Abstract
Objective
Fibronectin (FN) is a widely expressed molecule that can participate in development of osteoarthritis (OA) affecting cartilage, meniscus, and synovial membrane (SM). The alternatively spliced isoforms of FN in joint tissues other than cartilage have not been extensively studied previously. The present study compares FN splice variation in patients with varying degrees of osteoarthritic change.
Design
Joint tissues were collected from asymptomatic donors and patients undergoing arthroscopic procedures. Total RNA was amplified by PCR using primers flanking alternatively spliced Extra Domain A (EDA), Extra Domain B (EDB) and Variable (V) regions.
Results
EDB+, EDB− and EDA− and V+ variants were present in all joint tissues, while the EDA+ variant was rarely detected. Expression levels of EDB+ and EDV+ variants were similar in cartilage, synovium and meniscal tissues. Synovial expression of V+ FN in arthroscopy patients varied with degree of cartilage degeneration. Two V− isoforms, previously identified in cartilage, were also present in SM and meniscus.
Conclusions
Fibronectin splicing in meniscus and SM bears striking resemblance to that of cartilage. Expression levels of synovial V+ FN varied with degree of cartilage degeneration. V+ FN should be investigated as a potential biomarker of disease stage or progression in larger populations.
Keywords: Fibronectin, alternative splicing, cartilage, synovium, degeneration
Introduction
Osteoarthritis (OA), historically considered a disease of cartilage extracellular matrix (ECM) degeneration, is now appreciated to involve pathologic changes in all connective tissues of the joint, including the synovial membrane (SM), bone, and meniscus1. Changes in these tissues reflect ECM molecular reorganization, turnover and repair responses. One widely expressed molecule that may play a pivotal role in all of these processes is fibronectin (FN).
FN is a ubiquitously expressed glycoprotein found in ECM that plays important roles in cell adhesion, migration, and differentiation 2. Although encoded by a single gene, multiple alternative splicing products exist. Alternative splicing occurs at three major sites: the Extra Domain A (EDA), Extra Domain B (EDB) and Variable (V) regions2. Alternative splicing at the EDA and EDB regions results in inclusion or exclusion of one single protein domain in its entirety. In contrast, multiple splice sites within the V region exist and thus multiple V-region variants are observed. Splice variation is associated with different tissue distributions, developmental phases, and functional consequences. FN containing the EDA, EDB and portions of the V region are expressed during embryonic development, but are not highly expressed in adult tissues except during aging 3, wound-healing 4, malignancy5, 6 and inflammation 7. Splice variants containing the EDB region have been described as a marker of angiogenesis 8. The EDA region plays a role in inflammatory responses 9, and contributes to FN-mediated cellular adhesion and myofibroblast differentiation 10,11. The V region contains integrin and proteoglycan binding sites and thus also contributes to cell adhesion 12. This region, also called the type III connecting segment (IIICS), promotes secretion of FN dimers contributing to FN forms found in serum 13. In adult human cartilage, a unique splice variant is expressed lacking the entire V-region as well as flanking domains ([V +III15 +I10]− FN) 14. This isoform may in part prevent inflammatory cell adhesion and invasion into mature articular cartilage matrix 15.
In the setting of arthritis, variation due to alternative splicing has been observed. FN including the EDA and EDB domains are increased in cartilage from patients with both OA and rheumatoid arthritis (RA) 16, 17. EDA+ isoforms have been detected in synovial fluid (SF) from OA and RA patients,18 and alterations in V region splicing have been demonstrated in cartilage from OA patients26. However, splicing in joint tissues other than cartilage has not been well described previously, despite the importance of tissues such as synovium and meniscus in disease initiation and progression. The ability of FN fragments to potentiate cartilage matrix damage is well established 19, 20, and recombinant FN fragments can induce proteolytic enzyme production by cartilage explants in vitro 21. But the contribution of isoforms to these FN-fragment driven activities is not understood.
A better understanding of the tissue sources of FN splice variants within the joint and variation with disease-stage in OA is necessary to determine their contributions to pathogenesis in OA. As OA involves all joint tissues 1, the present studies were carried out to define FN splice variation in cartilage, SM and meniscal tissues, and determine whether splicing varies according to stage of disease.
