Abstract
Targeting angiogenesis, one of the hallmarks of carcinogenesis, using non-toxic phytochemicals has emerged as a translational opportunity for angioprevention and to control advanced stages of malignancy. Herein, we investigated the inhibitory effects and associated mechanism/s of action of Procyanidin B2-3,3″-di-O-gallate (B2G2), a major component of grape seed extract, on human umbilical vein endothelial cells (HUVECs) and human prostate microvascular endothelial cells (HPMECs). Our results showed that B2G2 (10–40 μM) inhibits growth and induces death in both HUVECs and HPMECs. Additional studies revealed that B2G2 causes a G1 arrest in cell cycle progression of HUVECs by down-regulating cyclins (D1 and A), CDKs (Cdk2 and Cdc2) and Cdc25c phosphatase and up-regulating CDK inhibitors (p21 and p27) expression. B2G2 also induced strong apoptotic death in HUVECs through increasing p53, Bax and Smac/Diablo expression while decreasing Bcl-2 and survivin levels. Additionally, B2G2 inhibited the growth factors-induced capillary tube formation in HUVECs and HPMECs. Interestingly, conditioned media (CCM) from prostate cancer (PCA) cells (LNCaP and PC3) grown under normoxic (~21% O2) and hypoxic (1% O2) conditions significantly enhanced the tube formation in HUVECs, which was compromised in presence of conditioned media from B2G2-treated PCA cells. B2G2 also inhibited the motility and invasiveness of both HUVECs and HPMECs. Mechanistic studies showed that B2G2 targets VEGFR2/PI3K/Akt and integrin signaling molecules which are important for endothelial cells survival, proliferation, tube formation and motility. Overall, we report that B2G2 inhibits several attributes of angiogenesis in cell culture; therefore, warrants further investigation for its efficacy for angioprevention and cancer control.
Keywords: Angioprevention, Apoptosis, B2G2, Cell cycle, Endothelial cells, Integrin, VEGFR2
INTRODUCTION
Angiogenesis refers to the formation of new blood vessels from preexisting ones and is required in many physiological and pathological conditions like embryonic development, wound healing, tissue regeneration and tumor growth [1, 2]. Developing tumors are fast growing mass of cells with high metabolic rate and their requirement for nutrients is far greater than normal tissues. Likewise, tumor cells also produce more metabolic wastes than normal cells [3, 4]. Therefore, in order to ensure a constant supply of nutrients and oxygen along with efficient removal of metabolic waste, developing solid tumor promotes angiogenesis for sustained growth and progression [3, 5, 6]. Angiogenesis is a complex multi-step process which is tightly regulated by endogenous pro- and anti-angiogenic factors [7, 8]. Tumor cells including prostate cancer (PCA) cells are known to secrete various pro-angiogenic factors and the secretion of these factors is further potentiated under low oxygen (hypoxic) conditions to enhance the blood supply [5, 9, 10]. Among the pro-angiogenic factors secreted by tumor cells, vascular endothelial growth factor (VEGF) is considered as the most important growth factor responsible for angiogenesis, and thus, for sustaining tumor growth. VEGF promotes angiogenesis by activating endothelial cells to enhance their proliferation, migration and invasiveness of these cells which results in the formation of new blood vessels [11–13]. Due to the fact that tumor cells depend heavily on angiogenesis for their growth and progression, angiogenesis has emerged as an attractive target for both cancer chemoprevention and therapy [7, 8, 14–16].
Several angiogenesis inhibitors have been identified due to the dedicated efforts of various groups to this field, and many of these inhibitors have already been approved by U.S. Food and Drug Administration (FDA) for their use either alone or in combination with chemotherapeutic drugs and several others are at different stages of clinical trials [17, 18]. Most of the identified angiogenesis inhibitors act by targeting VEGF/VEGF receptor (VEGFR) signaling, which in principle, is a sound strategy to inhibit solid tumor growth and yields positive result initially; however, in due course of time, tumor cells develop resistance through circumventing these molecules and continue to spread vascular networks [19]. Moreover, recent research has also shown that beside VEGF, tumor cells secrete other pro-angiogenic factors like basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF) and transforming growth factor (TGF) to promote angiogenesis [3, 20, 21]. Furthermore, these treatment options are often associated with the burden of high cost, serious side effects and unacceptable level of toxicity; therefore, broad spectrum angiogenesis inhibitors that are safe, non-toxic and cost-effective are needed [7, 22, 23].
As per the cancer incidence data, it has been observed that Asian populations show lower risk of acquiring various types of cancers including PCA than their Western counterparts, and this has been linked to their higher consumption of vegetarian diet including fruits and vegetables along with lesser consumption of red meat, animal fat and processed food [24–26]. The preventive and inhibitory effects of more consumption of fruits and vegetables on the risk of acquiring specific cancers has been attributed to the presence of key phytochemicals that modulate signaling pathways deregulated in cancer [2, 15]. Therefore, many phytochemicals have become prime focus of scientific investigations for their anti-cancer and angiopreventive activities, and are already reported to inhibit tumor angiogenesis in in vitro as well as in vivo models [7, 27–29]. In the present study, we evaluated the potential anti-angiogenic efficacy of one such phytochemical namely Procyanidin B2 3,3″-di-O-gallate (B2G2) (Fig 1A) in various established angiogenesis-related cell culture models. B2G2 has been identified by our laboratory as the most active constituent of grape seed extract (GSE), a naturally occurring dietary agent with proven potential against various malignancies including PCA [30–32]. Earlier, we have shown that B2G2 is effective against various PCA cells such as LNCaP, C4-2B, DU145 and PC-3 by causing growth inhibition and inducing apoptosis in these cells; however, its anti-angiogenic activity is still unknown [31, 32]. Our results suggest that B2G2 inhibits proliferation, capillary tube formation, motility and invasiveness of endothelial cells by inducing cell cycle arrest and apoptosis and by targeting VEGFR2/PI3K/Akt and Integrin signaling pathways, which indicate towards its strong and promising anti-angiogenic activity.
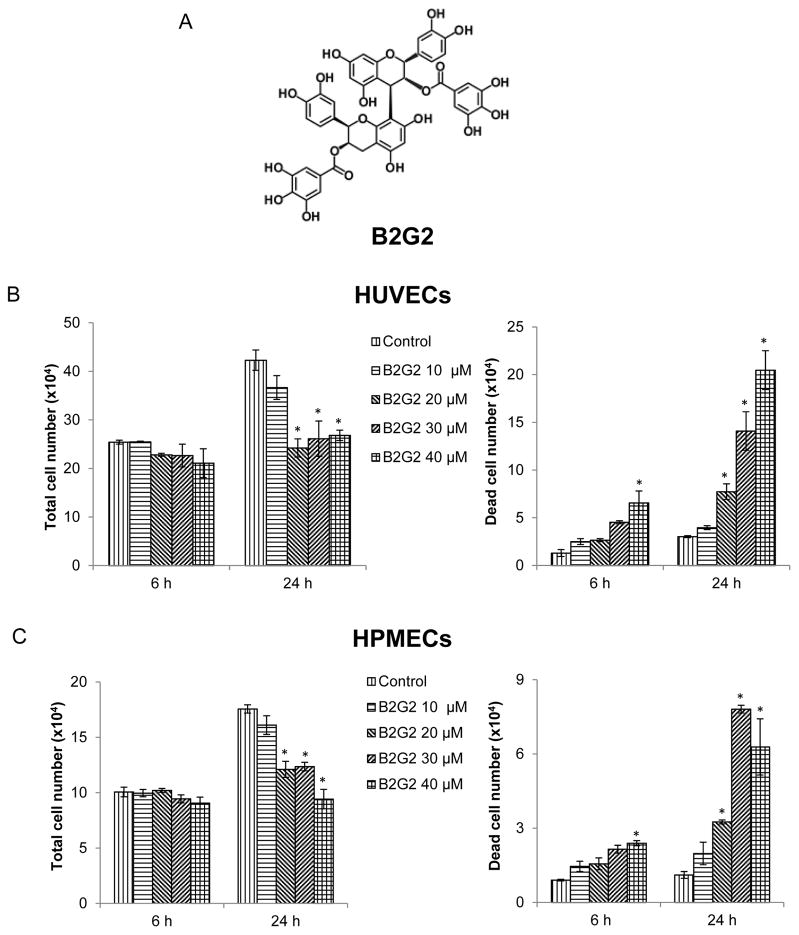
Fig. 1.
