Abstract
Background
Epidemiologic data on the association between body mass index (BMI) and heart failure (HF) risk among diabetic patients are rare.
Methods and Results
We performed a prospective cohort study of risk for HF among 31,155 patients with type 2 diabetes (11,468 men and 19,687 women). Cox proportional hazards regression models were used to estimate the association of different levels of BMI with HF risk. During a mean follow-up of 7.8 years, 5,834 subjects developed HF (2,379 men and 3,455 women). The multivariable-adjusted (age, race, smoking, income and type of insurance) hazard ratios of HF associated with BMI levels (18.5–22.9, 23–24.9, 25–29.9 [reference group], 30–34.9, 35–39.9, and ≥40 kg/m2) at baseline were 0.95, 1.00, 1.00, 1.16, 1.64, and 2.02 (Ptrend <0.001) for men, and 1.16, 1.16, 1.00, 1.23, 1.55, and 2.01 (Pnon-linear <0.001) for women, respectively. When we used an updated mean value of BMI, the association of HF risk with BMI did not change. When stratified by age, race, smoking status and use of anti-diabetic drugs, the positive associations among men and the J-shaped associations among women were still present.
Conclusions
Our study suggests a positive association between BMI and HF risk among men, and a J-shaped association between BMI and HF risk among women with type 2 diabetes.
Keywords: body mass index, heart failure, cohort study, diabetes mellitus
Diabetes is a major public health problem, affecting nearly 8% of the US population.1 Diabetes is an independent risk factor for heart failure (HF),2 and the presence of diabetes is designated by the current American Heart Association HF classification as stage A HF (patients at high risk for developing HF).3 Obesity is another important public health problem worldwide. In the US, two in three adults are currently classified as overweight or obese (body mass index [BMI] ≥ 25 kg/m2) and one-third of them are frankly obese (BMI ≥ 30 kg/m2).4 Among patients with diabetes in the US, approximately 45–65% of them are obese.5
In the general population, obesity is a well-established risk factor for cardiovascular diseases (CVD) including HF.6,7 However, whether and how obesity is associated with CVD risk among diabetic patients is seldom studied. Several studies have assessed the association between BMI and the risk of total or CVD mortality in patients with type 2 diabetes,5,8–17 but few studies have focused on the relationship between BMI and incident HF. Thus, the aim of the present study was to examine the association between BMI and the risk of incident HF among patients with type 2 diabetes in the Louisiana State University Hospital-Based Longitudinal Study (LSUHLS).
Methods
Study Population
Between 1997 and 2012, the LSU Health Care Services Division (LSUHCSD) operated seven public hospitals and affiliated clinics in Louisiana providing quality medical care to the residents of Louisiana regardless of their income or insurance coverage.18–25 Since 1997, administrative, anthropometric, laboratory (test code, test collection date, test result values, and abnormal flag), clinical diagnosis, and medication data collected at these facilities are available in electronic form for both inpatients and outpatients. In total, approximately 1.6 million patients (35% of the Louisiana population) were served by the LSUHCSD facilities. The LSUHLS was established in 2010 by using these data.18 We established a cohort of diabetic patients who used LSUHCSD hospitals between January 1, 1999 (this could make sure all diabetes cases are newly diagnosed because the LSU HCSD data warehouse is available in electronic form since January 1, 1997) and December 31, 2009, using the International Classification of Diseases (ICD)-9 (code 250). All diabetic patients in the LSUHCSD hospitals were diagnosed using the American Diabetes Association (ADA) criteria: a fasting plasma glucose ≥ 7.0 mmol/l, or 2-hour glucose ≥ 11.1 mmol/l after a 75-g 2-hour oral glucose tolerance test (OGTT); or a patient with one or more classic symptoms plus a random plasma glucose ≥ 11.1 mmol/l.26 A validation study has been carried out for the diagnosis of diabetes in the LSUHCSD hospitals, and 20,919 patients from a sample of 21,566 hospital discharge diagnoses based on ICD codes also had physician-confirmed diabetes using the ADA diabetes diagnosis criteria,26 and the agreement of diabetes diagnosis was 97%.
In the present study, we only included patients who had newly diagnosed diabetes. These patients had used the LSUHSCD system for an average of 5.0 years before the diagnosis of diabetes. After excluding patients with a history of HF and stroke at baseline, 31,155 patients of type 2 diabetes (11,468 men and 19,687 women) with complete data on any of the required variables for analysis were included in the present analyses. The ages of the participants were 30–94 years. The study and analysis plan were approved by the Pennington Biomedical Research Center and LSU Health Sciences Center Institutional Review Boards, LSU System. We did not obtain informed consent from participants involved in our study because we used anonymized data compiled from electronic medical records.
Baseline and follow-up measurements
The patient’s characteristics, including age of diabetes diagnosis, gender, race/ethnicity, family income, smoking status, types of health insurance, body weight, height, BMI, blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, HbA1c, estimated glomerular filtration rate (eGFR), and medication (antihypertensive drug, cholesterol lowering drug and anti-diabetic drug) within a half year of the diabetes diagnosis (baseline) and during follow-up after the diabetes diagnosis (follow-up) were extracted from the computerized hospitalization records. The updated mean values of HbA1c, LDL cholesterol, BMI, blood pressure and eGFR over time were calculated for each participant from baseline to each year of follow up. For example, at one year the updated mean is the average of the baseline and one year values and at three years it is the average of baseline, one year, two year, and three year values. In the case of an event during follow-up, the period for estimating updated mean value was from baseline to the year before this event occurred.27 Height and weight were measured without shoes and with light clothing according to a standardized protocol. BMI was calculated by dividing weight in kilograms by the square of height in meters. In the baseline analysis, we chose the BMI measures within a half year before the diagnosis of diabetes as baseline measurement. The mean values of BMI were calculated from the date of the diagnosis of diabetes to the end of the date of the diagnosis of HF or death, or June 31, 2013. The average number of BMI measurements during the follow-up period (from 1999 to the end of follow-up time – Year 2013 or to the year before HF or death event occurred) was 15.0.
