Abstract
Hepatic progenitor cells (HPC) play important roles in both liver regeneration and carcinogenesis. Combined hepatocellular-cholangiocarcinoma (CHC), a malignant primary liver tumor with poor prognosis, is thought to be of HPC origin. However, the prognostic significance of this etiology is not well defined. Therefore, we retrospectively investigated the relationship of HPC-related pathological features and long-term outcome in patients with CHC in our department. In a cohort of 80 patients identified between 1997 and 2003, including 70 patients who underwent resection with curative intent, overall survival (OS) and disease-free survival (DFS) were correlated with the proliferative activity of nontumor ductular reaction (DR) and the expression levels of HPC and biliary markers including α-fetoprotein (AFP), keratin 7 (K7), keratin 19 (K19), oval cell (OV)-6, epithelial cell adhesion molecule (EpCAM), and c-Kit in both tumor and nontumor liver. We found that nontumor ductular reactions (DRs), specifically the proliferating cell nuclear antigen (PCNA) labeling index of the ductular reaction (PI-DR), a surrogate for transit-amplifying compartments, was an independent prognostic factor for both OS and DFS. By contrast, intratumoral expression of only one marker, absence of AFP, was associated with OS. PI-DR was also independently associated with synchronous “multicentric occurrence” in hepatocellular carcinoma components, a feature of CHC that may predispose to metachronous multifocal tumorigenesis.
Conclusion
Proliferative ductular reaction related to HPC activation is associated with recurrence of CHC. Background HPC activation is strongly associated with multifocal occurrence and related tumor recurrence, highlighting the critical role of background liver disease, a “field effect,” in the recurrence of CHC.
Hepatic progenitor cells (HPCs), which reside in Canals of Hering (CoH), when activated can give rise to ductular reactions (DRs). DRs correlate with the degree of inflammation and fibrosis in the course of many chronic human liver diseases. Because most of these diseases are strong etiological factors for primary liver cancer, activation of the HPC compartment has been strongly linked to hepatic carcinogenesis.1 Progenitor cell-like features suggesting an HPC origin has been strongly associated with a poor prognosis in hepatocellular carcinoma (HCC).2-4
Combined hepatocellular-cholangiocarcinoma (CHC) is a malignant primary liver tumor that contains elements of both HCC and intrahepatic cholangiocarcinoma (ICC). Although relatively rare among liver cancers, CHC has garnered recent attention due to its distinct pathological features suggesting an HPC origin,5 aggressive biological behavior, and related poor clinical outcome. 6 Although a progenitor cell-like phenotype of CHC has been well established by morphological and immunohistochemical observations,7,8 and although certain clinical correlates of survival have been described, pathological correlates of clinical outcome have not been previously identified.
In the present study we performed a clinicopathological study on 80 patients with CHC who underwent hepatectomy to explore the prognostic predictive utility of intratumoral and nontumor pathological findings, focusing on HPC-related features. The study of nontumor transit-amplifying compartments is critical because such features may suggest a “field effect”9 that predicts the development of metachronous tumor recurrence. We found that proliferative DRs in nontumor tissue were independent prognostic factors for both overall survival (OS) and disease-free survival (DFS) and independently associated with multicentric occurrence.10
Patients and Methods
Patients and Clinical Data
From January 1997 to December 2003, patients who underwent hepatectomy in the Department of Hepatic Surgery and who were postoperatively confirmed as CHC were recruited for prospective follow-up. Informed consent was obtained from each patient under a protocol approved by the Hospital Research Ethics Committees. Patient demographics and baseline characteristics are presented in Table 1. The neutrophil-to-lymphocyte ratio (NLR) was measured as an indicator of inflammatory status, previously established as a prognostic indicator in HCC.11 Tumor stage was determined according to the 2009 UICC TNM classification system.12 We defined resection with curative intent as complete excision of the primary tumor with negative microscopic margins ≥2 cm from the tumor and with no residual tumors indicated by ultrasonography and computed tomography (CT) scan within 1 month of initial surgery.
Table 1.
Demographic and Baseline Characteristics of 80 Patients With Combined Hepatocellular-Cholangiocarcinoma
| Factors | Value | Percent |
|---|---|---|
| Demographic characteristics | ||
| Age | 49.2 ± 10.5 | |
| Gender (male/female) | 72/8 | 90.0/10.0 |
| HBsAg positive | 68 | 85.0 |
| Anti-HCV positive | 2 | 3.2 |
| HBsAg, anti-HCV positive | 1 | 1.6 |
| Chronic alcoholism | 11 | 13.8 |
| Tumor characteristics | ||
| Maximum tumor diameter | 6.2 ± 3.0 | |
| Tumor number (multiple)* | 26 | 32.5 |
| Confluent multinodularity† | 56 | 70.0 |
| Hemorrhage and necrosis | 53 | 66.3 |
| Tumor thrombosis | 13 | 16.3 |
| Microvascular invasion | 64 | 80.0 |
| Histological grade (HCC component) | ||
| Well/moderately | 35 | 43.8 |
| Poorly | 45 | 56.2 |
| Histological grade (ICC component) | ||
| Well/moderately | 70 | 87.5 |
| Poorly | 10 | 12.5 |
| Histology of chronic liver disease | ||
| Necroinflammation grade (score) | ||
| No (0) | 0 | 0.0 |
| Minimal (1-4) | 4 | 5.0 |
| Mild (5-8) | 49 | 61.3 |
| Moderate (9-12) | 25 | 31.3 |
| Marked (13-18) | 2 | 2.5 |
| Fibrosis stage | ||
| 0 | 2 | 2.5 |
| 1-4 | 27 | 33.7 |
| 5-6 | 51 | 63.8 |
| Laboratory values | ||
| AFP (ng/mL) | 413.8 (1.0-1000.0) | |
| CA19-9 (U/mL) | 17.3 (0.0-570.0) | |
| CEA (ng/mL) | 1.8 (0.0-50.9) | |
| AST (IU/L) | 39.9 (16.3-39.9) | |
| ALT (U/L) | 43.1 (14.3-700.7) | |
| ALP (IU/L) | 153.5 (40.0-382.0) | |
| NLR | 2.1 (0.6-11.1) |
AFP, alpha-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, Carcinoembryonic antigen; HBsAg, hepatitis B surface antigen; HCV, hepatitis C; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; NLR, neutrophil-to-lymphocyte ratio; TNM, tumor-node-metastasis.
Multifocal tumors diagnosed by preoperative image finding.
Pathologically diagnosed tumors showing multinodularity.
Follow-up and Detection of Recurrence
All patients were followed regularly every 2-3 months after surgery until study closure in July 2008 with serum α-fetoprotein (AFP)/CA19-9 and abdominal ultrasonography. Progressive elevation of serum AFP/CA19-9 levels and/or ultrasonographic detection of a new hepatic lesion prompted hospitalization for confirmation of diagnosis and appropriate management, including repeat resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), or supportive therapy. Recurrence was confirmed by contrast-enhanced imaging studies or cholangiography according to standard guidelines for HCC,13 ICC,14 or radiologic features of CHC described previously.15 OS was defined as the interval between the dates of surgery and death, whereas DFS was defined as the interval between the dates of surgery and recurrence. If recurrence was not diagnosed, patients were censored on the date of death or the last follow-up. Clinical follow-up was not disclosed to laboratory personnel until statistical analysis.
