Abstract
Loa loa infections have emerged as a serious public health problem in patients co-infected with Onchocerca volvulus or Wuchereria bancrofti because of severe adverse neurological reactions after treatment with ivermectin. Accurate diagnostic tests are needed for careful mapping in regions where mass drug administration is underway. Loop-mediated isothermal amplification (LAMP) has become a widely adopted screening method because of its operational simplicity, rapidity and versatility of visual detection readout options. Here, we present a multi-step bioinformatic pipeline to generate diagnostic candidates suitable for LAMP and experimentally validate this approach using one of the identified candidates to develop a species-specific LAMP assay for L. loa. The pipeline identified ~140 new L. loa specific DNA repeat families as putative biomarkers of infection. The consensus sequence of one family, repeat family 4 (RF4), was compiled from ~ 350 sequences dispersed throughout the L. loa genome and maps to a L. loa-specific region of the long terminal repeats found at the boundaries of Bel/Pao retrotransposons. PCR and LAMP primer sets targeting RF4 specifically amplified L. loa but not W. bancrofti, O. volvulus, Brugia malayi, human or mosquito DNA. RF4 LAMP detects the DNA equivalent of one microfilaria (100 pg) in 25–30 minutes and as little as 0.060 pg of L. loa DNA (~1/1600th of a microfilaria) purified from spiked blood samples in approximately 50 minutes. In summary, we have successfully employed a bioinformatic approach to mine the L. loa genome for species-specific repeat families that can serve as new DNA biomarkers for LAMP. The RF4 LAMP assay shows promise as a field tool for the implementation and management of mass drug administration programs and warrants further testing on clinical samples as the next stage in development towards this goal.
Introduction
Loa loa, the African eye worm, is a filarial nematode parasite responsible for the neglected tropical disease loiasis. The parasite is transmitted to humans by tabanid Chrysops flies (primarily C. silacea and C. dimidiate) [1]. Between three and 13 million people are infected [2] in forested and some savannah regions of Western and Central Africa [3] with approximately 30 million at risk [4, 5]. Historically, loiasis has received less attention than other filarial diseases since it is generally asymptomatic. In some individuals, the migration of adult worms (30–70 mm in length) through the subconjunctiva of the eye; transient, localized angioedema (Calabar swelling); and pruritus are observed, with more serious clinical manifestations occasionally evident with chronic infection [1]. Sheathed microfilariae (mf) predominantly migrate between the lungs and peripheral blood but have also been recovered from spinal fluid, urine and sputum [1]. While approximately 40% of the infections are amicrofilaraemic [6, 7], certain infected individuals harbor more than 30,000 mf per ml of blood [8–12]. The disease has emerged as a serious public health problem because of severe adverse effects (SAEs) when mf titers are as low as 8000/ml [9], and even death in individuals with high mf levels (> 50,000 mf/ml) after treatment with ivermectin for onchocericasis [1, 12] or lymphatic filariasis [3, 13–15]. The risk of co-infection necessitates careful mapping of L. loa, O. volvulus and W. bancrofti infections [3, 13–16]. L. loa infected individuals are treated with diethylcarbamazine, which is active against adults and mf [17] followed by albendazole to eliminate residual mf [18].
A history of eye worm and/or the occurrence of Calabar swelling are used as an indication of infection, but detection of mf is required for definitive diagnosis [1]. Parasitological diagnosis based on microscopy [19] is challenging as mf can only be detected in the peripheral blood between the daytime hours of 10:00–16:00 and many infections are occult [6, 7]. Differentiation from other species of mf is based on the presence of a sheath, and the staining pattern of nuclei in the tail. While microscopy is a valuable technique, morphological interpretation can be subjective and requires substantial expertise. More recently, hand-held mf detection devices, which score motility, have been described [11, 20]. However, these methods are not useful for occult infections, nor will they distinguish L. loa mf from other blood-borne mf.
Serological assays measuring L. loa specific antibody responses to the recombinant antigens Ll-SXP-1 [21] and repeat 3 of the L. loa nematode polyprotein antigen [22] have been proposed for the detection of occult infections [22, 23].
Several DNA targets have been described for use in PCR, including repeat 3 of the nematode polyprotein antigen [24–26] LLMF72 [27]; the LL3M9 repeat family [27, 28]; and the internal transcribed spacer 1 region of rDNA [29]. These targets are either present in low copy number, which can impact sensitivity, or are not species-specific.
Several isothermal amplification methods targeting DNA have been developed which offer significant advantages over PCR. Of these methods, loop-mediated isothermal amplification (LAMP) has become the most widely adopted. Its simplicity and visual detection format without the need for expensive equipment offers considerable advantages over PCR [30–32]. Two recent publications have described LAMP assays for L. loa using the PCR targets, LL3M9 and LLMF72 [33, 34]. However, these are not necessarily the ideal targets for this platform. LL3M9 contains multiple copies of a simple 6 bp repeat which is conserved in nematodes, and LLMF72 is a single copy gene. These attributes may affect specificity and sensitivity respectively.
The genomic era has brought with it the ability to devise comparative and subtractive strategies to identify, in silico, new diagnostic candidates from an organism’s complete genome. In the present study, we devise a multi-step bioinformatic pipeline to identify species-specific DNA repeat sequences that are particularly suited for LAMP and experimentally validate the approach using one of the biomarkers to develop a new diagnostic test for L. loa.
Materials and Methods
Materials
Genomic DNA samples were generously donated by the following: L. Loa, B.L. Makepeace and C. Hartley (University of Liverpool); B. malayi, L.A. McReynolds (New England Biolabs); O. volvulus and Homo sapiens, F. Perler (New England Biolabs); Aedes albopictus, Z. Li (New England Biolabs). Whole genome amplified (WGA) W. bancrofti DNA was obtained from the NIH/NIAID Filariasis Research Reagent Resource Center (http://www.filariasiscenter.org). Human whole blood was obtained from Innovative Research (Novi, MI). DNA quantity was determined using a Qubit dsDNA HS Assay kit in conjunction with a Qubit 2.0 Fluorometer as directed by the manufacturer (Life Technologies).
Due to limited quantities of genomic L. loa DNA, WGA L. loa DNA was generated using the PicoPLEX WGA Kit as instructed by the manufacturer (Rubicon Genomics) and used as the primary DNA source for L. loa LAMP assay development.