Materials and Methods
Cartilage, meniscus and SM specimens
Human articular cartilage, meniscus and SM tissues were collected from four asymptomatic organ donors via the Gift of Hope Organ & Tissue Donor Network (Elmhurst, IL, USA). Specimens were obtained within 24 hours of death, and donated according to Gift of Hope policies. Despite no history of clinical arthritis, cartilage degeneration and bone changes were observed within the joints: all four donors exhibited grade 2 osteoarthritic changes (cartilage fibrillation or fissuring with or without small osteophytes) as evaluated by the modified Collins score 22, although some areas of grade 0 (normal appearing, “non-lesional”) cartilage could be identified.
SM biopsies were obtained during arthroscopic knee procedures, from patients enrolled in the IRB approved Rush Arthritis and Knee Injury Repository; all patients gave informed consent. Patients with ligament tears are excluded from this repository, so the majority of specimens are derived from subjects undergoing partial meniscectomies for degenerative menisci, or loose body removals. Cartilage integrity in these patients was graded intra-operatively using the Outerbridge score, 23 which ranges from 0 (normal) to 4 (full-thickness cartilage erosion). SM specimens from 15 patients were chosen, representing the range of Outerbridge grades from 0 to 4 (three patients in each grade) (Figure 1A–E). Patient characteristics are presented in Table 1.
Figure 1. Spectrum of cartilage pathology in arthroscopy patients from whom synovial membrane specimens were obtained.
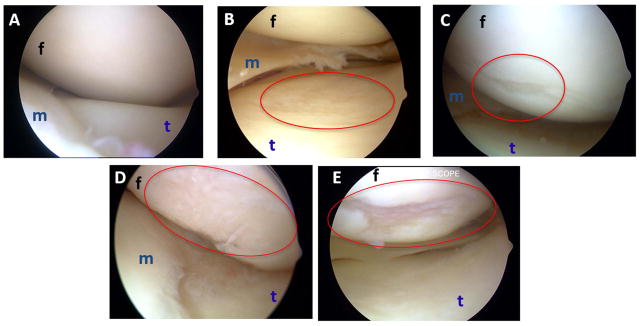
For arthroscopy patients donating synovial membrane, integrity of each articular surface in the knee was graded using the Outerbridge system32 by the surgeon intra-operatively. Pictures representative of each grade are shown (panels A–E). (A) Grade 0: normal cartilage. (B) Grade 1: softening, swelling, minor surface roughening. (C) Grade 2: fibrillation and/or fissuring in an area half an inch or less in diameter. (D) Grade 3: fibrillation and/or fissuring in an area greater than half an inch in diameter. (E) Grade 4: fissuring and erosion of cartilage down to subchondral bone. m: meniscus, f: femoral condyle, t: tibial plateau.
Table 1.
Characteristics of patients undergoing arthroscopic procedures who contributed synovial membrane for analysis.
| Outerbridge grade | Gender (M/F) | Ages |
|---|---|---|
| 0 | 2M/1F | 30,39,46 |
| 1 | 3M | 48,49,54 |
| 2 | 3M | 41,49,56 |
| 3 | 3M | 42,48,52 |
| 4 | 1M/2F | 46,60,65 |
Outerbridge grade refers to worst score of all cartilage surfaces in the knee (femoral, patellar, and tibial).
RNA extraction
SM from arthroscopy patients were collected from the operating room, rinsed in sterile PBS to remove contaminating synovial fluid, stabilized in RNAlater solution (Ambion, Inc), and then stored at −80°C until processing. Tissue specimens from the organ donors were treated similarly.
Total cellular RNA was isolated using Trizol reagent (Life Technologies, Rockville, MD), after homogenization. Total RNA was further purified using the RNA Easy kit (Qiagen, Valencia, CA). All RNA was treated with RNase-free DNase I (Qiagen).
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
1μg of total RNA from each sample was converted to complementary DNA (cDNA) using EcoDry Premix (Oligo dT, Clontech Laboratories, Inc.) Polymerase chain reaction (PCR) was performed with MyFi DNA Polymerase Mix (Bioline USA Inc.). Primers were designed flanking human FN alternatively spliced regions (EDA, EDB and V), and sequences reported previously 24. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as a control. The PCR cycle consisted of an initial denaturation of 95°C for 1 min followed by 94°C for 15 sec, annealing at 55°C–61°C for 15 sec, and extension at 72°C for 30 sec. This cycle was repeated 25–35 times. PCR products were mixed with orange loading dye (LI-COR; Biosciences, Lincoln, NE), separated in 1.2–2% agarose gels, and analyzed using an Odyssey Infrared Imaging System. Where relevant, signal intensities were determined using LI-COR imaging software, and density of specific transcript variants normalized to GAPDH for quantification. Comparison of signal intensity of amplification products from patients with different degrees of cartilage degeneration was performed with the Kruskal-Wallis test followed by Dunn multiple comparison test. Finally, PCR products amplified with the EDV primer pair generated a number of bands. Two unexpected bands were gel purified using QIAquick Gel Extraction Kit (Qiagen), and sequencing of these and other PCR products was performed by Big Dye Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, ABI, Foster City, CA). The sequences were analyzed in an ABI-3730 DNA Analyzer.
