Abstract
BACKGROUND
Annual updates on cancer occurrence and trends in the United States are provided through collaboration between the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR). This year’s report highlights the increased cancer risk associated with excess weight (overweight or obesity) and lack of sufficient physical activity (<150 minutes of physical activity per week).
METHODS
Data on cancer incidence were obtained from the CDC, NCI, and NAACCR; data on cancer deaths were obtained from the CDC’s National Center for Health Statistics. Annual percent changes in incidence and death rates (age-standardized to the 2000 US population) for all cancers combined and for the leading cancers among men and among women were estimated by joinpoint analysis of long-term trends (incidence for 1992–2008 and mortality for 1975–2008) and short-term trends (1999–2008). Information was obtained from national surveys about the proportion of US children, adolescents, and adults who are overweight, obese, insufficiently physically active, or physically inactive.
RESULTS
Death rates from all cancers combined decreased from 1999 to 2008, continuing a decline that began in the early 1990s, among men and among women in most racial and ethnic groups. Death rates decreased from 1999 to 2008 for most cancer sites, including the 4 most common cancers (lung, colorectum, breast, and prostate). The incidence of prostate and colorectal cancers also decreased from 1999 to 2008. Lung cancer incidence declined from 1999 to 2008 among men and from 2004 to 2008 among women. Breast cancer incidence decreased from 1999 to 2004 but was stable from 2004 to 2008. Incidence increased for several cancers, including pancreas, kidney, and adenocarcinoma of the esophagus, which are associated with excess weight.
CONCLUSIONS
Although improvements are reported in the US cancer burden, excess weight and lack of sufficient physical activity contribute to the increased incidence of many cancers, adversely affect quality of life for cancer survivors, and may worsen prognosis for several cancers. The current report highlights the importance of efforts to promote healthy weight and sufficient physical activity in reducing the cancer burden in the United States.
Keywords: cancer; incidence; mortality; Surveillance; Epidemiology, and End Results; the North American Association of Central Cancer Registries; National Program of Cancer Registries; United States; obesity; physical inactivity
INTRODUCTION
The Annual Report to the Nation has provided updated cancer incidence and mortality data for the United States since the initial report in 1998, which demonstrated the first continuous decline in cancer mortality rates since the 1930s.1 In addition, each report has included a special section focused on important public health topics, cancer in special populations, or cancers of special interest.2–13 This year’s report continues the collaborative effort between the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC), the National Cancer Institute (NCI), and the North American Association of Central Cancer Registries (NAACCR). This report provides updated information on incidence and mortality trends for all cancers combined, for childhood cancers, and for the top 15 cancers for each of 5 major racial and ethnic groups by sex (totaling 17 cancers among men and 18 cancers among women). This report also highlights several cancers associated with excess weight (overweight and obesity) and lack of sufficient physical activity and describes temporal and regional patterns of excess weight and insufficient physical activity among US children, adolescents, and adults.
MATERIALS AND METHODS
Cancers, Cancer Deaths, and Population Estimates
Population-based data on cancer incidence, including newly diagnosed cases, are based on information collected by central cancer registries in the CDC’s National Program of Cancer Registries (NPCR) and/or the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program. These registries, as members of the NAACCR, submit data annually to that organization for evaluation and use in this report. Site and histology for incident cancers were coded according to the International Classification of Diseases for Oncology (ICD-O) edition in use at the time of diagnosis, converted to the Third Edition coding,14 and categorized according to SEER site groups.15 Incidence rates were calculated for all sites combined, for childhood cancers, and for the top 15 cancers among men and women for each of 5 major racial and ethnic groups (white, black, Asian and Pacific Islander [API], American Indian/Alaska Native [AI/AN], and Hispanic). Information regarding race and Hispanic ethnicity was collected separately. Hispanic ethnicity includes men and women from all race categories identified as Hispanic. Rates for AI/ANs were based on cases and deaths in counties covered by the Indian Health Service’s Contract Health Service Delivery Area, because these rates most accurately reflect the true rates in these populations.10
Incidence data were not available uniformly for every period, geographic area, or racial and ethnic group in the United States. Long-term (1992–2008) incidence trends for all racial and ethnic groups combined were estimated using data from the SEER 13 registries, which provide coverage of 14% of the US population.16 Beginning in 1995, after the advent of the NPCR, coverage of the US population increased dramatically. Consequently, 5-year (2004–2008) average annual incidence rates and short-term (1999–2008) incidence trends for all racial and ethnic groups combined and for each of 5 major racial and ethnic populations were calculated using data from NPCR and SEER registries. For the period from 2004 to 2008, 48 registries (covering 96% of the US population) met the NAACCR data quality criteria, and for the period from 1999 to 2008, 41 registries (covering 86% of the US population) met these criteria.
Cause of death was based on death certificate information reported to state vital statistics offices and compiled into a national file through the CDC National Center for Health Statistics’ National Vital Statistics System.17 To maximize comparability among International Classification of Diseases (ICD) and ICD-O versions, cause of death was categorized according to SEER anatomic site groups.15 The underlying causes of death were selected according to the version of the ICD codes and selection rules in use at the time of death (ICD-6 to ICD-10). Death rates were calculated for all sites combined, for childhood cancers, and for the top 15 cancers for each of 5 major racial and ethnic groups by sex. We examined long-term (1975–2008) mortality trends for all racial and ethnic groups combined and 5-year (2004–2008) average annual mortality rates and short-term (1999–2008) mortality trends for all racial and ethnic groups combined and for each of 5 major racial and ethnic groups.
Population estimates used in the SEER*Stat software (available at: http://seer.cancer.gov/seerstat [accessed January 27, 2012]) were a modified version of the annual time series of July 1 county population estimates by age, sex, race, and ethnicity produced by the US Census Bureau.16 Modifications incorporated bridged, single-race estimates that were derived from multiple-race categories in the 2000 Census.18 For most states, population estimates as of July 1 of each year were used to calculate annual incidence rates, because it is presumed that these estimates reflect the average population of a defined geographic area for a calendar year. However, certain county population estimates were adjusted to account for populations displaced along the Gulf Coast of Louisiana, Alabama, Mississippi, and Texas in fall 2005 by hurricanes Katrina and Rita.16 National total population estimates were not affected by these adjustments. Other specific modifications included using additional local information to estimate the native Hawaiian population accurately and to derive population estimates for a newly created county in Colorado.16 These modified county-level population estimates, summed to the state and national levels, were used as denominators in rate calculations.16
Statistical Analyses of Incidence and Mortality Trends
Average annual incidence rates per 100,000 persons were age-adjusted to the 2000 US standard population by using the direct method.19 Corresponding 95% confidence intervals were calculated as modified gamma intervals.20 For stability and reliability, rates were not reported if the numerator included fewer than 16 observations.
Trends in age-standardized cancer incidence and death rates were analyzed using joinpoint regression, which involves fitting a series of joined straight lines on a logarithmic scale to the trends in the annual age-standardized rates (available at: http://www.srab.cancer.gov/join-point [accessed January 27, 2012]).21 Up to 3 joinpoints were allowed in models for the period from 1992 to 2008 (Table 1), up to 5 joinpoints were allowed in models for the period from 1975 to 2008 (Table 2), and up to 2 joinpoints were allowed in models for the period from 1999 to 2008 (Tables 3 and 4). The resulting trends of various periods were described by the slope of the line segment or the annual percent change (APC). The average APC (AAPC) was estimated as a geometric weighted average of the APCs, with the weights equal to the length of each line segment during the prespecified, fixed interval (available at: http://srab.cancer.gov/joinpoint/aapc.html [accessed January 27, 2012]).22 Long-term incidence trends were calculated using both observed and delay-adjusted SEER 13 data (Table 1); descriptions of these trends were based on the delay-adjusted data, except when noted. Delay adjustment is a statistical method that corrects for unreported (delayed) or updated cases and mostly affects cancers diagnosed in recent years and cancers diagnosed in nonhospital settings, eg, melanoma or leukemia.23 The t test was used to test whether the APC was statistically different from zero, and the Z test was used to test whether the AAPC was statistically different from zero; all statistical tests were 2-sided. In describing trends, the terms increase or decrease were used when the slope (APC or AAPC) of the trend was statistically significant (P < .05). For nonstatistically significant trends, terms such as stable, nonsignificant increase, and nonsignificant decrease were used.
Table 1.
Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Rate Trends With Joinpoint Analyses from 1992 to 2008 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
| Joinpoint Analyses (1992–2008)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | AAPCc | ||||||
| Sex/Cancer Site or Type | Years | APCd | Years | APCd | Years | APCd | Years | APCd | 1999–2008 | 2004–2008 |
| All sitese | ||||||||||
| Both sexes | 1992–1994 | −3.1f | 1994–1999 | 0.3 | 1999–2008 | −0.7f | −0.7g | −0.7g | ||
| (Delay-adjusted) | 1992–1994 | −3.2f | 1994–1999 | 0.4 | 1999–2005 | −0.8f | 2005–2008 | 0.1 | −0.5 | −0.1 |
| Men | 1992–1995 | −4.5f | 1995–2000 | 0.2 | 2000–2008 | −1.1f | −1.0g | −1.1g | ||
| (Delay-adjusted) | 1992–1994 | −5.6f | 1994–2008 | −0.6f | −0.6g | −0.6g | ||||
| Women | 1992–1994 | −0.4 | 1994–1998 | 1.2 | 1998–2004 | −0.8f | 2004–2008 | 0.0 | −0.4g | 0.0 |
| (Delay-adjusted) | 1992–1998 | 0.8f | 1998–2006 | −0.5f | 2006–2008 | 1.1 | −0.2 | 0.3 | ||
| Children (ages 0–14 y) | 1992–2008 | 0.4 | 0.4 | 0.4 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.5f | 0.5g | 0.5g | ||||||
| Children (ages 0–19 y) | 1992–2008 | 0.5f | 0.5g | 0.5g | ||||||
| (Delay-adjusted) | 1992–2008 | 0.6f | 0.6g | 0.6g | ||||||
| Top 17 cancers for menh | ||||||||||
| Prostate | 1992–1995 | −11.2f | 1995–2000 | 2.1 | 2000–2008 | −2.1f | −1.7g | −2.1g | ||
| (Delay-adjusted) | 1992–1995 | −11.1f | 1995–2000 | 2.0 | 2000–2008 | −1.9f | −1.5g | −1.9g | ||
| Lung and bronchus | 1992–2008 | −2.0f | −2.0g | −2.0g | ||||||
| (Delay-adjusted) | 1992–2008 | −1.9f | −1.9g | −1.9g | ||||||
| Colon and rectum | 1992–1995 | −2.6f | 1995–1998 | 1.5 | 1998–2008 | −2.7f | −2.7g | −2.7g | ||
| (Delay-adjusted) | 1992–1995 | −2.6f | 1995–1998 | 1.5 | 1998–2008 | −2.6f | −2.6g | −2.6g | ||
| Urinary bladder | 1992–2008 | −0.1 | −0.1 | −0.1 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| Melanoma of the skin | 1992–2008 | 2.4f | 2.4g | 2.4g | ||||||
| (Delay-adjusted) | 1992–2008 | 2.5f | 2.5g | 2.5g | ||||||
| Non-Hodgkin lymphoma | 1992–2008 | 0.1 | 0.1 | 0.1 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.2 | 0.2 | 0.2 | ||||||
| Kidney and renal pelvis | 1992–2008 | 2.2f | 2.2g | 2.2g | ||||||
| (Delay-adjusted) | 1992–2006 | 2.0f | 2006–2008 | 6.2f | 2.9g | 4.1g | ||||
| Oral cavity and pharynx | 1992–2006 | −1.4f | 2006–2008 | 3.0 | −0.4 | 0.8 | ||||
| (Delay-adjusted) | 1992–2006 | −1.4f | 2006–2008 | 3.8 | −0.2 | 1.2 | ||||
| Leukemia | 1992–2008 | −0.4f | −0.4g | −0.4g | ||||||
| (Delay-adjusted) | 1992–2008 | 0.3f | 0.3g | 0.3g | ||||||
| Pancreas | 1992–2002 | 0.0 | 2002–2008 | 1.4f | 0.9g | 1.4g | ||||
| (Delay-adjusted) | 1992–2002 | 0.0 | 2002–2008 | 1.8f | 1.2g | 1.8g | ||||
| Liver and intrahepatic bile duct | 1992–2008 | 3.4f | 3.4g | 3.4g | ||||||
| (Delay-adjusted) | 1992–2008 | 3.6f | 3.6g | 3.6g | ||||||
| Stomach | 1992–2008 | −1.9f | −1.9g | −1.9g | ||||||
| (Delay-adjusted) | 1992–2008 | −1.8f | −1.8g | −1.8g | ||||||
| Esophagus | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| Brain and other nervous system | 1992–2008 | −0.4f | −0.4g | −0.4g | ||||||
| (Delay-adjusted) | 1992–2008 | −0.3 | −0.3 | −0.3 | ||||||
| Myeloma | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.4f | 0.4g | 0.4g | ||||||
| Larynx | 1992–2008 | −3.0f | −3.0g | −3.0g | ||||||
| (Delay-adjusted) | 1992–2008 | −2.9f | −2.9g | −2.9g | ||||||
| Thyroid | 1992–1996 | −1.2 | 1996–2008 | 5.3f | 5.3g | 5.3g | ||||
| (Delay-adjusted) | 1992–1996 | −1.2 | 1996–2008 | 5.5f | 5.5g | 5.5g | ||||
| Top 18 cancers for womenh | ||||||||||
| Breast | 1992–1999 | 1.3f | 1999–2005 | −2.1f | 2005–2008 | 0.9 | −1.1g | 0.1 | ||
| (Delay-adjusted) | 1992–1999 | 1.3f | 1999–2005 | −2.0f | 2005–2008 | 1.1 | −1.0 | 0.3 | ||
| Lung and bronchus | 1992–1998 | 0.8f | 1998–2001 | −1.3 | 2001–2005 | 0.5 | 2005–2008 | −1.8f | −0.7 | −1.2g |
| (Delay-adjusted) | 1992–1997 | 0.7 | 1997–2008 | −0.3f | −0.3g | −0.3g | ||||
| Colon and rectum | 1992–1995 | −1.9f | 1995–1998 | 1.9 | 1998–2008 | −2.1f | −2.1g | −2.1g | ||
| (Delay-adjusted) | 1992–1995 | −1.8f | 1995–1998 | 1.9 | 1998–2008 | −2.0f | −2.0g | −2.0g | ||
| Corpus and uterus, NOS | 1992–2006 | −0.2 | 2006–2008 | 2.3 | 0.4 | 1.1 | ||||
| (Delay-adjusted) | 1992–2006 | −0.2 | 2006–2008 | 2.7 | 0.5 | 1.2 | ||||
| Thyroid | 1992–1998 | 3.8f | 1998–2008 | 6.5f | 6.5g | 6.5g | ||||
| (Delay-adjusted) | 1992–1998 | 3.8f | 1998–2008 | 6.6f | 6.6g | 6.6g | ||||
| Non-Hodgkin lymphoma | 1992–2004 | 1.3f | 2004–2008 | −1.3 | 0.1 | −1.3 | ||||
| (Delay-adjusted) | 1992–2004 | 1.3f | 2004–2008 | −0.9 | 0.3 | −0.9 | ||||
| Melanoma of the skin | 1992–2008 | 2.1f | 2.1g | 2.1g | ||||||
| (Delay-adjusted) | 1992–2008 | 2.2f | 2.2g | 2.2g | ||||||
| Ovarye | 1992–1996 | −1.6 | 1996–2001 | 0.1 | 2001–2004 | −2.7 | 2004–2008 | −0.8 | −1.2 | −0.8 |
| (Delay-adjusted)e | 1992–1996 | −1.6 | 1996–2001 | 0.2 | 2001–2004 | −2.6 | 2004–2008 | −0.3 | −1.0 | −0.3 |
| Kidney and renal pelvis | 1992–1998 | 1.2 | 1998–2008 | 3.1f | 3.1g | 3.1g | ||||
| (Delay-adjusted) | 1992–1998 | 1.1 | 1998–2008 | 3.3f | 3.3g | 3.3g | ||||
| Pancreas | 1992–2008 | 0.5f | 0.5g | 0.5g | ||||||
| (Delay-adjusted) | 1992–2000 | −0.1 | 2000–2008 | 1.4f | 1.2g | 1.4g | ||||
| Leukemia | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.6f | 0.6g | 0.6g | ||||||
| Urinary bladder | 1992–2004 | −0.2 | 2004–2008 | −1.7f | −0.9g | −1.7g | ||||
| (Delay-adjusted) | 1992–2008 | −0.3f | −0.3g | −0.3g | ||||||
| Cervix uteri | 1992–2008 | −2.7f | −2.7g | −2.7g | ||||||
| (Delay-adjusted) | 1992–2008 | −2.6f | −2.6g | −2.6g | ||||||
| Oral cavity and pharynx | 1992–2008 | −1.0f | −1.0g | −1.0g | ||||||
| (Delay-adjusted) | 1992–2008 | −1.0f | −1.0g | −1.0g | ||||||
| Brain and other nervous system | 1992–2008 | −0.2 | −0.2 | −0.2 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.0 | 0.0 | 0.0 | ||||||
| Stomach | 1992–2008 | −0.9f | −0.9g | −0.9g | ||||||
| (Delay-adjusted) | 1992–2008 | −0.8f | −0.8g | −0.8g | ||||||
| Myeloma | 1992–2008 | −0.2 | −0.2 | −0.2 | ||||||
| (Delay-adjusted) | 1992–2008 | 0.2 | 0.2 | 0.2 | ||||||
| Liver and intrahepatic bile duct | 1992–1996 | 7.2f | 1996–2008 | 2.0g | 2.0g | 2.0g | ||||
| (Delay-adjusted) | 1992–2008 | 3.0f | 3.0g | 3.0g | ||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: Surveillance, Epidemiology, and End Results (SEER) 13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico, the Alaska Native Tumor Registry, rural Georgia, and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound).