Bioinformatic Analysis
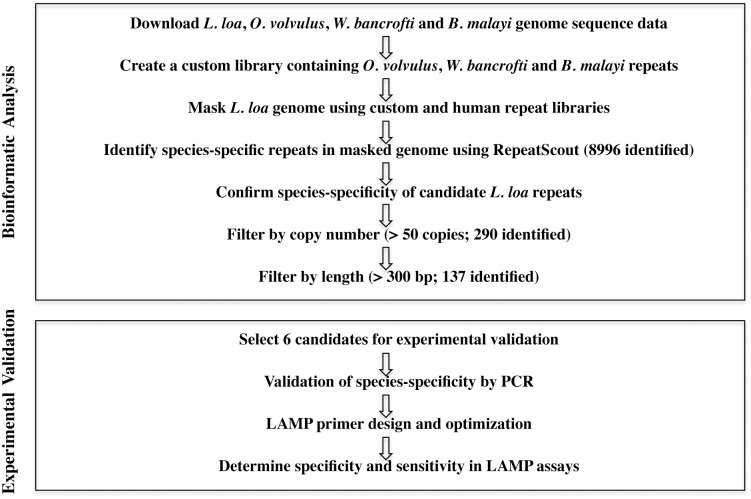
Genome sequence data for L. loa (V3), B. malayi, W. bancrofti and O. volvulus were downloaded from the Filarial worms Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/). For each genome, contig and supercontigs were assembled into a single file. RepeatScout (version 1.0.5) [35] was run on the O. volvulus, B. malayi and W. bancrofti genomes to identify repeat sequences using the following parameters: build _lmer_table-l 15 followed by RepeatScout using the default parameters, and the 15 mer table obtained using the build_lmer_table algorithm. Next, the repeats identified in B. malayi, W. bancrofti and O. volvulus using RepeatScout were combined into a single custom library. Then, RepeatMasker was run on the L. loa genome using the custom-repeat library followed by a human repeat library (http://repeatmasker.org) [36, 37]. RepeatScout was then run on the masked L. loa genome described above generating a library containing putatively specific L. loa consensus repeat sequences. To confirm their specificity, candidates were screened against the filarial genomes using the following blastn parameters: outfmt = 5, num_ alignments = ´10000000´, word_size = 15, e-values less than 10e-7. Only sequences absent from the O. volvulus, B. malayi and W. bancrofti genomes but represented by at least 51 copies in the L. loa genome were further processed. To facilitate LAMP primer design, only consensus sequences longer than 300 bp were retained and sorted by percent GC content (Fig 1).
Fig 1. Bioinformatic filtering pipeline to identify L. loa-specific repeat families.
Genome sequences were downloaded from the Filarial Worms sequencing project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/). The L. loa genome was masked using RepeatMasker then mined for L. loa-specific repeats using RepeatScout. The resulting consensus repeat sequences that consist of 51 or more members and that are 300 bp or more in length were selected for further evaluation.
Contigs (GenBank accession numbers: JPEI01001237.1, JPEI01001554.1, JPEI01001218.1, JPEI01001588.1, JPEI01001706.1) containing multiple repeat family 4 (RF4) members were aligned with themselves using Blastn (http://blast.ncbi.nlm.nih.gov/) or with other RF4 containing contigs (if only one family member was present) to confirm repeat length. Blastx analysis (http://blast.ncbi.nlm.nih.gov/) as well as the Gypsy Database 2.0 (www.gydb.org) and Censor at Repbase (www.girinst.org/censor/index.php, [38]) were used to characterize and map the conserved proteins identified adjacent to RF4 members in the L. loa genome.
Primer Design
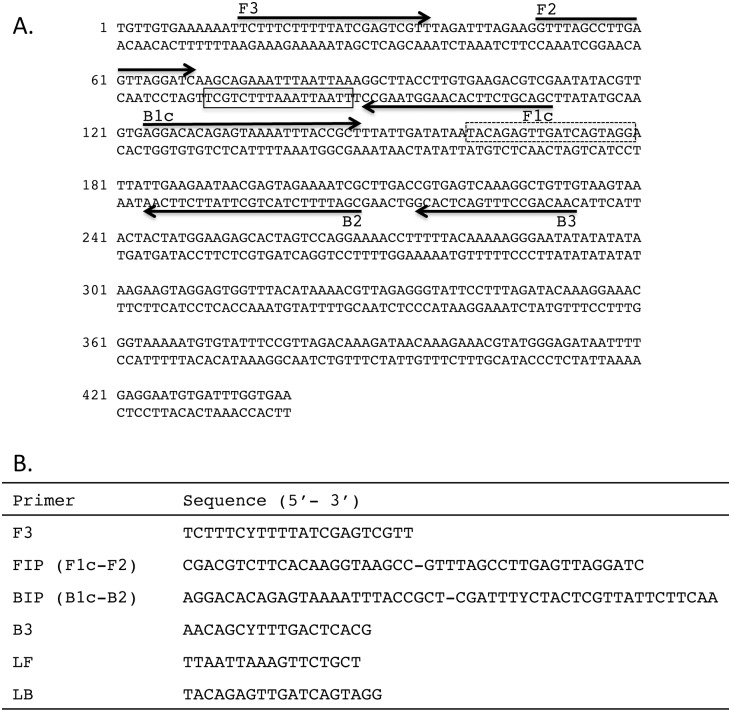
PCR primers were designed to amplify repeat families using the consensus sequences generated by RepeatScout. The forward and reverse degenerate primer sequences for L. loa RF4 are (5’ TCTTTCYTTTTATCGAGTCGTT 3’) and (5’ TCYTYAAAATTATCTCCCATACG 3’) respectively, where Y = C or T. For LAMP primer design, the L. loa RF4 consensus sequence was submitted to PrimerExplorer V4 (http://primerexplorer.jp/e/) generating primer sequences for F3, FIP, BIP and B3 (Fig 2). Loop primers (Fig 2) were designed manually using ‘‘A guide to LAMP primer design” available from the Eiken Chemical Co. (http://primerexplorer.jp/e/). Primers were synthesized (PCR and LAMP) and HPLC purified (LAMP) by Integrated DNA Technologies (Coralville, IA, USA).
Fig 2. L. loa LAMP primer set targeting RF4.
Location of the six LAMP primers within the 440 bp consensus sequence of RF4 is shown (A). Arrows indicate the direction of extension. Solid and dash line boxes represent the binding regions of the loop forward (LF) and loop back (LB) primers respectively. (B) Sequence of the RF4 LAMP primers. F3, BIP and B3 are degenerate oligonucleotides where Y = C or T.
PCR Assays
DNA (20 ng) was mixed with 12.5 μl of One Taq Hot Start 2X Master Mix with standard buffer (New England Biolabs) containing 0.2 mM each of the forward and reverse L. loa RF4 primers in a final volume of 25 μl. Reactions were denatured once at 94°C for 30 sec then cycled 30 times at 94°C for 30 sec, 51°C for 30 sec and 68°C for 30 sec followed by a final 5 min extension at 68°C.
As a positive control for the presence of intact DNA, a 244 bp actin fragment was PCR amplified from 1 ng of various DNAs using One Taq Hot Start 2X Master Mix with standard buffer (New England Biolabs) in 25 μl reactions containing 3.6 mM MgCl2 and 0.2 μM each of the forward and reverse actin primers [39]. When mosquito or human DNA was used as template, reactions contained 9 ng of DNA and 4.4 mM MgCl2. All reactions were denatured once at 95°C for 30 sec then cycled 35 times at 95°C for 30 sec, 50°C for 30 sec and 68°C for 30 sec followed by a final 5 min extension at 68°C.
