (Milan criteria38), but the results were unchanged with exclusion of all patients with hepatocellular carcinoma.
Conclusions
Among VA patients meeting eligibility criteria for liver transplantation, greater distance from a VATC or any transplant center was associated with lower likelihood of being put on a waitlist or receiving a transplant, and greater likelihood of death. The relationship between these findings and centralizing specialized care deserves further investigation.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
Footnotes
Supplemental content at jama.com
Author Contributions: Drs Goldberg and Kaplan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Goldberg, Backus, Halpern, Kaplan.
Acquisition, analysis, or interpretation of data: Goldberg, French, Forde, Groenveld, Bitterman, Backus, Halpern, Kaplan.
Drafting of the manuscript: Goldberg, Kaplan.
Critical revision of the manuscript for important intellectual content: Goldberg, French, Forde, Groenveld, Bitterman, Backus, Halpern, Kaplan.
Statistical analysis: Goldberg, French, Forde, Halpern, Kaplan.
Administrative, technical, or material support: Goldberg, Groenveld, Kaplan.
Study supervision: Halpern, Kaplan.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Sponsor: The Health Resources and Services Administration had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content of this article does not necessarily reflect the views or policies of the US Department of Health and Human Services, the US Department of Veterans Affairs, or the US government. The mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Additional Contributions: We thank Vincent Lo Re III, MD, MSCE (Division of Infectious Diseases, University of Pennsylvania), for advice with regard to use of the coding algorithm based on the International Classification of Diseases, Ninth Revision, and C. Brent Roberts, MPH (Center for Health Equity Research and Promotion at the Philadelphia VA Medical Center), for assisting with Organ Procurement and Transplantation Network and Corporate Data Warehouse cross-referencing. Neither Dr Lo Re nor Mr Roberts received compensation for their role in the study.
References
- 1.Livingston EH, Burchell I. Reduced access to care resulting from centers of excellence initiatives in bariatric surgery. Arch Surg. 2010;145(10):993–997. doi: 10.1001/archsurg.2010.218. [DOI] [PubMed] [Google Scholar]
- 2.Blue Cross and Blue Shield. Blue Distinction selection criteria overview. http://www.bcbs.com/why-bcbs/blue-distinction/blue-distinction-overview.html. Accessed September 6, 2013.
- 3.Carr BG, Branas CC. Time, distance, and access to emergency care in the United States. LDI Issue Brief. 2009;14(4):1–4. [PubMed] [Google Scholar]
- 4.Carr BG, Branas CC, Metlay JP, Sullivan AF, Camargo CA., Jr Access to emergency care in the United States. Ann Emerg Med. 2009;54(2):261–269. doi: 10.1016/j.annemergmed.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans RW, Kitzmann DJ. Contracting for services: liver transplantation in the era of mismanaged care. Clin Liver Dis. 1997;1(2):287–303. viii. doi: 10.1016/s1089-3261(05)70272-5. [DOI] [PubMed] [Google Scholar]
- 6.Gregory PM, Malka ES, Kostis JB, Wilson AC, Arora JK, Rhoads GG. Impact of geographic proximity to cardiac revascularization services on service utilization. Med Care. 2000;38(1):45–57. doi: 10.1097/00005650-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol. 2010;17(11):2824–2831. doi: 10.1245/s10434-010-1159-0. [DOI] [PubMed] [Google Scholar]
- 8.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27(28):4671–4678. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299(2):202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 10.Thabut G, Munson J, Haynes K, Harhay MO, Christie JD, Halpern SD. Geographic disparities in access to lung transplantation before and after implementation of the lung allocation score. Am J Transplant. 2012;12(11):3085–3093. doi: 10.1111/j.1600-6143.2012.04202.x. [DOI] [PubMed] [Google Scholar]
- 11.Zorzi D, Rastellini C, Freeman DH, Elias G, Duchini A, Cicalese L. Increase in mortality rate of liver transplant candidates residing in specific geographic areas: analysis of UNOS data. Am J Transplant. 2012;12(8):2188–2197. doi: 10.1111/j.1600-6143.2012.04083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J. Access to kidney transplantation among remote-and rural-dwelling patients with kidney failure in the United States. JAMA. 2009;301(16):1681–1690. doi: 10.1001/jama.2009.545. [DOI] [PubMed] [Google Scholar]
- 13.Scientific Registry of Transplant Recipients website. US hospitals with liver transplant centers. http://srtr.org/csr/current/Centers/TransplantCenters.aspx?organcode=LI. Accessed September 6, 2013.