Histopathological Diagnosis and Analysis
Histological staining methods and criteria for CHC diagnosis are presented in the Supporting Methods. Pathology specimens were independently evaluated by two experienced pathologists (W.M.C., M.H.Z.). The degree of inflammation and fibrosis was graded and staged according to the method of Ishak et al.16 Because some cases showed marked geographical histological heterogeneity in differentiation, the histopathological grade of each tumor component was defined by the poorest degree of differentiation identified within the tumor. Histological grade of tumors was determined according to described schemas.17 Multicentric occurrence (MO)10,18 was also assessed in HCC components. Briefly, MO was defined by the presence of either (1) at least two nodules that included an early tumor within a dysplastic nodule or without substantial destruction of the hepatic architecture (so-called “nodule in nodule” appearance) or (2) moderately or poorly differentiated tumors within a margin of a welldifferentiated tumor. Dysplastic foci or nodules were diagnosed following recent consensus guidelines.19
Immunohistochemistry and Evaluation
We performed immunohistochemistry on paraffin-embedded 4-μm sections of 80 CHCs from which both tumor and nontumor tissues (>2 cm away from tumor) were available, with antibodies summarized in Supporting Table 1. A standard two-step immunoperoxidase-labeled protocol with goat antimouse/rabbit horseradish peroxidase (HRP) (EnVision, Dako, Glostrup, Denmark) was applied stringently on all slides. An overview of how immunostaining was evaluated is detailed in the Supporting Methods.
Evaluation of Reactive Lesions in Nontumor Liver
Cell types and reactive lesions were identified according to Roskams et al.,20 with some terminology variations. In our study the term ductular reaction is specific for reactive ductules with biliary/HPC phenotype arranged in an irregularly shaped structure residing along parenchymal-stromal boundaries. The description of terminology, morphology, and evaluation schema for these reactive lesions is detailed in Supporting Table 2. In addition, proliferation rate in DR was evaluated by calculating the proliferating cell nuclear antigen (PCNA) labeling index. Specifically, 10 400× high-powered fields including epithelial-stromal boundaries within each section were randomly chosen and captured. The same fields were captured in sequential serial sections stained with K7 for quantification of the number of reactive ductular cells (RDCs) in reactive ductules. 21,22 The proliferation index of DR (PI-DR) was calculated as ratio between the number of PCNA immunoreactive nuclei and the total number of RDCs.
Double-Fluorescence Immunostaining
For antigen colocalization studies, double-fluorescence immunostaining of formalin-fixed, paraffin-embedded tissue was performed with a sequential fluorescent method as described.8 Alexa488-conjugated goat antimouse IgG (Invitrogen, Carlsbad, CA) and Alexa568-conjugated goat antirabbit IgG (Invitrogen) were used as secondary antibodies.
To reduce autofluorescence, tissue sections were treated as described.23 Immunofluorescence was observed with the Olympus IX-71. Under these conditions, single labeling appears green (Alexa488) or red (Alexa568), whereas colabeling appears to be yellow or orange.
Statistical Methods
We applied normality tests on all numeric variables before further analysis. Continuous normally distributed variables are summarized as mean ± standard deviation (SD) and represented graphically as mean with standard error of the mean (SEM) bar. Nonnormally distributed variables are summarized by median and range. To compare the means between groups, analysis of variance (ANOVA) or Student t test was performed. To determine differences of nonnormal variables between groups, medians were tested by Mann-Whitney U tests. The degree of association was determined by Spearman or Pearson correlation as appropriate. Methods for survival data analysis are described in the Supporting Methods. Collinearity was diagnosed among variables before all regression analyses. Reliability of the all models was then tested by residual or receiver operating characteristic (ROC) analysis. All analysis was carried out using SPSS software v. 12.0 (Chicago, IL) or R software (R Foundation, Vienna, Austria). P < 0.05 was considered significant.
Results
Clinical, Histological, and Follow-up Data
As shown in Fig. 1, 88 Chinese patients were recruited. Four patients presenting with recurrent tumors and four patients whose nontumor tissues (>2 cm away from the primary tumor) were not available were excluded. Of the remaining 80 patients, 70 had undergone surgery with curative intent. None had received any preoperative antitumor treatment. Pathological specimens from 80 patients showed features of CHC with representative histology shown in Supporting Fig. 1. Samples from 51 patients were assessed as “HCC-predominant,” 18 “ICC-predominant,” and 8 “intermediate area” predominant; the latter phenotype has been described as “CHC with stem-cell feature.”17 HCC and ICC components were equally dominant in three patients. “Antler-like” features representing a cholangiolocarcinoma (CLC) component was observed in 12 cases. Within ICC areas, four cases contained a component of squamous cell carcinoma and one case also contained sarcomatous changes. Within nontumor sections, 51 cases (63.8%) were confirmed as cirrhotic, whereas only two patients showed no fibrosis. Adjacent to tumors, foci or nodules (>1 mm) with dysplastic features were frequently observed. Large cell change (LCC) was found in 64 (80.0%) cases and small cell change (SCC) in 55 (68.8%). Coexisting dysplastic nodules (without distinguishing low grade or high grade) were found in 16 (20.0%) cases. Among patients undergoing noncurative resections (n = 10) the median survival was only 88 days. In the curative resection group (n = 70), probability of OS at 1, 3, and 5 years was 74.3%, 38.6%, and 30.0%, respectively. In all, 64 out of 70 (91.4%) patients developed intrahepatic recurrence (n = 63) and/or distant metastases (n = 4; three lung and one abdominal wall). The 1-, 3-, and 5-year probability of DFS was 41.4%, 18.6%, and 10.0%, respectively. After detection of intrahepatic recurrence, 31 patients received TACE and nine patients received repeat resection (two of whom after initial TACE). One patient received RFA. After repeat resections, seven patient samples were available for pathological evaluation, three of which revealed HCC with biliary differentiation, one HCC, two ICC, and one complete necrosis.
Fig. 1.