Reaction products were analyzed by electrophoresis on 1.5% agarose gels equilibrated with 1X Tris-borate/EDTA buffer [40].
LAMP Assays
LAMP reactions contained 1.6 μM each of primers FIP and BIP, 0.2 μM each of F3 and B3, 0.4 μM each of LF and FB, 1.4 mM of each dNTP, 20 mM Tris-HCl (pH 8.8), 50 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.1% Tween-20 and 8 U of Bst 2.0 DNA Polymerase (New England Biolabs) mixed with one of several template DNAs, or 10 mM Tris-HCl (pH 8), 0.1 mM EDTA for non-template controls (NTC), in a total volume of 25 μl. One μl of a 25X Valeramide (V) N,N-Diethylformamide (DEF) solution (final concentration of 34mM V/128 mM DEF) was added to some reactions to reduce non-specific amplification [41, 42]. Reactions were incubated at 61°C for 60–90 minutes in a Loopamp Realtime Turbidimeter (LA-320c, Eiken Chemical Co.). The instrument measures the change in turbidity at 650 nm caused by the precipitation of magnesium pyrophosphate produced by polymerase activity. Turbidity data were analyzed using the LA-320c software package that reports when the change in turbidity over time (dT/dt) reaches a value of 0.1, which we then assigned to be the threshold time (Tt).
To mimic a clinical situation, a series of blood samples spiked with L. loa DNA were prepared for analysis with LAMP. Four ng of genomic L. loa DNA were diluted to 20 μl with uninfected human whole blood then a set of two-fold serial dilutions was prepared from this spiked sample using additional blood. For the non-template control, a sample containing only uninfected human whole blood was prepared. DNA was extracted from each 10 μl dilution using the ChargeSwitch gDNA Blood Kit (Invitrogen) as directed by the manufacturer and the purified DNA was eluted in 25 μl of 10 mM Tris-HCl, pH 8.5. Two μl of each dilution was used in LAMP reactions containing the V/DEF chemical additive. The quantity of L. loa DNA amplified from spiked blood samples was determined from a standard curve generated by amplifying known amounts of genomic L. loa DNA using the same LAMP conditions.
Results
Identification and Evaluation of Diagnostic Candidates
The assembled L. loa genome [43] enabled an in silico search for new DNA biomarkers. A bioinformatic pipeline was constructed to identify species-specific L. loa repeats (Fig 1). The first step towards this objective was to mask the L. loa genome for repeats common to filarial parasites. Approximately 12% (11.92%) of the L. loa genome was masked using the custom filarial repeat library created from B. malayi, W. bancrofti and O. volvulus sequence data with RepeatScout. An additional 4% (3.74%) of the L. loa genome was masked using the human repeat library as well as with simple repeats identified by RepeatMasker. Following masking of the L. loa genome, a total of 8996 putatively specific L. loa consensus repeat sequences were identified using RepeatScout. To confirm species-specificity, diagnostic candidates were screened against the filarial genomes using Blastn. As copy number may influence assay sensitivity [44, 45], the data was filtered for those families consisting of 51 or more copies. The 290 families identified were then filtered by size (>300 bps) to ease LAMP primer design. A total of 137 L. loa repeat families were identified that fulfilled our criteria, representing potential new DNA biomarkers suitable for a LAMP based assay (S1 Text).
Six L. loa repeat families (RF0, RF4, RF39, RF683, RF972 and RF1628) were chosen for further evaluation based on copy number (368–774) to maximize sensitivity, as well as % GC content (33–35) to facilitate primer design (S1 Text). Multiple primer sets were designed to the RepeatScout consensus sequence generated for each repeat family and tested empirically in both PCR and LAMP amplification assays. All except RF4 were eventually eliminated from consideration (data not shown) due to either poor amplification (RF1628), complex banding patterns in PCR (RF0, RF39), or poor sensitivity in LAMP reactions (RF683, RF972).
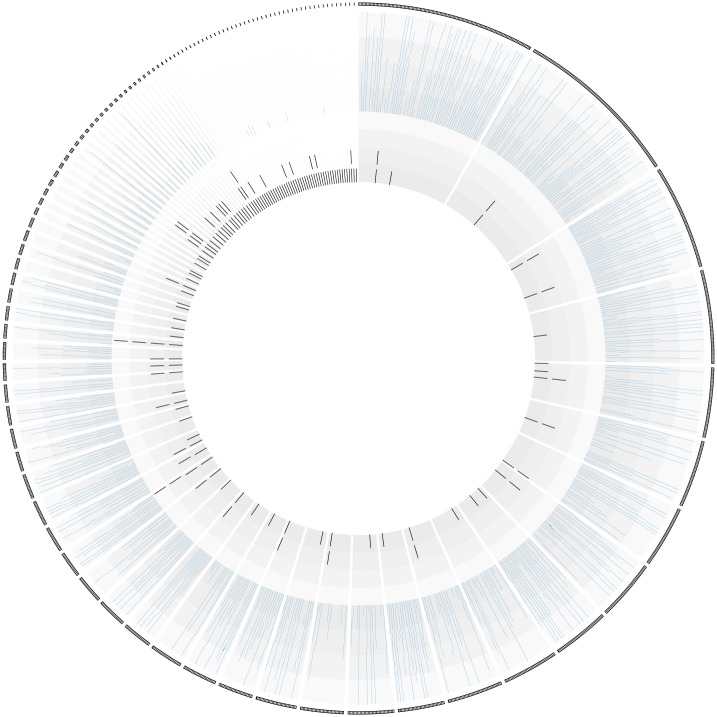
RepeatScout identified 368 members of RF4 in the L. loa genome [43] from which it compiled a 440 bp long, 33% GC rich consensus sequence (S1 Fig). Blastn analysis of a more recently released version of the L. loa genome generated using Single Molecule, Real-Time DNA sequencing [46] with the RF4 consensus sequence identified 350 members of this repeat. Spatial distribution of RF4 members in each of the contigs from the L. loa genome revealed that most occur as singletons. The remainder is found adjacent to each other, though not arranged in tandem arrays (Fig 3). Many of the larger contigs contain multiple copies of two (34 occurrences), three (five occurrences) or a maximum of four repeats (two occurrences).
Fig 3. Spatial distribution of RF4 members in the L. loa genome.
Circos representation of all the contigs from the L. loa genome (sorted by size around the outer edge) where copies of RF4 are located (black spokes around inner circle) and their position within each contig.