Effect of B2G2 on endothelial cells growth and proliferation. (A) Chemical structure of Procyanidin B2 3,3″-di-O-gallate (B2G2). (B–C) HUVECs and HPMECs were grown in complete EGM-2 media with 2% FBS at the density of 5 × 104 cell/well in six well plate. After 24 h of seeding, cells were treated with 10 to 40 μM concentrations of B2G2 for 6 h and 24 h. At the end of each time point, cells were harvested and counted as mentioned in ‘Materials and Methods’, and total cell number and percentage of dead cells are shown. Each value represents mean ± S.E. of three samples for each treatment. *p<0.05, significant with respect to control.
MATERIALS AND METHODS
Cell lines and reagents
Human umbilical vein endothelial cells (HUVECs, Cat # cAP-0001) and human prostate microvascular endothelial cells (HPMECs, Cat # cAP-0014) were purchased from Angio-Proteomie (Boston, MA) and cultured in EBM-2 medium supplemented with 2% fetal bovine serum (FBS) and supplements (hFGF, VEGF, IGF-1, hEGF, Hydrocortisone, Ascorbic acid, GA-1000 and Heparin) (EGM-2 bulletkit) (Lonza, Walkersville, MD) under standard culture conditions (37°C, 95% humidified air and 5% CO2). LNCaP and PC3 human PCA cell lines were from ATCC (Manassas, VA) and cultured under standard culture conditions. Antibodies for Bax (Cat # 2772), Bcl-2 (Cat # 4223), Smac/Diablo (Cat # 2954), cleaved poly-(ADP-ribose) polymerase (PARP) (Cat # 9546), Cyclin D1 (Cat # 2922), Cdc25c (Cat # 4688), Survivin (Cat # 2803), Integrin β1 (Cat # 9699), Integrin β3 (Cat # 13166), Integrin β5 (Cat # 3629), Integrin αv (Cat # 4711), ILK1 (Cat # 3862), Cdc42 (Cat # 2462), α-Actinin (Cat # 3134), Vinculin (Cat # 4650), ARP2 (Cat # 3128), ARP3 (Cat # 4738), VEGFR2 (Cat # 9698), PI3K (Cat # 4292), PDK1 (Cat # 5662), Akt (Cat # 4685), ERK1/2 (Cat # 9102), Src (Cat # 2109), FAK (Cat # 13009) and antibodies recognizing p-VEGFR2 (Cat # 2478), p-PI3K (Cat # 4228), p-PDK1 (Cat # 3061), p-Akt (Cat # 4060), p-ERK1/2 (Cat # 4370), p-Src (Cat # 2101), p-FAK (Cat # 3283) and peroxidase conjugated secondary antibodies were from Cell Signaling Technology (Beverly, MA). Antibodies for Cyclin A (Cat # sc-751), p21 (Cat # sc-397), p27 (Cat # sc-528), p53 (Cat # sc-6243), Cdk2 (Cat # sc-163), Cdk4 (Cat # sc-749), Cdc2 (Cat # sc-54) and β-actin (Cat # sc-1615) were from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence detection system was from GE healthcare (Buckinghamshire, UK). Matrigel were procured from BD Biosciences (San Jose, CA). B2G2 was synthesized according to the method published recently [32]. B2G2 stock solution was prepared in DMSO and stored at −80°C. An equal amount of DMSO (vehicle) was present in each treatment, including control; DMSO concentration did not exceed 0.1% (v/v) in any treatment. DMSO treated cells served as control in each experiment.
Cell viability assay
HUVECs and HPMECs were seeded (5 × 104/well) in 6-well culture plates in EGM-2 medium with 2% FBS and growth factors (VEGF, FGF, IGF and EGF) provided by the vendor. After 24 h, cells were treated with different concentrations of B2G2 (10, 20, 30 and 40 μM) for 6 or 24 h. At the end of each treatment time, cells were collected and total cell number and dead cell number were determined using a hemocytometer after trypan blue staining. Notably, based on cell death results showing minimal B2G2 effect at all concentrations after 6 h of its treatment, this time point was used in all biological studies (tube formation, invasion and migration) so that the experiments are not masked by cell death effect of B2G2 observed at 24 h (data shown in results section).
Cell cycle distribution and apoptosis
HUVECs were cultured in EGM-2 bullet kit and treated with different concentrations of B2G2 (10, 20, 30 and 40 μM) for 24 h. At the end of each treatment time, cells were collected and split equally in two parts. One part was subjected to Annexin V-PI staining for estimation of apoptotic cell death using Annexin V/Dead cell apoptosis kit (Invitrogen, Eugene, OR) and the other part was processed for cell cycle analysis following saponin/PI staining as described previously and were analyzed by FACS [32] using UCCC flow cytometry shared-resources facility.
Capillary Tube Formation Assay
HUVECs and HPMECs (3 × 104) were cultured in 1.0 ml EBM2 (containing 0.5% FBS with or without growth factors) in presence of B2G2 (20, 30 and 40 μM) on matrigel coated plates. Tubular structure formed by HUVECs and HPMECs, after 6 h and 8 h respectively, was photographed with Zeiss inverted microscope (at 100x) using Cannon Power Shot A640 camera and AxioVision Rel.4.7 software. In a related experiment, LNCaP and PC3 cells were treated with either DMSO or 30 μM dose of B2G2 under normoxic (~21% O2) and hypoxic (1% O2) conditions in culture medium with 0.5% FBS. Media was collected after 6 h, centrifuged and supernatant was labelled as either control conditioned media (CCM) or B2G2 conditioned media (BCM). These conditioned media were mixed with EBM2 media containing 0.5% FBS (75:25 ratio), and then added onto HUVECs and tube formation was studied as described above. The tubular structure formation by HUVECs and HPMECs was quantified by calculating the tube length (at 100x) with Zeiss inverted microscope using Cannon Power Shot A640 camera and AxioVision Rel.4.7 software as described previously [27].
Transwell Invasion Assay
The effect of B2G2 treatment on the invasiveness of HUVECs and HPMECs was assessed by matrigel invasion assay using BD BioCoat Matrigel invasion chambers (BD Biosciences, San Jose, CA). In this assay, the bottom chambers of transwell were filled with EGM-2 media containing 2% FBS supplemented with various growth factors, and in the top chambers HUVECs or HPMECs (3 × 104) were seeded in 500 μL EBM-2 media containing 0.5% FBS with or without B2G2 (20, 30 and 40 μM). After 10 h, non-invasive cells that remained on the upper side of the filter membrane were gently removed by scrubbing and the invasive cells on the lower side of the filter membrane were fixed with ice cold methanol for 5 min followed by staining with hematoxylin and eosin and quantified as described previously [28].