Joinpoint analyses with up to 3 joinpoints yielding up to 4 trend segments (Trends 1–4) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is a weighted average of the APCs that is calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, and ≥85 years; Census publication p25-1130).
All sites excludes myelodysplastic syndromes and borderline tumors; ovary excludes borderline tumors.
The APC is statistically significantly different from zero (2-sided t test; P <.05).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2004 through 2008 for all racial and ethnic groups combined (using data from the National Program of Cancer Registries [NPCR] and SEER Program areas reported by the North American Association of Central Cancer Registries [NAACCR] as meeting high-quality incidence data standards for 2004–2008). More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Table 2.
US Cancer Death Rate Trends With Joinpoint Analyses From 1975 to 2008 for the Most Common Cancers, by Sex, for All Racial and Ethnic Groups Combineda
| Joinpoint Analyses (1975–2008)b | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | AAPCc | ||||||||
| Sex/Cancer Site or Type | Years | APCd | Years | APCd | Years | APCd | Years | APCd | Years | APCd | Years | APCd | 1999–2008 | 2004–2008 |
| All sites | ||||||||||||||
| Both sexes | 1975–1984 | 0.5e | 1984–1991 | 0.3e | 1991–1994 | −0.5 | 1994–1998 | −1.3e | 1998–2001 | −0.8 | 2001–2008 | −1.6e | −1.4f | −1.6f |
| Men | 1975–1979 | 1.0e | 1979–1990 | 0.3e | 1990–1993 | −0.5 | 1993–2001 | −1.5e | 2001–2008 | −1.8e | −1.7f | −1.8f | ||
| Women | 1975–1991 | 0.6e | 1991–2001 | −0.6e | 2001–2008 | −1.5e | −1.3f | −1.5f | ||||||
| Children (ages 0–14 y) | 1975–1998 | −2.9e | 1998–2003 | 0.2 | 2003–2008 | −2.8e | −1.5f | −2.8f | ||||||
| Children (ages 0–19 y) | 1975–1996 | −2.7e | 1996–2008 | −1.3e | −1.3f | −1.3f | ||||||||
| Top 17 cancers for meng | ||||||||||||||
| Lung and bronchus | 1975–1978 | 2.5e | 1978–1984 | 1.2e | 1984–1990 | 0.4e | 1990–1993 | −1.1 | 1993–2005 | −1.9e | 2005–2008 | −2.7e | −2.2f | −2.5f |
| Prostate | 1975–1987 | 0.9e | 1987–1991 | 3.1e | 1991–1994 | −0.7 | 1994–2004 | −3.9e | 2004–2008 | −3.1e | −3.5f | −3.1f | ||
| Colon and rectum | 1975–1978 | 0.8 | 1978–1984 | −0.4 | 1984–1990 | −1.3e | 1990–2002 | −2.0e | 2002–2005 | −4.0e | 2005–2008 | −2.3e | −2.8f | −2.7f |
| Pancreas | 1975–1986 | −0.8e | 1986–2001 | −0.3e | 2001–2008 | 0.6e | 0.4f | 0.6f | ||||||
| Leukemia | 1975–1980 | 0.5 | 1980–1987 | −0.7e | 1987–1995 | 0.1 | 1995–2008 | −0.9e | −0.9f | −0.9f | ||||
| Non-Hodgkin lymphoma | 1975–1991 | 2.7e | 1991–1997 | 1.6e | 1997–2008 | −2.8e | −2.8f | −2.8f | ||||||
| Liver and intrahepatic bile duct | 1975–1985 | 1.5e | 1985–1996 | 3.8e | 1996–1999 | 0.6 | 1999–2008 | 2.5e | 2.5f | 2.5f | ||||
| Esophagus | 1975–1985 | 0.7e | 1985–1994 | 1.2e | 1994–2006 | 0.4e | 2006–2008 | −2.2 | −0.2 | −0.9 | ||||
| Urinary bladder | 1975–1983 | −1.4e | 1983–1987 | −2.8e | 1987–1993 | 0.2 | 1993–1997 | −1.1 | 1997–2008 | 0.0 | 0.0 | 0.0 | ||
| Kidney and renal pelvis | 1975–1992 | 1.1e | 1992–2008 | −0.4e | −0.4f | −0.4f | ||||||||
| Brain and other nervous system | 1975–1977 | 4.3 | 1977–1982 | −0.3 | 1982–1991 | 1.3e | 1991–2008 | −0.9e | −0.9f | −0.9f | ||||
| Stomach | 1975–1987 | −2.3e | 1987–1991 | −1.0 | 1991–2008 | −3.4e | −3.4f | −3.4f | ||||||
| Myeloma | 1975–1994 | 1.5e | 1994–2008 | −1.0e | −1.0f | −1.0f | ||||||||
| Melanoma of the skin | 1975–1989 | 2.3e | 1989–2008 | 0.3e | 0.3f | 0.3f | ||||||||
| Oral cavity and pharynx | 1975–1993 | −1.9e | 1993–2000 | −3.0e | 2000–2008 | −1.2e | −1.4f | −1.2f | ||||||
| Larynx | 1975–1994 | −0.8e | 1994–2008 | −2.5e | −2.5f | −2.5f | ||||||||
| Soft tissue including heart | 1975–1980 | 7.6e | 1980–1997 | 1.2e | 1997–2002 | −3.5e | 2002–2008 | 1.2 | −0.4 | 1.2 | ||||
| Top 18 cancers for womeng | ||||||||||||||
| Lung and bronchus | 1975–1982 | 6.0e | 1982–1990 | 4.2e | 1990–1995 | 1.7e | 1995–2003 | 0.3e | 2003–2008 | −1.0e | −0.4f | −1.0f | ||
| Breast | 1975–1990 | 0.4e | 1990–2008 | −2.2e | −2.2f | −2.2f | ||||||||
| Colon and rectum | 1975–1984 | −1.0e | 1984–2001 | −1.8e | 2001–2008 | −3.0e | −2.8f | −3.0f | ||||||
| Pancreas | 1975–1984 | 0.8e | 1984–2003 | 0.1 | 2003–2008 | 0.6e | 0.4f | 0.6f | ||||||
| Ovary | 1975–1982 | −1.2e | 1982–1992 | 0.3 | 1992–1998 | −1.2e | 1998–2002 | 1.0 | 2002–2008 | −1.9e | −1.0f | −1.9f | ||
| Leukemia | 1975–1980 | 0.8 | 1980–2000 | −0.4e | 2000–2008 | −1.5e | −1.4f | −1.5f | ||||||
| Non-Hodgkin lymphoma | 1975–1994 | 2.2e | 1994–1997 | 1.1 | 1997–2008 | −3.4e | −3.4f | −3.4f | ||||||
| Corpus and uterus, NOS | 1975–1989 | −1.6e | 1989–1997 | −0.7e | 1997–2008 | 0.3e | 0.3f | 0.3f | ||||||
| Brain and other nervous system | 1975–1992 | 0.9e | 1992–2008 | −1.0e | −1.0f | −1.0f | ||||||||
| Liver and intrahepatic bile duct | 1975–1978 | −1.5 | 1978–1988 | 1.4e | 1988–1995 | 3.9e | 1995–2001 | 0.3 | 2001–2004 | 2.7 | 2004–2008 | 0.4 | 1.1 | 0.4 |
| Myeloma | 1975–1993 | 1.5e | 1993–2002 | −0.5e | 2002–2008 | −2.9e | −2.1f | −2.9f | ||||||
| Stomach | 1975–1987 | −2.8e | 1987–1990 | −0.3 | 1990–2008 | −2.7e | −2.7f | −2.7f | ||||||
| Kidney and renal pelvis | 1975–1994 | 1.1e | 1994–2008 | −0.8e | −0.8f | −0.8f | ||||||||
| Cervix uteri | 1975–1982 | −4.4e | 1982–1996 | −1.6e | 1996–2003 | −3.8e | 2003–2008 | −0.6 | −2.0f | −0.6 | ||||
| Urinary bladder | 1975–1986 | −1.7e | 1986–2008 | −0.4e | −0.4f | −0.4f | ||||||||
| Esophagus | 1975–2001 | 0.0 | 2001–2008 | −1.9e | −1.5f | −1.9f | ||||||||
| Oral cavity and pharynx | 1975–1989 | −0.9e | 1989–2008 | −2.2e | −2.2f | −2.2f | ||||||||
| Gallbladder | 1975–1990 | −3.0e | 1990–2008 | −2.3e | −2.3f | −2.3f | ||||||||
Abbreviations: AAPC, average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total US, 1975 through 2008.
Joinpoint analyses with up to 5 joinpoints yielding up to 6 trend segments (Trends 1–6) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is a weighted average of the APCs that is calculated by joinpoint regression.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, and ≥85 years; Census publication p25-1130).
The APC is statistically significantly different from zero (2-sided t test; P <.05).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2004 through 2008 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Table 3.