Results
FN splice variants in different joint tissues
Figure 2 shows the fibronectin splicing profile of RNAs extracted from knee SM, meniscus, and articular cartilage from one of the four organ donors with Collins Grade 2 cartilage changes (fibrillation and fissuring). Cartilage was obtained from both lesional cartilage (LC) and normal appearing cartilage (NC) from the tibial surface, and SM was only obtained from three of these donors. mRNA splice variants containing the EDB region were detectable in cartilage, and were faintly detectable in meniscus (M) and synovial membrane (SM) as well. EDB+ FN was present in both normal and lesional cartilage. In contrast, the EDA+ transcript (410 bp band) was only faintly detectable in one SM specimen (Figure 2, lane 2) and was absent in meniscus and cartilage. Amplification with V-region primers demonstrated multiple variants. Bands corresponding to multiple V+ (1076 to 1243 bp, Figure 2) 25 and V− variants were detectable in all three tissues. A 473 bp band corresponding to the [V+III15+I10]− splice variant was detectable in meniscus, lesional (LC) and normal-appearing cartilage (NC), and faintly in SM. We also observed two bands of apparent sizes 638 and 719 bp in meniscus and cartilage from the donors. After amplification of RNA from normal cartilage using our V-region flanking primers, we purified and sequenced these two bands. These bands were confirmed to be [V+III15]− and [V+I10]− forms as described by Parker et al.26.
Figure 2. Fibronectin splice variants in meniscus (M), synovial membrane (SM), normal cartilage (NC) and lesional cartilage (LC).
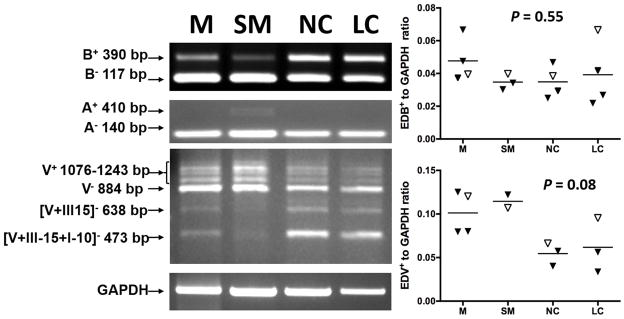
Tissues were obtained from four asymptomatic organ donors with Collins grade 2 degenerative changes in the joints. PCR reactions were carried out as described to amplify transcripts containing the alternative spliced regions EDA, EDB, and the V-region. EDB and EDA RT-PCR amplification revealed two forms, representing transcripts with or without the EDA or EDB domains. V region RT-PCR amplification revealed multiple V+ and V− transcripts, including the [V+III-15 +I10]−, [V+III15]− and [V+I10]− forms. A: Representative PCR products separated by agarose gel electrophoresis from a single donor are depicted. B: Relative expression of V+ variants normalized to GAPDH by densitometry. There was a tendency for SM expression to be higher than expression in NC (n=4, P=0.08). C: Relative expression of EDB+ variants normalized to GAPDH. Differences between tissues were not statistically significant (n=4, p=0.55).
Among the donor joint tissues, there was a trend for higher synovial expression of EDV+ transcripts (Figure 2b; mean EDV/GAPDH ratio±SD: 0.12±0.01) compared to normal cartilage (0.055±0.01, ANOVA P=0.04, Tukey’s multiple comparisons test P=0.08). Expression levels of EDB+ mRNA (Figure 2c) were similar in cartilage, meniscal and synovial tissues (Figure 2c). Levels of EDB+ and EDV+ transcripts did not differ between lesional and non-lesional areas of cartilage (P=0.97 for EDB+ and P=0.98 for EDV+).