Prospective follow-up
Follow-up information was obtained from the LSUHLS inpatient and outpatient database by using the unique number assigned to every patient who visits the LSUHCSD hospitals. Since 1997, diagnosis of HF in the LSUHCSD hospitals has been made by the treating physicians using the Framingham Criteria for Heart Failure diagnosis.28 After clinical diagnosis of HF, echocardiography has been used for each HF patient to support the clinical diagnosis, classify HF (ejection fraction ≤ 40% or >40%), and guide the treatment according to the classification. The diagnosis of HF was the primary endpoint of interest of the study, and was defined according to the ICD-9: HF (ICD-9 codes 402.01, 402.11, 402.91, and 428). We have conducted a validation study among 4,380 HF patients (not only diabetic patients but also non-diabetic patients) in the LSUHCSD hospitals. The physician-confirmed HF diagnosis was done at clinical visits following the first time when the patients were given a HF diagnosis by using both the Framingham Criteria for Heart Failure diagnosis and ejection fraction.28 Of 4,380 HF patients, 2,353 had ejection fraction ≤ 40%, and 2,027 had ejection fraction >40%; 2,246 (95%) of 2,353 HF patients with reduced ejection fraction were confirmed by using both the Framingham Criteria for Heart Failure diagnosis and ejection fraction (≤40%), and 1,430 (71%) of 2,027 HF patients were confirmed by using both the Framingham Criteria for Heart Failure diagnosis and ejection fraction (>40%).28,29 Follow-up of each cohort member continued until the date of HF, the date of the last visit if the subject stopped use of LSUHCSD hospitals, death or June 31, 2013.
Statistical analyses
The association between BMI and incident HF was analyzed by using Cox proportional hazards models. BMI was evaluated in the following 2 ways: (1) as 6 categories (BMI 18.5–22.9, 23–24.9, 25–29.9 [reference group], 30–34.9, 35–39.9, and ≥ 40 kg/m2), and (2) as a continuous variable. Since there was an interaction between BMI and sex on incident HF (χ2 = 8.086, df = 1, p<0.001), we stratified the sample by sex for analysis. All analyses were adjusted for age and race (Model 1); age, race, smoking, income, and type of insurance (Model 2); and variables in Model 2 and further for LDL cholesterol, systolic blood pressure, HbA1c, GFR, history of obstructive apnea, use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents at baseline (in baseline analyses) and during follow-up (in the follow-up analyses) (Model 3). The different categories of BMI were included in the models as dummy variables, and the significance of the trend over different categories was tested in the same models with the median of each category as a continuous variable. We used restricted cubic splines in Cox models to test whether there is a non-linear association of BMI as a continuous variable with HF risk.30 Non-linear trends were assessed with likelihood-ratio tests of restricted cubic splines.30 To avoid the potential bias due to severe diseases at baseline, additional analyses were carried out excluding the subjects who were diagnosed with HF and cancer during the first two years of follow-up. Statistical significance was considered to be P<0.05. All statistical analyses were performed with PASW for Windows, version 20.0 (IBM SPSS Inc, Chicago, III) and SAS for Windows, version 9.3 (SAS Institute, Cary, NC).
Results
General characteristics of the patients, stratified according to baseline BMI categories are presented in Table 1. The mean follow-up time was 7.8 years. The association of BMI with incident HF risk was positive among men and was J-shaped among women with type 2 diabetes (Figure). The multivariable-adjusted (age, race, smoking, income and type of insurance, Model 2) hazard ratios (HRs) (95% confidence intervals [CIs]) of incident HF associated with BMI levels (18.5–22.9, 23–24.9, 25–29.9 [reference group], 30–34.9, 35–39.9, and ≥ 40 kg/m2) at baseline were 0.95 (95% CI 0.78–1.15), 1.00 (0.84–1.19), 1.00, 1.16 (1.04–1.29), 1.64 (1.45–1.85), and 2.02 (1.79–2.29) for men (Ptrend <0.001), and 1.16 (1.00–1.37), 1.16 (0.97–1.38), 1.00, 1.23 (1.11–1.37), 1.55 (1.39–1.73), and 2.01 (1.81–2.23) for women (Pnon-linear <0.001), respectively (Table 2). When BMI was examined as a continuous variable, the multivariable-adjusted HRs for each 1-unit increase in BMI at baseline were 1.037 (95% CI 1.032–1.042) for men, and 1.032 (95% CI 1.028–1.036) for women. After further adjustment for LDL cholesterol, systolic blood pressure, HbA1c, GFR, history of obstructive apnea, use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents (Model 3), the association did not change.
Table 1.
Baseline characteristics of African American and white patients with type 2 diabetes according to body mass index categories
| Body mass index (kg/m2)
|
P value | ||||||
|---|---|---|---|---|---|---|---|
| 18.5–22.9 | 23–24.9 | 25.0–29.9 | 30–34.9 | 35–39.9 | ≥40 | ||
| No. of participants | 1,553 | 1,777 | 7,188 | 7,968 | 5,867 | 6,802 | - |
| No. of cases | 258 | 303 | 1,147 | 1,396 | 1,202 | 1,528 | - |
| Male, % | 49.3 | 48.5 | 44.4 | 39.6 | 31.8 | 24.0 | <0.001 |
| African American, % | 977 (62.9) | 1,039 (58.5) | 4,195 (58.4) | 4,468 (56.1) | 3,223 (54.9) | 3,580 (52.6) | <0.001 |
| Age, mean (SE), years | 53.0 (0.3) | 53.9 (0.2) | 53.8 (0.1) | 52.5 (0.1) | 51.0 (0.1) | 48.3 (0.1) | <0.001 |
| Income, mean (SE), $/family | 18,515 (670) | 19,034 (627) | 19,136 (313) | 18,882 (295) | 19,071 (344) | 18,840 (326) | 0. 955 |
| Systolic blood pressure, mean (SE), mm Hg | 138 (0.6) | 140 (0.6) | 142 (0.3) | 144 (0.3) | 146 (0.3) | 148 (0.3) | <0.001 |
| HbA1c, mean (SE), % | 8.01 (0.06) | 7.96 (0.06) | 7.85 (0.03) | 7.77 (0.03) | 7.74 (0.03) | 7.53 (0.03) | <0.001 |
| LDL cholesterol, mean (SE), mg/dL | 109 (1.0) | 113 (1.0) | 114 (0.5) | 114 (0.5) | 113 (0.5) | 111 (0.5) | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2), % | <0.001 | ||||||
| ≥90 | 53.1 | 52.6 | 47.6 | 45.6 | 46.0 | 48.5 | |
| 60–89 | 33.8 | 34.6 | 39.0 | 41.4 | 41.8 | 41.0 | |
| 30–59 | 10.8 | 11.1 | 11.6 | 11.7 | 11.0 | 9.5 | |
| 15–29 | 1.6 | 0.8 | 1.1 | 1.0 | 0.9 | 0.8 | |
| <15 | 0.8 | 0.9 | 0.8 | 0.3 | 0.3 | 0.3 | |
| Smoking status, % | <0.001 | ||||||
| Never smoking | 43.7 | 53.7 | 61.9 | 66.7 | 70.9 | 74.2 | |
| Past smoking | 8.1 | 6.9 | 7.0 | 6.8 | 7.2 | 7.3 | |
| Current smoking | 48.2 | 39.4 | 31.0 | 26.5 | 21.9 | 18.5 | |
| Type of insurance, % | <0.001 | ||||||
| Free | 70.6 | 72.5 | 75.2 | 77.8 | 80.0 | 83.0 | |
| Self-pay | 7.3 | 6.4 | 5.6 | 5.2 | 4.4 | 3.8 | |
| Medicaid | 7.4 | 5.9 | 4.8 | 4.2 | 5.0 | 5.1 | |
| Medicare | 12.6 | 13.2 | 12.1 | 10.5 | 8.2 | 5.9 | |
| Commercial | 2.1 | 1.9 | 2.3 | 2.3 | 2.4 | 2.2 | |
| Use of medications, % | |||||||
| Glucose-lowering medication | <0.001 | ||||||
| Oral hypoglycemic agents | 22.9 | 27.6 | 34.3 | 35.3 | 35.1 | 37.2 | |
| Insulin | 31.8 | 30.7 | 29.0 | 31.0 | 33.6 | 31.7 | |
| Lipid-lowering medication | 41.7 | 49.7 | 54.8 | 59.4 | 59.7 | 55.9 | <0.001 |
| Antihypertensive medication | 60.5 | 65.7 | 70.6 | 74.2 | 75.9 | 60.5 | <0.001 |
Values represent means or percentages. All continuous data except age adjusted for age, sex and race.