Cancer Incidence Rates for 2004–2008 and Fixed-Interval Trends From 1999 to 2008 for the Top Cancers by Sex, Race, and Ethnicity, for Areas in the United States With High-Quality Incidence Dataa
| All Racial and Ethnic Groups Combined |
Whiteb | Blackb | APIb | AI/AN (CHSDA)b | Hispanicb | Non-Hispanicb | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Cancer Site or Typec |
Rank | Rated | 1999– 2008 AAPCe |
2004– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
Rank | Rated | 1999– 2008 AAPCe |
| All sitesf | ||||||||||||||||||||||
| Both sexes | 472.6 | −0.6g | −0.6g | 471.6 | −0.7g | 485.5 | −0.7g | 302.0 | −0.7g | 388.2 | −0.6 | 368.2 | −0.9g | 481.9 | −0.5g | |||||||
| Men | 553.0 | −1.0g | −1.0g | 545.0 | −1.0g | 626.2 | −1.3g | 332.4 | −1.5g | 427.8 | −1.1g | 423.4 | −1.5g | 564.2 | −0.9g | |||||||
| Women | 416.5 | −0.4g | −0.4g | 420.8 | −0.4g | 394.2 | −0.1 | 284.0 | 0.1 | 362.1 | −0.3 | 333.5 | −0.4g | 423.9 | −0.3g | |||||||
| Children (ages 0–14 y) | 15.6 | 0.5 | 0.5 | 16.1 | 0.1 | 12.4 | 1.9g | 12.5 | 0.4 | 12.4 | 0.6 | 15.6 | 0.2 | 15.6 | 0.6g | |||||||
| Children (ages 0–19 y) | 17.2 | 0.6g | 0.6g | 17.8 | 0.4 | 13.2 | 1.7g | 13.7 | 1.3 | 13.5 | −0.1 | 17.0 | 0.7g | 17.3 | 0.9 | |||||||
| Top 17 cancers for men | ||||||||||||||||||||||
| Prostate | 1 | 152.9 | −1.6g | −1.6g | 1 | 142.8 | −1.9g | 1 | 230.8 | −1.7g | 1 | 79.7 | −2.7g | 1 | 101.2 | −2.5g | 1 | 126.7 | −2.2g | 1 | 155.1 | −1.5g |
| Lung and bronchus | 2 | 84.4 | −2.0g | −2.6g | 2 | 83.7 | −1.9g | 2 | 102.7 | −2.4g | 2 | 49.8 | −1.6g | 2 | 71.0 | −0.6 | 3 | 46.8 | −2.8g | 2 | 87.3 | −1.9g |
| Colon and rectum | 3 | 55.7 | −3.0g | −3.7g | 3 | 54.6 | −3.2g | 3 | 66.9 | −1.4g | 3 | 42.4 | −2.0g | 3 | 51.5 | −2.5 | 2 | 48.6 | −1.9g | 3 | 56.3 | −2.9g |
| Urinary bladder | 4 | 37.6 | −0.8g | −1.8g | 4 | 39.5 | −0.9g | 5 | 19.3 | 0.3 | 6 | 15.4 | −0.6 | 5 | 17.5 | −0.8 | 4 | 20.4 | −2.1g | 4 | 38.9 | −0.7g |
| Melanoma of the skin | 5 | 23.8 | 2.3g | 2.3g | 5 | 26.3 | 2.1g | 25 | 1.1 | −0.3 | 20 | 1.6 | −0.3 | 14 | 6.1 | −0.3 | 16 | 4.6 | −0.4 | 5 | 25.7 | 2.5g |
| Non-Hodgkin lymphoma | 6 | 23.4 | 0.0 | −0.8 | 6 | 23.9 | 0.0 | 6 | 16.8 | −0.5 | 7 | 14.9 | −1.0 | 6 | 16.0 | −0.8 | 5 | 19.6 | −0.6 | 6 | 23.7 | 0.1 |
| Kidney and renal pelvis | 7 | 20.7 | 2.5g | 1.4g | 7 | 20.8 | 2.4g | 4 | 22.6 | 3.1g | 9 | 9.9 | 3.4g | 4 | 27.4 | 2.3g | 6 | 19.4 | 1.9g | 7 | 20.9 | 2.6g |
| Oral cavity and pharynx | 8 | 16.2 | 0.0 | 0.0 | 9 | 16.3 | 0.3g | 9 | 16.3 | −2.5g | 8 | 10.7 | −0.5 | 9 | 13.3 | −1.5 | 11 | 10.4 | −2.2g | 8 | 16.8 | 0.3 |
| Leukemia | 9 | 16.1 | −1.0g | −2.2g | 8 | 16.5 | −1.0g | 12 | 12.5 | −0.8 | 11 | 8.8 | −1.7g | 10 | 12.6 | −1.4 | 9 | 11.8 | −1.5g | 9 | 16.3 | −0.9g |
| Pancreas | 10 | 13.4 | 0.8g | 0.8g | 10 | 13.2 | 0.9g | 7 | 16.7 | 0.5 | 10 | 9.7 | 0.2 | 11 | 11.1 | 0.6 | 10 | 11.5 | 0.1 | 10 | 13.6 | 0.9g |
| Liver and intrahepatic bile duct | 11 | 9.7 | 3.8g | 3.8g | 12 | 8.6 | 3.8g | 10 | 14.1 | 5.3g | 4 | 21.7 | −0.2 | 7 | 15.8 | 3.9g | 7 | 17.0 | 2.4g | 12 | 9.1 | 3.8g |
| Stomach | 12 | 9.5 | −2.0g | −2.0g | 13 | 8.5 | −2.3g | 8 | 16.4 | −1.3g | 5 | 16.8 | −2.9g | 8 | 13.9 | −3.0 | 8 | 13.8 | −3.1g | 11 | 9.1 | −2.1g |
| Esophagus | 13 | 8.7 | 0.1 | 0.1 | 11 | 8.8 | 0.7g | 14 | 9.7 | −4.6g | 14 | 4.1 | −0.3 | 12 | 7.0 | −0.3 | 15 | 5.6 | −0.7 | 13 | 9.0 | 0.2 |
| Brain and other nervous system | 14 | 7.9 | −0.3g | −0.3g | 14 | 8.4 | −0.3g | 15 | 4.6 | −0.3 | 13 | 4.1 | 0.1 | 16 | 5.2 | 1.5 | 13 | 6.0 | −0.8g | 14 | 8.1 | −0.2 |
| Myeloma | 15 | 7.2 | 0.2 | −1.4 | 16 | 6.6 | 0.0 | 11 | 13.6 | 0.3 | 15 | 4.0 | 1.2g | 13 | 6.5 | −2.7 | 12 | 6.6 | −0.6 | 15 | 7.2 | 0.2 |
| Larynx | 16 | 7.0 | −2.4g | −2.4g | 15 | 6.7 | −2.6g | 13 | 10.9 | −2.8g | 18 | 2.2 | −5.9g | 15 | 6.0 | 0.3 | 14 | 5.7 | −3.2g | 16 | 7.1 | −2.3g |
| Thyroid | 17 | 5.5 | 6.3g | 6.3g | 18 | 5.8 | 6.3g | 19 | 3.0 | 5.7g | 12 | 5.0 | 4.9g | 19 | 3.2 | 1.3 | 17 | 4.2 | 4.4g | 17 | 5.7 | 6.5g |
| Top 18 cancers for women | ||||||||||||||||||||||
| Breast | 1 | 121.2 | −1.2g | 0.2 | 1 | 122.3 | −1.2g | 1 | 116.1 | 0.3 | 1 | 84.9 | 0.4 | 1 | 89.2 | −0.6 | 1 | 92.3 | −0.5 | 1 | 123.9 | −1.2g |
| Lung and bronchus | 2 | 55.7 | 0.0 | −0.8g | 2 | 57.2 | 0.1 | 2 | 51.4 | 0.0 | 3 | 28.1 | 0.0 | 2 | 51.7 | −0.1 | 3 | 27.0 | −0.4 | 2 | 58.1 | 0.1 |
| Colon and rectum | 3 | 41.4 | −2.4g | −3.0g | 3 | 40.3 | −2.5g | 3 | 49.7 | −1.8g | 2 | 32.7 | −1.6g | 3 | 41.5 | −1.3 | 2 | 34.2 | −2.2g | 3 | 42.0 | −2.4g |
| Corpus and uterus, NOS | 4 | 24.1 | 0.3 | 0.3 | 4 | 24.6 | 0.1 | 4 | 21.7 | 1.8g | 5 | 16.3 | 1.9g | 4 | 20.3 | 1.2 | 4 | 19.9 | 1.2g | 4 | 24.5 | 0.3 |
| Thyroid | 5 | 16.3 | 7.3g | 7.3g | 6 | 17.1 | 7.3g | 9 | 10.0 | 6.7g | 4 | 16.9 | 6.5g | 8 | 11.0 | 5.0g | 5 | 16.1 | 6.7g | 6 | 16.5 | 7.3g |
| Non-Hodgkin lymphoma | 6 | 16.3 | −0.2 | −1.2g | 7 | 16.8 | −0.2 | 7 | 11.6 | 0.1 | 6 | 10.4 | 0.2 | 6 | 14.0 | 0.0 | 6 | 15.2 | 0.0 | 7 | 16.4 | −0.2 |
| Melanoma of the skin | 7 | 15.4 | 2.5g | 2.5g | 5 | 17.4 | 2.6g | 27 | 1.0 | 1.0 | 21 | 1.3 | −1.8 | 15 | 5.2 | 3.8 | 17 | 4.3 | 0.1 | 5 | 16.7 | 2.8g |
| Ovary | 8 | 12.7 | −1.7g | −1.7g | 8 | 13.1 | −1.8g | 11 | 9.5 | −0.8 | 8 | 9.3 | −0.9 | 7 | 11.4 | −1.9 | 8 | 11.5 | −0.9 | 8 | 12.8 | −1.8g |
| Kidney and renal pelvis | 9 | 10.9 | 2.8g | 1.9g | 9 | 10.9 | 2.7g | 6 | 11.7 | 3.7g | 14 | 4.9 | 3.6g | 5 | 16.8 | 2.7g | 9 | 11.2 | 2.6g | 9 | 10.8 | 2.8g |
| Pancreas | 10 | 10.4 | 0.9g | 0.9g | 10 | 10.0 | 1.0g | 5 | 13.8 | 0.4 | 10 | 8.1 | −0.4 | 9 | 10.1 | −0.4 | 10 | 9.9 | 0.2 | 10 | 10.4 | 0.9g |
| Leukemia | 11 | 9.7 | −0.6g | −0.6g | 11 | 9.9 | −0.7g | 13 | 7.7 | −1.2g | 12 | 5.8 | 0.0 | 12 | 7.2 | −2.1 | 11 | 8.5 | −0.5 | 11 | 9.7 | −0.6g |
| Urinary bladder | 12 | 9.4 | −1.1g | −2.2g | 12 | 9.8 | −1.0g | 14 | 6.7 | −0.8 | 15 | 3.8 | −0.9 | 18 | 4.4 | −4.5 | 14 | 5.4 | −1.7 | 12 | 9.7 | −0.9g |
| Cervix uteri | 13 | 8.1 | −2.3g | −0.5 | 13 | 7.7 | −2.1g | 8 | 10.6 | −3.3g | 11 | 7.4 | −3.0g | 10 | 9.8 | −3.7 | 7 | 12.2 | −3.6g | 13 | 7.6 | −2.2g |
| Oral cavity and pharynx | 14 | 6.2 | −0.1 | 0.9 | 14 | 6.2 | 0.0 | 15 | 5.3 | −1.1 | 13 | 5.1 | −2.0g | 14 | 5.3 | −0.7 | 18 | 4.1 | −1.0 | 14 | 6.3 | 0.0 |
| Brain and other nervous system | 15 | 5.7 | −0.2 | −0.2 | 15 | 6.1 | −0.3 | 17 | 3.6 | 0.0 | 16 | 3.1 | 0.3 | 17 | 4.4 | 2.2 | 16 | 4.8 | −1.1g | 15 | 5.9 | −0.3 |
| Stomach | 16 | 4.7 | −1.3g | −1.3g | 17 | 4.0 | −1.5g | 12 | 8.2 | −1.7g | 7 | 9.4 | −3.0g | 13 | 6.8 | −4.2g | 12 | 8.4 | −2.0g | 17 | 4.3 | −1.5g |
| Myeloma | 17 | 4.6 | −0.6g | −1.6g | 16 | 4.1 | −0.7g | 10 | 9.8 | −0.3 | 17 | 2.8 | −1.3 | 16 | 4.6 | −7.6g | 15 | 4.8 | −1.3g | 16 | 4.6 | −0.6g |
| Liver and intrahepatic bile duct | 18 | 3.3 | 1.9g | 2.7g | 18 | 2.9 | 1.5 | 16 | 4.0 | 2.7g | 9 | 8.2 | 0.1 | 11 | 7.6 | 3.8 | 13 | 6.4 | 1.1g | 18 | 3.0 | 2.0g |
AAPC indicates average annual percent change; AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) Program areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods: 2004–2008 for all racial and ethnic groups combined, white, black, API, AI/AN, Hispanic, and non-Hispanic (48 states: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming), and AAPCs from 2004 to 2008 and from 1999 to 2008 for all racial and ethnic groups combined, white, black, API, AI/AN, Hispanic, and non-Hispanic (41 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming).
White, black, API, and AI/AN (CHSDA counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2004 to 2008 for all racial and ethnic groups combined (using data from NPCR and SEER Program areas reported by the NAACCR as meeting high-quality incidence data standards for 2004–2008). More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Rates are per 100,000 persons and are age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000).
The AAPC is a weighted average of the annual percent change (APC) and is calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age standardized to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, …, 80–84, ≥85 years; Census publication 25–1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
For all sites, myelodysplastic syndromes are included for the rate calculations but not for the APC calculations; they are excluded from cancer-specific analysis. Ovary excludes borderline tumors.
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
Table 4.
US Cancer Death Rates for 2004–2008 and Fixed-Interval Trends From 1999 to 2008 for the Top Cancers by Sex, Race, and Ethnicitya
| All Racial and Ethnic Groups Combined |
Whiteb | Blackb | APIb | AI/AN (CHSDA Counties)b |
Hispanicb,c | Non-Hispanicb,c | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Cancer Site or Typed |
Rank | Ratee | 1999– 2008 AAPCf |
2004– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
Rank | Ratee | 1999– 2008 AAPCf |
| All sites | ||||||||||||||||||||||
| Both sexes | 181.3 | −1.5g | −1.5g | 180.0 | −1.4g | 220.8 | −2.0g | 110.9 | −1.4g | 159.6 | −0.4 | 121.1 | −1.7g | 185.9 | −1.4g | |||||||
| Men | 223.0 | −1.8g | −1.8g | 220.0 | −1.7g | 295.3 | −2.4g | 134.7 | −1.6g | 190.0 | −0.4 | 149.2 | −2.3g | 228.6 | −1.7g | |||||||
| Women | 153.2 | −1.3g | −1.5g | 152.8 | −1.3g | 177.7 | −1.5g | 94.1 | −1.1g | 138.4 | −0.4 | 101.6 | −1.4g | 157.1 | −1.2g | |||||||
| Children (ages 0–14 y) | 2.3 | −1.5g | −2.9g | 2.4 | −1.5g | 2.3 | −1.1 | 1.9 | −4.2g | 1.4 | –h | 2.3 | −2.3g | 2.3 | −1.4g | |||||||
| Children (ages 0–19 y) | 2.6 | −1.7g | −1.7g | 2.6 | −1.5g | 2.5 | −2.1g | 2.1 | −3.3g | 1.9 | −1.4 | 2.7 | −1.3g | 2.5 | −1.9g | |||||||
| Top 17 cancers for mend | ||||||||||||||||||||||
| Lung and bronchus | 1 | 67.4 | −2.1g | −2.6g | 1 | 66.9 | −2.0g | 1 | 85.4 | −2.8g | 1 | 36.7 | −1.3g | 1 | 50.5 | 0.0 | 1 | 32.0 | −3.2g | 1 | 70.3 | −2.0g |
| Prostate | 2 | 24.4 | −3.6g | −3.6g | 2 | 22.4 | −3.4g | 2 | 54.9 | −3.7g | 4 | 10.5 | −3.2g | 2 | 20.7 | −1.1 | 2 | 18.5 | −3.4g | 2 | 24.7 | −3.5g |
| Colon and rectum | 3 | 20.7 | −3.0g | −3.0g | 3 | 20.1 | −3.0g | 3 | 30.5 | −1.9g | 3 | 13.3 | −2.3g | 3 | 19.8 | −1.3 | 3 | 15.5 | −2.2g | 3 | 21.1 | −3.0g |
| Pancreas | 4 | 12.5 | 0.4g | 0.4g | 4 | 12.4 | 0.5g | 4 | 15.6 | −0.4 | 6 | 8.4 | 0.8 | 5 | 10.5 | 0.1 | 5 | 9.2 | 0.3 | 4 | 12.7 | 0.5g |
| Leukemia | 5 | 9.7 | −0.9g | −0.9g | 5 | 9.9 | −0.9g | 7 | 8.6 | −0.5 | 8 | 5.0 | −1.7 | 9 | 6.4 | 1.4 | 8 | 6.0 | −1.6g | 5 | 9.8 | −0.8g |
| Non-Hodgkin lymphoma | 6 | 8.6 | −2.8g | −2.8g | 6 | 8.9 | −2.7g | 10 | 6.1 | −2.6g | 7 | 5.4 | −2.6g | 10 | 5.1 | −4.2 | 7 | 6.3 | −2.5g | 6 | 8.7 | −2.7g |
| Liver and intrahepatic bile duct | 7 | 7.9 | 2.6g | 2.6g | 9 | 7.2 | 2.6g | 5 | 11.5 | 3.6g | 2 | 14.7 | −0.9 | 4 | 11.9 | 3.1 | 4 | 11.6 | 1.5g | 9 | 7.6 | 2.6g |
| Esophagus | 8 | 7.8 | −0.2 | −0.9 | 8 | 7.9 | 0.4 | 8 | 8.5 | −4.3g | 9 | 3.2 | −1.0 | 8 | 6.7 | 0.1 | 10 | 4.1 | −1.1 | 7 | 8.0 | −0.1 |
| Urinary bladder | 9 | 7.7 | 0.1 | 0.1 | 7 | 8.0 | 0.2 | 13 | 5.5 | −0.6 | 11 | 2.7 | −0.8 | 13 | 3.6 | –h | 11 | 3.8 | −1.5 | 8 | 7.9 | 0.3g |
| Kidney and renal pelvis | 10 | 5.9 | −0.2 | −0.6 | 10 | 6.0 | −0.1 | 11 | 6.0 | −0.5 | 12 | 2.6 | 1.2 | 6 | 8.9 | −1.3 | 9 | 5.2 | −0.7 | 10 | 5.9 | −0.3 |
| Brain and other nervous system | 11 | 5.2 | −1.0g | −1.0g | 11 | 5.6 | −0.9g | 15 | 3.1 | −0.9 | 13 | 2.4 | −0.9 | 14 | 2.9 | 5.6g | 13 | 3.2 | −0.9 | 11 | 5.4 | −0.9g |
| Stomach | 12 | 5.2 | −3.4g | −3.4g | 13 | 4.5 | −3.5g | 6 | 10.7 | −3.1g | 5 | 9.2 | −3.7g | 7 | 8.5 | −4.4 | 6 | 7.7 | −3.7g | 12 | 5.0 | −3.5g |
| Myeloma | 13 | 4.4 | −1.1g | −1.1g | 14 | 4.2 | −1.0g | 9 | 8.2 | −1.1g | 14 | 2.1 | 1.4 | 11 | 4.0 | −1.7 | 12 | 3.3 | −2.1 | 13 | 4.5 | −0.9g |
| Melanoma of the skin | 14 | 4.0 | 0.8g | 0.8g | 12 | 4.6 | 1.0g | 21 | 0.5 | 0.4 | 20 | 0.4 | –h | 16 | 1.4 | –h | 17 | 1.0 | 0.1 | 14 | 4.3 | 1.1g |
| Oral cavity and pharynx | 15 | 3.9 | −1.1g | −1.1g | 15 | 3.7 | −0.6g | 12 | 6.0 | −3.3g | 10 | 3.0 | −3.1g | 12 | 3.8 | −1.3 | 14 | 2.4 | −3.2g | 15 | 4.0 | −0.9g |
| Larynx | 16 | 2.2 | −2.5g | −2.5g | 16 | 2.0 | −2.3g | 14 | 4.4 | −2.9g | 16 | 0.8 | −3.4 | 15 | 2.0 | –h | 15 | 1.8 | −3.7g | 16 | 2.2 | −2.3g |
| Soft tissue, including heart | 17 | 1.5 | −0.3 | 1.1g | 18 | 1.5 | −0.2 | 16 | 1.4 | −0.8 | 15 | 1.0 | 1.5 | 18 | 1.0 | –h | 16 | 1.0 | −1.8 | 18 | 1.5 | −0.1 |
| Top 18 cancers for womend | ||||||||||||||||||||||
| Lung and bronchus | 1 | 40.1 | −0.3 | −0.9g | 1 | 41.2 | −0.2 | 1 | 38.8 | −0.7g | 1 | 18.5 | −0.4 | 1 | 33.9 | 0.6 | 2 | 14.3 | −0.5 | 1 | 42.2 | −0.1 |