FN splice variation in synovial membrane from patients undergoing arthroscopic meniscectomy, with various stages of cartilage degeneration
Given the emerging importance of synovial membrane in OA 27, we next examined FN splice variation specifically in this tissue. Samples from 15 patients undergoing meniscal arthroscopy for degenerative meniscal tears (n=13) or loose body removals (n=2) were evaluated. Patients’ ages ranged from 30 to 65 years, 12 male and 3 female (Table 1). Cartilage integrity in these patients was graded by the surgeon 28 as presented in Figure 1A–E. In each patient, all cartilage surfaces of the knee joint were evaluated, and patients were stratified according to the worst score. Synovial specimens were chosen from fifteen patients representing the spectrum of degeneration from normal to grade 4 (3 patients from each grade). All 15 expressed V− variants including most prominently the 884 bp V− form, as well as the “cartilage-specific” [V+III15+I10]−, [V+III15]− and [V+I10]− variants. Figure 3A shows EDV PCR products from a representative set of five patients. We further quantified the density of V+ isoforms (1076 to 1243 bp isoforms) normalized to GAPDH. There was a trend for synovial V+ variant expression to increase with increasing cartilage Outerbridge score (Figure 3C, n=3 in each group, P=0.09). Figure 3B depicts synovial EDB PCR products from a representative set of five patients with Outerbridge scores ranging from 0 to 4. EDB+ to GAPDH ratios were quantified in all 15 patients, but there was no significant association with stage of cartilage degeneration (Figure 3D, P=0.45). All tissues expressed EDA− variants; only one synovial specimen expressed EDA+ variant (data not shown).
Figure 3. FN splice variants in synovial membrane (SM) from patients with various degrees of underlying cartilage degeneration who were undergoing arthroscopic procedures.
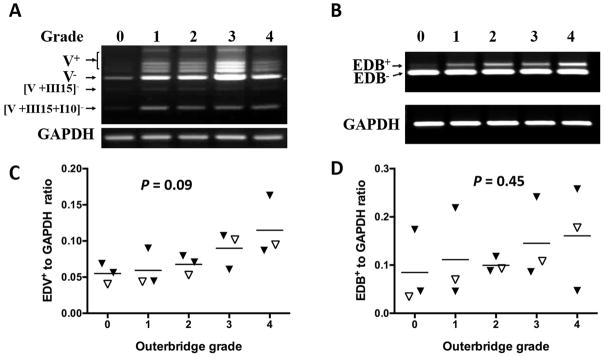
Grade refers to Outerbridge grade determined by the surgeon intra-operatively (0–4; 0 = normal, 4= full thickness cartilage erosion). A: All synovial specimens expressed various V+ and V− forms, as well as the cartilage-specific [V+III15]− and [V+III15+I10]− variants. B: EDB+ and EDB− were present in all SM. C: Relative expression of V+ variants normalized to GAPDH by densitometry. D: Relative expression of EDB+ variants normalized to GAPDH, analyzed by densitometry. P value calculated by ANOVA. Open triangles in panels C and D represent samples shown in images in panels A and B, respectively.
Comparison of synovial FN expression between patients and asymptomatic donors
Although different grading systems were applied to grade osteoarthritis changes in patients (Outerbridge23) and donors (modified Collins22), a limited comparison was done between synovial expression levels of EDB+ and EDV+ variants in patients with Outerbridge grade 2 changes (n=3, fibrillation and/or fissuring in an area half an inch or less in diameter), and donors, all of whom had modified Collins grade 2 changes (cartilage fibrillation or fissuring with or without small osteophytes). Synovial EDB+/GAPDH ratios were higher in grade 2 arthroscopy patients (0.10±0.02) compared with asymptomatic donors (0.04±0.01, P<0.01), while EDV+/GAPDH ratios were lower in patients (0.07±0.01) compared to the donors (0.11±0.01, P=0.03).
Discussion
Fibronectin is a versatile protein that is present in multiple forms due to alternative splicing of three regions: Extra Domain A (EDA), Extra Domain B (EDB) and Variable (V) regions. In humans, the V region can be spliced in at least five different ways leading to multiple products. To add to this diversity, in human cartilage the adjacent III-15 and I-10 domains can be spliced out, resulting in [V+III15+I10]−, [V+III15]− and [V+I10]− forms 14, 26. In human intervertebral disc tissues 24, we previously reported increased EDB+ and EDV+ variant species in the annulus fibrosus (AF) tissues that were associated with degree of disc degeneration 24. Because there are similarities between the intervertebral disc and articular joints 28, we conducted the current study to further investigate potential associations between FN splicing and knee joint OA.