Figure.
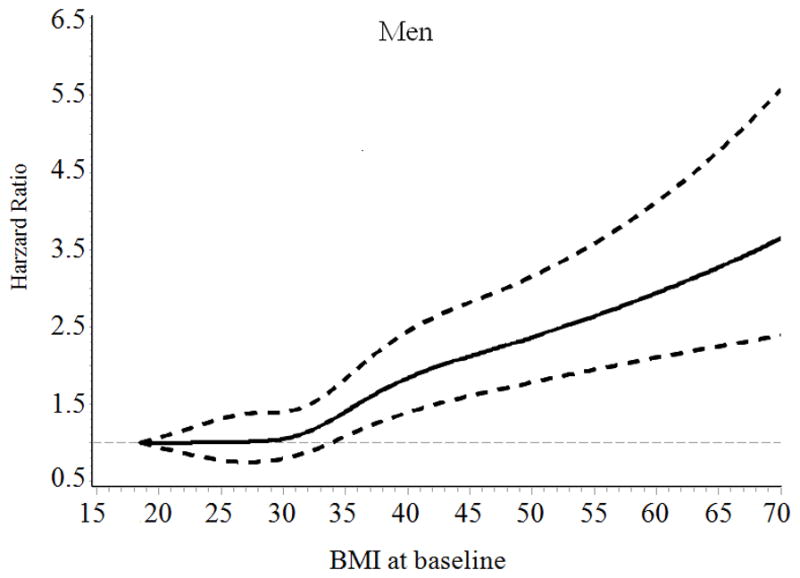
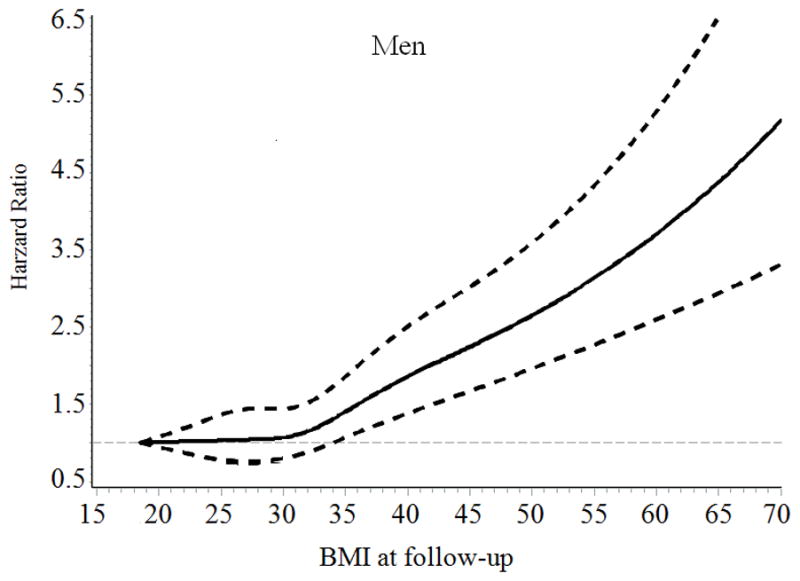
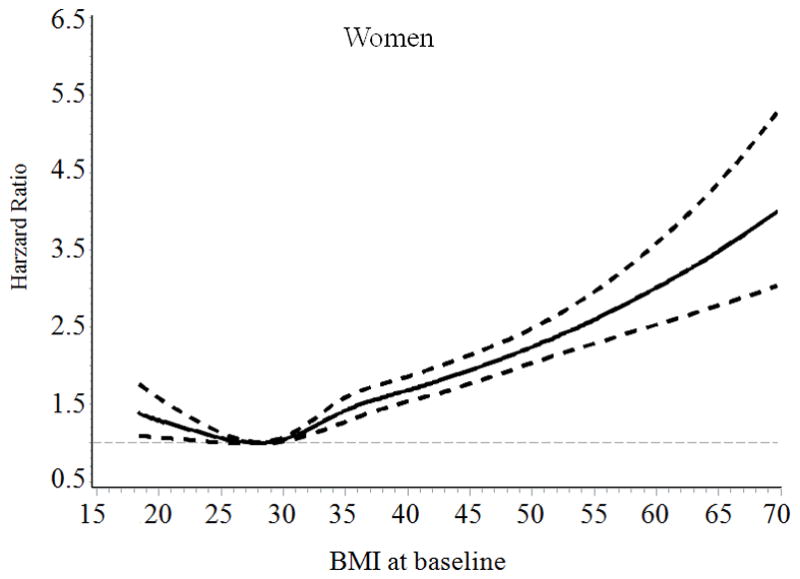
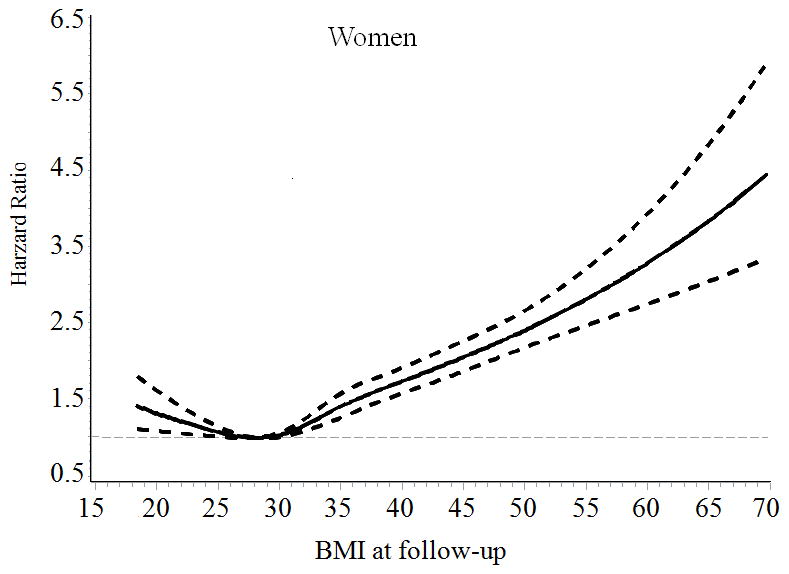
Hazard ratios of heart failure by BMI at baseline and during follow-up among men and women with type 2 diabetes. Hazard ratios are adjusted for age, race, type of health insurance, income, and smoking
Table 2.