RF4 comprises part of the Long Terminal Repeats (LTRs) in Bel/Pao retrotransposons [47]. Examination of the genomic sequence surrounding RF4 members demonstrated that they span part of a larger (~ 600 bp) repetitive sequence within the L. loa genome (S2 Fig). Blastx analysis of the sequence in the vicinity of these 600 bp repetitive regions revealed conserved proteins exhibiting high identity with the Mabel family of Bel/Pao retrotransposons in B. malayi (Gypsy Database 2.0: www.gydb.org; Censor at Repbase: (www.girinst.org/censor/index.php, [38]) suggesting that RF4 comprises part of the LTR at the ends of Bel/Pao retrotransposons in L. loa. A detailed examination of one L. loa scaffold, (GenBank acc. # JPEI01001237.1) revealed two 600 bp LTRs bordering the sequence coding for the putative proteins of this 7.9 kb retrotransposon (S2 Fig). Alignment of the LTR and RF4 consensus sequences (data not shown) demonstrated that two non-contiguous regions of the LTR (nts 75–150 and 230–600) are species-specific and appear to have been fused by RepeatScout to generate the RF4 consensus sequence (S2 Fig). Blastn analysis of the L. loa LTR consensus sequence demonstrated that the regions of the LTR extending from nts 1 to ~70 and from ~170–290 exhibit homology with the B. malayi, O. volvulus and W. bancrofti genomes and thus were excluded from the RF4 consensus. The LAMP primers map between nts 360–600, well within the species-specific region of the L. loa LTR.
Specificity of RF4 in DNA Amplification Assays
The species-specificity of RF4 was confirmed by PCR. A single band of the expected size (~480 bp) was amplified from L. loa but not from W. bancrofti, O. volvulus, B. malayi, H. sapiens or A. albopictus DNA (Fig 4A). The single band amplified from L. loa DNA indicates that RF4 is dispersed throughout the genome rather than organized in tandem arrays. The integrity of these DNA samples was confirmed in PCR experiments using primers designed to amplify a conserved actin gene. A single amplification product of 244 bp, the expected fragment size was obtained (Fig 4B).
Fig 4. Species-specificity of RF4 as determined by PCR.
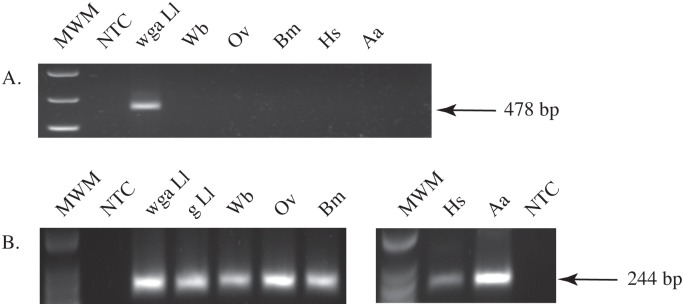
Agarose gels showing specific amplification of L. loa RF4 (A) or a conserved 244 bp actin gene fragment (B) from WGA L. loa (WGA Ll), genomic L. loa (g Ll), W. bancrofti (Wb), O. volvulus (Ov), B. malayi (Bm), A. albopictus (Aa), and H. sapiens (Hs) DNA. PCR (A) and low molecular weight (B) markers (MWM) were used (New England Biolabs).
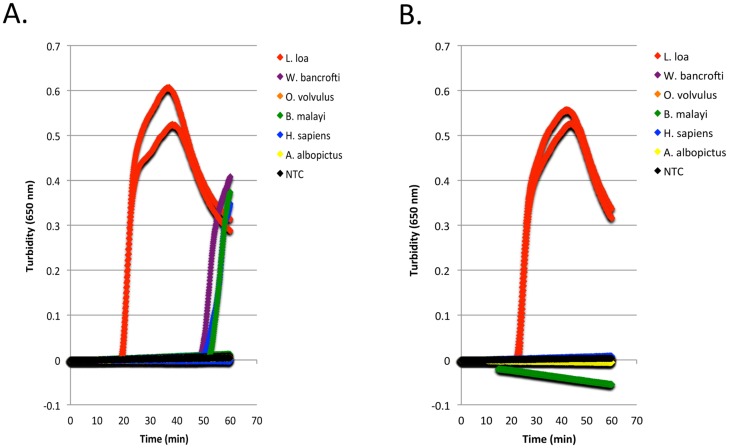
The L. loa RF4 RepeatScout consensus sequence generated by aligning 326 sequences (Fig 1A and S1 Fig) was submitted to Primer Explorer for LAMP primer design. Loop forward and back primers were designed manually (Fig 1B). The specificity of RF4 was evaluated in LAMP assays using various genomic DNAs. As part of the assay optimization process, amplification in the presence and absence of the chemical additives, Valeramide and N,N,-diethylformamide was evaluated as background amplification occasionally occurs. Turbidity reached a threshold value of 0.1 in approximately 20 minutes when 0.2 ng L. loa DNA was added to reactions in the absence of additive (Fig 5A) compared to approximately 25 minutes in the presence of additive (Fig 5B). However, in reactions lacking additive, turbidity exceeding a threshold of 0.1 could be detected in approximately 50–60 minutes in some of the heterologous DNA samples whereas in reactions containing additive, turbidity remained negative (Fig 5B). No amplification was observed in the additive-plus or additive-minus non-template controls.
Fig 5. Species-specificity of the L. loa RF4-based LAMP assay.
Turbidity curves generated with various DNAs (200 pg) amplified in the absence (A) or presence (B) of the V/DEF additive using the L. loa LAMP primer set with Bst 2.0. Each graph shows the results of two experiments.
The L. loa RF4-based LAMP Assay is Sensitive
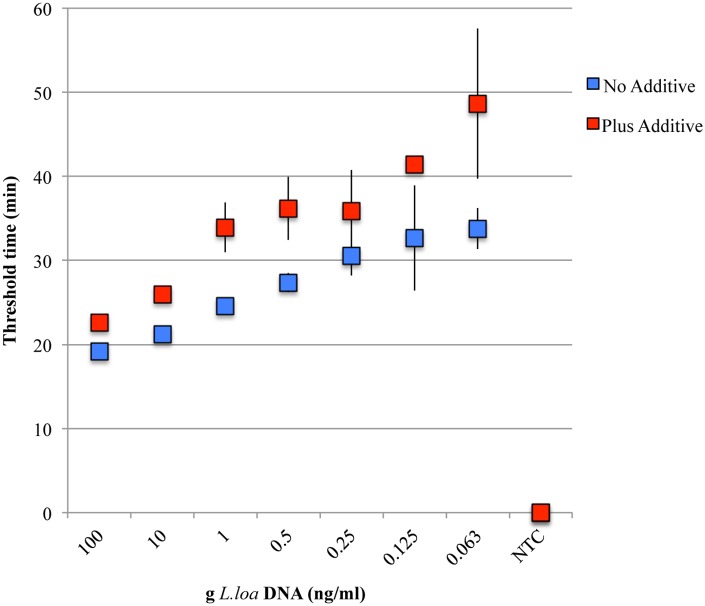
The sensitivity of the L. loa RF4 primer set was evaluated in the presence or absence of chemical additive (Fig 6). In the absence of additive, dilutions of genomic L. loa DNA amplified with the RF4 LAMP primer set reached a turbidity threshold of 0.1 ranging from 20 minutes for the highest concentration of template DNA (100 ng/ml) to ~ 35 minutes for the lowest. In the presence of additive, more time was required to reach the turbidity threshold of 0.1 for each DNA dilution. However, at the lowest concentration of DNA tested (0.063 ng/ml) turbidity reached the threshold of 0.1 in ~ 50 minutes; well within the cutoff time for the assay (60 minutes). At the lower concentrations of DNA (≤ 0.125 ng/ml), not all triplicates amplified. This was observed in reactions irrespective of additive status. No turbidity was observed in the NTCs regardless of the presence or absence of chemical additive.