| Breast | 2 | 23.5 | −2.0g | −2.0g | 2 | 22.8 | −2.1g | 2 | 32.0 | −1.4g | 2 | 12.2 | −0.9 | 2 | 17.2 | −1.7 | 1 | 15.1 | −1.5g | 2 | 24.2 | −1.8g |
| Colon and rectum | 3 | 14.5 | −2.9g | −2.9g | 3 | 14.0 | −3.0g | 3 | 20.4 | −2.9g | 3 | 9.9 | −0.9 | 3 | 14.0 | 0.1 | 3 | 10.3 | −2.0g | 3 | 14.8 | −2.6g |
| Pancreas | 4 | 9.4 | 0.4g | 0.4g | 4 | 9.2 | 0.4g | 4 | 12.5 | 0.1 | 4 | 7.0 | −0.2 | 4 | 8.3 | 1.2 | 4 | 7.5 | −0.2 | 4 | 9.6 | 0.4g |
| Ovary | 5 | 8.4 | −1.1g | −2.4g | 5 | 8.8 | −1.1g | 6 | 7.0 | −1.3g | 7 | 5.0 | 0.5 | 6 | 6.7 | −0.7 | 5 | 5.9 | −0.5 | 5 | 8.6 | −1.1g |
| Leukemia | 6 | 5.4 | −1.5g | −1.5g | 7 | 5.5 | −1.4g | 9 | 4.9 | −1.5g | 9 | 2.9 | −1.1 | 10 | 3.6 | –h | 9 | 3.9 | −1.5g | 6 | 5.4 | −1.4g |
| Non-Hodgkin | 7 | 5.4 | −3.5g | −3.5g | 6 | 5.6 | −3.5g | 12 | 3.8 | −2.6g | 8 | 3.4 | −2.7g | 7 | 4.4 | −3.0 | 8 | 4.3 | −2.8g | 7 | 5.4 | −3.4g |
| lymphoma | ||||||||||||||||||||||
| Corpus and uterus, NOS | 8 | 4.2 | 0.2g | 0.2g | 8 | 3.9 | 0.1 | 5 | 7.2 | 0.5 | 10 | 2.5 | 1.9g | 12 | 3.1 | –h | 10 | 3.2 | 0.0 | 8 | 4.2 | 0.3g |
| Brain and other nervous system | 9 | 3.5 | −1.0g | −1.0g | 9 | 3.8 | −0.9g | 16 | 2.0 | −1.5g | 12 | 1.6 | 0.2 | 15 | 1.9 | –h | 13 | 2.4 | −0.7 | 9 | 3.6 | −0.9g |
| Liver and intrahepatic bile duct | 10 | 3.2 | 1.2g | 0.6 | 10 | 3.0 | 1.6g | 11 | 3.9 | 0.3 | 5 | 6.3 | −1.4 | 5 | 6.7 | −0.1 | 6 | 5.2 | 0.8 | 10 | 3.1 | 1.1g |
| Myeloma | 11 | 2.8 | −2.3g | −2.3g | 12 | 2.6 | −2.5g | 7 | 5.6 | −2.4g | 13 | 1.4 | −3.0 | 13 | 2.4 | −7.9g | 12 | 2.4 | −2.6g | 11 | 2.8 | −2.3g |
| Stomach | 12 | 2.7 | −3.0g | −3.0g | 13 | 2.3 | −3.1g | 8 | 5.0 | −3.8g | 6 | 5.4 | −4.1g | 9 | 3.9 | −5.6g | 7 | 4.5 | −2.8g | 13 | 2.5 | −3.2g |
| Kidney and renal pelvis | 13 | 2.7 | −0.5 | −1.3g | 11 | 2.7 | −0.8 | 14 | 2.6 | −0.9g | 15 | 1.2 | 0.7 | 8 | 4.1 | 0.1 | 14 | 2.3 | −0.1 | 12 | 2.7 | −0.5 |
| Cervix uteri | 14 | 2.4 | −1.9g | −0.2 | 15 | 2.2 | −1.8g | 10 | 4.3 | −2.9g | 11 | 2.1 | −3.7g | 11 | 3.4 | 0.1 | 11 | 3.1 | −2.5g | 14 | 2.4 | −1.8g |
| Urinary bladder | 15 | 2.2 | −0.4 | −0.4 | 14 | 2.2 | −0.3 | 13 | 2.7 | −1.1 | 16 | 0.9 | −2.7 | 19 | 1.1 | –h | 16 | 1.2 | 0.5 | 15 | 2.3 | −0.3 |
| Esophagus | 17 | 1.6 | −1.5g | −1.5g | 17 | 1.6 | −1.0g | 15 | 2.4 | −4.3g | 17 | 0.9 | 0.2 | 16 | 1.5 | –h | 18 | 0.8 | −3.5g | 17 | 1.7 | −1.4g |
| Oral cavity and pharynx | 18 | 1.4 | −1.7g | −1.7g | 18 | 1.4 | −1.5g | 17 | 1.5 | −3.3g | 14 | 1.3 | −1.4 | 17 | 1.2 | –h | 19 | 0.8 | −2.6 | 18 | 1.5 | −1.6g |
| Gallbladder | 20 | 0.8 | −2.1g | −2.1g | 20 | 0.7 | −2.4g | 19 | 1.0 | −0.8 | 18 | 0.8 | −2.4 | 14 | 2.2 | −7.5 | 15 | 1.2 | −4.5g | 20 | 0.7 | −2.0g |
Abbreviations: AAPC, average annual percent change; API, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Center for Health Statistics public-use data file for the total US, 1975–2008.
White, black, API, and AI/AN (CHSDA counties) populations include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Data for Hispanic and non-Hispanic exclude the District of Columbia, Minnesota, New Hampshire, and North Dakota.
Cancers are listed in descending rank order of sex-specific, age-adjusted incidence rates for 2004 to 2008 for all racial and ethnic groups combined. More than 15 cancers may appear under men and women to include the top 15 cancers in each racial and ethnic group.
Rates are per 100,000 persons and are age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000).
The AAPC is a weighted average of the annual percent change and is calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
The statistic could not be calculated. The average annual percent change is based on <10 cases for at least 1 year within the time interval.
Excess Weight and Lack of Sufficient Physical Activity and Associated Cancers
A rigorous review of more than 7000 studies on the relation between nutrition, physical activity, excess weight, and cancer risk concluded that there is convincing evidence of an association between excess weight and increased risk of several cancers, including adenocarcinoma of the esophagus, colon and rectum cancer, kidney cancer, pancreas cancer, postmenopausal female breast cancer, and endometrial cancer.24 In this report, incidence rates are presented for these 6 cancers. Postmenopausal breast cancer was approximated by estimating rates among women aged ≥50 years. Because most cancers (95%) of the corpus uterus are diagnosed in the endometrium, uterine cancer rates were used to describe endometrial cancer. Incidence rates are also presented for 3 cancers for which the review concluded that the evidence of an association between physical inactivity and increased risk of cancer is considered convincing (colon cancer) or probable (postmenopausal breast and endometrial cancers).24
The prevalence of overweight and obesity among US children, adolescents, and adults was obtained from published estimates from the National Health and Nutrition Examination Survey (NHANES).25–29 In this report, we used body mass index (BMI), which is calculated as weight in kilograms divided by height in meters squared, to define healthy weight, overweight, and obesity.30 In adults, BMI in the range from 18.5 to 24.9 kg/m2 is considered healthy weight, BMI in the range from 25.0 to 29.9 kg/m2 is considered overweight, and BMI ≥30 kg/m2 is considered obese.25 In children and adolescents, the definitions of overweight and obese are based on the 2000 CDC BMI-for-age-and-sex growth charts; BMI in the range from ≥85th percentile to <95th percentile is considered overweight, whereas BMI ≥95th percentile is considered obese.26 In this report, the definition of excess weight includes overweight and obese.
The proportion of US youth participating in physical activity was obtained from published estimates from the Youth Risk Behavior Surveillance System (YRBSS).31 In this report, physical activity levels were based on Healthy People 2020 objectives32 and were defined as youth doing any kind of physical activity that increased their heart rate and made them breathe hard some of the time for a total of at least 60 minutes per day on 7 days (active), 1 to 6 days (insufficiently active), or 0 days (inactive) during the 7 days before the survey. The proportion of US adults participating in physical activity was obtained from the National Health Interview Survey (NHIS).33 In this report, physical activity levels (during leisure time) among adults were based on recent guidelines34 and were defined as engaging in at least 150 minutes of moderate-intensity aerobic activity, at least 75 minutes of vigorous-intensity aerobic activity, or an equivalent combination of moderate-intensity and vigorous-intensity physical activity per week (active); some aerobic activity but not enough to meet the active definition (insufficiently active); or no moderate-intensity or vigorous-intensity aerobic activity for at least 10 minutes at a time (inactive). State-specific estimates of the prevalence of obesity and physical inactivity were obtained from the YRBSS31 for US youth and from the Behavioral Risk Factor Surveillance System(BRFSS) for US adults.35,36
Published estimates of relative risk were obtained from recent comprehensive meta-analyses of the association between excess weight and the risk of adenocarcinoma of the esophagus24 and cancers of the colon and rectum,37 kidney,24 pancreas,24 female breast (postmenopausal),38 and endometrium.39 These estimates were given as the risk associated with a specified unit change in BMI. Assuming a linear association between BMI and cancer risk, we calculated estimates for the risk associated with overweight and obese. Published estimates of relative risk were obtained from recent comprehensive meta-analyses of the association between physical activity and risk of cancers of the colon,40 female breast (postmenopausal),41 and endometrium.42 These estimates originally were presented as the risk of the most active relative to the least active; we calculated the inverse to present the risk of the least active relative to the most active.
RESULTS
Long-Term (1992–2008) Cancer Incidence Trends for All Racial and Ethnic Groups Combined
Trend analysis based on SEER 13 data indicated that overall delay-adjusted cancer incidence in all racial and ethnic groups and sexes combined was stable from 1999 to 2008 (Table 1). Among men, overall cancer incidence decreased on average by 0.6% annually from 1994 to 2008. Overall cancer incidence among women decreased 0.5% annually from 1998 to 2006, but rates did not change from 2006 to 2008. Overall cancer incidence among children ages 0 to 14 years increased 0.5% per year and, among children ages 0 to 19 years, incidence increased 0.6% per year from 1999 to 2008, continuing trends from 1992. Among men, incidence rates for 5 of the 17 most common cancers decreased from 1999 to 2008: prostate, lung and bronchus (lung), colon and rectum (colorectal), stomach, and larynx. In contrast, rates among men increased from 1999 to 2008 for 7 cancers: kidney and renal pelvis (kidney), pancreas, liver and intrahepatic bile duct (liver), thyroid, melanoma of the skin (melanoma), leukemia, and myeloma. Among women, incidence rates decreased from 1999 to 2008 for 6 of the 18 most common cancers: lung, colorectal, urinary bladder (bladder), cervix uteri (cervix), oral cavity and pharynx (oral cavity), and stomach. Incidence rates among women increased from 1999 to 2008 for 6 cancers: thyroid, melanoma, kidney, pancreas, leukemia, and liver. Incidence rates were unchanged from 1999 to 2008 for all other cancers.
Long-Term (1975–2008) Cancer Mortality Trends for All Racial and Ethnic Groups Combined
Overall cancer death rates have been declining among children since the 1970s and among adults since the 1990s (Table 2). Trends in death rates for the most recent 10-year period (1999–2008) show an average 1.7% decrease per year among men and an average 1.3% decrease per year among women as well as among children ages 0 to 19 years. Death rates decreased 1.5% per year among children ages 0 to 14 years. Death rates for 11 of the 17 most common cancers among men and for 14 of the 18 most common cancers among women (lung, colon and rectum, kidney, brain, stomach, oral cavity, leukemia, non-Hodgkin lymphoma, and myeloma among both men and women; prostate and larynx among men; and breast, ovary, urinary bladder, esophagus, and gallbladder among women) decreased during the most recent 10-year (1999–2008) and 5-year (2004–2008) periods. During the same periods, death rates increased for pancreatic cancer among both men and women, for liver cancer and melanoma of the skin among men, and for corpus uterus (uterine) cancer among women. After decades of decline, cervical cancer death rates changed little in the most recent period.
Cancer Incidence Rates (2004–2008) and Short-Term (1999–2008) Trends by Race and Ethnicity
Five-year (2004–2008) average annual incidence rates and short-term (1999–2008) trends were based on combined data from SEER and NPCR registries submitted to the NAACCR (Table 3). These data were not adjusted for delayed reporting. Cancer incidence rates decreased from 1999 to 2008 among both men and women of all racial and ethnic groups combined, although the decrease was not statistically significant among AI/AN men and women combined and among black, API, and AI/AN women. Among children (ages 0–19 years), cancer incidence rates increased in all racial and ethnic groups combined, although this increase was not observed among white, API, or AI/AN children. Prostate cancer incidence rates decreased in each racial and ethnic group. For all racial and ethnic groups combined, female breast cancer incidence rates were stable from 2004 to 2008. Among men, lung cancer incidence rates decreased from 1999 to 2008 in all racial and ethnic groups, but this decrease was not statistically significant among AI/AN men. Although lung cancer incidence rates among women in all racial and ethnic groups combined were stable over the 10-year period, rates decreased significantly in the most recent 5 years. Colorectal cancer incidence rates decreased among both men and women in all racial and ethnic groups but did not decrease significantly among AI/AN men or women. Uterine cancer incidence rates increased among black, API, and Hispanic women.