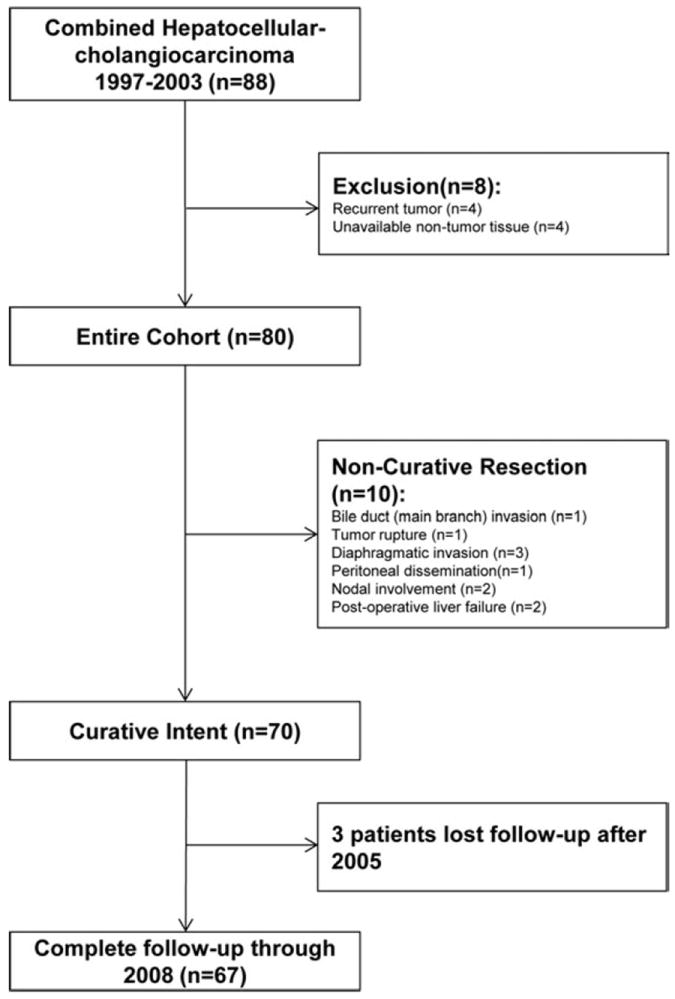
Flow chart of patient enrollment, grouping, and follow-up.
DR Is Observed in Nontumor Tissue From CHC Patients: Relationship With Background Transit-Amplifying Compartments
In nontumor K7 staining sections of the entire group (n = 80), all patients showed increased intensity of DR (Fig. 2A), with five patients in 1+ (≤10%), 11 patients in 2+ (10%-25%), 22 patients in 3+ (26%-50%), 42 patients in 4+ (≥50%). In the curative resection group, 37 of 70 (52.9%) showed 4+ (≥50%) in intensity of DR. In terms of PI-DR, which measured the proliferation status of DR, 21 of 80 patients (26.3%) had PI-DR ≥50%. In the curative resection group, PI-DR ≥50% was found in 19/70 patients (27.1%). Importantly, we observed some cases with intensive K7 staining DR (4+) showing a low level of PCNA staining, as shown in Fig. 2B. The observation of this dissociation of K7 expression and proliferation within DR was supported by statistical analyses across the cohort (r = 0.074, P = 0.512, Fig. 2C). Around portal areas of nontumor sections stained with K7, HPCs can be observed with intensive staining in the CoH location. Intermediate hepatobiliary cells (IHBCs) were found around HPCs or in continuity with reactive ductules (Fig. 3A). Epithelial cell adhesion molecule (EpCAM) or oval cell (OV)-6-positive hepatocytes were also observed (Fig. 3A,B). K7-DR was only found associated with number of HPCs (r = 0.423, P < 0.001). By contrast, cases with high PI-DR were found not only to have increased number of HPCs (r = 0.714, P < 0.001), but also more IHBCs (r = 0.506, P < 0.001) as well as higher OV-6 expression in parenchyma (r = 0.514, P < 0.001). A subgroup of DR expressing Bmi-1 was also found with higher PI-DR (P < 0.001). To reduce the potential impact of unspecific proliferation stimulators or confounding factors, correlations were corrected for the PCNA labeling index within hepatocytes, necroinflammation score, stage of fibrosis, gender, age at surgery, status of virus infection, and chronic alcoholism. After correction, PI-DR was still significantly associated with all these factors (Fig. 3C). Cumulatively, these associations suggest that HPC activation is a dominant feature in background liver.
Fig. 2.
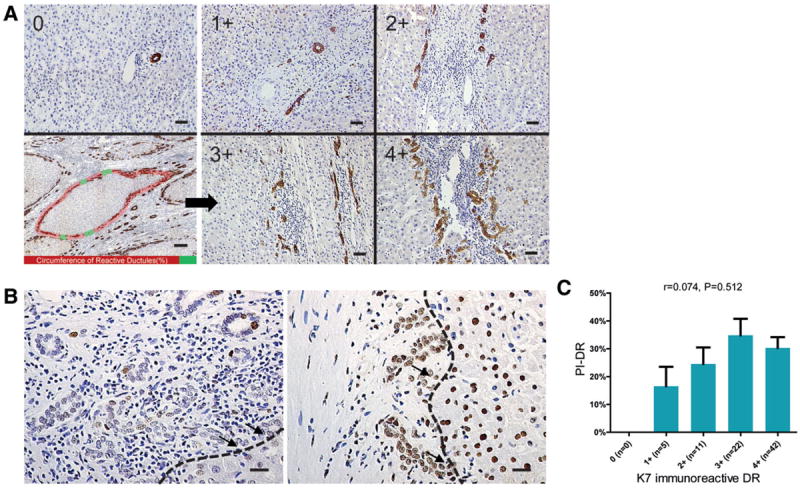
PCNA labeling index of ductular reaction (PI-DR) is not associated with K7 immunoreactive ductular reaction (K7-DR) in nontumor liver of patients with CHC. (A) Left panel shows a representative example of a portal area without ductular reaction (upper, scale bar = 25 μm). Schema for intensity evaluation of K7-DR in nontumor tissue is also presented (lower, scale bar = 300 μm). Red shading represents reactive ductules circumference, whereas green shading represents nonreactive ductules circumference. Ratio of red to overall shading is semiquantified like the right panel showing the schema described in Supporting Table 2. (B) Light micrograph of portal area displaying PCNA staining for DR represents a low level of proliferation activity (left) and a high level of proliferation activity (right). Arrows show reactive ductules connecting with liver parenchymal (scale bar = 25 μm). (C) Means with SEM error bars of PCNA labeling indices of DR at each level of K7-DR are shown. Spearman correlation analysis provides correlation coefficients (r) and P-value.
Fig. 3.