Hazard ratios for heart failure according to different levels of body mass index at baseline and during follow-up among patients with type 2 diabetes
| Body mass index during (kg/m2)
|
Each 1 kg/m2 increase | ||||||
|---|---|---|---|---|---|---|---|
| 18.5–22.9 | 23–24.9 | 25.0–29.9 | 30–34.9 | 35–39.9 | ≥40 | ||
| Baseline | |||||||
| Men | 766 | 861 | 3,191 | 3,153 | 1,866 | 1,631 | - |
| No. of cases | 123 | 150 | 573 | 621 | 465 | 447 | - |
| Person-years | 5,802 | 6,410 | 24,226 | 23,577 | 12,885 | 11,166 | - |
| Hazard ratios (95% CIs) | |||||||
| Model 1a | 0.96 (0.80–1.17) | 1.01 (0.85–1.20) | 1.00 | 1.15 (1.03–1.28) | 1.62 (1.43–1.83) | 2.01 (1.77–2.27) | 1.036 (1.031–1.041) |
| Model 2b | 0.95 (0.78–1.15) | 1.00 (0.84–1.19) | 1.00 | 1.16 (1.04–1.29) | 1.64 (1.45–1.85) | 2.02 (1.79–2.29) | 1.037 (1.032–1.042) |
| Model 3c | 0.98 (0.81–1.19) | 1.03 (0.86–1.22) | 1.00 | 1.11 (1.00–1.24) | 1.45 (1.28–1.64) | 1.71 (1.50–1.94) | 1.027 (1.022–1.033) |
| Women | 787 | 916 | 3,997 | 4,815 | 4,001 | 5,171 | - |
| No. of cases | 135 | 153 | 574 | 775 | 737 | 1,081 | - |
| Person-years | 6,397 | 7,522 | 33,527 | 40,004 | 32,162 | 40,134 | - |
| Hazard ratios (95% CIs) | |||||||
| Model 1a | 1.21 (1.01–1.45) | 1.17 (0.98–1.39) | 1.00 | 1.22 (1.10–1.36) | 1.53 (1.38–1.71) | 1.98 (1.78–2.19) | 1.031 (1.027–1.035) |
| Model 2b | 1.16 (1.00–1.37) | 1.16 (0.97–1.38) | 1.00 | 1.23 (1.11–1.37) | 1.55 (1.39–1.73) | 2.01 (1.81–2.23) | 1.032 (1.028–1.036) |
| Model 3c | 1.23 (1.02–1.48) | 1.20 (1.01–1.43) | 1.00 | 1.19 (1.07–1.32) | 1.46 (1.31–1.63) | 1.76 (1.58–1.96) | 1.024 (1.020–1.029) |
| Follow-up | |||||||
| Men | 704 | 853 | 3,305 | 3,254 | 1,859 | 1,493 | - |
| No. of cases | 115 | 154 | 598 | 637 | 451 | 424 | - |
| Person-years | 5,269 | 6,477 | 25,172 | 24,213 | 12,966 | 9,967 | - |
| Hazard ratios (95% CIs) | |||||||
| Model 1a | 0.97 (0.80–1.18) | 1.00 (0.85–1.19) | 1.00 | 1.13 (1.02–1.26) | 1.60 (1.42–1.81) | 2.12 (1.87–2.40) | 1.039 (1.034–1.045) |
| Model 2b | 0.96 (0.79–1.17) | 1.00 (0.84–1.19) | 1.00 | 1.15 (1.03–1.29) | 1.63 (1.44–1.84) | 2.17 (1.91–2.46) | 1.040 (1.035–1.046) |
| Model 3c | 1.00 (0.82–1.22) | 1.00 (0.84–1.19) | 1.00 | 1.10 (0.99–1.23) | 1.42 (1.26–1.61) | 1.81 (1.58–2.07) | 1.030 (1.025–1.036) |
| Women | 715 | 894 | 4,025 | 4,956 | 3,987 | 5,110 | - |
| No. of cases | 139 | 130 | 591 | 777 | 719 | 1,099 | - |
| Person-years | 5,869 | 7,416 | 33,542 | 41,195 | 32,016 | 39,707 | - |
| Hazard ratios (95% CIs) | |||||||
| Model 1a | 1.26 (1.05–1.51) | 0.96 (0.80–1.16) | 1.00 | 1.15 (1.04–1.28) | 1.47 (1.32–1.64) | 2.00 (1.81–2.21) | 1.034 (1.030–1.038) |
| Model 2b | 1.22 (1.02–1.47) | 0.95 (0.79–1.14) | 1.00 | 1.16 (1.05–1.29) | 1.50 (1.35–1.68) | 2.03 (1.84–2.25) | 1.035 (1.031–1.039) |
| Model 3c | 1.28 (1.07–1.54) | 1.00 (0.83–1.21) | 1.00 | 1.13 (1.02–1.25) | 1.40 (1.26–1.56) | 1.71 (1.54–1.90) | 1.026 (1.021–1.030) |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted for age and race.
Adjusted for age, race, types of insurance, income, and smoking.
Adjusted for age, race, types of insurance, income, smoking, LDL cholesterol, systolic blood pressure, HbA1c, glomerular filtration rate, history of obstructive sleep apnea, use of antihypertensive drugs, glucose-lowering agents, and cholesterol-lowering agents at baseline (in baseline analyses) and during follow-up (in the follow-up analyses).