The incidence rate for all cancers combined was 33% higher among men than among women (Table 3). Black men had the highest cancer incidence rate from 2004 to 2008 of any racial and ethnic group, with overall rates 15% higher than those of white men and nearly double the rates of API men. The top 3 most commonly diagnosed cancers among men in each racial and ethnic group, except Hispanic men, were, in rank order, prostate, lung, and colorectal cancer; among Hispanic men, the incidence rate of colorectal cancer was slightly higher than the incidence of lung cancer. Among women, overall cancer incidence rates were highest among non-Hispanic and white women. Breast cancer was the most commonly diagnosed cancer among women in each racial and ethnic group. Incident lung and colorectal cancers ranked second and third, respectively, among women of all racial and ethnic groups combined and for white, black, and AI/AN women. However, these rankings were reversed among API and Hispanic women. Incident uterine cancer ranked fourth for all women except API women, in whom the fourth most common cancer was thyroid. Thyroid cancer is now the fifth most common incident cancer among women of all racial and ethnic groups combined, with the highest rates observed among white and API women. Beyond the top 3 most commonly diagnosed cancers among men and the top 4 most commonly diagnosed cancers among women, cancer rankings varied considerably by race and ethnicity.
Current Cancer Death Rates (2004–2008) and Short-Term (1999–2008) Trends by Race and Ethnicity
Overall cancer death rates declined from 1999 to 2008 among men, women, and children in all racial and ethnic groups combined (Table 4). Overall cancer death rates declined in each racial and ethnic group except AI/AN men, women, and children, among whom declines were not significant. Similarly, among men, death rates for the most common cancers (lung, colorectal, and prostate) decreased in all racial and ethnic groups except among AI/AN men, in whom the rates remained unchanged. Among women, death rates for breast and colorectal cancers decreased among white, black, and Hispanic women; nonsignificant declines were observed for breast and colorectal cancer among API women and for breast cancer among AI/AN women. Lung cancer death rates decreased from 2004 to 2008 among women of all racial and ethnic groups combined. Death rates increased for liver cancer among white, black, and Hispanic men and white women, for pancreas cancer among white men and women, and for melanoma among white men.
Special Section: Excess Weight, Lack of Sufficient Physical Activity, and Associated Cancers
From 2007 to 2008, one-third of US children and adolescents were considered overweight or obese based on measured weight and height data from NHANES (Table 5). The prevalence of obesity was greater among children ages 6 to 19 years than among those ages 2 to 5 years. In analyses by racial and ethnic group, the prevalence of obesity was highest among Hispanic boys and non-Hispanic black girls. From 2005 to 2008, children and adolescents who lived in households headed by those with a college degree had the lowest prevalence of obesity.
Table 5.
Current Prevalence of Overweight and Obesity Among US Children and Adolescents and Adults: National Health and Nutrition Examination Survey, 2007–2008a
| Children and adolescents (ages 2–19 years)b | Boys | Girls | ||
| % Overweight | % Obese | % Overweight | % Obese | |
| All children and adolescents | 14 | 18 | 15 | 16 |
| By age, years | ||||
| 2–5 | 11 | 10 | 11 | 11 |
| 6–11 | 15 | 21 | 17 | 18 |
| 12–19 | 16 | 19 | 17 | 17 |
| By racial and ethnic group | ||||
| Non-Hispanic white | 14 | 16 | 14 | 15 |
| Non-Hispanic black | 16 | 17 | 16 | 23 |
| Hispanic | 16 | 24 | 19 | 17 |
| By education of household head | ||||
| College graduate | NA | 12 | NA | 8 |
| Some college | NA | 16 | NA | 15 |
| High school graduate | NA | 18 | NA | 20 |
| Less than high school | NA | 21 | NA | 20 |
| Adults (aged ≥20 years)c | Men | Women | ||
| % Overweight | % Obese | % Overweight | % Obese | |
| All adults | 40 | 32 | 29 | 36 |
| By age, years | ||||
| 20–39 | 36 | 28 | 26 | 34 |
| 40–59 | 44 | 34 | 28 | 38 |
| ≥60 | 41 | 37 | 35 | 34 |
| By racial and ethnic group | ||||
| Non-Hispanic white | 41 | 32 | 28 | 33 |
| Non-Hispanic black | 31 | 37 | 29 | 50 |
| Hispanic | 45 | 34 | 33 | 43 |
| By education | ||||
| College graduate | NA | 27 | NA | 23 |
| Some college | NA | 36 | NA | 38 |
| High school graduate | NA | 35 | NA | 40 |
| Less than high school | NA | 32 | NA | 42 |
Abbreviations: NA, not available.
Overweight and obese were defined by body mass index (BMI), which is calculated as weight in kilograms divided by height in meters squared. Estimates are based on measured height and weight.
In children and adolescents, the definitions of overweight and obese are based on the 2000 Centers for Disease Control and Prevention (CDC) BMI-for-age-and-sex growth charts; BMI ranging from the ≥85th percentile to the <95th percentile is considered overweight, and BMI ≥95th percentile is considered obese. Sources: Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–24826; and Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005–2008. NCHS Data Brief. 2010;(51):1–8.27
In adults, BMI in the range from 25.0 to 29.9 kg/m2 is considered overweight, and BMI ≥ 30 kg/m2 is considered obese. Sources: Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–24125; and Ogden CL et al. Obesity and socioeconomic status in adults: United States, 2005–2008. NCHS Data Brief. 2010;(50):1–8.28
From 2007 to 2008 two-thirds of US adults were considered overweight or obese based on measured weight and height data from NHANES (Table 5). The prevalence of obesity was highest among men aged ≥60 years and among women aged 40 to 59 years. In analyses by racial and ethnic group, the prevalence of obesity was highest among non-Hispanic black men and women and among Hispanic women. From 2005 to 2008, adults with a college degree had the lowest prevalence of obesity.
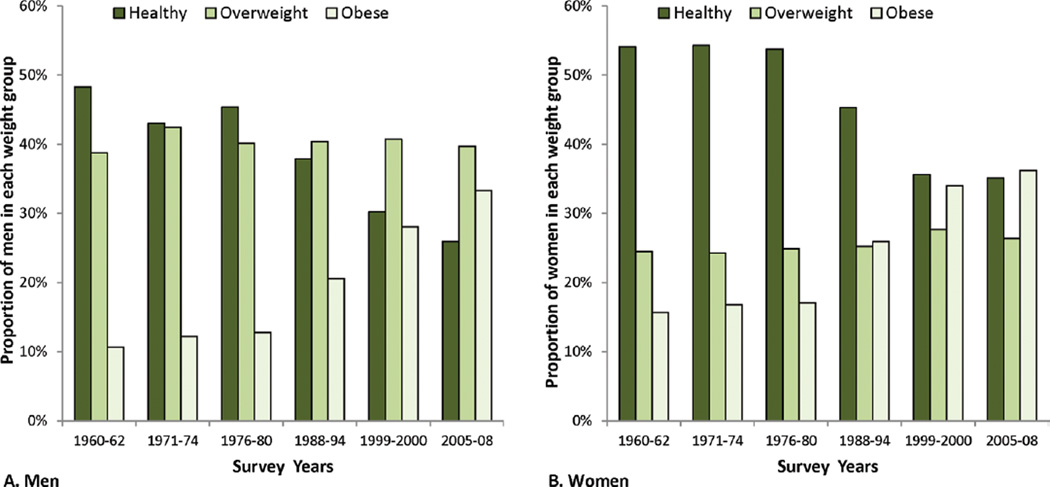
Data from NHANES indicate that the prevalence of obesity among US adults increased slowly from the 1960s to the 1980s then increased sharply until 1999/2000 (Fig. 1). From 1999 to 2008, the prevalence of obesity remained stable in women and increased slightly in men (most of the increase occurred early in this period).43
Figure 1.
The distribution of body mass index (BMI) is illustrated over time for adult (ages 20–74 years) (A) men and (B) women by sex and survey year: United States, 1960 to 2008. Body mass index (BMI) is calculated as weight in kilograms divided by height in meters squared. BMI in the range 18.5–24.9 kg/m2 is considered healthy weight (dark green bars), BMI in the range 25–29.9 kg/m2 is considered overweight (medium green bars) and BMI ≥ 30 kg/m2 is considered obese (light green bars). Prevalence was age-adjusted to the 2000 US standard population using five age groups: 20–34 years, 35–44 years, 45–54 years, 55–64 years, and 65–74 years. Data are from the National Health and Nutrition Examination Survey except data for 1960–1962 which are from the National Health Examination Survey. Source: National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying (Table 71). Hyattsville, MD. 2011.29
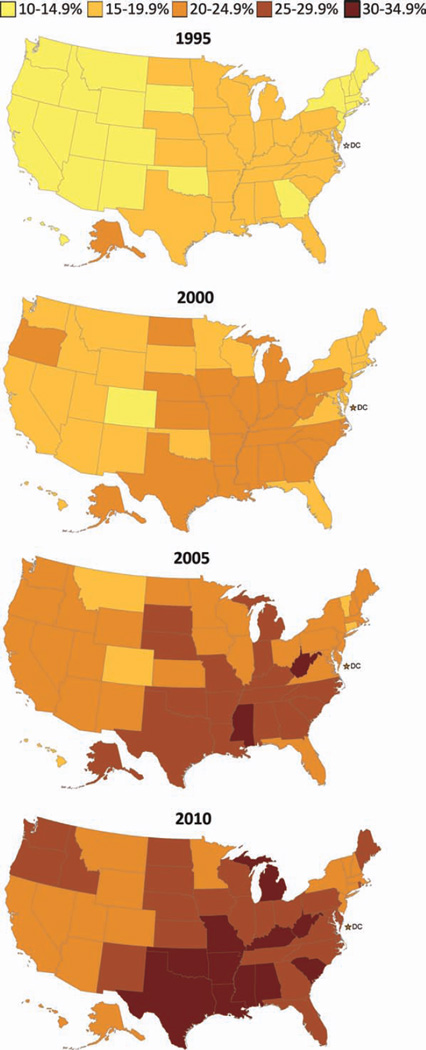
On the basis of self-reported weight and height data from the BRFSS, the prevalence of obesity among US adults increased in all states from 1995 to 2010 (Fig. 2). Rates were consistently higher among Southern and Midwestern states.
Figure 2.
These maps illustrate the proportion of US adults who were obese (body mass index [BMI] ≥30 kg/m2) according to the Behavioral Risk Factor Surveillance (BRFSS) for the years 1995, 2000, 2005, and 2010. BMI is calculated as weight in kilograms divided by height in meters squared. BMI is based on self-reported weight and height; prevalence is age-adjusted to the 2000 US standard population. Source: BRFSS public use file. (See Web Table 1).
On the basis of data from YRBSS, in 2009, 75% of US high school boys and 89% of US high school girls did not engage in recommended levels of physical activity; 17% of boys and 30% of girls were considered physically inactive (Table 6). The prevalence of physical inactivity increased with grade level. In analyses by racial and ethnic group, the prevalence of physical inactivity was highest among non-Hispanic black boys and girls.
Table 6.
Current Level of Aerobic Physical Activity Among US Youth (Youth Risk Behavior Surveillance System, 2009)a and Adults (National Health Interview Survey, 2008)b
| High school studentsa | Boys | Girls | ||||
| % Active | % Insufficiently Active |
% Inactive | % Active | % Insufficiently Active |
% Inactive | |
| All high school students | 25 | 58 | 17 | 11 | 59 | 30 |
| By school grade | ||||||
| 9 | 28 | 55 | 17 | 14 | 59 | 27 |
| 10 | 25 | 59 | 16 | 13 | 57 | 30 |
| 11 | 23 | 61 | 16 | 10 | 60 | 30 |
| 12 | 22 | 59 | 19 | 9 | 58 | 33 |
| By racial and ethnic group | ||||||
| Non-Hispanic white | 26 | 58 | 16 | 12 | 63 | 25 |
| Non-Hispanic black | 24 | 55 | 21 | 10 | 46 | 44 |
| Hispanic | 21 | 62 | 17 | 11 | 58 | 31 |
| Adults (aged ≥18 years)b | Men | Women | ||||
| % Active | % Insufficiently Active |
% Inactive | % Active | % Insufficiently Active |
% Inactive | |
| All adults | 47 | 19 | 34 | 40 | 22 | 38 |
| By age, years | ||||||
| 18–24 | 59 | 17 | 24 | 46 | 20 | 34 |
| 25–34 | 52 | 18 | 30 | 48 | 21 | 31 |
| 35–44 | 48 | 19 | 32 | 43 | 23 | 34 |
| 45–64 | 45 | 18 | 37 | 39 | 24 | 38 |
| ≥65 | 37 | 19 | 44 | 26 | 19 | 56 |
| By racial and ethnic group | ||||||
| Non-Hispanic white | 52 | 18 | 31 | 44 | 22 | 34 |
| Non-Hispanic black | 42 | 17 | 42 | 28 | 20 | 52 |
| Hispanic | 35 | 19 | 46 | 32 | 19 | 49 |
| By education | ||||||
| College graduate | 62 | 20 | 18 | 56 | 23 | 22 |
| Some college | 51 | 22 | 28 | 42 | 24 | 35 |
| High school graduate | 39 | 16 | 45 | 32 | 21 | 47 |
| Less than high school | 28 | 17 | 55 | 23 | 16 | 60 |
Among youth, categories of physical activity were defined as doing any kind of physical activity that increased their heart rate and made them breathe hard some of the time for a total of at least 60 minutes daily on 7 days (active), 1 to 6 days (insufficiently active), or 0 days (inactive) during the 7 days before the survey. Source: Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance—United States, 2009.31
Among adults, active (during leisure time) was defined as at least 150 minutes of moderate-intensity aerobic activity, at least 75 minutes of vigorous-intensity aerobic activity, or an equivalent combination of moderate-intensity and vigorous-intensity physical activity per week; insufficiently active was defined as some aerobic activity but not enough to meet the active definition; inactive was defined as no moderate-intensity or vigorous-intensity aerobic activity for at least 10 minutes at a time. Adapted from: Carlson SA, Fulton JE, Schoenborn SA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39(4):305–313.33 Additional estimates provided by authors.
On the basis of data from the NHIS, in 2008, 53% of US men and 60% of US women did not engage in recommended levels of aerobic physical activity; more than one-third were considered physically inactive (Table 6). The prevalence of aerobic physical inactivity increased with age and was highest among Hispanic men and non-Hispanic black women. Adults with a college degree had the lowest prevalence of aerobic physical inactivity.
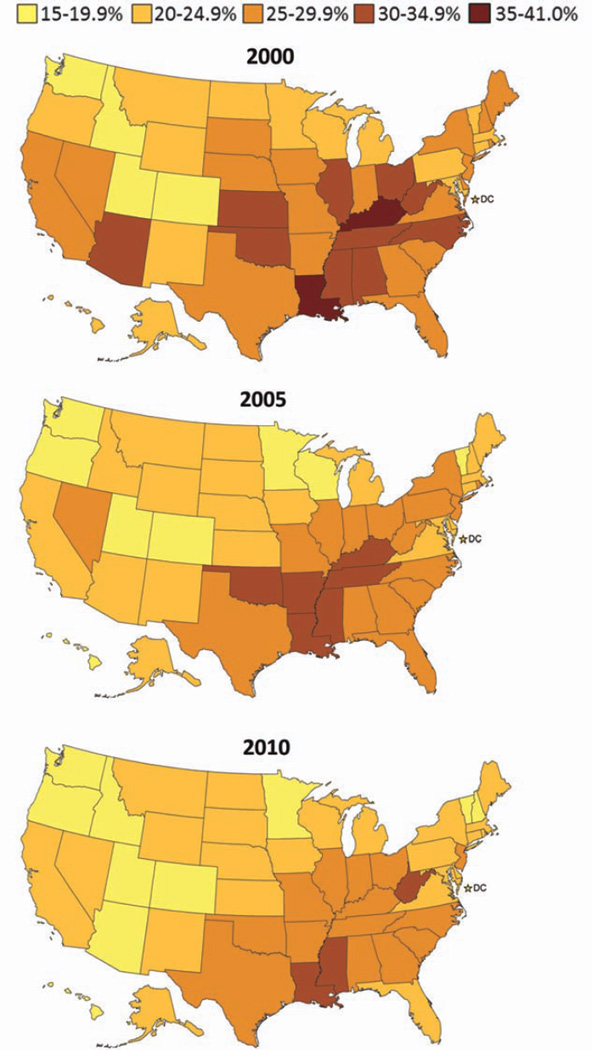
On the basis of data from the BRFSS, from 2000 to 2010, prevalence rates of aerobic physical inactivity among adults stayed the same or decreased in most states and were lowest for states in the West and highest for states in the South (Fig. 3).