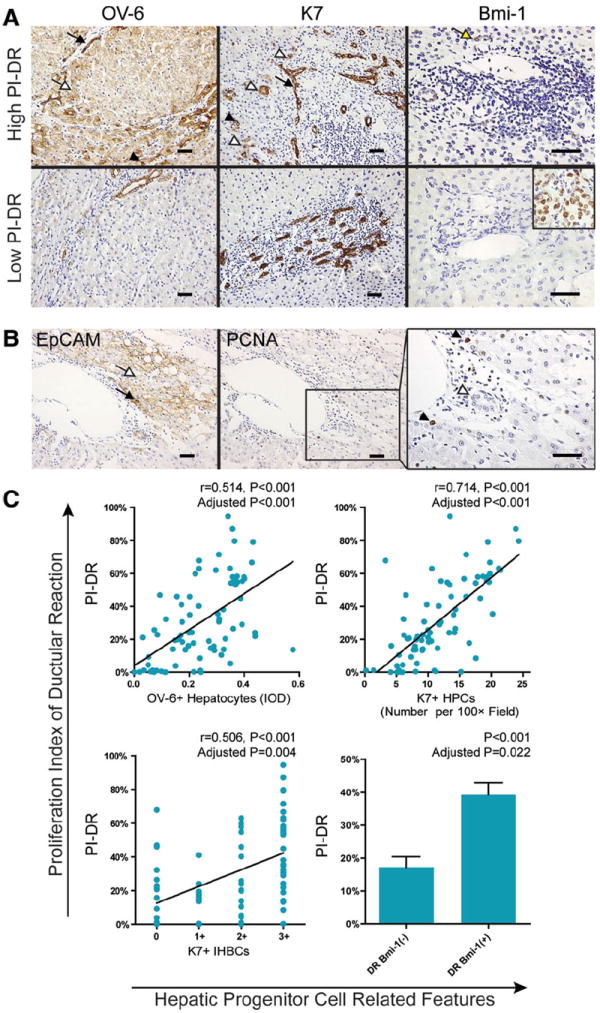
PI-DR is closely associated with background transit-amplifying compartments. (A) Representative photomicrographs of patients with a high level PI-DR or a low level PI-DR. Nontumor sections stained with OV-6 (left), AFP (middle), and Bmi-1 (right) are shown. On K7/OV-6 staining section, hepatic progenitor cells (HPCs, black arrowhead), K7+ intermediate hepatobiliary cells (IHBCs, white arrowhead), OV-6+ hepatocytes (white arrow), and reactive ductular cells (RDCs, black arrow) can be observed. On Bmi-1 staining section, RDCs with positive nuclear stain can be observed (yellow arrow). A representative picture of tumor cell staining from the same case serves as an internal positive control for Bmi-1-negative staining in nontumor portal area (scale bar = 50 μm). (B) Sequential sections that stained with EpCAM (left) and PCNA (middle) is presented. In the EpCAM staining section, an EpCAM + HPC (black arrow) and a cluster of EpCAM + hepatocytes (white arrow) shows membranous staining. A weak cytoplasmic staining can also be observed. In a high-power field of PCNA staining (right), positive nuclear staining of PCNA can be observed (black arrowhead) in parenchyma, whereas a bile duct (BD) shows no staining (white arrowhead) (scale bar = 50 μm). (C) Scatterplot with fitting line shows PI-DR strongly correlates with OV-6+ hepatocytes measured by IOD in parenchyma (upper left) and K7+ HPCs counting (upper right). Pearson correlation analysis provides correlation coefficient (r) and P-value. A split scattergram with fitting line shows PI-DR correlates with semiquantification of IHBCs in nontumor parenchyma (lower left). Spearman correlation analysis provides correlation coefficient (r) and P-value. Means with SEM error bars of PI-DR at subgroups without or with Bmi-1 staining in DR are shown (lower right). P-values were corrected for overall hepatocytes proliferation, total necroinflammation score, fibrosis stage, age at surgery, status of virus infection, gender, and chronic alcoholism.
Consistent with prior studies,1,24 both PI-DR and K7-DR correlated with hepatic inflammation as measured by the Ishak grade score (Table 2). Correlations were also found between PI-DR/K7-DR and fibrosis stage. In addition, a higher level of PI-DR was found associated with older age at surgery, nonalcoholism, impaired hepatocyte replication measured by hepatocyte p21Waf1/Cip1 labeling index (p21-LI), coexistence of LCC, and adjacent SCC. Multivariate analysis showed that PI-DR was independently associated with fibrosis stage, hepatocyte replicative arrest, age, and coexistence of LCC. By contrast, K7-DR only correlated with necroinflammation.
Table 2.
Correlation of Ductular Reactions With Clinical and Histological Variables in 80 Patients With Combined Hepatocellular-Cholangiocarcinoma
| PI-DR
|
K7-DR
|
|||||
|---|---|---|---|---|---|---|
| Correlation | P | Adjusted P* | Correlation | P | Adjusted P* | |
| Age at surgery | 0.252 | 0.024 | 0.017 | 0.081 | NS | NS |
| Gender | 0.005 | NS | NS | -0.095 | NS | NS |
| Chronic viral hepatitis | −0.178 | NS | NS | -0.083 | NS | NS |
| Chronic alcoholism | −0.230 | 0.041 | NS | 0.089 | NS | NS |
| Total necroinflammation score | 0.244 | 0.029 | NS | 0.317 | 0.004 | 0.007 |
| Interface hepatitis | 0.280 | 0.012 | NS | 0.433 | <0.001 | 0.001 |
| Confluent necrosis | 0.264 | 0.018 | NS | 0.229 | 0.041 | NS |
| Focal (spotty) lytic necrosis, apoptosis, and focal inflammation | 0.143 | NS | NS | 0.087 | NS | 0.036 |
| Portal inflammation | 0.083 | NS | NS | 0.277 | 0.001 | NS |
| Fibrosis stage | 0.231 | 0.039 | 0.012 | 0.265 | 0.018 | NS |
| Hepatocyte replicative arrest (p21-LI) | 0.297 | 0.008 | 0.048 | 0.216 | NS | NS |
| Periportal hepatocyte proliferation (PCNA-LI) | 0.759 | <0.001 | <0.001 | 0.082 | NS | NS |
| Overall hepatocyte proliferation (PCNA-LI) | 0.218 | NS | NS | -0.132 | NS | NS |
| Replicative arrest proliferation ratio | −0.034 | NS | NS | 0.081 | NS | NS |
| Coexisting dysplastic nodules | 0.187 | NS | NS | 0.162 | NS | NS |
| Coexisting small cell change | 0.255 | 0.022 | 0.051 | 0.169 | NS | NS |
| Coexisting large cell change | 0.318 | 0.004 | 0.010 | 0.150 | NS | NS |
K7-DR, keratin 7 immunoreactive ductular reaction; p21-LI, p21 labeling index; PCNA-LI, proliferating cell nuclear antigen labeling index; PI-DR, proliferation index of ductular reaction.
Corrected for age at surgery, gender, chronic viral hepatitis, chronic alcoholism, total necroinflammation score, fibrosis stage.
PI-DR, but Not Intratumoral HPC/Biliary Marker Expression, Is Associated With DFS
We then asked if these HPC-related features can predict OS or DFS without or with correcting for demographic, clinical, and pathologic parameters in the curative resection group (n = 70) (Table 3). In univariate analysis, the OS was significantly associated with MO, absence of AFP expression by the tumor, PI-DR, K7-DR, and PCNA-LI of preportal hepatocytes. DFS was associated with MO, microvascular invasion (MVI), NLR, necroinflammation score, and PI-DR, but not K7-DR. Thus, specific features of the tumor (MO and MVI) as well as markers of nontumor liver were associated with both OS and DFS on univariate analysis. The multivariate Cox proportional hazards models for OS and DFS are detailed in Supporting Fig. 3. As Model A shown in Table 3, MO, K7-DR, PI-DR, and absence of AFP remained significant predictors for OS. Histological grade of the ICC component showed a trend for association with OS (P = 0.057). By contrast, DFS was only associated with MVI and PI-DR.
Table 3.