FN splicing varies according to cell type, and can be altered in many disease states. However, with the exception of cartilage, patterns of splice variation in other human joint tissues have not been extensively characterized. We first investigated FN splice variants in SM, meniscus and cartilage obtained from four asymptomatic organ donors (Figure 2). Synovial splice patterns were further explored in biopsies from arthroscopy patients with various degrees of cartilage integrity loss (Figure 3). This analysis yielded a number of interesting findings.
EDB+ splice variants were detected in meniscus, SM and cartilage tissues from asymptomatic donors (Figure 2), and transcript levels of and EDB+ variants did not differ significantly among these tissues (n=4, p=0.55). In normal adult mice, expression of this isoform has been demonstrated in cartilaginous structures 29, but meniscal expression has not been specifically described. To our knowledge, this is the first demonstration of the presence of EDB+ FN transcripts in human meniscal tissues, consistent with a previous report of this isoform in human articular cartilage 17. EDB+ FN was also found in synovium of patients undergoing arthroscopic procedures with and without evidence of early cartilage degeneration (Figure 3), and levels were higher than levels in synovium from the asymptomatic donors. A previous study demonstrated protein expression of EDB+ FN in SM from RA and OA patients, but did not compare levels with those in asymptomatic tissues 30. The increased synovial levels in symptomatic patients compared with asymptomatic donors is intriguing, but needs to be confirmed by larger studies.
Expression of the EDB domain in adult tissues has been described as a marker of angiogenesis 8, as EDB is upregulated around newly forming vascular structures in malignant and non-malignant tissues 31, 32. In OA, the normally avascular articular cartilage becomes invaded by new vascular channels at the interface with the subchondral bone 33 and angiogenesis is also observed in the meniscus 34 and SM 35. Use of a monoclonal antibody directed against the EDB domain of FN is being investigated as a therapeutic agent to target tumor vasculature 36, and inhibition of angiogenesis has demonstrated utility in a rodent model of OA 37. All tissues in our study expressed the EDB+ variant, including tissues from patients without visible cartilage damage, and non-lesional areas of cartilage from the donors. As expression of EDB+ variants was found in multiple joint tissues regardless of the presence or absence of cartilage damage, expression of this variant may not be related to structural disease in OA. The function of this splice variant in normal adult human cartilage is unknown.
Our analysis of V region splice variation yielded several interesting findings. First, several V− splice variants were detectable in all joint tissues. Specifically, Figures 2 and 3 show significant SM and meniscus expression of three variants previously described as “cartilage-specific”: the [V120 +III15 +I10]− [V+III15]−, and [V+I10]− isoforms 15, 26. Expression of these three variants was observed in SM from all fifteen arthroscopy patients. These biopsies were obtained from the suprapatellar pouch by direct visualization, and care was taken to ensure that only SM was included. Moreover, processing and analysis of synovial mRNA from patients was performed separately from the donor tissues, to prevent contamination from cartilage or meniscal mRNA specimens. Parker and colleagues described cartilage expression of the [V+III15]− and [V+I10]− isoforms in osteoporotic patients, with more limited expression in OA patients 26. Non-cartilaginous joint tissues were not analyzed in their study. The potential functional role for these V− variants in SM is unclear. Another novel finding was the variation of synovial expression levels of V+ transcripts according to tissue source and degree of underlying joint degeneration. V+ variants were identified in all three joint tissues (Figure 2), and were slightly higher in SM compared with cartilage. In SM from arthroscopy patients (Figure 3), expression levels of V+ variants tended to increase with increasing levels of cartilage damage. This finding is consistent with the hypothesis that the synovial reaction in OA is related to cartilage damage precipitated by aging or joint injury 27. The V region of FN contains an α4β1 integrin binding site 12, and is likely to be important in cell-cell interactions and cellular trafficking during wound healing. It is possible that the synovial expression of the V+ isoforms during early and intermediate stages of disease reflects a synovial response to cartilage damage.
We found no evidence of EDA+ variant expression at the mRNA level in meniscal or cartilage tissues, and little evidence in synovium, with the exception of low expression in one donor and one patient. Hines et al 38 noted immunostaining for EDA+ FN protein in the synovial lining cells of patients with RA, but not in synovium from four OA patients. These findings are consistent with our current results, as it is possible that expression in the SM occurs in a small minority of OA patients or at very low levels and may be overlooked if analyzing very small numbers of specimens.