Transwell Migration assay
The effect of B2G2 treatment on the migratory potential of HUVECs and HPMECs was assessed by transwell migration assay using Falcon 24-well cell culture inserts (BD Biosciences, San Jose, CA) with 8-μm pores. In this assay, the bottom chambers of transwell were filled with EGM-2 media containing 2% FBS supplemented with various growth factors, and in the top chambers HUVECs or HPMECs (3 × 104) were seeded in 500 μL EBM-2 media containing 0.5% FBS with or without B2G2 (20, 30 and 40 μM). After 6 h, cells that did not migrate through the pores and therefore remained on the upper side of the filter membrane were gently removed by scrubbing and the migrated cells on the lower side of the filter membrane were fixed with ice cold methanol for 5 min followed by staining with hematoxylin and eosin and quantified as described previously [28].
Immunoblot analysis
HUVECs were treated with desired concentrations of B2G2 for 6 h and 24 h and total cell lysates were prepared in non-denaturing lysis buffer (10 mM Tris–HCl, pH 7.4,150 mM NaCl, 1% Triton X-100, 1 mM ethylene diamine tetra acetic acid, 1 mM ethylene glycol-bis (amino ethyl ether)-tetra acetic acid, 0.5% NP-40, 0.3 mM phenyl methyl sulfonyl fluoride, 0.2 mM sodium orthovanadate and 5 U/ml aprotinin). Protein concentration in lysates was determined using DC Protein assay system (Bio Rad, Hercules, CA). For immunoblot analyses, 50–80 μg protein lysates were run on sodium dodecyl sulfate–polyacrylamide gels and blotted onto nitrocellulose membranes that were probed for desired proteins using specific primary antibodies (dilution - 1:1000) followed by peroxidase-conjugated appropriate secondary antibody (dilution – 1: 2000) and visualized by enhanced chemiluminescence detection system (GE biosciences). Membranes were also stripped and re-probed again for protein/s of interest or β-actin antibody to check protein loading; however, only representative loading control blots are shown.
Statistical Analysis
Statistical analysis was performed using SigmaStat 2.03 software (Jandel Scientific, San Rafael, CA). Data was analyzed using one way ANOVA (Tukey test) and a statistically significant difference was considered to be at p<0.05.
RESULTS
Effect of B2G2 on cell viability in HUVECs and HPMECs
To assess the biological effect of B2G2 treatment on endothelial cells, we determined total and dead cell number by trypan blue exclusion assay at 6 h and 24 h following B2G2 treatment. As shown in Fig 1B and Fig 1C (left panels), there was no significant decrease in total cell number by selected B2G2 doses at 6 h in both HUVECs and HPMECs; however, at 24 h, a significant decrease in total cell number was observed in 20, 30 and 40 μM B2G2 treated cells. Regarding cell death, the results showed that at 6 h, although not strong, only 40 μM dose of B2G2 caused a statistically significant cell death; however, at 24 h time point, all B2G2 concentrations (20, 30 and 40 μM) caused a significant cell death (Fig 1B and 1C, right panels).
Effect of B2G2 on cell cycle progression and apoptosis in HUVECs
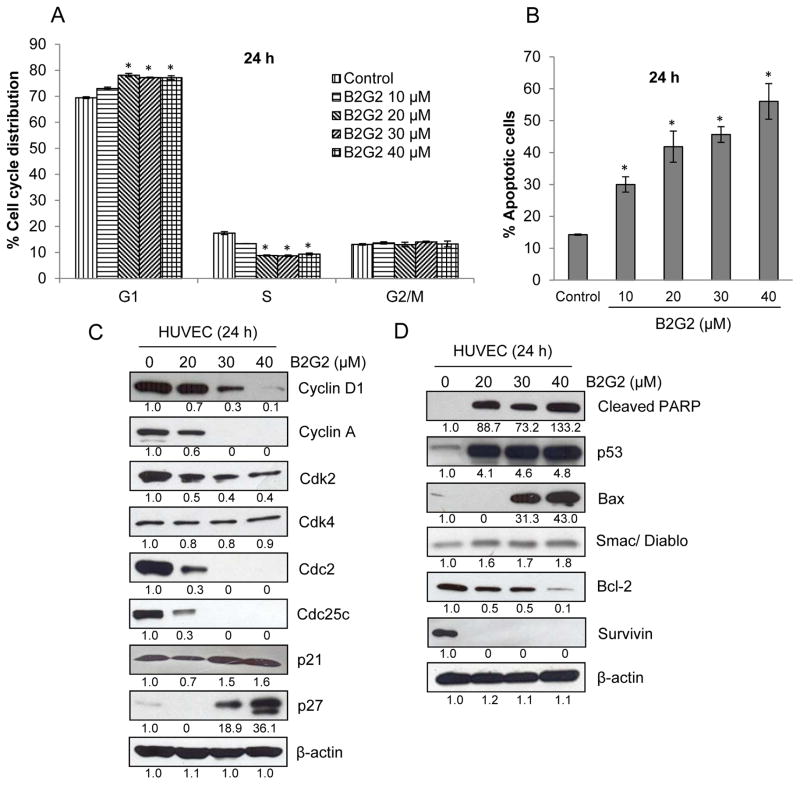
Earlier studies have reported that various anti-angiogenic and cytotoxic chemotherapeutic agents cause cell cycle arrest and apoptosis in tumor endothelial cells [16, 33, 34]. Accordingly, to understand the mechanism underlying B2G2-mediated growth inhibition and endothelial cell death, we next examined its effect on cell cycle progression and apoptosis in HUVECs by saponin/PI and Annexin V-PI staining, respectively [34]. The results showed that B2G2 (20, 30 and 40 μM) treatment for 24 h caused a significant G1 phase arrest which was associated with a significant decrease in S phase population (Fig 2A). Furthermore, all the tested doses of B2G2 (10, 20, 30 and 40 μM) induced significant apoptotic cell death in HUVECs after 24 h of treatment (Fig 2B).
Fig. 2.
Effect of B2G2 on cell cycle distribution and apoptosis in HUVECs. (A–B) HUVECs were grown in complete EGM-2 media with 2% FBS at the density of 4 × 105 cell/60 mm plate. After 24 h of seeding, cells were treated with 10 to 40 μM concentrations of B2G2 for 24 h. At the end of experiment, cells were harvested and cell cycle distribution and apoptosis were analyzed by flow cytometry as described in ‘Materials and Methods’. Each value represents mean ± S.E. of three samples for each treatment. *p<0.05, significant with respect to control. (C–D) HUVECs were treated with B2G2 for 24 h as described above and total cell lysates were prepared and analyzed for cell cycle regulators (Cyclin D1, Cyclin A, Cdk2, Cdk4, Cdc2, Cdc25c, p21 and p27) and apoptosis-related molecules (cleaved PARP, p53, Bax, Smac/Diablo, Bcl-2, and Survivin) by immunoblotting. To check for protein loading, blots were stripped and re-probed with an antibody specific for β-actin.