Univariate and Multivariate Analyses of Factors Associated With Survival and Recurrence in 70 Patients With Combined Hepatocellular-Cholangiocarcinoma Underwent Curative Surgery
| Factors | Overall Survival (OS)
|
Disease-Free Survival (DFS)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate P | Multivariate
|
Univariate P | Multivariate
|
||||||||
| Model A†
|
Model B†
|
Model A†
|
Model B†
|
||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||||
| Characteristics of demography, tumor & laboratory tests | Age: ≥50 vs. <50 years* | 0.490 | NA | NA | 0.803 | NA | NA | ||||
| Gender: male vs. female | 0.568 | NA | NA | 0.712 | NA | NA | |||||
| HBsAg : P vs. N | 0.544 | NA | NA | 0.147 | NA | NA | |||||
| Tumor size: >5.5 vs. ≤5.5 cm* | 0.262 | NA | NA | 0.545 | NA | NA | |||||
| Tumor number.: multiple vs. single | 0.332 | NA | NA | 0.089 | NS | NS | |||||
| TNM stage: I vs. II vs. III | 0.084 | NS | NS | 0.114 | NA | NA | |||||
| Serum AFP: >100 vs. ≤100 ng/mL | 0.382 | NA | NA | 0.094 | NS | NS | |||||
| Serum CA19-9 >37 vs. ≤37 U/mL | 0.253 | NA | NA | 0.688 | NA | NA | |||||
| AST/ALT ratio <1 vs. 1-2 vs. >2 | 0.415 | NA | NA | 0.276 | NA | NA | |||||
| NLR >2 vs. ≤2* | 0.526 | NA | NA | 0.034 | NS | NS | |||||
| Histology: nontumor | Ishak grade ≥7 vs. <7 | 0.211 | NA | NA | 0.017 | NS | NS | ||||
| Ishak stage ≥5 vs. <5 | 0.474 | NA | NA | 0.323 | NA | NA | |||||
| PI-DR ≥50% vs. <50% | 0.009 | 2.294 (1.203,4.375) | 0.012 | NA | 0.003 | 1.875 (1.050,3.349) | 0.034 | NA | |||
| PCNA-LIperiportal hepatocytes ≥25% vs. <25% | 0.019 | NA/NS‡ | NA/NS‡ | 0.058 | NA/NS‡ | NA/NS‡ | |||||
| PCNA-LIcentrilobular hepatocytes ≥0.7% vs. <0.7%* | 0.368 | NA | NA | 0.816 | NA | NA | |||||
| K7-DR ≥50% vs. <50% | 0.019 | 1.952 (1.088,3.502) | 0.025 | 1.731 (0.969,3.091) | 0.064 | 0.209 | NA | NA | |||
| OV-6Parachymal Expression H vs. L* | 0.004 | NA | 2.017 (1.070,3.801) | 0.030 | 0.008 | NA | 1.709 (1.020,2.862) | 0.042 | |||
| AFPParachymal Expression H vs. L* | 0.002 | NA | 0.558 (0.307,1.015) | 0.056 | 0.053 | NA | NS | ||||
| Histology: tumor§ | Histological grade (HCC) poorly vs. well/moderately | 0.536 | NA | NA | 0.192 | NA | NA | ||||
| Histological grade (ICC) poorly vs. well/moderately | 0.093 | 2.351 (0.974,5.671) | 0.057 | 2.530 (1.045,6.124) | 0.040 | 0.964 | NA | NA | |||
| “Multicentric Occurrence” in HCC P vs. N | 0.003 | 2.199 (1.167,4.145) | 0.015 | 2.138 (1.112,4.112) | 0.023 | 0.030 | NS | NS | |||
| Microvascular Invasion P vs. N | 0.066 | NS | NS | 0.004 | 2.136 (1.077,4.238) | 0.030 | 2.276 (1.156,4.482) | 0.017 | |||
| K7 Expression H vs. L | 0.544 | NA | NA | 0.544 | NA | NA | |||||
| AFP Expression P vs. N | 0.019 | 0.422 (0.204,0.874) | 0.020 | NS | 0.516 | NA | NA | ||||
| K19 Expression H vs. L | 0.133 | NA | NA | 0.805 | NA | NA | |||||
| OV-6 Expression H vs. L | 0.642 | NA | NA | 0.666 | NA | NA | |||||
| c-Kit Expression H vs. L | 0.744 | NA | NA | 0.752 | NA | NA | |||||
| EpCAM Expression H vs. L | 0.603 | NA | NA | 0.253 | NA | NA | |||||
P, positive; N, Negative; H, High; L, Low; NA, not adopted; NS, not significant; AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; DR, ductular reaction; EpCAM, epithelial cell adhesion molecule; HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma; K19, Keratin 19; K7-DR, Keratin 7 immunoreactive ductular reaction; NLR, neutrophil-to-lymphocyte ratio; PCNA-LI, proliferating cell nuclear antigen labeling index; PI-DR, proliferation index of ductular reaction; TNM, tumor-node-metastasis.
Medians were used for cutoff values.
Model A: PI-DR adopted; Model B: OV-6Parachymal Expression and AFPParachymal Expression adopted.
Factors with P < 0.100 were selected for further multivariate analysis. Considering significant correlations were found between PCNA-LIperiportal hepatocytes and PI-DR, PCNA-LIperiportal hepatocytes was not a candidate for Cox proportional hazards model for the first time. After stable models were established, the factor was put into the model.
In terms of HPC/Biliary markers expression in tumor, if it failed to define a correlation with OS or DFS using the strategy presented in the Supporting Methods, HR (95% CI) and P value were calculated by an optimal cutoff value for presentation.
Background “Progenitor Dominant” Regeneration Pattern Is Associated With Worsened DFS
In parallel to OV-6, AFP is an immature hepatocyte marker representing hepatocyte dedifferentiation that was expressed mostly in hepatocytes near parenchymal-stromal boundaries (Fig. 4A). Scattered AFP-positive hepatocytes could also be observed in centrilobular and intermediate zones in some cases. However, double immunostaining showed that coexpression of AFP and OV-6 or EpCAM in hepatocytes was extremely rare (2/80, 2.5%) (Fig. 4A). The observations were supported by the finding that OV-6 and AFP expression (IOD) in hepatocytes were inversely correlated (r = −0.343, P = 0.002, Fig. 4B). Additionally, lower hepatocyte AFP expression is significantly associated with higher hepatocyte P21-LI (r = −0.337, P = 0.002). This finding supports the concept that progenitor activation and hepatocyte dedifferentiation are the two processes contributing to DR origin and related regeneration. 1,25 We therefore classified the predominant liver regeneration pattern in each case based on the expression patterns of AFP and OV-6 in parenchyma as follows (Fig. 4B): (1) OV-6high AFPlow were regarded as “progenitor dominant” regeneration; (2) OV-6lowAFPhigh were regarded as “dedifferentiation dominant” regeneration; (3) OV-6lowAFPlow were regarded as “mild” regeneration; (4) OV-6highAFPhigh were regarded as “progenitor/dedifferentiation synergistic” regeneration. In survival analyses within the curative resection group, the “progenitor dominant” regeneration pattern was associated with the poorest OS (Fig. 4D), highest recurrence rate (Fig. 4E), and was characterized by the greatest level of PI-DR (Fig. 4C). In Model B (Table 3), OV-6 expression in hepatocytes was an independent predictor for both OS and DFS. Thus, an OV-6high AFPlow background “progenitor dominant” regeneration pattern may help define a “field effect” that is strongly associated with DFS.