In this study, arthroscopy patients with normal cartilage, or with low grades of cartilage degeneration, were younger than those with higher grades of degeneration (Table 1). Because of the small sample number, we cannot rule out an effect of age on V+ splice variation in SM. Furthermore, the regulating factors for alternative splicing and the functional importance of these variants remain to be determined. In addition, although synovial expression of EDB+ and EDV+ variants differed in patients and asymptomatic donors, the value of this comparison is limited, owing to the small number of specimens, and the slight differences in the cartilage grading systems applied to patients and donors. The Collins grade applied to donor tissues accounts for both cartilage degeneration and bone changes, while the Outerbridge system applied to arthroscopy patients solely grades cartilage defects. Nevertheless, the current analysis extends our understanding of FN splice variation in two important ways. First, two specific V− isoforms previously described only in cartilage, were in fact expressed in multiple joint tissues including meniscus and SM. These findings highlight the emerging importance of the meniscus and the SM as active tissues in OA 1, 27. Second, synovial expression of V+ isoforms appeared to vary with degree of cartilage damage in patients undergoing arthroscopic knee procedures. These expression patterns suggest potential utility of FN isoforms as markers of disease stage in OA and in other forms of arthritis. Given the functional consequences of FN splicing on cellular responses and interactions with extracellular matrix, our observations may guide further investigation regarding the impact of FN splicing on reparative and inflammatory processes occurring in multiple joint tissues affected by arthritis and joint injury.
Acknowledgments
The authors gratefully acknowledge the patients for donation of joint tissues for this study; Arkady Margulis, MD for coordination of the post-mortem tissue donations from the Gift of Hope; Drs. Charles Bush-Joseph and Nikhil Verma for tissue collection from the Orthopedic practices at Rush University Medical Center; and the Cancer Genomics Facility staff of the Kimmel Cancer Center, Thomas Jefferson University for assistance with sequencing. We thank Anjali Nair and Dr. Nancy Ruel for technical assistance, and Martin Heyworth, MD for critically editing the manuscript. Yejia Zhang, MD, PhD is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, 1K08 HD049598). Carla Scanzello, MD, PhD is supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS, 1K08 AR057859).
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarzbauer JE, Desimone DW. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb Perspect Biol. 2011;3:a005041. doi: 10.1101/cshperspect.a005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnuson VL, Young M, Schattenberg DG, et al. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991;266:14654–62. [PubMed] [Google Scholar]
- 4.Muro AF, Chauhan AK, Gajovic S, et al. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–60. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybak JN, Roesli C, Kaspar M, et al. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67:10948–57. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 6.Scarpino S, Stoppacciaro A, Pellegrini C, et al. Expression of EDA/EDB isoforms of fibronectin in papillary carcinoma of the thyroid. J Pathol. 1999;188:163–7. doi: 10.1002/(SICI)1096-9896(199906)188:2<163::AID-PATH335>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Berndt A, Borsi L, Luo X, et al. Evidence of ED-B+ fibronectin synthesis in human tissues by non-radioactive RNA in situ hybridization. Investigations on carcinoma (oral squamous cell and breast carcinoma), chronic inflammation (rheumatoid synovitis) and fibromatosis (Morbus Dupuytren) Histochem Cell Biol. 1998;109:249–55. doi: 10.1007/s004180050224. [DOI] [PubMed] [Google Scholar]
- 8.Castellani P, Viale G, Dorcaratto A, et al. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–8. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- 9.Gondokaryono SP, Ushio H, Niyonsaba F, et al. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82:657–65. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 10.Liao YF, Gotwals PJ, Koteliansky VE, et al. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–74. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 11.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostafavi-Pour Z, Askari JA, Whittard JD, Humphries MJ. Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol. 2001;20:63–73. doi: 10.1016/s0945-053x(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzbauer JE, Spencer CS, Wilson CL. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol. 1989;109:3445–53. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod JN, Burton-Wurster N, Gu DN, Lust G. Fibronectin mRNA splice variant in articular cartilage lacks bases encoding the V, III-15, and I-10 protein segments. J Biol Chem. 1996;271:18954–60. doi: 10.1074/jbc.271.31.18954. [DOI] [PubMed] [Google Scholar]