We next examined the effect of B2G2 on various cell cycle regulators, namely Cyclins, Cdks, Cdk inhibitors (CDKIs) and Cdc25c phosphatase to delineate their involvement in the observed cell cycle arrest by B2G2. As shown in Fig 2C, B2G2 treatment decreased the protein levels of Cyclins D1 and A; Cdk2 and Cdc2 (also known as Cdk1); and Cdc25c in HUVECs, with no effect on Cdk4. Furthermore, B2G2 treatment increased the levels of CDKIs namely p21waf1 and p27kip1 in HUVECs after 24 h of treatment (Fig 2C). The effect of B2G2 was dose-dependent on the expression of Cyclin D1, Cyclin A, Cdk2, Cdc2, Cdc25c, p21waf1 and p27kip1. PARP cleavage is a molecular marker of apoptotic cell death, and therefore, we next examined B2G2 effect on cleaved PARP levels, which showed that B2G2 induces strong PARP cleavage in HUVECs (Fig 2D). We also examined the effect of B2G2 on pro- and anti-apoptotic molecules in HUVECs after 24 h of its treatment. As shown in Fig 2D, the levels of pro-apoptotic proteins p53, Bax and Smac/diablo were increased, whereas a profound decrease was observed in levels of anti-apoptotic proteins Bcl-2 and survivin in HUVECs following B2G2 treatment.
Effect of B2G2 on capillary tube formation by HUVECs and HPMECs
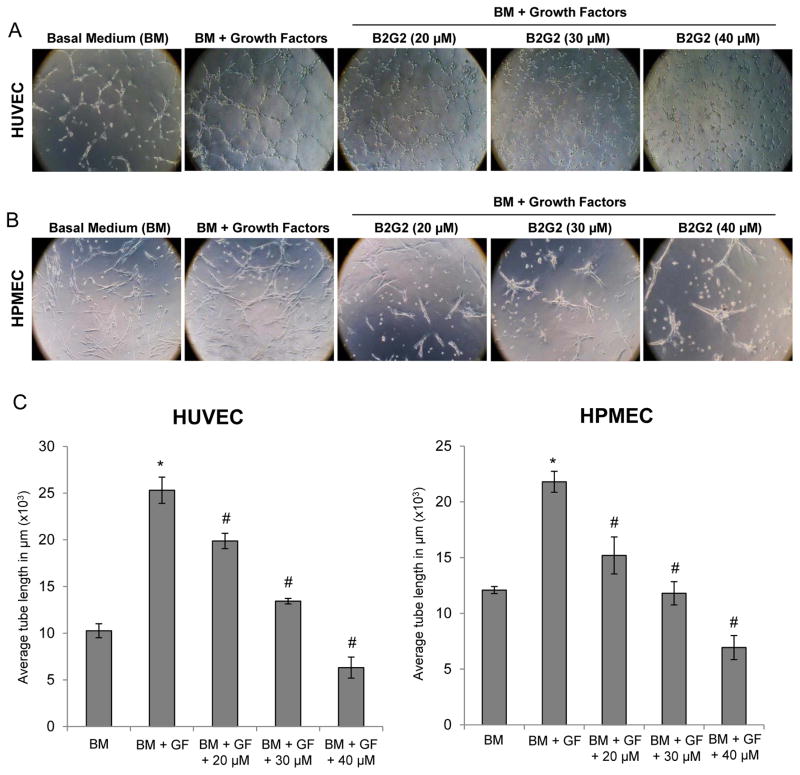
To understand the effect of B2G2 on capillary tube formation by endothelial cells, we used two-dimensional matrigel assay and examined the effects of B2G2 (at earlier time point when it had almost no cell death effect) on tubular structure formed by HUVECs and HPMECs. As shown in Fig 3A and 3B, supplementation of growth factors to basal medium resulted in a strong increase in capillary tube formation by both HUVECs and HPMECs, and B2G2 treatment significantly inhibited the capillary tube formation by these endothelial cells in a dose-dependent manner. Quantification for average tube length showed that B2G2 treatments at 20–40 μM concentrations resulted in 21–75% and 30–68% inhibition in tube formation by HUVECs and HPMECs, respectively (Fig 3C).
Fig. 3.
Effect of B2G2 on capillary tube formation by HUVECs and HPMECs. (A–B) Representative images depicting effect of B2G2 treatment (20–40 μM) on capillary tube formation by HUVECs and HPMECs on matrigel after 6 h and 8 h of cell seeding, respectively. Tubular structures were photographed at 100x magnification. (C) Tube length was measured as described in ‘Materials and Methods’. Tube length data is presented as mean ± standard error of three samples for each treatment. *p<0.05, significant with respect to BM treated group; #p<0.05, significant with respect to BM + GF treated group.
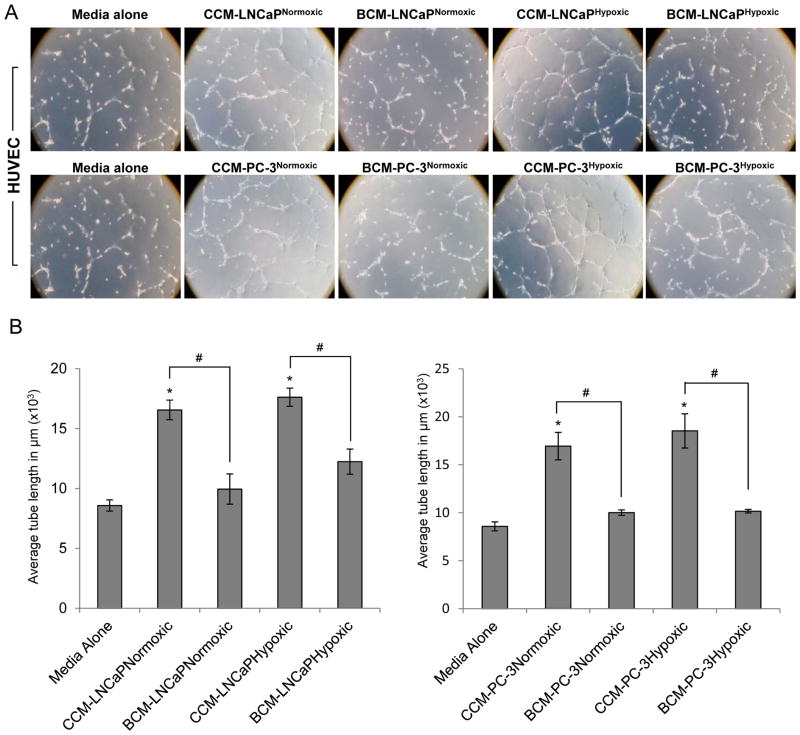
Furthermore, it is also known in literature that to promote neo-angiogenesis, tumor cells secrete various pro-angiogenic factors, which act in a paracrine manner to promote endothelial cell survival and tubular structure formation, and that the secretion of these factors is more profound under hypoxic conditions [9, 10]. Therefore, LNCaP and PC3 PCA cells were cultured under normoxic and hypoxic conditions with or without B2G2, and control (CCM) and B2G2-treated conditioned media (BCM) were collected and examined for their tube formation ability of HUVECs. As shown in Fig 4A, CCM from LNCaP and PC-3 PCA cells promoted the tube formation by HUVECs and the effect was more profound for the conditioned media collected from PCA cells cultured under hypoxic conditions. Most notably, with BCM, the tube formation was disrupted and only broken tubular structures were observed irrespective of normoxic or hypoxic conditions. Quantification for average tube length showed that compared to CCM, BCM from LNCaP cells cultured under normoxic and hypoxic conditions resulted in 39 and 30% decrease, respectively, in tube formation by HUVECs. Similarly, BCM from PC-3 cells cultured under normoxic and hypoxic conditions showed 40 and 45% decrease, respectively, compared to CCM, in tube formation by HUVECs (Fig 4B). However, it is quite possible that the observed inhibitory effect of BCM on capillary tube formation by HUVECs is due to the presence of B2G2; and this aspect needs to be further studied in future, probably through culturing cells further without B2G2 and then collect the B2G2-treated conditioned media or through removing B2G2 from the conditioned media using appropriate filter.