Fig 6. Sensitivity of the L. loa RF4-based LAMP assay.
Dilutions of genomic L. loa DNA were amplified with the RF4 primer set and Bst 2.0 DNA polymerase in the absence (blue) or presence (red) of the V/DEF additive. Two ul of each dilution was added to LAMP reactions. The average threshold time, defined as the time at which the change in turbidity over time (dT/dt) reaches a value of 0.1, is plotted against the concentration of L. loa DNA (ng/ml). All reactions were performed in triplicate. Error bars represent the standard deviation at each point.
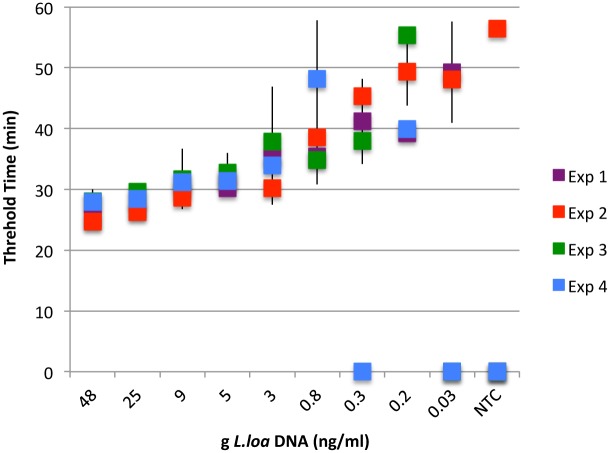
To mimic a clinical situation, assays were performed on DNA extracted from a two-fold dilution series of genomic L. loa DNA spiked blood samples (Fig 7). Following extraction, the concentration of L. loa DNA in the samples ranged from ~ 0.03–50 ng/ml which is equivalent to 0.3–500 mf/ml blood. Three experiments were performed using 2 μl of each DNA dilution, equivalent to ~ 1-1/1600th of an mf per LAMP reaction. Robust amplification was observed in samples containing equivalent amounts of template DNA down to 0.8 ng/ml of parasite DNA. Below this dilution, concordance between experiments was somewhat less but L. loa DNA was still easily detected within 60 minutes. The turbidity threshold of 0.1 was reached in 25–30 minutes for 100 pg with slightly more time required as the concentration of template DNA decreased (Fig 7). Turbidity was observed in only 1 of 12 NTCs tested over the course of the 4 experiments.
Fig 7. Detection of L. loa DNA in spiked blood samples.
A two-fold dilution series of genomic L. loa DNA was prepared using uninfected human whole blood. NTCs only contained uninfected human whole blood. After DNA isolation, two μl of each dilution (or NTC) was used in LAMP reactions containing the V/DEF additive with Bst 2.0 DNA polymerase. For each experiment, all samples were assayed in triplicate. Average threshold times and standard deviations are plotted against ng DNA/ml of elution buffer.
Discussion
The recent availability of genome sequence from L. loa [43, 46] has enabled the development of a multi-step bioinformatic pipeline to perform a genome wide search for new diagnostic candidates. We have taken advantage of sequence information from the closely related filarial species B. malayi, W. bancrofti and O. volvulus as well as from human to identify species-specific repeats in the L. loa genome. We focused our efforts on repeat families with a copy number greater than or equal to 51 to maximize assay sensitivity and customized the process to select sequences ideally suited for LAMP, although the candidates were evaluated using both PCR and LAMP. The filarial genomes differ in repeat content with the L. loa genome being less repetitive (9.3%) than that of B. malayi (12.1%), but more repetitive than that of W. bancrofti (6.2%) [43].
We have previously developed a LAMP diagnostic assay for brugian filariasis using the Brugia Hha I repeat as the biomarker [39]. This amplification system was shown to be extremely sensitive in detecting Brugia DNA [39], with levels of sensitivity comparable to the Hha I PCR amplification system [48, 49]. Several repeat families have been employed as biomarkers, including the Ssp I repeat, to detect W. bancrofti in humans and vectors [50–53]. The W. bancrofti genome is estimated to contain more than 2000 copies of the Ssp I repeat, comprising ~0.5% of the genome [43]. Recently a LAMP assay based on Ssp I capable of detecting the equivalent of 1/1000th of the DNA amount contained in a single microfilaria was published [54].
Using our genome filtering method, we identified ~9000 species-specific repeat families, ~300 of which are present with a copy number of 51 or more in the genome, and of these, 137 are of potential diagnostic value. Interestingly, the previously identified L. loa repeat LL3M9 used in both PCR [2] and LAMP assays [33] was filtered out in the process since orthologs were found in B. malayi and W. bancrofti. Our bioinformatic pipeline did not select the other L. loa sequence used as a LAMP biomarker, namely LLMF72, because it is a single copy gene. Of the six diagnostic candidates we validated in PCR and LAMP amplification studies, all were found to be species-specific with RF4 showing the most promise. Our analysis revealed RF4 to be part of a Bel/Pao LTR retroelement family present in metazoan genomes [47, 55]. This is consistent with its organization in the genome. This family was originally characterized with the discovery of element sequences such as Pao [56], Bel [57], Tas [58] and the various Cer-like sequences described in Caenorhabditis elegans [59]. The most prominent repeats in the L. loa and W. bancrofti genomes are the BEL retrotransposons that comprise 1.3% and 1.5% of the genome respectively [43]. To be of diagnostic value it is imperative that the DNA biomarker be stable and highly conserved in different geographic isolates. RepeatScout identified ~350 sequences related to RF4 in each of the two drafts available of the L. loa genome prepared from parasite isolates collected from different geographical locations namely Cameroon [43] and the Central African Republic [46] supporting its use in diagnostic tests. The RF4 consensus sequence generated by Repeat Scout is 440 bp long (33% GC).
High levels of specificity were achieved in RF4-based LAMP and PCR assays. LAMP primers amplified L. loa DNA, but not DNA isolated from the closely related filarial parasites O. volvulus, B. malayi or W.bancrofti, or from human or insect. Specificity was significantly enhanced, with negligible effect on sensitivity, in the presence of the chemical additives Valeramide and N,N,-diethylformamide. Combinations of small amides are thought to destabilize DNA secondary structure while leaving proper primer annealing alone [41, 60]. A similar specificity profile was obtained in PCR reactions, highlighting the versatility of this target for molecular diagnostic studies.
When highly purified DNA was used as template, LAMP amplification of RF4 was evident within 60 minutes at the lowest concentration of DNA tested (0.063 ng/ml; 2 μl is equivalent to 1/800 th of an mf), and most robust at 0.25 ng/ml and above. When LAMP was performed on varying amounts of L. loa DNA purified from spiked blood samples, robust amplification was observed in reactions containing 2 μl of 0.8 ng/ml or more parasite DNA. When the LAMP F3 and B3 primers were used to PCR amplify DNA from the spiked blood samples, 5 pg (1/20th of mf) of starting material could be detected on agarose gels (data not shown). Therefore, RF4 can be used in both LAMP and PCR platforms, although LAMP provides greater sensitivity.