Figure 3.
These maps illustrate the proportion of US adults who were physically inactive according to the Behavioral Risk Factor Surveillance System (BRFSS) for the years 2000, 2005, and 2010. Physically inactive is defined as no moderate or vigorous-intensity aerobic activity for at least 10 minutes at a time; prevalence is age-adjusted to the 2000 US standard population. Source: BRFSS public use file. (See Web Table 1).
Incidence rates and trends for cancers associated with excess weight and lack of sufficient physical activity are presented in Table 7 (long-term trends for all racial and ethnic groups combined) and Table 8 (5-year rates and short-term trends by racial and ethnic group). Although colorectal cancer incidence decreased from 1999 to 2008, rates of postmenopausal breast cancer stabilized from 2005 to 2008 after declining from 1999 to 2005, and incidence of some cancers increased (Table 7). Kidney cancer incidence increased from 1999 to 2008 approximately 2.9% per year among men (accelerating to 4.1% from 2004 to 2008) and 3.3% among women (Table 7); increases were evident in all racial and ethnic groups (Table 8). Pancreas cancer increased approximately 1.2% per year from 1999 to 2008 (accelerating slightly from 2004 to 2008) among both men and women (Table 7), although this increase was confined to whites (Table 8). A significant increase from 1999 to 2008 in uterine cancer was observed among black, API, and Hispanic women; a nonsignificant increase was observed among white and AI/AN women (Table 8). From 1992 to 2008, adenocarcinoma of the esophagus increased 2.6% per year among men and 3.3% per year among women (Table 7); these increases were restricted to white and Hispanic men and white women (Table 8). State-specific 5-year (2004–2008) average annual incidence rates for cancers associated with excess weight and lack of sufficient physical activity are presented in Table 9.
Table 7.
Surveillance, Epidemiology, and End Results (SEER) Cancer Incidence Rate Trends With Joinpoint Analyses From 1992 to 2008 for the Cancers Associated With Excess Weight and Lack of Sufficient Physical Activity, by Sex, for All Racial and Ethnic Groups Combineda
| Joinpoint Analyses (1992–2008)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | AAPCc | ||||||
| Sex/Cancer Site or Typed | Years | APCe | Years | APCe | Years | APCe | Years | APCe | 1999–2008 | 2004–2008 |
| Both sexes | ||||||||||
| Adenocarcinoma of esophagus | 1992–1999 | 5.4f | 1999–2008 | 1.2 | 1.2 | 1.2 | ||||
| (Delay-adjusted) | 1992–1999 | 5.4f | 1999–2008 | 1.3 | 1.3 | 1.3 | ||||
| Colon and rectum | 1992–1995 | −2.1f | 1995–1998 | 1.7 | 1998–2008 | −2.4f | −2.4g | −2.4g | ||
| (Delay-adjusted) | 1992–1995 | −2.1f | 1995–1998 | 1.7 | 1998–2008 | −2.3f | −2.3g | −2.3g | ||
| Colon | 1992–1995 | −2.1f | 1995–1998 | 1.5 | 1998–2008 | −2.4f | −2.4g | −2.4g | ||
| (Delay-adjusted) | 1992–1995 | −2.1f | 1995–1998 | 1.5 | 1998–2008 | −2.3f | −2.3g | −2.3g | ||
| Pancreas | 1992–2000 | −0.1 | 2000–2008 | 1.1f | 0.9g | 1.1g | ||||
| (Delay-adjusted) | 1992–2001 | 0.0 | 2001–2008 | 1.5f | 1.2g | 1.5g | ||||
| Kidney and renal pelvis | 1992–1999 | 1.4f | 1999–2008 | 3.0f | 3.0g | 3.0g | ||||
| (Delay-adjusted) | 1992–1997 | 1.1f | 1997–2005 | 2.6f | 2005–2008 | 4.4f | 3.2g | 3.9g | ||
| Men | ||||||||||
| Adenocarcinoma of esophagus | 1992–2008 | 2.5f | 2.5g | 2.5g | ||||||
| (Delay-adjusted) | 1992–2008 | 2.6f | 2.6g | 2.6g | ||||||
| Colon and rectum | 1992–1995 | −2.6f | 1995–1998 | 1.5 | 1998–2008 | −2.7f | −2.7g | −2.7g | ||
| (Delay-adjusted) | 1992–1995 | −2.6f | 1995–1998 | 1.5 | 1998–2008 | −2.6f | −2.6g | −2.6g | ||
| Colon | 1992–1995 | −2.9f | 1995–1998 | 1.5 | 1998–2008 | −2.8f | −2.8g | −2.8g | ||
| (Delay-adjusted) | 1992–1995 | −2.9f | 1995–1998 | 1.4 | 1998–2008 | −2.7f | −2.7g | −2.7g | ||
| Pancreas | 1992–2002 | 0.0 | 2002–2008 | 1.4f | 0.9g | 1.4g | ||||
| (Delay-adjusted) | 1992–2002 | 0.0 | 2002–2008 | 1.8f | 1.2g | 1.8g | ||||
| Kidney and renal pelvis | 1992–2008 | 2.2f | 2.2g | 2.2g | ||||||
| (Delay-adjusted) | 1992–2006 | 2.0f | 2006–2008 | 6.2f | 2.9g | 4.1g | ||||
| Women | ||||||||||
| Adenocarcinoma of esophagus | 1992–2008 | 3.2f | 3.2g | 3.2g | ||||||
| (Delay-adjusted) | 1992–2008 | 3.3f | 3.3g | 3.3g | ||||||
| Colon and rectum | 1992–1995 | −1.9f | 1995–1998 | 1.9 | 1998–2008 | −2.1f | −2.1g | −2.1g | ||
| (Delay-adjusted) | 1992–1995 | −1.8f | 1995–1998 | 1.9 | 1998–2008 | −2.0f | −2.0g | −2.0g | ||
| Colon | 1992–2000 | −0.2 | 2000–2008 | −2.3f | −2.0g | −2.3g | ||||
| (Delay-adjusted) | 1992–2000 | −0.2 | 2000–2008 | −2.2f | −1.9g | −2.2g | ||||
| Pancreas | 1992–2008 | 0.5f | 0.5g | 0.5g | ||||||
| (Delay-adjusted) | 1992–2000 | −0.1 | 2000–2008 | 1.4f | 1.2g | 1.4g | ||||
| Kidney and renal pelvis | 1992–1998 | 1.2 | 1998–2008 | 3.1f | 3.1g | 3.1g | ||||
| (Delay-adjusted) | 1992–1998 | 1.1 | 1998–2008 | 3.3f | 3.3g | 3.3g | ||||
| Breast (aged ≥50 y) | 1992–1999 | 1.7f | 1999–2004 | −2.9f | 2004–2008 | 0.0 | −1.6g | 0.0 | ||
| (Delay-adjusted) | 1992–1999 | 1.6f | 1999–2005 | −2.6f | 2005–2008 | 1.0 | −1.4g | 0.1 | ||
| Corpus and uterus, NOS | 1992–2006 | −0.2 | 2006–2008 | 2.3 | 0.4 | 1.1 | ||||
| (Delay-adjusted) | 1992–2006 | −0.2 | 2006–2008 | 2.7 | 0.5 | 1.2 | ||||
Abbreviations: AAPC indicates average annual percent change; APC, annual percent change; NOS, not otherwise specified.
Source: SEER 13 areas covering about 14% of the US population (Connecticut, Hawaii, Iowa, Utah, and New Mexico, the Alaska Native Tumor Registry, rural Georgia and the metropolitan areas of San Francisco, Los Angeles, San Jose-Monterey, Detroit, Atlanta, and Seattle-Puget Sound).
Joinpoint analyses with up to 3 joinpoints yielding up to 4 trend segments (Trends 1–4) were based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130; US Bureau of the Census, Current Population Reports, p25-1130. Washington, DC: US Government Printing Office, 2000). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is a weighted average of the APCs calculated by joinpoint regression.
Cancers are sorted according to International Classification of Diseases for Oncology Third Edition code. Adenocarcinoma of the esophagus is restricted to esophageal cancers with microscopically confirmed histology codes in the range from 8140 to 8575. Myelodysplastic syndromes are excluded from cancer-specific analyses.
The APC is based on rates that were age-adjusted to the 2000 US standard population (19 age groups: <1, 1–4, 5–9, …, 80–84, ≥85 years; Census publication p25-1130).
The APC is statistically significantly different from zero (2-sided t test; P <.05).
The AAPC is statistically significantly different from zero (2-sided Z test; P <.05).
Table 8.
Cancer Incidence Rates for 2004–2008 and Fixed-Interval Trends From 1999 to 2008 for Cancers Associated With Excess Weight and Lack of Sufficient Physical Activity by Sex, Race, and Ethnicity for Areas in the United States With High-Quality Incidence Dataa
| All Racial and Ethnic Groups |
Whiteb | Blackb | APIb | AI/AN (CHSDA)b |
Hispanicb | Non- Hispanicb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Cancer Site or Typec |
Rated | 1999– 2008 AAPCe |
2004– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
Rated | 1999– 2008 AAPCe |
| Both sexes | |||||||||||||||
| Adenocarcinoma of esophagus | 3.0 | 1.9f | 2.0f | 3.3 | 2.0f | 0.8 | 0.9 | 0.6 | 3.6 | 2.0 | −0.1 | 1.5 | 2.3f | 3.1 | 2.0f |
| Colon and rectum | 47.7 | −2.6f | −3.2f | 46.7 | −2.8f | 56.5 | −1.6f | 36.9 | −2.0f | 46.0 | −1.7 | 40.5 | −1.9f | 48.3 | −2.6f |
| Colon | 34.7 | −2.6f | −3.3f | 33.8 | −2.8f | 43.3 | −1.9f | 24.7 | −2.3f | 32.2 | −2.2 | 28.6 | −2.2f | 35.1 | −2.6f |
| Pancreas | 11.7 | 0.7f | 0.5f | 11.5 | 0.9f | 15.1 | 0.4 | 8.8 | −0.1 | 10.6 | 0.0 | 10.6 | 0.1 | 11.8 | 0.8f |
| Kidney and renal pelvis | 15.3 | 2.6f | 1.7f | 15.4 | 2.5f | 16.2 | 3.4f | 7.1 | 3.5f | 21.5 | 2.5f | 14.8 | 2.3f | 15.4 | 2.8f |
| Men | |||||||||||||||
| Adenocarcinoma of esophagus | 5.7 | 1.9f | 1.9f | 6.2 | 2.0f | 1.4 | 0.9 | 1.0 | 2.7 | 3.3 | −0.8 | 2.9 | 2.7f | 5.9 | 2.0f |
| Colon and rectum | 55.7 | −3.0f | −3.7f | 54.6 | −3.2f | 66.9 | −1.4f | 42.4 | −2.0f | 51.5 | −2.5 | 48.6 | −1.9f | 56.3 | −2.9f |
| Colon | 39.1 | −3.0f | −3.7f | 38.2 | −3.2f | 50.0 | −1.7f | 26.9 | −2.9f | 34.4 | −3.2f | 32.9 | −2.0f | 39.6 | −3.0f |
| Pancreas | 13.4 | 0.8f | 0.8f | 13.2 | 0.9f | 16.7 | 0.5 | 9.7 | 0.2 | 11.1 | 0.6 | 11.5 | 0.1 | 13.6 | 0.9f |
| Kidney and renal pelvis | 20.7 | 2.5f | 1.4f | 20.8 | 2.4f | 22.6 | 3.1f | 9.9 | 3.4f | 27.4 | 2.3f | 19.4 | 1.9f | 20.9 | 2.6f |
| Women | |||||||||||||||
| Adenocarcinoma of esophagus | 0.8 | 1.9f | 1.9f | 0.8 | 2.1f | 0.4 | 1.0 | 0.2 | –g | 0.8 | –g | 0.4 | −0.8 | 0.8 | 2.1f |
| Colon and rectum | 41.4 | −2.4f | −3.0f | 40.3 | −2.5f | 49.7 | −1.8f | 32.7 | −1.6f | 41.5 | −1.3 | 34.2 | −2.2f | 42.0 | −2.4f |
| Colon | 31.3 | −2.4f | −3.1f | 30.4 | −2.4f | 39.0 | −2.1f | 23.1 | −1.8f | 30.3 | 7.0 | 25.3 | −2.4f | 31.7 | −2.4f |
| Pancreas | 10.4 | 0.9f | 0.9f | 10.0 | 1.0f | 13.8 | 0.4 | 8.1 | −0.4 | 10.1 | −0.4 | 9.9 | 0.2 | 10.4 | 0.9f |
| Kidney and renal pelvis | 10.9 | 2.8f | 1.9f | 10.9 | 2.7f | 11.7 | 3.7f | 4.9 | 3.6f | 16.8 | 2.7f | 11.2 | 2.6f | 10.8 | 2.8f |
| Breast (age 50+) | 327.9 | −1.3f | 0.4 | 332.6 | −1.5f | 305.7 | 0.3 | 211.2 | 0.2 | 243.2 | −0.9 | 244.9 | −0.6 | 334.3 | −1.3f |
| Corpus and uterus, NOS | 24.1 | 0.3 | 0.3 | 24.6 | 0.1 | 21.7 | 1.8f | 16.3 | 1.9f | 20.3 | 1.2 | 19.9 | 1.2f | 24.5 | 0.3 |
Abbreviations: AAPC, average annual percent change; AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; CHSDA, Indian Health Service Contract Health Services Delivery Area; NOS, not otherwise specified.
Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) Program areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods, including 2004–2008 rates for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (48 states: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming); 2004–2008 and 1999–2008 AAPCs for all races/ethnicities, white, black, AI/AN, API, Hispanic, and non-Hispanic (41 states: Alabama, Alaska, Arizona, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Texas, Utah, Vermont, Washington, West Virginia, Wisconsin, and Wyoming).
White, black, API, and AI/AN (CHSDA counties) include Hispanic and non-Hispanic; the race and ethnicity categories are not mutually exclusive.
Cancers are sorted according to International Classification of Diseases for Oncology Third Edition (ICD-O-3) code. Adenocarcinoma of the esophagus is restricted to esophageal cancers with microscopically confirmed histology codes in the range from 8140 to 8575. Myelodysplastic syndromes are excluded from cancer-specific analyses.
Rates are per 100,000 persons and are age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication no. p25-1130; US Bureau of the Census, Current Population Reports, P25-1130. Washington, DC: US Government Printing Office, 2000).