Fig. 4.
Effect of B2G2 on Prostate cancer cells-induced capillary tube formation by HUVECs. (A) LNCaP and PC3 cells were treated with B2G2 (30 μM) and grown under normoxic (~21% O2) and hypoxic (1% O2) conditions and conditioned media was collected as described in ‘Materials and Methods’. HUVECs were seeded on matrigel along with 0.5% FBS containing media or conditioned media and EBM-2 (75:25 ratio) and capillary tube formation was analyzed after 6 h. Representative images depicting formation of capillary tubes are shown at 100x magnification. In this experiment, HUVECs incubated with 0.5% FBS containing LNCaP or PC-3 media alone served as a negative control. (B) Tube length was measured as described in ‘Materials and Methods’. Tube length data is presented as mean ± standard error of three samples for each treatment. *p<0.05, significant with respect to control group; #p<0.05, significant with respect to CCM treated group.
Effect of B2G2 on motility of HUVECs and HPMECs
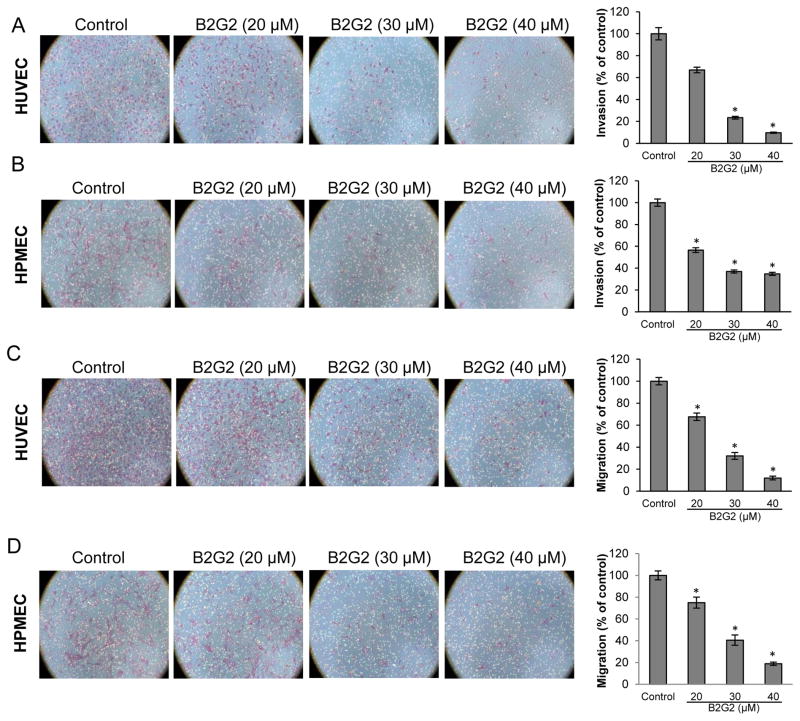
Endothelial cell motility i.e. invasion and migration is essential for the formation of new blood vessels during neo-angiogenesis, making it a critical event for tumor growth [35]. Accordingly, next we studied the effect of B2G2 treatment (at earlier time point when it had almost no cell death effect) on the invasiveness of HUVECs and HPMECs using matrigel transwell invasion assay. As shown in Fig 5A, B2G2 treatment inhibited the number of HUVECs invading through the matrigel by 33%, 76% and 90% at 20, 30 and 40 μM doses, respectively. Similarly, B2G2 treatment also inhibited the number of HPMECs invading through the matrigel by 43%, 63% and 65% at 20, 30 and 40 μM doses, respectively (Fig 5B).
Fig. 5.
Effect of B2G2 on invasion and migration of HUVECs and HPMECs. Representative images and graphical representation depicting the effect of B2G2 (20, 30 and 40 μM) on invasion (A and B) and migration (C and D) of HUVECs and HPMECs. Cells were allowed to invade and migrate for 10 h and 6 h, respectively, and non-invasive and non-migratory cells were removed by scraping with cotton swab. Invasive and migratory cells at the bottom of the membrane were fixed, stained and counted. Data are shown as percentage of cell invasion and migration compared to control (with control value as 100%). Each value represents mean ± S.E. of three samples for each treatment. All images are shown at 100x magnification. * p<0.05
Next, we examined the effect of B2G2 treatment on the migratory properties of HUVECs and HPMECs using transwell migration assay. As shown in Fig 5C, B2G2 treatment (at earlier time point when it had almost no cell death effect) inhibited the migration of HUVECs by 32%, 67% and 88% at 20, 30 and 40 μM doses, respectively. Similarly, B2G2 treatment also inhibited the migration of HPMECs by 25%, 59% and 81% at 20, 30 and 40 μM doses, respectively (Fig 5D). Taken together, B2G2 inhibited endothelial cell motility (invasion and migration) in a dose-dependent manner.
Effect of B2G2 on VEGFR-PI3K-Akt and Integrin signaling in HUVECs
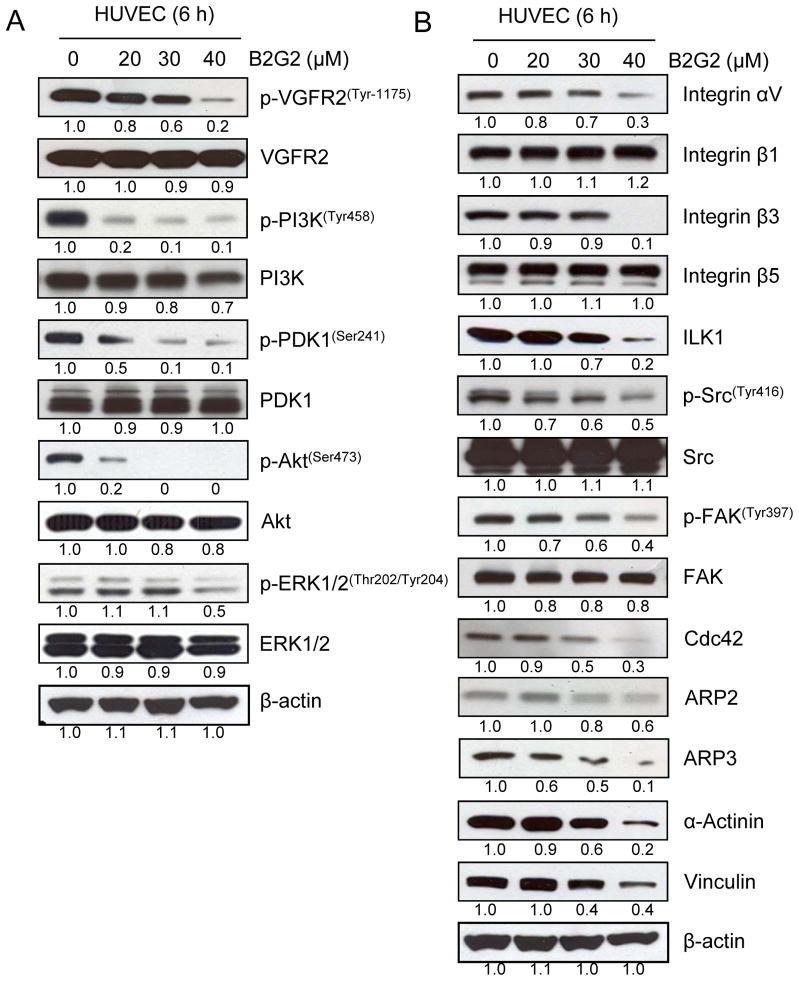
VEGF has been considered as a prime regulator of angiogenesis and controls proliferation, motility and tube formation in endothelial cells [36]. Since VEGF regulates angiogenesis, targeting this biological process via the inhibition of VEGFR2/PI3K/Akt signaling axis represents a promising strategy for cancer chemoprevention and therapy [11, 27]. As shown in Fig 6A, significant inhibition in phosphorylation of VEGFR2, PI3K, PDK1 and Akt, without noticeable change in total levels, in HUVECs was observed following B2G2 treatment for 6 h indicating that the observed inhibition in tube formation, invasion and motility by B2G2 could be due to its ability to inhibit VEGFR signaling. Importantly, at this time point (6 h), B2G2 did not show much cytotoxicity in HUVECs (Fig 1B). B2G2 treatment also moderately (mainly at 40 μM dose) decreased the phosphorylation of ERK1/2 in HUVECs (Fig 6A).