When we performed additional analyses by using an updated mean value of BMI, we found a positive association between BMI and HF risk among men (Ptrend<0.001) and a J-shaped association between BMI and HF risk among women (Pnon-linear <0.001) (Table 2). Women who had low body weight (BMI<23) and obesity (BMI ≥ 30) had an increased risk of HF compared with overweight women (BMI 25–29.9). After excluding subjects who were diagnosed with HF and cancer during the first two years of follow-up (n= 1812), the positive association between BMI and HF risk among men and the J-shaped association between BMI and HF risk among women did not change (data not shown).
When stratified by age, race, smoking status and use of anti-diabetic drugs, the multivariable-adjusted positive association between BMI and HF risk was present among men in most of the subgroups (Table 3), and the multivariable-adjusted J-shaped association between BMI and HF risk was present among women in some of the subgroups (Table 4). As ejection fraction is an important marker for HF diagnosis and treatment, we also conducted additional analyses of BMI with incident HF stratified by ejection fraction ≤ 40% and >40% (Table 5). The positive association was found in incident HF defined as preserved ejection fraction ≤ 40% among men or HF defined as preserved ejection fraction >40% among both men and women.
Table 3.
Hazard ratios (95% confidence intervals) for heart failure among men according to different levels of BMI at baseline and during follow-up among various subpopulations
| Body mass index (kg/m2)
|
||||||
|---|---|---|---|---|---|---|
| <23 | 23–24.9 | 25–29.9 | 30–34.9 | 35–39.9 | ≥40 | |
| Baseline | ||||||
| Race | ||||||
| African American | 0.97 (0.76–1.24) | 1.11 (0.88–1.40) | 1.00 | 1.17 (1.00–1.37) | 1.58 (1.32–1.89) | 1.92 (1.59–2.31) |
| White | 0.92 (0.67–1.27) | 0.86 (0.66–1.13) | 1.00 | 1.16 (0.99–1.36) | 1.71 (1.45–2.02) | 2.17 (1.83–2.57) |
| Age groups, yr | ||||||
| <50 | 1.15 (0.85–1.55) | 1.02 (0.74–1.39) | 1.00 | 0.99 (0.81–1.23) | 1.47 (1.18–1.83) | 1.83 (1.49–2.25) |
| 50–59 | 0.63 (0.43–0.93) | 0.98 (0.72–1.33) | 1.00 | 1.30 (1.09–1.55) | 1.62 (1.34–1.97) | 2.02 (1.66–2.46) |
| 60–94 | 1.10 (0.78–1.54) | 1.09 (0.82–1.45) | 1.00 | 1.13 (0.93–1.38) | 1.67 (1.34–2.09) | 1.77 (1.36–2.32) |
| Smoking status | ||||||
| Never | 1.10 (0.85–1.42) | 1.08 (0.87–1.35) | 1.00 | 1.19 (1.04–1.36) | 1.76 (1.52–2.04) | 2.17 (1.86–2.52) |
| Ever or current | 0.77 (0.57–1.03) | 0.87 (0.66–1.15) | 1.00 | 1.13 (0.93–1.36) | 1.41 (1.13–1.76) | 1.73 (1.38–2.17) |
| Using glucose-lowering agents | ||||||
| No | 0.82 (0.61–1.10) | 0.97 (0.75–1.26) | 1.00 | 1.03 (0.85–1.23) | 1.30 (1.05–1.60) | 1.65 (1.34–2.04) |
| Oral hypoglycemic agents | 1.08 (0.72–1.64) | 1.08 (0.75–1.54) | 1.00 | 1.17 (0.95–1.46) | 1.56 (1.22–1.98) | 2.20 (1.72–2.80) |
| Insulin | 0.97 (0.70–1.35) | 0.92 (0.68–1.26) | 1.00 | 1.35 (1.12–1.63) | 2.05 (1.69–2.48) | 2.37 (1.94–2.90) |
| Follow-up | ||||||
| Race | ||||||
| African American | 1.02 (0.80–1.30) | 1.03 (0.82–1.30) | 1.00 | 1.13 (0.97–1.32) | 1.54 (1.29–1.84) | 2.10 (1.74–2.53) |
| White | 0.84 (0.60–1.18) | 0.97 (0.75–1.26) | 1.00 | 1.20 (1.03–1.40) | 1.75 (1.48–2.07) | 2.32 (1.95–2.75) |
| Age groups, yr | ||||||
| <50 | 1.14 (0.83–1.56) | 0.91 (0.66–1.25) | 1.00 | 1.02 (0.83–1.25) | 1.29 (1.04–1.61) | 2.07 (1.69–2.54) |
| 50–59 | 0.68 (0.46–1.00) | 0.91 (0.66–1.24) | 1.00 | 1.29 (1.08–1.54) | 1.80 (1.49–2.18) | 1.98 (1.62–2.42) |
| 60–94 | 1.09 (0.79–1.52) | 1.25 (0.95–1.64) | 1.00 | 1.08 (0.89–1.31) | 1.61 (1.28–2.03) | 1.97 (1.48–2.61) |
| Smoking status | ||||||
| Never | 1.10 (0.85–1.42) | 1.14 (0.92–1.42) | 1.00 | 1.15 (1.01–1.32) | 1.79 (1.55–2.07) | 2.26 (1.94–2.63) |
| Ever or current | 0.80 (0.60–1.07) | 0.80 (0.61–1.07) | 1.00 | 1.19 (0.99–1.43) | 1.28 (1.02–1.61) | 1.99 (1.58–2.51) |
| Using glucose-lowering agents | ||||||
| No | 0.79 (0.59–1.07) | 1.04 (0.81–1.34) | 1.00 | 1.00 (0.83–1.20) | 1.38 (1.12–1.70) | 1.72 (1.38–2.14) |
| Oral hypoglycemic agents | 1.20 (0.79–1.81) | 1.25 (0.88–1.76) | 1.00 | 1.32 (1.07–1.64) | 1.84 (1.44––2.34) | 2.39 (1.85–3.09) |
| Insulin | 1.00 (0.72–1.39) | 0.73 (0.52–1.03) | 1.00 | 1.26 (1.05–1.51) | 1.71 (1.41–2.07) | 2.47 (2.03–3.00) |
Adjusted for age, race, income, type of insurance, and smoking at baseline (in the baseline analyses) and during follow-up (in the follow-up analyses) other than the variable for stratification.
Among individuals who had ejection fraction measured at baseline.
Table 4.