The AAPC is a weighted average of the annual percent change (APC) and is calculated by joinpoint analyses with up to 2 joinpoints yielding up to 3 trend segments based on rates per 100,000 persons and age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication p25-1130). For joinpoint analysis, the Joinpoint Regression Program was used (version 3.5, April 2011; Surveillance Research Program, National Cancer Institute, Bethesda, Md).
The AAPC is statistically significant from zero (2-sided Z test; P < 0.5).
The statistic could not be calculated. The average annual percent change is based on <10 cases for at least 1 year within the time interval.
Table 9.
State-Specific Cancer Incidence Rates for 2004–2008 for Cancers Associated With Excess Weight and Lack of Sufficient Physical Activity for Areas in the United States With High-Quality Incidence Dataa,b,c
| Men and Women Combined | Women | ||||||
|---|---|---|---|---|---|---|---|
| State | Adenocarcinoma of Esophagus |
Colon and Rectum |
Colon | Pancreas | Kidney and Renal Pelvis |
Breast (Aged ≥50 y) |
Corpus and Uterus, NOS |
| Alabama | 2.5 | 50.5 | 37.7 | 11.6 | 15.1 | 310.1 | 18.3 |
| Alaska | 3.5 | 50.2 | 37.6 | 12.4 | 16.5 | 369.5 | 22.6 |
| Arizona | 2.4 | 37.5 | 27.5 | 10.1 | 14.2 | 293.2 | 19.1 |
| Arkansas | 2.3 | 48.0 | 34.8 | 10.9 | 16.0 | 293.0 | 18.5 |
| California | 2.2 | 44.2 | 31.7 | 11.5 | 13.2 | 335.5 | 22.3 |
| Colorado | 3.0 | 42.1 | 30.7 | 10.8 | 13.2 | 336.3 | 19.5 |
| Connecticut | 3.2 | 49.2 | 35.3 | 13.7 | 15.3 | 363.7 | 28.7 |
| Delaware | 3.2 | 50.3 | 36.8 | 12.4 | 15.7 | 337.5 | 28.0 |
| District of Columbia | – d | – d | – d | – d | – d | – d | – d |
| Florida | 2.7 | 45.1 | 33.1 | 11.3 | 14.1 | 302.6 | 21.4 |
| Georgia | 2.2 | 46.7 | 33.6 | 11.5 | 14.2 | 319.4 | 18.3 |
| Hawaii | 1.4 | 49.2 | 32.9 | 12.6 | 12.5 | 317.9 | 28.5 |
| Idaho | 3.4 | 41.8 | 30.8 | 11.8 | 14.0 | 329.0 | 22.7 |
| Illinois | 3.3 | 53.9 | 39.1 | 12.8 | 16.8 | 334.4 | 26.7 |
| Indiana | 3.7 | 50.7 | 37.4 | 11.5 | 17.0 | 315.4 | 25.8 |
| Iowa | 4.0 | 53.5 | 39.5 | 11.4 | 16.7 | 330.4 | 28.4 |
| Kansas | 2.8 | 48.8 | 35.6 | 10.9 | 15.2 | 338.0 | 23.6 |
| Kentucky | 3.4 | 55.7 | 39.8 | 11.5 | 17.9 | 326.0 | 23.8 |
| Louisiana | 2.4 | 53.9 | 39.1 | 13.3 | 19.0 | 318.2 | 17.0 |
| Maine | 4.7 | 51.6 | 37.9 | 12.6 | 16.0 | 352.7 | 30.9 |
| Maryland | – d | – d | – d | – d | – d | – d | – d |
| Massachusetts | 4.3 | 48.4 | 35.1 | 12.3 | 16.0 | 357.1 | 29.7 |
| Michigan | 3.4 | 47.3 | 34.5 | 12.6 | 15.4 | 322.4 | 27.6 |
| Minnesota | 3.5 | 46.8 | 34.3 | 10.1 | 15.6 | 347.4 | 27.7 |
| Mississippi | 2.1 | 53.9 | 40.0 | 11.7 | 17.4 | 299.4 | 19.5 |
| Missouri | 3.2 | 50.3 | 37.2 | 11.8 | 17.0 | 325.6 | 24.1 |
| Montana | 3.6 | 44.9 | 32.1 | 11.1 | 12.6 | 331.7 | 24.1 |
| Nebraska | 3.4 | 55.2 | 39.7 | 11.3 | 15.9 | 336.2 | 26.3 |
| Nevada | – d | – d | – d | – d | – d | – d | – d |
| New Hampshire | 5.1 | 47.1 | 33.6 | 12.3 | 14.3 | 361.7 | 29.5 |
| New Jersey | 2.9 | 51.3 | 36.9 | 12.8 | 15.6 | 344.9 | 29.5 |
| New Mexico | 2.4 | 40.4 | 28.4 | 10.5 | 12.7 | 298.3 | 20.2 |
| New York | 2.8 | 48.8 | 35.4 | 12.9 | 15.4 | 329.8 | 29.1 |
| North Carolina | 2.7 | 46.8 | 34.2 | 11.6 | 16.5 | 330.8 | 21.6 |
| North Dakota | 3.2 | 54.4 | 38.8 | 11.6 | 15.2 | 344.3 | 26.3 |
| Ohio | 3.7 | 49.9 | 36.5 | 11.6 | 15.6 | 327.3 | 27.3 |
| Oklahoma | 3.0 | 48.8 | 35.3 | 10.9 | 16.4 | 347.5 | 20.4 |
| Oregon | 3.7 | 43.9 | 31.8 | 11.4 | 14.4 | 363.7 | 24.4 |
| Pennsylvania | 3.7 | 52.6 | 38.0 | 12.6 | 16.5 | 336.9 | 30.6 |
| Rhode Island | 4.2 | 50.7 | 36.5 | 10.9 | 17.2 | 359.8 | 30.7 |
| South Carolina | 2.3 | 47.4 | 35.4 | 11.6 | 14.6 | 324.5 | 20.0 |
| South Dakota | 3.5 | 47.9 | 35.0 | 9.8 | 14.6 | 325.2 | 24.7 |
| Tennessee | 2.6 | 48.8 | 35.3 | 10.8 | 15.6 | 315.1 | 19.2 |
| Texas | 2.4 | 45.2 | 32.7 | 11.2 | 16.9 | 308.3 | 18.7 |
| Utah | 2.5 | 36.3 | 26.4 | 9.6 | 10.9 | 304.1 | 22.0 |
| Vermont | 3.7 | 44.1 | 30.3 | 12.5 | 15.0 | 349.1 | 31.2 |
| Virginia | 2.6 | 45.1 | 33.1 | 11.7 | 14.3 | 332.5 | 22.5 |
| Washington | 3.4 | 43.0 | 30.8 | 11.9 | 15.1 | 359.7 | 24.4 |
| West Virginia | 3.7 | 55.0 | 39.3 | 10.5 | 16.6 | 306.6 | 29.0 |
| Wisconsin | 3.7 | 46.4 | 34.2 | 11.9 | 15.7 | 338.6 | 27.6 |
| Wyoming | 3.9 | 44.7 | 32.8 | 10.0 | 14.1 | 316.8 | 20.7 |
Abbreviations: NOS, not otherwise specified.
Rates are per 100,000 persons and are age standardized to the 2000 US standard population (19 age groups: ages <1 year, 1–4 years, 5–9 years, …, 80–84 years, ≥85 years; Census publication no. p25-1130; US Bureau of the Census, Current Population Reports, P25-1130. Washington, DC: US Government Printing Office, 2000).
Cancers are sorted according to International Classification of Diseases for Oncology Third Edition (ICD-O-3) code. Adenocarcinoma of the esophagus is restricted to esophageal cancers with microscopically confirmed histology codes in the range from 8140 to 8575. Myelodysplastic syndromes are excluded from cancer-specific analyses.
Source: National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) Program areas reported by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for 2004–2008 (48 states: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming).
Data not available.
On the basis of results from published comprehensive meta-analyses of BMI, each 5-kg/m2 increase in BMI is associated with 30% to 60% increased risk of endometrial cancer,39 adenocarcinoma of the esophagus,24 and kidney cancer24 and with a 13% to 18% increased risk of colorectal cancer,37 pancreatic cancer,24 and postmenopausal breast cancer38 (Table 10). On the basis of results from published comprehensive meta-analyses of physical activity, lack of sufficient physical activity has been associated with a 30% to 40% increased risk of colon cancer,40 postmenopausal breast cancer,41 and endometrial cancer42 (Table 10).
Table 10.
Relative Risk (RR) Associated with Excess Weight and Lack of Sufficient Physical Activity
| Cancer Site and Type |
Summary RR From Comprehensive Meta-Analysis (95% CI) per Given Unit Increase in BMI |
RR: Overweight (BMI 25–29 kg/m2) vs BMI <25 kg/m2a |
RR: Obese (BMI ≥30 kg/m2) vs BMI <25 kg/m2a |
| Adenocarcinoma of esophagusb | 1.11 (1.07–1.15) per 1 kg/m2 increase in BMI | 1.55 | 2.10 |
| Colorectalc | 1.18 (1.14–1.21) per 5 kg/m2 increase in BMI | 1.18 | 1.36 |
| Pancreasb | 1.14 (1.07–1.22) per 5 kg/m2 increase in BMI | 1.14 | 1.28 |
| Kidneyb | 1.31 (1.24–1.39) per 5 kg/m2 increase in BMI | 1.31 | 1.62 |
| Postmenopausal breastd | 1.05 (1.03–1.07) per 2 kg/m2 increase in BMI | 1.13 | 1.25 |
| Endometriale | 1.60 (1.52–1.68) per 5 kg/m2 increase in BMI | 1.60 | 2.20 |
| Cancer Site and Type |
Summary RR From Comprehensive Meta-Analysis (95% CI) for Level of Physical Activity |
RR: Inactive vs Most Activef |
|
| Colong | 0.76 (0.72–0.81): Most active (recreational or occupational) vs least active | 1.32 | |
| Postmenopausal breasth | 0.75 (CI not given): Most active (recreational, occupational, or household) vs least active | 1.33 | |
| Endometriali | 0.73 (0.58–0.93): Highest activity (recreational) vs lowest activity | 1.37 | |
Abbreviations: BMI, body mass index; CI, confidence interval; RR, relative risk.
Published estimates were given as the risk associated with a specified unit change in BMI. Assuming a linear association between BMI and cancer risk, estimates were calculated for the risk associated with overweight and obese.
Source: World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007.24
Source: Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30.37
Source: World Cancer Research Fund/American Institute for Cancer Research. The Associations Between Food, Nutrition and Physical Activity and the Risk of Breast Cancer: WCRF/AICR Systematic Literature Review Continuous Update Report. Washington, DC: American Institute for Cancer Research; 2008.38
Source: Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:3119–3130.39
Published estimates were presented as the risk of the most active relative to the least active; the inverse was calculated to present the risk of the least active relative to the most active.
Source: Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616.40
Source: Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–47.41
Source: Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103:933–938.42
DISCUSSION
This Annual Report to the Nation documents continued declines in mortality from all cancers combined. These declines indicate progress across the cancer continuum, including primary prevention, which involves education about risk factors and promotion of healthy behaviors, increased screening and early detection, and improved treatment. Differences by racial and ethnic group suggest differences in risk behaviors as well as access to and use of screening and treatment.44–48
Lung cancer incidence and mortality continue to decline among both men and women, reflecting the success of tobacco-control strategies to prevent initiation, accelerate declines in consumption, and promote cessation.11,45,49 Although a decline in lung cancer has been observed among men for years, the beginning of a decline was first documented among women in last year’s report13 and is driven largely by declines in states with strong, long-running, comprehensive tobacco-control programs.49
Prostate cancer incidence has fluctuated through the years, with a large peak in 1992 corresponding to an increase in prostate-specific antigen (PSA) testing,50 but has decreased significantly, although not uniformly, since 1992.15 Prostate cancer death rates also have decreased substantially over time. The contribution of PSA testing to this decline and the risks and benefits for individual men remain uncertain.50
Trends in breast cancer incidence over time reflect long-term changes in reproductive and other risk factors, introduction and increased prevalence of mammography screening, and use of hormones among postmenopausal women.51 After sharp declines in breast cancer incidence among women from 2002 to 2003 associated with reduced use of hormone replacement therapy,13,52 breast cancer incidence rates have stabilized since 2004. The prevalence of mammography screening among women aged ≥40 years that peaked at 70% in 2000 has since plateaued,53 which is consistent with the stabilization in breast cancer incidence rates.