Fig. 6.
Effect of B2G2 on VEGFR2/PI3K/Akt and Integrin signaling pathways in HUVECs. HUVECs were treated with B2G2 (20–40 μM) for 6 h and total cell lysates were prepared and analyzed for mentioned signaling molecules by immunoblotting. To check for protein loading, blots were stripped and re-probed with an antibody specific for β-actin.
Endothelial cell motility is essential during angiogenesis and requires the activation of signaling pathways (such as Integrins) that converge on to actin remodeling. Besides chemotactic motility in response to cytokines, endothelial cells also migrate in response to ECM components which is primarily dependent on interaction between Integrins and ECM [35, 37–39]. Therefore, we also examined the effect of B2G2 on the expression level of Integrins and their downstream signaling molecules which are involved in actin remodeling. As shown in Fig 6B, B2G2 treatment down-regulated the expression of Integrins αv and β3 at 40 μM dose, while no effect was observed on Integrins β1 and β5 expression in HUVECs. Furthermore, B2G2 treatment also decreased the ILK1 level, more profoundly at 40 μM dose (Figure 6B). Furthermore, the phosphorylation of kinases namely Src kinase and FAK, regulating the actin remodeling and the formation of focal adhesions, was also decreased following B2G2 treatment without affecting their total levels (figure 6B). Moreover, engagement of Integrins to ECM stimulates Cdc42 to induce actin polymerization through ARP2, ARP3, α-Actinin and Vinculin, and all of these molecules were down-regulated following B2G2 treatment (Fig 6B). Together, these results suggested the inhibitory effect of B2G2 on Integrin-mediated actin polymerization and remodeling in HUVECs, and thus inhibiting their motility as well.
DISCUSSION AND CONCLUSION
Angiogenesis is a multistep process involving endothelial cell growth, survival, invasion, migration, capillary tube formation, organization, and maturation into blood vessels in the tumor microenvironment [11, 36]. Therefore, use of non-toxic agents targeting one or more of the above mentioned processes can impair angiogenesis, and hence, halt tumor growth and metastasis [27, 40–42]. Consistent with these observations, in the present study, B2G2 showed potential anti-angiogenic activity in vitro by inhibiting proliferation, capillary tube formation, invasion and migration in HUVECs and HPMECs endothelial cells. However, the potential mechanisms behind the B2G2 mediated inhibitory effects on angiogenesis was analyzed only in HUVECs, as HUVEC angiogenesis model is well-established, and it is the most used in vitro model to study pro- or anti-angiogenic potential of a given compound, and also the fact that molecular pathways involved in various steps of angiogenesis have been well characterized in these cells [43–45].
Here, we showed that B2G2 caused strong anti-proliferative effect in HUVECs and HPMECs in a dose-dependent manner and the mechanistic study revealed that the observed effect could be attributed to the cell cycle arrest at G1 phase. Up-regulation of cell cycle inhibitory proteins namely p53, p21 and p27, along with down-regulation of Cdks (1, 2 and 4), Cyclins (D1 and A) and Cdc25c phosphatase was also observed in HUVECs following B2G2 treatment which could be responsible for the observed cell cycle arrest in these cells [41, 46, 47]. The up-regulation of p53 can also cause apoptosis in cells by increasing the expression of pro-apoptotic protein Bax as observed in HUVECs by B2G2 treatment [48]. Furthermore, results from the present study showed that B2G2 up-regulates the expression of Bax and Smac/Diablo (pro-apoptotic molecules) along with the down-regulation of Bcl-2 and Survivin (anti-apoptotic molecules) expression in HUVECs, followed by enhanced cleavage of PARP by B2G2 [48, 49]. The observed apoptosis in HUVECs is significant due to the fact that during chemotherapy, apoptosis of endothelial cells in tumors precedes apoptosis of tumor cells, and even when the tumor cells have acquired resistance to a particular drug, apoptosis of endothelial cells leads to inhibition of tumor growth [34]. Therefore, endothelial cells in the tumor microenvironment offer a unique translational opportunity as these cells are genetically non-transformed and are also less susceptible to acquire mutations which make them more prone to cytotoxic chemotherapy [29, 33].
During tumor angiogenesis, the formation and merging of capillary tubes by endothelial cells is an important event and its inhibition halts the formation and recruitment of new blood vessels to tumors. We observed that B2G2 strongly inhibited the capillary tube formation by HUVECs and HPMECs. It is important to highlight here that the inhibition in capillary tube formation was observed following 6 h of B2G2 treatment, and at this time point, B2G2 at all the doses did not show any considerable cytotoxicity in both the endothelial cells clearly indicating that the observed inhibition was not due to cell death. Furthermore, it has been previously reported that during hypoxic conditions, tumor cells secrete pro-angiogenic factors like VEGF, FGF, IGF, EGF, etc. in significant amount which act as chemo-attractant for endothelial cells and induce them to invade and migrate towards tumor microenvironment by modulating the extra cellular matrix (ECM) components [9, 10, 36, 50]. Therefore, invasion and migration of endothelial cells in response to pro-angiogenic factors are also essential and rate limiting events during neo-angiogenesis [35, 38]. In the present study, we found that B2G2 potently inhibited HUVECs and HPMECs motility in response to growth factors. Altogether, B2G2 targets various key attributes of angiogenesis process by: (i) inhibiting endothelial cell proliferation, (ii) causing cell cycle arrest and apoptosis, (iii) inhibiting capillary tube formation, and (iv) inhibiting invasion and migration of endothelial cells.