Fig. 4.
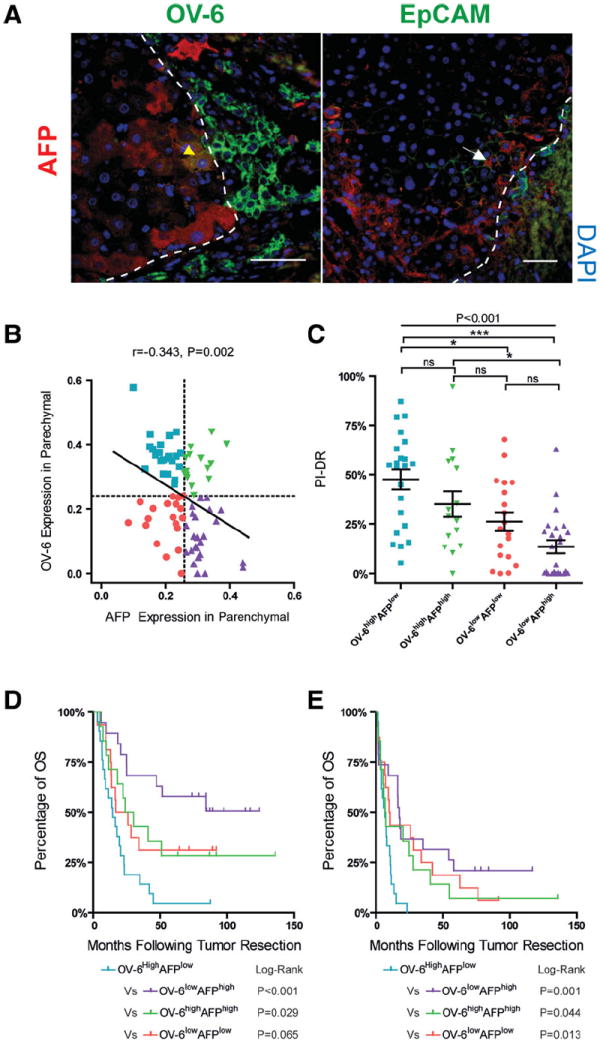
Liver regeneration patterns defined by OV-6 and AFP expression in nontumor parenchyma are associated with both OS and DFS. (A) AFP and OV-6/EpCAM coexpression hepatocyte is rare in nontumor tissue. Double-immunofluorescence staining for OV-6/AFP or EpCAM/AFP is shown. Because AFP shows cytoplasmic staining and OV-6 shows both cytoplasmic and membranous staining, the colocalization appears to be yellow (yellow arrow). Colocalization is not ascertained (white arrow) for hepatocytic AFP and EpCAM because it is impossible to exclude the possibility that membranous staining of EpCAM is from surrounding hepatocytes. (B) Scatterplots with fitting line show negative correlation between OV-6 and AFP expression (IOD) in nontumor tissue and how the liver regeneration pattern is defined. Pearson correlation provides correlation coefficient (r) and Pvalue. (C) Scatterplots with mean ± SEM bars of PCNA indices for DR at each regeneration pattern are shown; significance (*, ***) provided by Bonferroni’s multiple comparison test (ns, not significant). (D,E) Kaplan-Meier curves of each regeneration pattern represents a subpopulation of patients with different OS (D) and DFS (E). P-values were obtained by log-rank multiple comparison tests.
Tumoral HPCs Features Are Associated With Higher PI-DR: Proliferative DRs as a Field Effect
Intratumoral cells with an HPC/DR-like phenotype, 26 coexpressing HPC/biliary markers (e.g., K7, K19, OV-6, or EpCAM), and immature hepatocyte markers (e.g., AFP) were common by double immunostaining (53/80, 66.3%) (Fig. 5A). As shown in Fig. 5B, we then found an interesting field effect that PI-DR in nontumor liver was associated with expression of several intratumoral HPC/biliary markers including K7 (P = 0.010), K19 (P = 0.037), OV-6 (P = 0.001), and c-Kit (P = 0.005), and also the number of K7+ HPC-like tumor cells (r = 0.232, P = 0.039) (Fig. 5C). Increased PI-DR was also associated with a greater intratumoral proliferation index (PI-T) (P = 0.003).
Fig. 5.
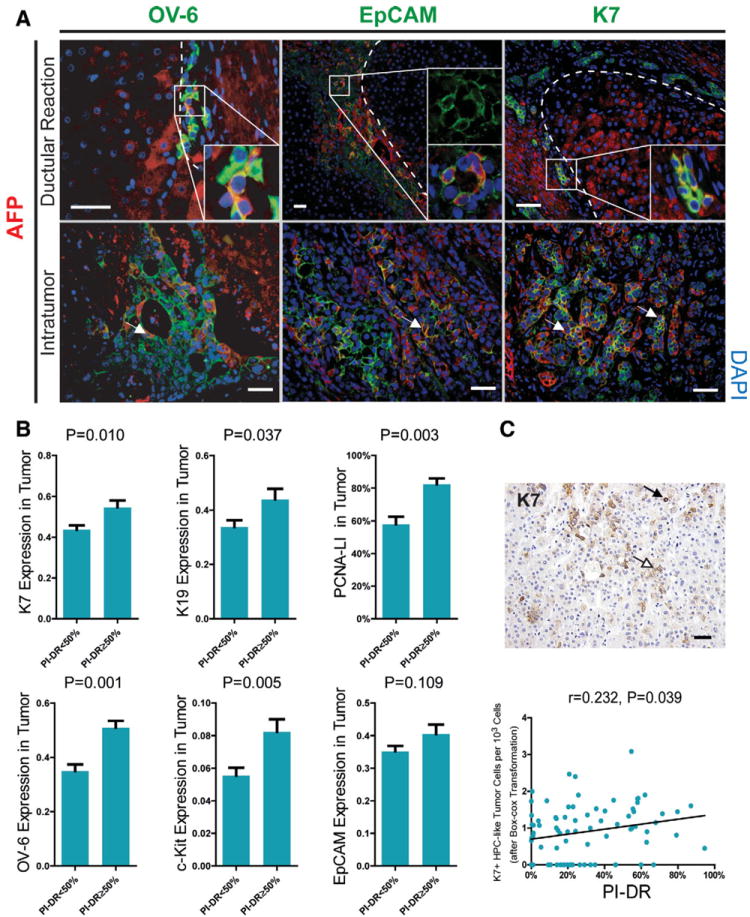
The field effect of nontumor proliferative DR on intratumoral HPCs/biliary markers expression. (A) Tumor cells mimic phenotype of cells within DR, defined by coexpression of HPC/biliary markers (K7, OV-6, or EpCAM) and immature hepatocyte marker (AFP). Double-immunofluorescence staining for OV-6/AFP, EpCAM/AFP, or K7/AFP ductular reactions and tumors are shown. With cytoplasmic staining, colocalization of OV-6/K7 and AFP appears to be yellow/orange. Cells showing weak cytoplasmic EpCAM staining colocalizing with cytoplasmic AFP appears to be orange in cytoplasm and green in membrane (scale bar = 50 μm). (B) Intratumoral K7, K19, OV-6, c-Kit, EpCAM (measured by IOD) at different levels of proliferative DR are shown using mean with SEM error bar. PCNA indices in tumor are also presented by mean percentage with SEM error bar. (C) A field of K7 staining tumor section showing hepatic progenitor-like tumor cell (K7-positive HPC-like tumor cell, black arrow) is presented. Hepatocyte-like tumor cell (white arrow) can also be observed in the same field (upper, scale bar = 25 μm). Scatterplot with fitting line shows PI-DR correlates with K7+ HPC-like cells counting in tumor. The number of K7+ HPC-like tumor cells is presented after box-cox transformation with λ = 0.3. Spearman correlation analysis provides correlation coefficient (r) and P-value (lower).