- 14.Goldberg DS, French B, Thomasson A, Reddy KR, Halpern SD. Current trends in living donor liver transplantation for primary sclerosing cholangitis. Transplantation. 2011;91(10):1148–1152. doi: 10.1097/TP.0b013e31821694b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner R, Edwards E, Freeman R, et al. United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368–373. doi: 10.1097/MLR.0b013e31827da95a. [DOI] [PubMed] [Google Scholar]
- 18.Murray KF, Carithers RL, Jr, AASLD AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134(6):1764–1776. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 20.Lo Re V, III, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20(7):689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Veteran Affairs. VA CMS data repository. http://www.virec.research.va.gov/VACMS. Accessed September 20, 2012.
- 22.Beehler GP, Rodrigues AE, Mercurio-Riley D, Dunn AS. Primary care utilization among veterans with chronic musculoskeletal pain: a retrospective chart review. Pain Med. 2013;14(7):1021–1031. doi: 10.1111/pme.12126. [DOI] [PubMed] [Google Scholar]
- 23.Katz IR, McCarthy JF, Ignacio RV, Kemp J. Suicide among veterans in 16 states, 2005 to 2008: comparisons between utilizers and nonutilizers of Veterans Health Administration (VHA) services based on data from the National Death Index, the National Violent Death Reporting System, and VHA administrative records. Am J Public Health. 2012;102(suppl 1):S105–S110. doi: 10.2105/AJPH.2011.300503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillespie BW, Merion RM, Ortiz-Rios E, et al. A2ALL Study Group. Database comparison of the adult-to-adult living donor liver transplantation cohort study (A2ALL) and the SRTR US Transplant Registry. Am J Transplant. 2010;10(7):1621–1633. doi: 10.1111/j.1600-6143.2010.03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballman KV. Genetics and genomics: gene expression microarrays. Circulation. 2008;118(15):1593–1597. doi: 10.1161/CIRCULATIONAHA.107.714600. [DOI] [PubMed] [Google Scholar]
- 26.Backus LI, Belperio PS, Loomis TP, Yip GH, Mole LA. Hepatitis C virus screening and prevalence among US veterans in Department of Veterans Affairs care. JAMA Intern Med. 2013;173(16):1549–1552. doi: 10.1001/jamainternmed.2013.8133. [DOI] [PubMed] [Google Scholar]
- 27.Backus LI, Gavrilov S, Loomis TP, et al. Clinical case registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 30.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43(2):345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 31.French B, Heagerty PJ. Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008;27(24):5005–5025. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittermann T, Makar G, Goldberg D. Exception point applications for 15 points: an unintended consequence of the share 15 policy. Liver Transpl. 2012;18(11):1302–1309. doi: 10.1002/lt.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 35.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792–799. doi: 10.1001/jama.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julapalli VR, Kramer JR, El-Serag HB. American Association for the Study of Liver Diseases. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11(11):1370–1378. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 37.Bryce CL, Angus DC, Arnold RM, et al. Sociodemographic differences in early access to liver transplantation services. Am J Transplant. 2009;9(9):2092–2101. doi: 10.1111/j.1600-6143.2009.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organ Procurement and Transplantation Network. Policies and bylaws. http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf. Accessed September 2, 2013.
- 39.Centers for Medicare & Medicaid Services. Medicare national coverage determinations manual. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/ncd103c1_Part2.pdf. Accessed September 6, 2013. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.