Among various growth factors, VEGF is one of the most important regulators of angiogenesis process, and therefore, identified as a potential anti-angiogenic target [51]. VEGF exerts its effects after binding with its homologous membrane tyrosine kinase receptors, VEGFRs, of which, VEGFR2 has the most important role in VEGF-induced angiogenesis [52]. VEGFR2 upon activation further activates PI3K/Akt and ERK pathways, which are considered responsible for endothelial cell proliferation, and pharmacological inhibition of PI3K has been reported to suppress VEGF-mediated vascular responses and survival of endothelial cells [53–55]. The pro-survival effect of PI3K is due to its ability to activate Akt via PDK1 kinase, and the Akt pathway is a major suppressor of apoptotic stimuli in the cells [53]. In the present study, we observed that B2G2 treatment strongly inhibit the phosphorylation of VEGFR2 at Tyr1175, a reliable marker for its activity, and further downstream signaling molecules mentioned above like PI3K, PDK1, AKT and ERK1/2s were also found to be inhibited in HUVECs. Interestingly, we observed strong B2G2-mediated decrease in the phosphorylation of PI3K, PDK1 and Akt, even at doses where VEGFR2 phosphorylation was not significantly affected; that clearly suggests that B2G2 could target upstream kinases other than VEGFR2 (IGFR, EGFR, etc.) which needs to be further investigated. Furthermore, the observed decrease in VEGFR2 phosphorylation suggests the possibility that B2G2 could compete with VEGFR2 ligand (i.e. VEGF) in a specific or non-specific fashion, and reduces VEGFR2 activation, which needs to be evaluated in future by molecular modeling tools and receptor-ligand binding studies.
In addition to proliferation, VEGFR2/PI3K/Akt pathway also regulates endothelial cell migration by modulating the function of various proteins involved in actin-nucleation and polymerization such as Cdc42, Vinculin, α-Actinin and ARP2/3 [53, 56]. Our data showed that B2G2 down-regulates the expression of Cdc42, Vinculin, α-Actinin and ARP2/3 in HUVECs suggesting that inhibition of actin remodeling may be one of the potential mechanisms for the inhibition of endothelial cell migration by B2G2. Furthermore, endothelial cell migration is regulated by interaction between Integrins and ECM components [37, 38, 57, 58]. Integrins are a family of heterodimeric transmembrane receptors that bind to ECM, and αVβ3 and αVβ5 integrins have been identified as major players in endothelial cell migration [38, 39, 57]. Importantly, several endogenous inhibitors of angiogenesis such as endostatin exert their function by inhibiting Integrin signaling, and monoclonal antibodies directed against αvβ3 Integrin also inhibit endothelial cell migration and angiogenesis indicating that Integrins can also be a target for cancer angioprevention [37–39, 58, 59]. Our results showed that B2G2 down-regulates the expression of αV and β3 integrins in HUVECs, indicating that B2G2 inhibits integrin signaling as well. Therefore, we further analyzed the effect of B2G2 on Integrin downstream signaling molecules, and our results showed that ILK1, a major regulator of integrin signaling, was also down-regulated by B2G2 treatment [60]. Interestingly, it has been observed previously that αvβ3 integrin and VEGFR2 interact with each other, and FAK is the converging signaling point for these receptors [35, 38, 57, 61, 62]. FAK along with Src kinase regulates the assembly and disassembly of focal adhesions, the region where endothelial cells are connected to ECM and are necessary for endothelial cell migration [61–63]. Our results showed that B2G2 inhibits the phosphorylation and activation of FAK and Src kinase, and as mentioned earlier, Vinculin and α-Actinin were also down-regulated by B2G2. Together, these molecular results suggest that this phytochemical interferes with the assembly of focal adhesions by acting against Integrin and VEGFR2 signaling pathways, and thus inhibits endothelial cell migration.
In summary, the present study is the first to demonstrate that Procyanidin B2-3,3″-di-O-gallate (B2G2) is a potential anti-angiogenic phytochemical, which involves growth inhibition, cell cycle arrest, induction of apoptosis, inhibition of capillary tube formation, inhibition of invasion and migration of HUVECs and HPMECs. The molecular events associated with these effects include modulation of cell cycle and apoptosis regulators and inhibition of VEGFR2/PI3K/Akt and Integrin signaling pathways. Though all the presented in vitro studies here suggest a potential anti-angiogenic property of B2G2, further in vivo studies are necessary to establish its inhibitory effect on vascular tube formation in prostate tumors which would help establish its usefulness in cancer prevention and control.
Acknowledgments
This work was supported by NCI RO1 grant CA91883 (to CA). Authors also acknowledge the CCSG P30CA046934 grant for supporting the UCD Shared Resources used in this study.
Footnotes
AUTHOR CONTRIBUTIONS
Rahul Kumar: Designed/performed study, collected data, analyzed data, and wrote the manuscript
Gagan Deep: Designed/performed study, collected data, analyzed data, and wrote the manuscript
Michael F. Wempe: Performed study, contributed important reagents
Rajesh Agarwal: Designed study, coordinated the research, and critically revised the manuscript
Chapla Agarwal: Conceived the study, elaborated its design, performed study, and contributed to finalize manuscript.
CONFLICT OF INTEREST
No potential conflicts of interest were identified by any authors of this manuscript.
References
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Gacche RN, Meshram RJ. Angiogenic factors as potential drug target: Efficacy and limitations of anti-angiogenic therapy. Biochimica et biophysica acta. 2014;1846(1):161–179. doi: 10.1016/j.bbcan.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5(16):1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 4.Naumov GN, Folkman J, Straume O. Tumor dormancy due to failure of angiogenesis: role of the microenvironment. Clinical & experimental metastasis. 2009;26(1):51–60. doi: 10.1007/s10585-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 5.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Current opinion in genetics & development. 2007;17(1):71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz CC, Taylor CT. Targeting the HIF pathway in inflammation and immunity. Current opinion in pharmacology. 2013;13(4):646–653. doi: 10.1016/j.coph.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Agarwal R. Tumor angiogenesis: a potential target in cancer control by phytochemicals. Current cancer drug targets. 2003;3(3):205–217. doi: 10.2174/1568009033481985. [DOI] [PubMed] [Google Scholar]
- 8.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators of inflammation. 2013;2013:127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sooriakumaran P, Kaba R. Angiogenesis and the tumour hypoxia response in prostate cancer: a review. International journal of surgery. 2005;3(1):61–67. doi: 10.1016/j.ijsu.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013;114(5):967–974. doi: 10.1002/jcb.24438. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 12.Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Seminars in oncology. 2014;41(2):235–251. doi: 10.1053/j.seminoncol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Dimova I, Popivanov G, Djonov V. Angiogenesis in cancer - general pathways and their therapeutic implications. Journal of BUON : official journal of the Balkan Union of Oncology. 2014;19(1):15–21. [PubMed] [Google Scholar]
- 14.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxidants & redox signaling. 2008;10(3):475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 15.Detchokul S, Frauman AG. Recent developments in prostate cancer biomarker research: therapeutic implications. British journal of clinical pharmacology. 2011;71(2):157–174. doi: 10.1111/j.1365-2125.2010.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sissung TM, Thordardottir S, Gardner ER, Figg WD. Current status of thalidomide and CC-5013 in the treatment of metastatic prostate cancer. Anti-cancer agents in medicinal chemistry. 2009;9(10):1058–1069. doi: 10.2174/187152009789735017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA: a cancer journal for clinicians. 2010;60(4):222–243. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samant RS, Shevde LA. Recent advances in anti-angiogenic therapy of cancer. Oncotarget. 2011;2(3):122–134. doi: 10.18632/oncotarget.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Science signaling. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 20.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews. Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 21.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. Journal of immunology research. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulder K, Scarfe A, Chua N, Spratlin J. The role of bevacizumab in colorectal cancer: understanding its benefits and limitations. Expert Opin Biol Ther. 2011;11(3):405–413. doi: 10.1517/14712598.2011.557657. [DOI] [PubMed] [Google Scholar]