- 15.Kozaki T, Matsui Y, Gu J, et al. Recombinant expression and characterization of a novel fibronectin isoform expressed in cartilaginous tissues. J Biol Chem. 2003;278:50546–53. doi: 10.1074/jbc.M307432200. [DOI] [PubMed] [Google Scholar]
- 16.Chevalier X, Claudepierre P, Groult N, et al. Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 1996;23:1022–30. [PubMed] [Google Scholar]
- 17.Chevalier X, Groult N, Hornebeck W. Increased expression of the Ed-B-containing fibronectin (an embryonic isoform of fibronectin) in human osteoarthritic cartilage. Br J Rheumatol. 1996;35:407–15. doi: 10.1093/rheumatology/35.5.407. [DOI] [PubMed] [Google Scholar]
- 18.Peters JH, Carsons S, Kalunian K, et al. Preferential recognition of a fragment species of osteoarthritic synovial fluid fibronectin by antibodies to the alternatively spliced EIIIA segment. Arthritis Rheum. 2001;44:2572–85. doi: 10.1002/1529-0131(200111)44:11<2572::aid-art438>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Homandberg GA, Meyers R, Aydelotte M, et al. Isolation and characterization of an abundant elastase inhibitor from NaCl extracts of bovine nasal septa and articular cartilage. Connect Tissue Res. 1992;28:289–305. doi: 10.3109/03008209209016821. [DOI] [PubMed] [Google Scholar]
- 20.Xie D, Homandberg GA. Fibronectin fragments bind to and penetrate cartilage tissue resulting in proteinase expression and cartilage damage. Biochim Biophys Acta. 1993;1182:189–96. doi: 10.1016/0925-4439(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 21.Saito S, Yamaji N, Yasunaga K, et al. The fibronectin extra domain A activates matrix metalloproteinase gene expression by an interleukin-1-dependent mechanism. J Biol Chem. 1999;274:30756–63. doi: 10.1074/jbc.274.43.30756. [DOI] [PubMed] [Google Scholar]
- 22.Muehleman C, Bareither D, Huch K, et al. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- 23.Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res. 1975;110:177–96. doi: 10.1097/00003086-197507000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DG, Markova D, Adams SL, et al. Fibronectin splicing variants in human intervertebral disc and association with disc degeneration. Spine. 2010;35:1581–8. doi: 10.1097/BRS.0b013e3181c6ef1a. [DOI] [PubMed] [Google Scholar]
- 25.Hynes RO. Fibronectins. New York, NY: Springer-Verlag; 1990. p. 546. [Google Scholar]
- 26.Parker AE, Boutell J, Carr A, Maciewicz RA. Novel cartilage-specific splice variants of fibronectin. Osteoarthritis Cartilage. 2002;10:528–34. doi: 10.1053/joca.2002.0792. [DOI] [PubMed] [Google Scholar]
- 27.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what’s a nice nucleus like you doing in a joint like this? Bone. 2012;50:771–6. doi: 10.1016/j.bone.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters JH, Chen GE, Hynes RO. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes Commun. 1996;4:127–148. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- 30.Kriegsmann J, Berndt A, Hansen T, et al. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2004;24:25–33. doi: 10.1007/s00296-003-0316-1. [DOI] [PubMed] [Google Scholar]
- 31.Kaczmarek J, Castellani P, Nicolo G, et al. Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer. 1994;59:11–6. doi: 10.1002/ijc.2910590104. [DOI] [PubMed] [Google Scholar]
- 32.Nicolo M, Biro A, Cardillo-Piccolino F, et al. Expression of extradomain-B-containing fibronectin in subretinal choroidal neovascular membranes. Am J Ophthalmol. 2003;135:7–13. doi: 10.1016/s0002-9394(02)01839-1. [DOI] [PubMed] [Google Scholar]
- 33.Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010;49:1852–61. doi: 10.1093/rheumatology/keq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashraf S, Wibberley H, Mapp PI, et al. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70:523–9. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 35.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 36.Borsi L, Balza E, Bestagno M, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102:75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 37.Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63:2700–10. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 38.Hino K, Shiozawa S, Kuroki Y, Ishikawa H, Shiozawa K, Sekiguchi K, Hirano H, Sakashita E, Miyashita K, Chihara K. EDA-containing fibronectin is synthesized from rheumatoid synovial fibroblast-like cells. Arthritis Rheum. 1995;38:678–83. doi: 10.1002/art.1780380516. [DOI] [PubMed] [Google Scholar]