Hazard ratios (95% confidence intervals) for heart failure among women according to different levels of BMI at baseline and during follow-up among various subpopulations
| Body mass index (kg/m2)
|
||||||
|---|---|---|---|---|---|---|
| <23 | 23–24.9 | 25–29.9 | 30–34.9 | 35–39.9 | ≥40 | |
| Baseline | ||||||
| Race | ||||||
| African American | 1.13 (0.89–1.44) | 1.12 (0.88–1.41) | 1.00 | 1.18 (1.03–1.36) | 1.46 (1.26–1.68) | 1.91 (1.67–2.19) |
| White | 1.19 (0.89–1.58) | 1.19 (0.92–1.55) | 1.00 | 1.31 (1.11–1.54) | 1.70 (1.44–2.01) | 2.18 (1.86–2.56) |
| Age groups, yr | ||||||
| <50 | 1.21 (0.86–1.70) | 1.09 (0.77–1.52) | 1.00 | 1.12 (0.91–1.36) | 1.25 (1.03–1.53) | 1.53 (1.28–1.84) |
| 50–59 | 1.42 (1.04–1.93) | 1.24 (0.92–1.66) | 1.00 | 1.23 (1.03–1.47) | 1.60 (1.34–1.91) | 2.11 (1.78–2.49) |
| 60–94 | 0.96 (0.71–1.32) | 1.18 (0.89–1.57) | 1.00 | 1.25 (1.04–1.49) | 1.62 (1.35–1.96) | 2.15 (1.78–2.60) |
| Smoking status | ||||||
| Never | 1.17 (0.93–1.46) | 1.11 (0.90–1.37) | 1.00 | 1.21 (1.07–1.37) | 1.50 (1.32–1.70) | 1.93 (1.72–2.18) |
| Ever or current | 1.18 (0.85–1.63) | 1.30 (0.95–1.79) | 1.00 | 1.30 (1.05–1.61) | 1.73 (1.39–2.16) | 2.25 (1.82–2.78) |
| Using glucose-lowering agents | ||||||
| No | 1.13 (0.86–1.49) | 1.03 (0.78–1.37) | 1.00 | 1.10 (0.92–1.32) | 1.46 (1.22–1.76) | 1.93 (1.61–2.30) |
| Oral hypoglycemic agents | 1.12 (0.74–1.70) | 1.28 (0.91–1.80) | 1.00 | 1.33 (1.08–1.64) | 1.50 (1.21–1.86) | 2.02 (1.64–2.48) |
| Insulin | 1.14 (0.83–1.55) | 1.23 (0.92–1.65) | 1.00 | 1.25 (1.05–1.49) | 1.58 (1.33–1.88) | 2.05 (1.74–2.42) |
| Follow-up | ||||||
| Race | ||||||
| African American | 1.22 (0.96–1.56) | 1.01 (0.79–1.29) | 1.00 | 1.18 (1.03–1.36) | 1.48 (1.28–1.71) | 2.06 (1.80–2.37) |
| White | 1.20 (0.91–1.58) | 0.86 (0.65–1.14) | 1.00 | 1.15 (0.98–1.34) | 1.53 (1.30–1.80) | 2.04 (1.75–2.38) |
| Age groups, yr | ||||||
| <50 | 1.44 (1.01–2.04) | 0.95 (0.65–1.38) | 1.00 | 1.20 (0.98–1.47) | 1.32 (1.08–1.62) | 1.71 (1.42–2.06) |
| 50–59 | 1.44 (1.06–1.97) | 1.10 (0.80–1.49) | 1.00 | 1.15 (0.96–1.37) | 1.45 (1.21–1.73) | 2.19 (1.86–2.58) |
| 60–94 | 1.02 (0.76–1.37) | 0.88 (0.66–1.18) | 1.00 | 1.09 (0.92–1.30) | 1.61 (1.34–1.93) | 1.87 (1.55–2.26) |
| Smoking status | ||||||
| Never | 1.22 (0.98–1.53) | 1.00 (0.80–1.24) | 1.00 | 1.16 (1.02–1.31) | 1.47 (1.30–1.66) | 1.97 (1.76–2.22) |
| Ever or current | 1.23 (0.89–1.69) | 0.86 (0.60–1.22) | 1.00 | 1.19 (0.96–1.46) | 1.62 (1.30–2.01) | 2.23 (1.81–2.74) |
| Using glucose-lowering agents | ||||||
| No | 1.33 (1.03–1.74) | 0.89 (0.66–1.21) | 1.00 | 1.12 (0.94–1.33) | 1.43 (1.19–1.72) | 1.87 (1.57–2.23) |
| Oral hypoglycemic agents | 1.00 (0.67–1.50) | 0.95 (0.67–1.35) | 1.00 | 1.05 (0.86–1.29) | 1.46 (1.18–1.80) | 1.94 (1.59–2.37) |
| Insulin | 1.23 (0.88–1.71) | 1.06 (0.77–1.45) | 1.00 | 1.26 (1.06–1.49) | 1.51 (1.27–1.80) | 2.16 (1.84–2.54) |
Adjusted for age, race, income, type of insurance, and smoking at baseline (in the baseline analyses) and during follow-up (in the follow-up analyses) other than
the variable for stratification.
Table 5.