- 23.Blanchet B, Billemont B, Barete S, Garrigue H, Cabanes L, Coriat R, Frances C, Knebelmann B, Goldwasser F. Toxicity of sorafenib: clinical and molecular aspects. Expert opinion on drug safety. 2010;9(2):275–287. doi: 10.1517/14740330903510608. [DOI] [PubMed] [Google Scholar]
- 24.Hardin J, Cheng I, Witte JS. Impact of consumption of vegetable, fruit, grain, and high glycemic index foods on aggressive prostate cancer risk. Nutrition and cancer. 2011;63(6):860–872. doi: 10.1080/01635581.2011.582224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Hammer TJ, Rabe KG, Anderson KE, Olson JE, Sinha R, Petersen GM. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer causes & control : CCC. 2011;22(12):1613–1625. doi: 10.1007/s10552-011-9838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakai K, Matsuo K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Sasazuki S, Shimazu T, Sawada N, Inoue M, Tsugane S Research Group for the D., Evaluation of Cancer Prevention Strategies in J. Lung cancer risk and consumption of vegetables and fruit: an evaluation based on a systematic review of epidemiological evidence from Japan. Japanese journal of clinical oncology. 2011;41(5):693–708. doi: 10.1093/jjco/hyr027. [DOI] [PubMed] [Google Scholar]
- 27.Deep G, Gangar SC, Rajamanickam S, Raina K, Gu M, Agarwal C, Oberlies NH, Agarwal R. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: targeting VEGF-VEGFR signaling. PloS one. 2012;7(4):e34630. doi: 10.1371/journal.pone.0034630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavitha CV, Agarwal C, Agarwal R, Deep G. Asiatic acid inhibits pro-angiogenic effects of VEGF and human gliomas in endothelial cell culture models. PloS one. 2011;6(8):e22745. doi: 10.1371/journal.pone.0022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24(7):1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 30.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. The Journal of nutrition. 2009;139(9):1806S–1812S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3′-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2007;28(7):1478–1484. doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi A, Raina K, Shrestha SP, Miller B, Thompson JA, Wempe MF, Agarwal R, Agarwal C. Procyanidin B2 3,3-di-O-gallate, a Biologically Active Constituent of Grape Seed Extract, Induces Apoptosis in Human Prostate Cancer Cells Via Targeting NF-kappaB, Stat3, and AP1 Transcription Factors. Nutrition and cancer. 2013 doi: 10.1080/01635581.2013.783602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390(6658):404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J. Angiogenesis and apoptosis. Seminars in cancer biology. 2003;13(2):159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 35.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100(6):782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106(4):148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 37.Akalu A, Cretu A, Brooks PC. Targeting integrins for the control of tumour angiogenesis. Expert opinion on investigational drugs. 2005;14(12):1475–1486. doi: 10.1517/13543784.14.12.1475. [DOI] [PubMed] [Google Scholar]
- 38.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harbor perspectives in medicine. 2011;1(1):a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu YJ, Muldoon LL, Gahramanov S, Kraemer DF, Marshall DJ, Neuwelt EA. Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. Journal of neuro-oncology. 2012;110(1):27–36. doi: 10.1007/s11060-012-0942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamias A, Dimopoulos MA. Angiogenesis in human cancer: implications in cancer therapy. European journal of internal medicine. 2003;14(8):459–469. doi: 10.1016/j.ejim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Bhat TA, Nambiar D, Pal A, Agarwal R, Singh RP. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo--implications for angioprevention. Carcinogenesis. 2012;33(2):385–393. doi: 10.1093/carcin/bgr282. [DOI] [PubMed] [Google Scholar]
- 42.Bhat TA, Nambiar D, Tailor D, Pal A, Agarwal R, Singh RP. Acacetin inhibits in vitro and in vivo angiogenesis and downregulates Stat signaling and VEGF expression. Cancer prevention research (Philadelphia, Pa) 2013;6(10):1128–1139. doi: 10.1158/1940-6207.CAPR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ucuzian AA, Greisler HP. In vitro models of angiogenesis. World journal of surgery. 2007;31(4):654–663. doi: 10.1007/s00268-006-0763-4. [DOI] [PubMed] [Google Scholar]
- 44.Hughes SE. Functional characterization of the spontaneously transformed human umbilical vein endothelial cell line ECV304: use in an in vitro model of angiogenesis. Experimental cell research. 1996;225(1):171–185. doi: 10.1006/excr.1996.0168. [DOI] [PubMed] [Google Scholar]
- 45.Vailhe B, Vittet D, Feige J-J. In Vitro Models of Vasculogenesis and Angiogenesis. Lab Invest. 2001;81(4):439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- 46.Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 47.Gallorini M, Cataldi A, di Giacomo V. Cyclin-dependent kinase modulators and cancer therapy. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2012;26(6):377–391. doi: 10.1007/BF03261895. [DOI] [PubMed] [Google Scholar]
- 48.Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G. Targeting p53 to mitochondria for cancer therapy. Cell cycle. 2008;7(13):1949–1955. doi: 10.4161/cc.7.13.6222. [DOI] [PubMed] [Google Scholar]
- 49.Farnebo M, Bykov VJ, Wiman KG. The p53 tumor suppressor: a master regulator of diverse cellular processes and therapeutic target in cancer. Biochemical and biophysical research communications. 2010;396(1):85–89. doi: 10.1016/j.bbrc.2010.02.152. [DOI] [PubMed] [Google Scholar]
- 50.Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. Journal of molecular medicine. 2003;81(1):20–31. doi: 10.1007/s00109-002-0397-4. [DOI] [PubMed] [Google Scholar]
- 51.Shinkaruk S, Bayle M, Lain G, Deleris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Current medicinal chemistry. Anti-cancer agents. 2003;3(2):95–117. doi: 10.2174/1568011033353452. [DOI] [PubMed] [Google Scholar]
- 52.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 53.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Frontiers in molecular neuroscience. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horowitz A, Seerapu HR. Regulation of VEGF signaling by membrane traffic. Cellular signalling. 2012;24(9):1810–1820. doi: 10.1016/j.cellsig.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortega N, Hutchings H, Plouet J. Signal relays in the VEGF system. Frontiers in bioscience : a journal and virtual library. 1999;4:D141–152. doi: 10.2741/A417. [DOI] [PubMed] [Google Scholar]
- 56.Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 57.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Experimental cell research. 2006;312(5):651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Cherrington JM, Strawn LM, Shawver LK. New paradigms for the treatment of cancer: the role of anti-angiogenesis agents. Advances in cancer research. 2000;79:1–38. doi: 10.1016/s0065-230x(00)79001-4. [DOI] [PubMed] [Google Scholar]
- 59.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Experimental cell research. 2006;312(5):594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends in cell biology. 1999;9(8):319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 61.Somanath PR, Malinin NL, Byzova TV. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis. 2009;12(2):177–185. doi: 10.1007/s10456-009-9141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nature cell biology. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 63.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. The Journal of cell biology. 2002;157(1):149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]