There are multiple reasons why LAMP has been adopted as a diagnostic platform to detect infectious agents including those responsible for neglected tropical diseases [19]. The main advantages of LAMP over PCR include its operational simplicity and isothermal nature. In PCR, thermal cycling is required to denature the template, anneal primers and extend the amplicon. LAMP employs Bst DNA polymerase, which provides both strand displacement and target amplification at a single temperature in a simple heat block or water bath at 60–65°C [30, 31]. Rapidity and versatility in readout options also make LAMP a particularly appealing technology. Cost is another factor with a recent estimate for a W. bancrofti LAMP test being $0.82 compared with more than $2.20 for PCR [54, 61]. In the present study, real-time turbidity was used for assay design and optimization yielding positive results within 60 minutes. However, in future studies we will use the more field-friendly hydroxy naphthol blue [62] and recently developed pH sensitive dyes [32] for detection.
In summary, we describe a LAMP diagnostic assay for L. loa based on a new DNA biomarker RF4 that generates a robust read-out within 60 minutes. The assay shows promise as a field tool for implementation and management of MDA programs and warrants further testing on clinical samples as the next stage in development towards this goal. Furthermore, we have successfully devised a method to mine the genome of L. loa for new biomarkers. We evaluated only a few repeat families resulting from our bioinformatics pipeline for their potential as diagnostic targets. While RF4 looks particularly promising, the bioinformatic output can be further analyzed to determine if more sensitive targets exist for LAMP and other amplification platforms. This comparative genomic approach can be applied to identify new diagnostic candidates for other filarial diseases and/or used to improve the sensitivity and specificity of existing molecular diagnostic methods.
Supporting Information
Sequences belonging to RF4 were identified in the L. loa genome and aligned by RepeatScout. The RF4 consensus sequence used for PCR and LAMP primer design is shown above the alignment. Within each supercontig containing an RF4 member, the location of the repeat is denoted in the sequence ID. Using the first sequence ID in the alignment as an example (Supercontig_3.5529_249_346/1-97), a 97 bp partial repeat is located between nucleotides 249–346 of supercontig_3.5529.
(TIF)
A) Diagram showing the location and orientation of the LTRs (black arrows) and organization of the GAG polyprotein (GAG), aspartic protease (AP), reverse transcriptase (RT), Ribonuclease H (Rnase H) and intregase (INT) of the BEL/PAO retrotransposon within the 12.3 Kb L. loa scaffold, 7180000007063_1 (GenBank acc.#JPEI01001237.1). B) MAP and alignment of the 600 bp L. loa LTR with the RF4 consensus sequence and the region spanning the LAMP primers. RepeatScout excluded the region of the L. loa LTR extending from bp 2–70 from the RF4 consensus due to homology with the B. malayi and W. bancrofti genomes. The region extending from bp 150–230 were excluded from RF4 because of homology with the W. bancrofti and O. volvulus genomes.
(TIF)
The consensus sequences are in fasta format. A descriptor of each consensus sequence follows the ">" symbol. It consists of a numerical name, copy number, %GC and length of the consensus repeat. Using the descriptor of the first consensus sequence in the list as an example (>R = 0_774_0_0_0_CG_35_bps_550), the name of this sequence is repeat 0. 774 copies of this family were identified by RepeatScout in the L. loa genome. The triplicate zeros indicate that no copies of this family were identified in the O. volvulus, B. malayi or W. bancrofti genomes. This consensus sequence is 35% GC rich and 550 bps long.
(DOCX)
Acknowledgments
We gratefully acknowledge Don Comb and Jim Ellard for their continued support of filarial research at New England Biolabs. We also thank Zhiru Li, and Andy Alhassan for helpful discussions. We are grateful to Bill Jack, Barton Slatko, Larry McReynolds, Jeremy Foster, Ashley Luck and Silvia Libro for critical reading of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funder, New England Biolabs, provided support in the form of salaries for authors CKSC, CBP, NAT, LE and TCE, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Boussinesq M. Loiasis. Ann Trop Med Parasitol. 2006;100(8):715–31. 10.1179/136485906X112194 . [DOI] [PubMed] [Google Scholar]
- 2. Fink DL, Fahle GA, Fischer S, Fedorko DF, Nutman TB. Toward molecular parasitologic diagnosis: enhanced diagnostic sensitivity for filarial infections in mobile populations. J Clin Microbiol. 2011;49(1):42–7. Epub 2010/10/29. 10.1128/JCM.01697-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly-Hope LA, Bockarie MJ, Molyneux DH. Loa loa ecology in central Africa: role of the Congo River system. PLoS Negl Trop Dis. 2012;6(6):e1605 10.1371/journal.pntd.0001605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoure HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, et al. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis. 2011;5(6):e1210 10.1371/journal.pntd.0001210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanji S, Akotshi DO, Mutro MN, Tepage F, Ukety TO, Diggle PJ, et al. Validation of the rapid assessment procedure for loiasis (RAPLOA) in the Democratic Republic of Congo. Parasites & vectors. 2012;5:25 10.1186/1756-3305-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pion DS, Gardon J, Kamgno J, Gardon-Wendel N, Chippaux JP, Boussinesq M. Structure of the microfilarial reservoir of Loa loa in the human host and its implications for monitoring the programmes of Community-Directed Treatment with Ivermectin carried out in Africa. Parasitology. 2004;129(Pt 5):613–26. . [DOI] [PubMed] [Google Scholar]
- 7. Pion SD, Demanou M, Oudin B, Boussinesq M. Loiasis: the individual factors associated with the presence of microfilaraemia. Ann Trop Med Parasitol. 2005;99(5):491–500. 10.1179/136485905X51300 . [DOI] [PubMed] [Google Scholar]
- 8. Boussinesq M, Gardon J, Kamgno J, Pion SD, Gardon-Wendel N, Chippaux JP. Relationships between the prevalence and intensity of Loa loa infection in the Central province of Cameroon. Ann Trop Med Parasitol. 2001;95(5):495–507. . [DOI] [PubMed] [Google Scholar]
- 9. Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350(9070):18–22. 10.1016/S0140-6736(96)11094-1 . [DOI] [PubMed] [Google Scholar]
- 10. Gardon J, Kamgno J, Folefack G, Gardon-Wendel N, Bouchite B, Boussinesq M. Marked decrease in Loa loa microfilaraemia six and twelve months after a single dose of ivermectin. Trans R Soc Trop Med Hyg. 1997;91(5):593–4. . [DOI] [PubMed] [Google Scholar]