Hazard ratios (95% confidence intervals) of different subtypes of heart failure according to ejection fraction with different levels of BMI at baseline and during follow-up
| Body mass index (kg/m2)
|
||||||
|---|---|---|---|---|---|---|
| <23 | 23–24.9 | 25–29.9 | 30–34.9 | 35–39.9 | ≥40 | |
| Baseline | ||||||
| Total heart failure | ||||||
| Men | 0.95 (0.78–1.15) | 1.00 (0.84–1.19) | 1.00 | 1.16 (1.04–1.29) | 1.64 (1.45–1.85) | 2.02 (1.79–2.29) |
| Women | 1.16 (1.00–1.37) | 1.16 (0.97–1.38) | 1.00 | 1.23 (1.11–1.37) | 1.55 (1.39–1.73) | 2.01 (1.81–2.23) |
| Ejection Fraction ≤40% | ||||||
| Men | 0.83 (0.59–1.16) | 1.11 (0.83–1.47) | 1.00 | 1.12 (0.92–1.35) | 1.49 (1.21–1.85) | 1.62 (1.30–2.04) |
| Women | 1.53 (1.01–2.30) | 1.58 (1.08–2.31) | 1.00 | 1.38 (1.08–1.78) | 1.32 (1.01–1.73) | 1.44 (1.11–1.87) |
| Ejection Fraction >40% | ||||||
| Men | 0.69 (0.47–1.01) | 0.82 (0.59–1.14) | 1.00 | 1.20 (0.99–1.45) | 1.99 (1.63–2.43) | 2.84 (2.34–3.46) |
| Women | 0.87 (0.65–1.17) | 0.97 (0.75–1.27) | 1.00 | 1.11 (0.95–1.30) | 1.65 (1.42–1.92) | 2.39 (2.07–2.75) |
| Follow-up | ||||||
| Total heart failure | ||||||
| Men | 0.96 (0.79–1.17) | 1.00 (0.84–1.19) | 1.00 | 1.15 (1.03–1.29) | 1.63 (1.44–1.84) | 2.17 (1.91–2.46) |
| Women | 1.22 (1.02–1.47) | 0.95 (0.79–1.14) | 1.00 | 1.16 (1.05–1.29) | 1.50 (1.35–1.68) | 2.03 (1.84–2.25) |
| Ejection Fraction ≤40% | ||||||
| Men | 0.84 (0.60–1.18) | 1.10 (0.83–1.46) | 1.00 | 1.00 (0.83–1.21) | 1.57 (1.28–1.93) | 1.59 (1.26–2.00) |
| Women | 1.46 (0.97–2.19) | 1.22 (0.83–1.81) | 1.00 | 1.00 (0.78–1.29) | 1.43 (1.11–1.84) | 1.25 (0.97–1.62) |
| Ejection Fraction >40% | ||||||
| Men | 0.58 (0.38–0.89) | 0.94 (0.69–1.29) | 1.00 | 1.41 (1.17–1.70) | 1.90 (1.54–2.33) | 3.47 (2.84–4.22) |
| Women | 0.87 (0.65–1.18) | 0.76 (0.57–1.02) | 1.00 | 1.12 (0.96–1.30) | 1.50 (1.29–1.76) | 2.53 (2.20–2.91) |
Adjusted for age, race, income, type of insurance, and smoking at baseline (in the baseline analyses) and during follow-up (in the follow-up analyses).
Discussion
Our study found a positive association between BMI and HF risk among men and a J-shaped association between BMI and HF risk among women patients with type 2 diabetes. A significantly increased risk of HF was observed among men with BMI ≥ 30 kg/m2, and among women with BMI ≥ 30 kg/m2 and BMI 18.5–22.9 kg/m2, compared with those with BMI 25–29.9 kg/m2.
Several previous studies have assessed the association between BMI and all-cause or CVD-mortality among patients with diabetes, and the results are inconsistent, including inverse associations,5,8–10 positive associations,11,12 U-shaped associations,13–15 or no association.16,17 The limitations of these studies included short follow-up duration,14–16 a small number of deaths,5,17 suboptimal control for smoking status and preexisting chronic conditions,5,14–17 and use of BMI up to several decades before diabetes diagnosis.14,16,17 Considering all of the above limitations, a recently published paper from two large prospective cohort studies concluded a J-shaped association between BMI and mortality among all diabetic participants and among those who had ever smoked, and a direct linear relationship among those who had never smoked.31 However, as the subjects in this study were female nurses and male health professionals who were at higher social-economic status, the results are limited to be generalized to the overall population.
Our study found a positive association between BMI and HF risk among men and a J-shaped association between BMI and HF risk among women with type 2 diabetes. Diabetic patients with BMI ≥ 30 kg/m2 have a significantly increased risk of HF as compared with those with BMI 25–29.9 kg/m2. There is substantial evidence supporting the biologic plausibility of a positive association between excess adiposity and the risk of CVD including HF,6,7 and the mechanism was suggested that adipose tissue can release a large number of cytokines and bioactive mediators which play important roles in the pathogenesis of many obesity related CVDs.32 It is noteworthy that among women with diabetes, a significantly increased risk of HF was also observed among those with BMI 18.5–22.9 kg/m2 as compared with those with BMI 25–29.9 kg/m2. The reason for this increased risk of HF among low-normal weight patients might be that diabetic patients with normal weight have metabolically obese normal-weight (MONW), who have hyperinsulinemia, insulin resistance, and dyslipidemia.33 There is another hypothesis that lean individuals may have a stronger genetic predisposition of type 2 diabetes.34
We have conducted the following analyses to ensure the rigor of the results. First, at baseline, people with a history of stroke or HF before the diagnosis of diabetes were excluded. Second, after further control of potential physiologic effects of excess fatness, such as hypertension, HbA1c, and dyslipidemia, the association did not change. Third, we performed a sensitivity analysis by excluding subjects who were diagnosed as HF and cancer during the first two years of follow-up, as these subjects might have been seriously sick or had reduced BMI, and we found the association did not change. Finally, we stratified by race, age, smoking status, and the use of glucose-lowering agents, and the association between BMI and incident HF risk was similar among the different sub-groups.
In recent years, some studies have focused on the role of smoking in the analyses of body weight with mortality because smoking is associated with decreased body weight but with an increased risk of death.35 The attenuated relationship between BMI and mortality among smokers has been observed both in the general population36 and in patients with diabetes.31 However, it is unclear whether this difference between smokers and non-smokers represents a biological difference or is largely due to bias.35 Contrary to the above studies, our study found no effect modification according to smoking status. Future studies are needed to clarify whether there is biologic difference related to smoking.
There are several strengths in our study. First, it is the first large-sample study with long follow-up time focusing on BMI and incident HF among diabetic patients. Second, we have used BMI measurements at baseline and updated mean values, which can avoid potential bias from a single baseline measurement. Third, participants in this study used the same public health care system which minimizes bias related to accessibility to health care. Finally, all data in analyses (like BMI) are from administrative databases which minimizes differential recall bias. This study also has limitations. First, our analysis was not performed on a representative sample of diabetic patients, which limits the generalizability of the results. However, LSUHCSD hospitals were public hospitals and covered over 1.6 million patients, most of whom are low income persons in Louisiana. Thus, the results of the present study will have wide applicability for diabetic patients with low income in the US. Second, although diagnosis of HF in the LSUHCSD hospitals has been combined with both the Framingham Criteria for Heart Failure diagnosis and echocardiogram, there are limitations in the diagnosis of HF with preserved ejection fraction, which have been discussed by a recent review.37 Third, although our analyses adjusted for an extensive set of confounding factors, residual confounding due to the measurement error in the assessment of confounding factors, unmeasured factors such as physical activity, education, and dietary factors, cannot be excluded.
In summary, this large hospital-based cohort study suggested a positive association between BMI and HF risk among men and a J-shaped association between BMI and HF risk among women with type 2 diabetes, regardless of age, race, smoking status, and use of glucose-lowering agents.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by Louisiana State University’s Improving Clinical Outcomes Network (LSU ICON), the Louisiana Clinical Data Research Network (LACDRN), and 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science (LA CaTS) Center.