MO of CHC Is Associated With Proliferative Activity in DR
The MO feature was observed in 32 patients, including six patients showing a “nodule in nodule” appearance. Univariate analysis (Supporting Fig. 6) showed that the presence of MO was associated with both higher PI-DR and OV-6 expression in nontumor sections. Patients with MO had smaller tumor size, consistent with MO described originally in early HCC.10 By contrast, we found no association between MO and MVI. Multivariate analysis with the presence of MO as a dependent variable showed independent associations with PI-DR, tumor size, stage of fibrosis, and confluent multinodularity (Supporting Table 3).
Discussion
CHC is a rare malignant tumor accounting for less than 3% of all primary carcinomas in our department. Few data exist regarding predictors of recurrence risk after resection with curative intent in CHC. In our large cohort with long-term clinical follow-up, we identified a strongly significant relationship between nontumor periportal DR and overall survival in CHC after curative treatment. Furthermore, the risk of intrahepatic tumor recurrence after resection strongly correlated with the PI-DR. PI-DR appears to coincide with a field effect based on a noncancerous cellular responsiveness that predisposes to metachronous tumor recurrence in CHC.
In the course of various chronic liver diseases, HPCs residing within CoH can be activated for parenchymal repair.20 DR is thought to consist of transit-amplifying progeny of hepatic progenitors.27,28 The PI-DR, which measures the proliferative capacity of a transit-amplifying compartment, likely represents a surrogate marker of HPC activation because high levels of proliferation rate within DRs has been demonstrated in both oval cell-mediated liver regeneration in an animal model29 and human liver disease.30 This hypothesis is supported by the strong correlation of PI-DR with HPC quantity (via enhanced HPC expansion) as well as with the number of K7+ IHBCs, a population thought to be derived from HPCs undergoing hepatocellular differentiation. That is also evidenced by PI-DR strongly correlating with quantification of OV-6-positive hepatocytes. Additionally, an association found between PI-DR and DR expressing Bmi-1, an important regulator of adult stem cell self-renewal and tumorigenesis,31 potentially supports the progenitor function of DR.
HPC activation may be triggered by an increasing inability of hepatocytes to replicate due to aging32 or due to replicative senescence as a results of mito-inhibition. 24,33 In our study, both older age at the time of surgery and impaired hepatocyte replication were associated with PI-DR, further supporting our hypothesis. Because PI-DR was independently associated with recurrence risk in a cohort with curative intent, activation of HPCs may be one of the critical drivers of CHC recurrence.
Alternatively, not all DRs may be of HPC origin.34 Dedifferentiation of mature hepatocytes into biliary epithelial cells, also described as “ductular metaplasia of hepatocytes,” has been found to occur in some animal models.35 It is difficult to rule out the possibility that ductular metaplasia of hepatocytes contributes to DRs in our observational study. We observed that intensive DRs in low proliferative activity were not usually in continuity with IHBCs that could potentially indicate the reverse process.36 This hypothesis is supported by the observation that the presence of AFPpositive hepatocytes is associated with diminished replication arrest of hepatocytes and DR proliferation. Being separated from various HPC/biliary markers, AFP expression in hepatocytes might be an additional marker of hepatocyte dedifferentiation predisposing to a ductular metaplasia. By categorizing the relative contribution of progenitor activation and hepatocellular dedifferentiation to regeneration patterns and evaluating the impact of the regeneration pattern on outcome, we identified that a “progenitor dominant” regeneration pattern, which is closely related with intensive transit-amplifying DR, is associated with the poorest OS and DFS, helping define an HPC associated field effect that strongly correlates DFS.
Necroinflammation, one possible driving source of HPC activation,37,38 was also significantly associated with risk of intrahepatic recurrence in our study. However, PI-DR was associated with early recurrence, whereas hepatic inflammation was mainly predictive of late recurrence (Supporting Fig. 5), suggesting some degree of independence of these two mechanisms. The cross-sectional nature of our study precludes establishment of a causal association between HPC activation and CHC recurrence after resection with curative intent, but our data suggest that quantification of HPC activation may assist risk stratification for recurrence.
Recurrence may result from either intrahepatic metastasis from the main tumor by way of the portal system or multifocal tumorigenesis.39 MVI, a risk factor for intrahepatic metastasis, was confirmed to be a strong predictor of recurrence. Independently from MVI, MO features frequently identified in HCC components may suggest a dominant feature of CHC. Increased recurrent risk of cases with MO features may be attributed to a high predisposition to metachronous multicentric recurrence. Interestingly, MO positively correlated with PI-DR, suggesting a potential relationship between transit-amplifying compartments and multicentric tumorigenesis. That was supported by a correlation between PI-DR and coexistence of small cell change, which is thought to be a precancerous lesion of HCC. Although the evidence from humans is still fragmentary, HPC/DR is an attractive candidate as a tumorigenic target or stimulus. A “maturation arrest” mechanism suggesting participation of transit-amplifying HPC in carcinogenesis through malignant transformation has been well documented. Libbrecht et al.40 described a close association between HPC marker expression and preneoplastic lesion of HCC. Recently, an increased risk of hepatocarcinogenesis in HCV-infected patients whose biopsies showed foci of IHBCs may have common mechanisms with our proposed progenitor dominant regeneration with highest recurrence risk.36 Alternatively, Lennerz et al.41 found a vanishing of DR with stepwise hepatocarcinogenesis in cirrhotic liver that may suggest the involvement of DR in HCC development, a novel hypothetical mechanism that DR may contribute to the generation of cancer-associated fibroblasts cross-talking with the microenvironment. These studies and our findings cumulatively suggest HPC activation may contribute to liver cancer recurrence by driving “field cancerization.” 42 Thus, hepatic progenitor cells may be a doubleedged sword promoting both liver regeneration and carcinogenesis in humans.
Mechanistic interpretation of our recurrence data is limited because few resections of recurrent tumor were performed. Of the seven patients who received repeat hepatectomy for recurrence more than 12 months after their initial surgery, four were found to have heterologous tumor phenotypes (two ICC, one HCC, one HCC with biliary differentiation). These possible metachronous primary tumors that arose most likely as a consequence of the hepatic inflammatory microenvironment may also have been of HPC origin. An indepth study of a large clinical series with repeat hepatectomy after K19+ HCC recurrence has been initiated to explore the associations among progenitor cells and synchronous and metachronous occurrence.