- 11. Bennuru S, Pion SD, Kamgno J, Wanji S, Nutman TB. Repurposed automated handheld counter as a point-of-care tool to identify individuals 'at risk' of serious post-ivermectin encephalopathy. PLoS Negl Trop Dis. 2014;8(9):e3180 10.1371/journal.pntd.0003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chippaux JP, Boussinesq M, Gardon J, Gardon-Wendel N, Ernould JC. Severe adverse reaction risks during mass treatment with ivermectin in loiasis-endemic areas. Parasitol Today. 1996;12(11):448–50. . [DOI] [PubMed] [Google Scholar]
- 13. Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S. Filariasis in Africa—treatment challenges and prospects. Clin Microbiol Infect. 2011;17(7):977–85. 10.1111/j.1469-0691.2011.03586.x . [DOI] [PubMed] [Google Scholar]
- 14. Okorie PN, Ademowo GO, Saka Y, Davies E, Okoronkwo C, Bockarie MJ, et al. Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis. 2013;7(9):e2416 10.1371/journal.pntd.0002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uttah E, Ibeh DC. Multiple filarial species microfilaraemia: a comparative study of areas with endemic and sporadic onchocerciasis. J Vector Borne Dis. 2011;48(4):197–204. . [PubMed] [Google Scholar]
- 16. Molyneux DH. Filaria control and elimination: diagnostic, monitoring and surveillance needs. Trans R Soc Trop Med Hyg. 2009;103(4):338–41. 10.1016/j.trstmh.2008.12.016 . [DOI] [PubMed] [Google Scholar]
- 17. Klion AD, Ottesen EA, Nutman TB. Effectiveness of diethylcarbamazine in treating loiasis acquired by expatriate visitors to endemic regions: long-term follow-up. J Infect Dis. 1994;169(3):604–10. . [DOI] [PubMed] [Google Scholar]
- 18. Klion AD, Massougbodji A, Horton J, Ekoue S, Lanmasso T, Ahouissou NL, et al. Albendazole in human loiasis: results of a double-blind, placebo-controlled trial. J Infect Dis. 1993;168(1):202–6. . [DOI] [PubMed] [Google Scholar]
- 19. Alhassan A, Li Z, Poole CB, Carlow CK. Expanding the MDx toolbox for filarial diagnosis and surveillance. Trends Parasitol. 2015. 10.1016/j.pt.2015.04.006 . [DOI] [PubMed] [Google Scholar]
- 20. D'Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, et al. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med. 2015;7(286):286re4 10.1126/scitranslmed.aaa3480 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klion AD, Vijaykumar A, Oei T, Martin B, Nutman TB. Serum immunoglobulin G4 antibodies to the recombinant antigen, Ll-SXP-1, are highly specific for Loa loa infection. J Infect Dis. 2003;187(1):128–33. 10.1086/345873 . [DOI] [PubMed] [Google Scholar]
- 22. Azzibrouck GB, Akue JP, Lenoble DR. Production and immunological characterization of a recombinant subunit of a Loa loa polyprotein antigen. Parasitology. 2010;137(7):1119–28. 10.1017/S0031182009991740 . [DOI] [PubMed] [Google Scholar]
- 23. Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. J Clin Microbiol. 2008;46(7):2298–304. 10.1128/JCM.00490-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toure FS, Egwang TG, Wahl G, Millet P, Bain O, Georges AJ. Species-specific sequence in the repeat 3 region of the gene encoding a putative Loa loa allergen: a diagnostic tool for occult loiasis. Am J Trop Med Hyg. 1997;56(1):57–60. . [DOI] [PubMed] [Google Scholar]
- 25. Toure FS, Kassambara L, Williams T, Millet P, Bain O, Georges AJ, et al. Human occult loiasis: improvement in diagnostic sensitivity by the use of a nested polymerase chain reaction. Am J Trop Med Hyg. 1998;59(1):144–9. . [DOI] [PubMed] [Google Scholar]
- 26. Toure FS, Mavoungou E, Deloron P, Egwang TG. Comparative analysis of 2 diagnostic methods of human loiasis: IgG4 serology and nested PCR. Bull Soc Pathol Exot. 1999;92(3):167–70. . [PubMed] [Google Scholar]
- 27. Fink DL, Kamgno J, Nutman TB. Rapid molecular assays for specific detection and quantitation of Loa loa microfilaremia. PLoS Negl Trop Dis. 2011;5(8):e1299 10.1371/journal.pntd.0001299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klion AD, Raghavan N, Brindley PJ, Nutman TB. Cloning and characterization of a species-specific repetitive DNA sequence from Loa loa . Mol Biochem Parasitol. 1991;45(2):297–305. . [DOI] [PubMed] [Google Scholar]
- 29. Jimenez M, Gonzalez LM, Carranza C, Bailo B, Perez-Ayala A, Muro A, et al. Detection and discrimination of Loa loa, Mansonella perstans and Wuchereria bancrofti by PCR-RFLP and nested-PCR of ribosomal DNA ITS1 region. Exp Parasitol. 2011;127(1):282–6. 10.1016/j.exppara.2010.06.019 . [DOI] [PubMed] [Google Scholar]
- 30. Notomi T, Mori Y, Tomita N, Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53(1):1–5. 10.1007/s12275-015-4656-9 . [DOI] [PubMed] [Google Scholar]
- 31. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):E63 Epub 2000/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanner NA, Zhang Y, Evans TC Jr. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. BioTechniques. 2015;58(2):59–68. 10.2144/000114253 . [DOI] [PubMed] [Google Scholar]
- 33. Fernandez-Soto P, Mvoulouga PO, Akue JP, Aban JL, Santiago BV, Sanchez MC, et al. Development of a highly sensitive loop-mediated isothermal amplification (LAMP) method for the detection of Loa loa . PloS one. 2014;9(4):e94664 10.1371/journal.pone.0094664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drame PM, Fink DL, Kamgno J, Herrick JA, Nutman TB. Loop-mediated isothermal amplification for rapid and semiquantitative detection of Loa loa infection. J Clin Microbiol. 2014;52(6):2071–7. 10.1128/JCM.00525-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21 Suppl 1:i351–8. 10.1093/bioinformatics/bti1018 . [DOI] [PubMed] [Google Scholar]
- 36. Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wootton JC, Federhen S. Statistics of local complexity in amino acid sequences and sequence databases. Comput Chem. 1993;17(2):149–63. 10.1016/0097-8485(93)85006-X [DOI] [Google Scholar]
- 38. Kohany O, Gentles AJ, Hankus L, Jurka J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics. 2006;7:474 10.1186/1471-2105-7-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poole CB, Tanner NA, Zhang Y, Evans TC Jr, Carlow CK. Diagnosis of brugian filariasis by loop-mediated isothermal amplification. PLoS Negl Trop Dis. 2012;6(12):e1948 10.1371/journal.pntd.0001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41. Tanner N, Evans TC. Compositions and Methods for Reducing Background DNA Amplification. Google Patents; 2013. [Google Scholar]