Footnotes
Disclosures
None.
References
- 1.Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the u.S. Population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in us men and women: Nhanes i epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the acc/aha 2005 guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 8.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: An analysis of the proactive study population. Int J Cardiol. 2012;162:20–26. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Kokkinos P, Myers J, Faselis C, Doumas M, Kheirbek R, Nylen E. Bmi-mortality paradox and fitness in african american and caucasian men with type 2 diabetes. Diabetes Care. 2012;35:1021–1027. doi: 10.2337/dc11-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwen LN, Kim C, Karter AJ, Haan MN, Ghosh D, Lantz PM, Mangione CM, Thompson TJ, Herman WH. Risk factors for mortality among patients with diabetes: The translating research into action for diabetes (triad) study. Diabetes Care. 2007;30:1736–1741. doi: 10.2337/dc07-0305. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among finnish adults with diabetes. Diabetes Care. 2005;28:799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 12.Katzmarzyk PT, Hu G, Cefalu WT, Mire E, Bouchard C. The importance of waist circumference and bmi for mortality risk in diabetic adults. Diabetes Care. 2013;36:3128–3130. doi: 10.2337/dc13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.So WY, Yang X, Ma RC, Kong AP, Lam CW, Ho CS, Cockram CS, Ko GT, Chow CC, Wong V, Tong PC, Chan JC. Risk factors in v-shaped risk associations with all-cause mortality in type 2 diabetes-the hong kong diabetes registry. Diabetes Metab Res Rev. 2008;24:238–246. doi: 10.1002/dmrr.792. [DOI] [PubMed] [Google Scholar]
- 14.Khalangot M, Tronko M, Kravchenko V, Kulchinska J, Hu G. Body mass index and the risk of total and cardiovascular mortality among patients with type 2 diabetes: A large prospective study in ukraine. Heart. 2009;95:454–460. doi: 10.1136/hrt.2008.150524. [DOI] [PubMed] [Google Scholar]
- 15.Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A, Philip S, Wild S, Sattar N. Association between bmi measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. 2013;36:887–893. doi: 10.2337/dc12-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen LN, Karter AJ, Waitzfelder BE, Crosson JC, Marrero DG, Mangione CM, Herman WH. Predictors of mortality over 8 years in type 2 diabetic patients: Translating research into action for diabetes (triad) Diabetes Care. 2012;35:1301–1309. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoppini G, Verlato G, Leuzinger C, Zamboni C, Brun E, Bonora E, Muggeo M. Body mass index and the risk of mortality in type ii diabetic patients from verona. Int J Obes Relat Metab Disord. 2003;27:281–285. doi: 10.1038/sj.ijo.802199. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Wang Y, Chen L, Horswell R, Xiao K, Besse J, Johnson J, Ryan DH, Hu G. Increasing prevalence of diabetes in middle or low income residents in louisiana from 2000 to 2009. Diabetes Res Clin Pract. 2011;94:262–268. doi: 10.1016/j.diabres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Li W, Wang Y, Chen L, Horswell R, Xiao K, Besse J, Johnson J, Ryan DH, Hu G. Increasing prevalence of hypertension in low income residents within louisiana state university health care services division hospital system. Eur J Intern Med. 2012;23:e179–184. doi: 10.1016/j.ejim.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Horswell R, Wang Y, Li W, Besse J, Xiao K, Chen H, Keller JN, Heymsfield SB, Ryan DH, Katzmarzyk PT. Body mass index and the risk of dementia among louisiana low income diabetic patients. PLoS One. 2012;7:e44537. doi: 10.1371/journal.pone.0044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Katzmarzyk PT, Horswell R, Li W, Xiao K, Besse J, Xie W, Johnson J, Heymsfield S, Ryan DH, Hu G. Racial disparities in diabetic complications in an underinsured population. J Clin Endocrinol Metab. 2012;97:4446–4453. doi: 10.1210/jc.2012-2378. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Cefalu WT, Ryan DH, Hu G. Blood pressure and stroke risk among diabetic patients. J Clin Endocrinol Metab. 2013;98:3653–3662. doi: 10.1210/jc.2013-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. Hba1c and coronary heart disease risk among diabetic patients hba1c and chd risk in diabetic patients. Diabetes Care. 2014;37:428–435. doi: 10.2337/dc13-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Li W, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Aggressive blood pressure control increases coronary heart disease risk among diabetic patients. Diabetes Care. 2013;36:3287–3296. doi: 10.2337/dc13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Hba1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care. 2013;36:3591–3598. doi: 10.2337/dc13-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 27.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (ukpds 36): Prospective observational study. Bmj. 2000;321:412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in framingham heart study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 30.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 31.Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–244. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 33.Conus F, Rabasa-Lhoret R, Peronnet F. Characteristics of metabolically obese normal-weight (monw) subjects. Appl Physiol Nutr Metab. 2007;32:4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 34.Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, Grallert H, Navarro P, Li M, Qi L, Steinthorsdottir V, Scott RA, Almgren P, Arking DE, Aulchenko Y, Balkau B, Benediktsson R, Bergman RN, Boerwinkle E, Bonnycastle L, Burtt NP, Campbell H, Charpentier G, Collins FS, Gieger C, Green T, Hadjadj S, Hattersley AT, Herder C, Hofman A, Johnson AD, Kottgen A, Kraft P, Labrune Y, Langenberg C, Manning AK, Mohlke KL, Morris AP, Oostra B, Pankow J, Petersen AK, Pramstaller PP, Prokopenko I, Rathmann W, Rayner W, Roden M, Rudan I, Rybin D, Scott LJ, Sigurdsson G, Sladek R, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Uitterlinden AG, Vivequin S, Weedon MN, Wright AF, Hu FB, Illig T, Kao L, Meigs JB, Wilson JF, Stefansson K, van Duijn C, Altschuler D, Morris AD, Boehnke M, McCarthy MI, Froguel P, Palmer CN, Wareham NJ, Groop L, Frayling TM, Cauchi S. Stratifying type 2 diabetes cases by bmi identifies genetic risk variants in lama1 and enrichment for risk variants in lean compared to obese cases. PLoS genetics. 2012;8:e1002741. doi: 10.1371/journal.pgen.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manson JE, Stampfer MJ, Hennekens CH, Willett WC. Body weight and longevity. A reassessment. JAMA. 1987;257:353–358. [PubMed] [Google Scholar]
- 36.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersson C, Vasan RS. Epidemiology of heart failure with preserved ejection fraction. Heart failure clinics. 2014;10:377–388. doi: 10.1016/j.hfc.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