It has long been controversial that CHCs are derived from HPCs. To date, direct evidence that tumors with mixed features are derived from HPCs/oval cells came only from rodent studies.31,43 Prior evidence from human studies was limited and/or speculative.5,8 The greatest evidence in humans that liver malignancy are of HPC origin has been the demonstration of a high frequency of expression of HPC/stem cell markers and an associated poorer prognosis.2,4,44 In this study, the notion “field effect” we have extended, which is a morphological and statistical correlation between nontumor HPC activation and intratumoral heterogeneity, has strengthened this association. Although this remains to be further identified, the extended “field effect” notion may be equivalent or attributed to the “model of reciprocal heterotypic signaling” reviewed recently.45 The lack of association of prognosis and intratumoral HPC/biliary markers expression suggests that the poor prognosis of tumors with HPC features is not only due to aggressive tumor biology, but also due to the transitamplifying HPCs related field effect in nontumor liver associated with subsequent tumor development.
In conclusion, various factors contribute to OS and DFS of patients with CHC after resection with curative intent. Background transit-amplifying components may act as an internal mechanism contributing at least in part to CHC recurrence by promoting MO. Our findings provide a potential interpretation of how DRs/HPCs activation in nontumor tissue contribute to the risk of recurrence of CHCs, further indicating HPCs as a possible target of carcinogenesis from a clinical aspect, and also suggesting that HPCs may act as potential therapeutic target to prevent recurrence of CHC, for instance, with interferon-based treatment.46 However, further study is warranted.
Supplementary Material
Acknowledgments
We thank Kyong-Mi Chang for careful reading of the article, and Hiroyoshi Doi and James Keith for numerous helpful contributions.
Supported by the Grant 2008ZX10002-025 from the State Key Project on Infectious Diseases of China. Additional support was provided by National Natural Science Foundation of China (No. 81030041), Key Basic Research Project of China (No. 2010CB945600, 2011CB966200), and China Scholarship Council (No. 2010658011).
Abbreviations
- AFP
α-fetoprotein
- CHC
combined hepatocellular-cholangiocarcinoma
- CLC
cholangiolocarcinoma
- DFS
disease-free survival
- DR
ductular reaction
- HCC
hepatocellular carcinoma
- HPC
hepatic progenitor cell
- ICC
intrahepatic cholangiocarcinoma
- IHBC
intermediate hepatobiliary cell
- IOD
integrated optical density
- K7
keratin 7
- K19
keratin 19
- MO
multicentric occurrence
- MVI
microvascular invasion
- OS
overall survival
- PCNA
proliferating cell nuclear antigen
- RDC
reactive ductular cell
Footnotes
Additional Supporting Information may be found in the online version of this article.
Potential conflict of interest: Nothing to report.
References
- 1.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 2.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 3.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, et al. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–2046. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 7.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, et al. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takenaka K, Adachi E, Nishizaki T, Hiroshige K, Ikeda T, Tsuneyoshi M, et al. Possible multicentric occurrence of hepatocellular carcinoma: a clinicopathological study. Hepatology. 1994;19:889–894. [PubMed] [Google Scholar]
- 11.Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–1762. doi: 10.1007/s00268-008-9552-6. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7. Chichester, UK, Hoboken, NJ: Wiley-Blackwell; 2010. International Union against Cancer; pp. 110–114. [Google Scholar]
- 13.Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):VI1–9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki K, Takayasu K, Kawano T, Muramatsu Y, Moriyama N, Wakao F, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology. 1993;18:1090–1095. [PubMed] [Google Scholar]
- 16.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FT. WHO classification of tumours of the digestive system. 4. Lyon, France: IARC; 2010. World Health Organization, International Agency for Research on Cancer. [Google Scholar]
- 18.Kojiro M. Pathology of hepatocellular carcinoma. Malden, MA: Blackwell; 2006. pp. 97–104. [Google Scholar]
- 19.International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 20.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 21.Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, et al. Regeneration of hepatocyte ‘buds’ in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 22.Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159–1170. doi: 10.1016/j.jhep.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viegas MS, Martins TC, Seco F, do Carmo A. An improved and costeffective methodology for the reduction of autofluorescence in direct immunofluorescence studies on formalin-fixed paraffin-embedded tissues. Eur J Histochem. 2007;51:59–66. [PubMed] [Google Scholar]
- 24.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 25.Eleazar JA, Memeo L, Jhang JS, Mansukhani MM, Chin S, Park SM, et al. Progenitor cell expansion: an important source of hepatocyte regeneration in chronic hepatitis. J Hepatol. 2004;41:983–991. doi: 10.1016/j.jhep.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Theise N, Chua M, Reid LM. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008;48:1598–1607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- 27.Roskams T. Progenitor cell involvement in cirrhotic human liver diseases: from controversy to consensus. J Hepatol. 2003;39:431–434. doi: 10.1016/s0168-8278(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 28.Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035–1045. doi: 10.1002/hep.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen CH, Jauho EI, Santoni-Rugiu E, Holmskov U, Teisner B, Tygstrup N, et al. Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am J Pathol. 2004;164:1347–1359. doi: 10.1016/S0002-9440(10)63221-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon SM, Gerasimidou D, Kuwahara R, Hytiroglou P, Yoo JE, Park YN, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;53:964–973. doi: 10.1002/hep.24122. [DOI] [PubMed] [Google Scholar]
- 31.Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007;133:937–950. doi: 10.1053/j.gastro.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Delladetsima J, Alexandrou P, Giaslakiotis K, Psichogiou M, Hatzis G, Sypsa V, et al. Hepatic progenitor cells in chronic hepatitis C: a phenomenon of older age and advanced liver disease. Virchows Arch. 2010;457:457–466. doi: 10.1007/s00428-010-0957-x. [DOI] [PubMed] [Google Scholar]
- 33.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 35.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziol M, Nault JC, Aout M, Barget N, Tepper M, Martin A, et al. Intermediate hepatobiliary cells predict an increased risk of hepatocarcinogenesis in patients with hepatitis C virus-related cirrhosis. Gastroenterology. 2010;139:335–343 e332. doi: 10.1053/j.gastro.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 38.Svegliati-Baroni G, Faraci G, Fabris L, Saccomanno S, Cadamuro M, Pierantonelli I, et al. Insulin resistance and necroinflammation drives ductular reaction and epithelial-mesenchymal transition in chronic hepatitis C. Gut. 2011;60:108–115. doi: 10.1136/gut.2010.219741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1053/jhep.1997.v25.pm0008985270. [DOI] [PubMed] [Google Scholar]
- 40.Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76–84. doi: 10.1016/s0168-8278(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 41.Lennerz JK, Chapman WC, Brunt EM. Keratin 19 epithelial patterns in cirrhotic stroma parallel hepatocarcinogenesis. Am J Pathol. 2011;179:1015–1029. doi: 10.1016/j.ajpath.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, et al. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2010;51:1401–1409. doi: 10.1002/hep.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Lim R, Knight B, Patel K, McHutchison JG, Yeoh GC, Olynyk JK. Antiproliferative effects of interferon alpha on hepatic progenitor cells in vitro and in vivo. Hepatology. 2006;43:1074–1083. doi: 10.1002/hep.21170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