- 42. Chakrabarti R, Schutt CE. The enhancement of PCR amplification by low molecular weight amides. Nucleic Acids Res. 2001;29(11):2377–81. 10.1093/nar/29.11.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Desjardins CA, Cerqueira GC, Goldberg JM, Dunning Hotopp JC, Haas BJ, Zucker J, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat Genet. 2013;45(5):495–500. 10.1038/ng.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonzalez A, Prediger E, Huecas ME, Nogueira N, Lizardi PM. Minichromosomal repetitive DNA in Trypanosoma cruzi: its use in a high-sensitivity parasite detection assay. Proc Natl Acad Sci U S A. 1984;81(11):3356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McReynolds LA, DeSimone SM, Williams SA. Cloning and comparison of repeated DNA sequences from the human filarial parasite Brugia malayi and the animal parasite Brugia pahangi . Proc Natl Acad Sci U S A. 1986;83(3):797–801. Epub 1986/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tallon LJ, Liu X, Bennuru S, Chibucos MC, Godinez A, Ott S, et al. Single molecule sequencing and genome assembly of a clinical specimen of Loa loa, the causative agent of loiasis. BMC Genomics. 2014;15:788 10.1186/1471-2164-15-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Copeland CS, Mann VH, Morales ME, Kalinna BH, Brindley PJ. The Sinbad retrotransposon from the genome of the human blood fluke, Schistosoma mansoni, and the distribution of related Pao-like elements. BMC Evol Biol. 2005;5:20 10.1186/1471-2148-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fischer P, Supali T, Wibowo H, Bonow I, Williams SA. Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA. Am J Trop Med Hyg. 2000;62(2):291–6. Epub 2000/05/17. . [DOI] [PubMed] [Google Scholar]
- 49. Lizotte MR, Supali T, Partono F, Williams SA. A polymerase chain reaction assay for the detection of Brugia malayi in blood. Am J Trop Med Hyg. 1994;51(3):314–21. Epub 1994/09/01. . [DOI] [PubMed] [Google Scholar]
- 50. Abbasi I, Githure J, Ochola JJ, Agure R, Koech DK, Ramzy RM, et al. Diagnosis of Wuchereria bancrofti infection by the polymerase chain reaction employing patients' sputum. Parasitol Res. 1999;85(10):844–9. . [DOI] [PubMed] [Google Scholar]
- 51. Siridewa K, Karunanayake EH, Chandrasekharan NV, Abeyewickreme W, Franzen L, Aslund L, et al. Cloning and Characterization of a Repetitive DNA Sequence Specific for Wuchereria bancrofti . Am J Trop Med Hyg. 1994;51(4):495–500. [PubMed] [Google Scholar]
- 52. Vasuki V, Hoti SL, Sadanandane C, Jambulingam P. A simple and rapid DNA extraction method for the detection of Wuchereria bancrofti infection in the vector mosquito, Culex quinquefasciatus by Ssp I PCR assay. Acta Trop. 2003;86(1):109–14. . [DOI] [PubMed] [Google Scholar]
- 53. Zhong M, McCarthy J, Bierwert L, Lizotte-Waniewski M, Chanteau S, Nutman TB, et al. A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples. Am J Trop Med Hyg. 1996;54(4):357–63. Epub 1996/04/01. . [DOI] [PubMed] [Google Scholar]
- 54. Takagi H, Itoh M, Kasai S, Yahathugoda TC, Weerasooriya MV, Kimura E. Development of loop-mediated isothermal amplification method for detecting Wuchereria bancrofti DNA in human blood and vector mosquitoes. Parasitol Int. 2011;60(4):493–7. Epub 2011/09/21. 10.1016/j.parint.2011.08.018 . [DOI] [PubMed] [Google Scholar]
- 55. Malik HS, Henikoff S, Eickbush TH. Poised for contagion: evolutionary origins of the infectious abilities of invertebrate retroviruses. Genome Res. 2000;10(9):1307–18. . [DOI] [PubMed] [Google Scholar]
- 56. Xiong Y, Burke WD, Eickbush TH. Pao, a highly divergent retrotransposable element from Bombyx mori containing long terminal repeats with tandem copies of the putative R region. Nucleic Acids Res. 1993;21(9):2117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davis PS, Judd BH. Nucleotide sequence of the transposable element, BEL, of Drosophila melanogaster . Drosophila Information Service. 1995;76:134–6. [Google Scholar]
- 58. Aeby P, Spicher A, de Chastonay Y, Müller F, Tobler H. Structure and genomic organization of proretrovirus-like elements partially eliminated from the somatic genome of Ascaris lumbricoides . EMBO J. 1986;5(12):3353–60. PMC1167333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bowen NJ, McDonald JF. Genomic analysis of Caenorhabditis elegans reveals ancient families of retroviral-like elements. Genome Res. 1999;9(10):924–35. . [DOI] [PubMed] [Google Scholar]
- 60. Chularerk P, Desowitz RS. A simplified membrane filtration technique for the diagnosis of microfilaremia. Journal of Parasitology. 1970;56(3):623–4. Epub 1970/06/01. . [PubMed] [Google Scholar]
- 61. Han ET. Loop-mediated isothermal amplification test for the molecular diagnosis of malaria. Expert Rev Mol Diagn. 2013;13(2):205–18. 10.1586/erm.12.144 . [DOI] [PubMed] [Google Scholar]
- 62. Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques. 2009;46(3):167–72. Epub 2009/03/26. 10.2144/000113072 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences belonging to RF4 were identified in the L. loa genome and aligned by RepeatScout. The RF4 consensus sequence used for PCR and LAMP primer design is shown above the alignment. Within each supercontig containing an RF4 member, the location of the repeat is denoted in the sequence ID. Using the first sequence ID in the alignment as an example (Supercontig_3.5529_249_346/1-97), a 97 bp partial repeat is located between nucleotides 249–346 of supercontig_3.5529.
(TIF)
A) Diagram showing the location and orientation of the LTRs (black arrows) and organization of the GAG polyprotein (GAG), aspartic protease (AP), reverse transcriptase (RT), Ribonuclease H (Rnase H) and intregase (INT) of the BEL/PAO retrotransposon within the 12.3 Kb L. loa scaffold, 7180000007063_1 (GenBank acc.#JPEI01001237.1). B) MAP and alignment of the 600 bp L. loa LTR with the RF4 consensus sequence and the region spanning the LAMP primers. RepeatScout excluded the region of the L. loa LTR extending from bp 2–70 from the RF4 consensus due to homology with the B. malayi and W. bancrofti genomes. The region extending from bp 150–230 were excluded from RF4 because of homology with the W. bancrofti and O. volvulus genomes.
(TIF)
The consensus sequences are in fasta format. A descriptor of each consensus sequence follows the ">" symbol. It consists of a numerical name, copy number, %GC and length of the consensus repeat. Using the descriptor of the first consensus sequence in the list as an example (>R = 0_774_0_0_0_CG_35_bps_550), the name of this sequence is repeat 0. 774 copies of this family were identified by RepeatScout in the L. loa genome. The triplicate zeros indicate that no copies of this family were identified in the O. volvulus, B. malayi or W. bancrofti genomes. This consensus sequence is 35% GC rich and 550 bps long.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.