Colorectal cancer incidence and death rates continue to decline, most likely attributable to significant improvements in the use of colorectal cancer screening, which can prevent cancer development through removal of precancerous adenomatous polyps.12,54 Still, more than one-third of US adults do not receive recommended colorectal screening.44,55 Innovative systems-level changes are needed to make screening available, affordable, and routine for all adults ages 50 to years and would prevent even more colorectal cancer cases and deaths.44
This report highlights cancers associated with excess weight and lack of sufficient physical activity. For more than 30 years, excess weight, insufficient physical activity, and an unhealthy diet have been considered second only to tobacco as preventable causes of disease and death in the United States.56 However since the 1960s, tobacco use has declined by one-third, whereas obesity rates have doubled, significantly impacting the relative contributions of these factors to the disease burden.57–59 Excess weight and lack of sufficient physical activity have been linked to increased risk of cardiovascular disease, hypertension, diabetes, and arthritis as well as many cancers.60,61 Specifically, excess weight has been convincingly associated with increased risk of adenocarcinoma of the esophagus, colon and rectal cancer, pancreas cancer, kidney cancer, and, in women, endometrial and postmenopausal breast cancers.24,37–39 Considerable evidence suggests that excess weight also may be associated with increased risk of other cancers, including gallbladder, liver, thyroid, and hematopoietic cancers.24 Lack of sufficient physical activity is associated with increased risk of colon, endometrial, and postmenopausal breast cancers and also may be associated with premenopausal breast cancer.24,34,40–42,62 This report indicates that, over the past 10 years, although incidence rates have decreased for colorectal cancer, the rates have stabilized for postmenopausal breast cancer and have increased for several of these cancers, including pancreas, kidney, liver, and thyroid cancers and adenocarcinoma of the esophagus. Endometrial cancer incidence is increasing among black, API, and Hispanic women. Although all of these cancers are influenced by multiple factors, the high prevalence of excess weight and insufficient physical activity63 likely contributed to these observed increases and to the lack of decline in breast cancer.64
Excess weight and lack of sufficient physical activity also may adversely affect cancer prognosis and quality of life among cancer survivors. Research indicates that excess weight is associated with poorer survival among patients with breast cancer65–67 and colorectal cancer.68–70 Recent research suggests that physical activity after diagnosis of breast or colon cancer is associated with reduced all-cause and cancer-specific mortality (R. Ballard-Barbash, personal communication). For prostate cancer, obesity is associated with mortality and incidence of late-stage disease but not with incidence of early-stage disease.71 Screening rates for breast and cervical cancers are lower among women who are obese, which may lead to diagnosis at later stage of disease.72,73
Although trends in the prevalence of excess weight and physical inactivity in the United States seem to be stabilizing or improving, current levels, particularly the unprecedented high levels of obesity among young individuals,74,75 are concerning and can impact future disease rates. Unhealthy behaviors among young individuals may lead to unhealthy behaviors in adulthood76,77 as well as adverse health profiles78–80 and an increased risk of cancer later in life.77,81–83 Continued progress in reducing cancer incidence and mortality will be difficult without success in promoting healthy weight and physical activity, particularly among youth.24,80,81
The International Agency for Research on Cancer concluded that from one-quarter to one-third of common cancers in industrialized nations were caused by the joint effect of excess weight and lack of sufficient physical activity.84 The World Cancer Research Fund/American Institute for Cancer Research concluded that approximately one-third of common cancers in the United States could be prevented by following healthy patterns of physical activity and diet, including maintaining a healthy weight.85 These estimates of attributable risk are based, in part, on the cancer sites considered, the size of the relative risk used, the definitions of physical activity and excess weight, and the prevalence of these risk factors.86 Although most estimates of attributable risk are similar, some are higher or lower.30,62,64,87,88 Despite some differences in the magnitude of the estimates, researchers agree that excess weight and lack of sufficient physical activity are important, avoidable causes of cancer in the United States and other industrialized nations. Maintaining a healthy weight throughout life may be among the most important ways to prevent cancer, particularly for individuals who do not smoke. Eating a healthy diet and engaging in sufficient physical activity protect against cancer directly, and indirectly by protecting against weight gain. These healthy lifestyle behaviors also prevent diseases, such as cardiovascular disease and diabetes.60,61,89
Given the very broad influences of excess weight and physical activity on human biology, many mechanisms have been considered and examined to explain the observed associations between cancer and excess weight and physical inactivity. These mechanisms are complex, interrelated, and not completely understood. It has been demonstrated that excess weight and physical activity affect the synthesis and metabolism of sex hormones, insulin and related growth factors, immune response, and oxidative stress.30,34,62,90–92 For example, postmenopausal obesity is related to higher exposures to free estrogens and androgens,93 and extensive evidence on reproductive epidemiologic risk factors supports a strong role for steroid hormones in the etiology of breast, ovarian, and endometrial cancers.30 Excess weight and physical inactivity also raise levels of circulating insulin; and chronic hyperinsulinemia is associated with the pathogenesis of several cancers related to excess weight, including colorectal, breast, pancreatic, and endometrial cancers.94–96 Excess weight also may influence the risk of cancer through effects on tumor growth regulators, including mammalian target of rapamycin (mTOR) and 5′ adenosine monophosphate (AMP)-kinase, and adipokines, including adiponectin and leptin.30,90,91,97 Moreover, a large and growing body of in vitro and animal model studies indicates that physiologic factors linked to excess weight and physical inactivity may play a role in cancer etiology by influencing cell growth, differentiation, and apoptosis as well as tissue invasion and angiogenesis.97 Research is beginning to examine the role of genetics interacting with the exposures of excess weight and physical inactivity and cancer outcomes.98 Some research suggests that physical activity may influence cell cycle changes differently than calorie restriction.92,99 Emerging research indicates that, independent of physical activity, the amount of time spent in sedentary behaviors, such as sitting, may adversely affect health outcomes, including cancer.100–102
Fundamentally, excess weight is caused by an energy imbalance in which energy intake exceeds energy expended.74,103 Excess of calories and lack of physical activity each contribute to excess weight.84 In addition, evidence suggests that sustained breastfeeding may help protect infants and young children from becoming overweight or obese.85,104 Although seemingly simple, the causes of the high rates of excess weight and insufficient physical activity in the US today are complex and include individual characteristics as well as societal and environmental factors.63,103,105 These include changes over the last few decades, such as low rates and short duration of breastfeeding; the increased availability of inexpensive, energy-dense food; larger food portion sizes; increased consumption of sugar-sweetened beverages; a car-dominated society; widening distances between home and work or shopping; and a trend toward more sedentary office jobs rather than physically demanding jobs.105–107
Recognizing the inter-relationships between individual, societal and environmental factors in promoting lifelong health and preventing disease, the National Prevention Strategy has identified 4 strategic directions, including healthy communities, clinical and community preventive services, an empowered populace, and health equity, as well as 7 targeted priorities.108 Two of these priorities address healthy eating and active living as well as issues related to personal, social, economic, and environmental factors that influence these health behaviors. To improve healthy eating, the National Prevention Strategy recommends increasing access to healthy and affordable foods in communities, implementing organizational and programmatic nutrition standards and policies, improving nutritional quality of the food supply, helping individuals recognize and make healthy food and beverage choices, supporting policies and programs to promote breastfeeding, and enhancing food safety. To encourage physical activity, the National Prevention Strategy recommends encouraging community design and development that supports physical activity; promoting and strengthening school and early learning policies and programs that increase physical activity; facilitating access to safe, accessible, and affordable places for physical activity; supporting workplace policies and programs that increase physical activity; and assessing physical activity levels and providing education, counseling, and referrals.
To monitor progress and identify opportunities for improvement, the CDC has published a series of reports to provide state-specific information on current behavioral indicators as well as indicators of policy and environmental supports for fruits and vegetables,109 physical activity,110 children’s food environment,111 and breastfeeding support.112 Some of these state supports are highlighted in Table 11. These include strategies to promote and reinforce healthy behaviors by requiring physical education and recess in schools; state-level policies to support full-time personnel to develop, implement, monitor, and maintain physical activity interventions and programs; strategies to improve the food environment by increasing access to healthy foods; food policy councils that can support improved food environments for healthy eating through consideration of the local food system; and state regulations to support breastfeeding in childcare centers. Investing in policies to support public health has been associated with decreases in mortality, including cancer.113
Table 11.
State-Specific Policies Supporting Healthy Eating and Active Living
| State | Regular Recess Required or Recommended in Elementary Schoola |
PE Required in Elementary, Middle, and High Schoolb |
At Least 1 State FTE Focused on Physical Activityc |
State-Level Healthier Food Retail Policiesd |
State Food Policy Councile |
Optimal Regulations Supporting Lactation in Childcare Centersf |
|---|---|---|---|---|---|---|
| Alabama | ✓ | |||||
| Alaska | ||||||
| Arizona | ✓ | ✓ | ✓ | |||
| Arkansas | ✓ | ✓ | ✓ | ✓ | ||
| California | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Colorado | ✓ | |||||
| Connecticut | ✓ | ✓ | ✓ | ✓ | ||
| Delaware | ✓ | ✓ | NA | ✓ | ||
| District of Columbia | ✓ | ✓ | NA | ✓ | NA | |
| Florida | NA | |||||
| Georgia | ✓ | |||||
| Hawaii | ✓ | ✓ | ✓ | |||
| Idaho | ✓ | ✓ | ✓ | |||
| Illinois | ✓ | ✓ | ✓ | |||
| Indiana | ✓ | ✓ | ||||
| Iowa | ✓ | ✓ | ✓ | |||
| Kansas | ✓ | ✓ | ||||
| Kentucky | ✓ | |||||
| Louisiana | ✓ | ✓ | NA | ✓ | ||
| Maine | ✓ | ✓ | ✓ | |||
| Maryland | ✓ | |||||
| Massachusetts | ✓ | ✓ | ✓ | |||
| Michigan | ✓ | ✓ | ✓ | ✓ | ||
| Minnesota | ✓ | ✓ | ||||
| Mississippi | ✓ | ✓ | ✓ | ✓ | ||
| Missouri | ✓ | ✓ | ||||
| Montana | ✓ | ✓ | ||||
| Nebraska | ✓ | ✓ | ||||
| Nevada | ✓ | NA | ✓ | |||
| New Hampshire | ✓ | NA | ||||
| New Jersey | ✓ | ✓ | ||||
| New Mexico | ✓ | ✓ | NA | ✓ | ||
| New York | ✓ | ✓ | ✓ | ✓ | ||
| North Carolina | ✓ | ✓ | ✓ | ✓ | ||
| North Dakota | ✓ | ✓ | ||||
| Ohio | ✓ | ✓ | ||||
| Oklahoma | ✓ | ✓ | ||||
| Oregon | ✓ | |||||
| Pennsylvania | ✓ | ✓ | ||||
| Rhode Island | ✓ | ✓ | ||||
| South Carolina | ✓ | ✓ | ✓ | |||
| South Dakota | ||||||
| Tennessee | ✓ | ✓ | ✓ | |||
| Texas | ✓ | ✓ | ||||
| Utah | ✓ | ✓ | ✓ | ✓ | ||
| Vermont | ✓ | ✓ | ✓ | |||
| Virginia | ✓ | ✓ | NA | ✓ | ||
| Washington | ✓ | ✓ | ||||
| West Virginia | ✓ | ✓ | Pending | |||
| Wisconsin | ✓ | ✓ | ✓ | |||
| Wyoming | NA | |||||
Abbreviations: FTE, full-time equivalent; NA, information not available.
State education agency personnel answered “yes” to the question, “Does your state require or recommend that elementary schools provide students with regularly scheduled recess?”110
State education agency personnel answered, “yes”, to all of the following questions: 1) “Has your state adopted a policy stating that elementary schools will teach physical education?”; 2) “Has your state adopted a policy stating that middle or junior high schools will teach physical education?”; and 3) “Has your state adopted a policy stating that senior high schools will teach physical education?”110
The state health department physical activity representative reported the number of state health department FTEs, primarily focused on state-based physical activity issues. The representative was contacted by the Wisconsin Department of Health Services for participation in a web-based, “State Physical Activity Capacity” survey hosted by the Wisconsin Department of Health Services. The representative responded to the following question: “What is the total number of FTEs in your state that are primarily focused on statewide physical activity issues? (example: if you have only 1 person and they are 0.5 FTE physical activity and 0.5 FTE nutrition, list the number as 0.5 FTE).”110
State-level food retail policies (legislation, executive action) enacted (yes/6no) between January 1, 2001, and August 1, 2009, and qualified if they supported any of the following goals: 1) the building and/or placement of new food retail outlets (eg, new supermarkets in underserved areas, loan financing program for small business development); 2) renovation and equipment upgrades of existing food retail outlets (eg, purchasing refrigerators for small corner stores to allow for the sale of fresh produce); and 3) increases in and promotion of foods encouraged by the 2005 Dietary Guidelines for Americans stocked or available at food retail outlets (eg, increase display or shelf space for encouraged foods such as fruits and vegetables; assistance in marketing of these healthier foods, such as through point of decision information).109
State councils designated with a check mark have a named point of contact on the Community Food Security Coalition website as of the accessed date. Those listed include councils of various types, with different approaches and at various stages of development. Regional or multistate councils are not designated in this source.109
States’ regulations in support of breastfeeding in child care centers were scored using the average appropriate fluids rating (1A1) as determined by the National Resource Center for Health and Safety in Child Care and Early Education. Cutoff points (1, inappropriate; 2, not optimal; 3, less optimal; 4, optimal) were set.112
The economic burden caused by excess weight and physical inactivity is substantial. According to recent data, per capita medical spending in the United States in 2008 for an obese individual was 42% higher ($1429) per year compared with someone of normal weight, resulting in a national burden of $147 billion: approximately 9.1% of all medical spending.114 In addition to medical costs, indirect costs of obesity include decreased years of disability-free life, increased mortality before retirement, earlier retirement, higher disability pensions, increased work absenteeism, and reduced productivity.115 Physical inactivity, independent of obesity, results in excessive direct medical costs,116,117 accounting for 2.4% of total US health care expenditures in 1995.118
Limitations
High-quality cancer surveillance in the United States now covers the entire population for mortality and 96% of the population for incidence; however, certain limitations in data sources, data collection, and analyses may have influenced the findings of this report. First, state and national population estimates are provided annually by the Census Bureau to estimate postcensal populations. Differences between the numerator (incidence data) and denominator (US Census population data) can occur in the designation of characteristics like age, race, ethnicity, and place of residency. Postcensal population estimates based on numbers updated by birth and death data are more subject to error than estimates based on the actual Census count; errors in these estimates may increase as time passes from the original recording of Census data. In addition, the NCI modified these Census estimates to account for changes in 2005 county-level populations because of displacement of individuals after Hurricanes Katrina and Rita in the most affected counties of Louisiana, Mississippi, Alabama, and Texas.
Second, as routinely noted in previous Annual Reports to the Nation,1–13 the broad racial and ethnic groups categorized for our analyses may mask variations in the cancer burden by country of origin or by other unique characteristics of high-risk or low-risk populations. Also, cancer rates for racial and ethnic groups may be affected by difficulties in ascertaining race and ethnicity information from medical records, death certificates, and Census reports.119
Third, the analysis of trends should be carefully interpreted for several reasons. Changes in incidence may result from changes in the prevalence of risk factors, the introduction or increased use of screening or diagnostic techniques, or a combination of these. The AAPC was used as a summary measure to average trends over a 5-year or 10-year period using joinpoint regression; joinpoint models identify recent changes in the magnitude and direction of trends but may give the impression of a continuous increase or decrease over time when this is not the case. Furthermore, delayed case reporting may affect incidence trends if the most recent joinpoint segments overestimate recent declines or underestimate recent increases; methods to adjust for delayed reporting23 were used in our report only in the analysis of SEER 13 data. The largest effects of adjusting for delayed reporting are observed for cancers diagnosed in non-hospital settings, such as melanoma and leukemia. This report presents trends based both on data from the SEER 13 registries and on combined data from the NAACCR, which includes SEER and NPCR registries. Both data sets have strengths and limitations and provide valuable insight into cancer trends in the United States. Longer-term trends can be examined using the SEER 13 registries, and these data also have been delay adjusted. However, combined data from the SEER and NPCR registries cover 96% of the US population and may better capture geographic and population differences in risk factors and incidence.
Fourth, US Department of Veterans Affairs (VA) hospitals traditionally are a critical source of data for cancers diagnosed among veterans, who represent approximately 3% to 8% of cancer diagnoses among men. A 2007 policy change regarding the transfer of VA cancer data to state central cancer registries has resulted in incomplete reporting of VA hospital cases in some, but not all, state registries beginning in the third quarter of the 2004 diagnosis year through the current period. Consequently, it is believed that cancer incidence rates among men from 2005 to 2008 are underestimated by 0.8% to 1% for all cancers combined based on an analysis of data from the SEER 9 registries. The level of underreporting varied from 0.2% to 7% according to cancer site, race, and age group.15,120 With the enactment of special data-sharing agreements with the VA, progress in collecting data from VA hospitals has been made in many states, and this will result in more complete and accurate national cancer incidence estimates.
Fifth, state-specific estimates of the prevalence of obesity from the BRFSS likely were underestimated because of bias of self-reported weight and height. Although self-reported BMI is highly correlated with measured BMI (r > 0.9),121 it often is underestimated; because, typically, height is over reported, and weight is under reported, particularly by women.122–126 Furthermore, the sensitivity of self-reported data to correctly classify obesity was 74% in the New York BRFSS.126 Low sensitivity of self-reported data to correctly classify obesity may account for the lower prevalence of obesity estimated from the BRFSS, which uses self-reported data, compared with the NHANES, which uses measured data.
Future Directions
The continued declines in cancer death rates for all sites combined and for the leading cancer sites among men and women overall and in nearly all racial and ethnic groups indicate progress in cancer prevention and control. These decreases are largely a result of tobacco prevention and control efforts, screening and early detection of some cancers, and improvements in treatment for many cancers.127 Although incidence is decreasing for many cancer sites, incidence of other cancers is increasing, including several associated with excess body weight.
Risk factors like tobacco use, excess alcohol consumption, poor diet, excess body weight, and physical inactivity contribute to the burden of many cancers.24,128 It is encouraging to note that many of these risk factors are modifiable. Reductions in these risk factors over time should be reflected in reductions in cancer incidence. For example, declines in lung cancer incidence parallel declines in smoking.11,49 Achieving and sustaining healthy lifestyle behaviors are essential to reducing the burden of cancer.
The increased cancer risk associated with excess weight and lack of sufficient physical activity has been the focus of this report. The US Dietary and Physical Activity Guidelines advise individuals in the general population to prevent and/or reduce overweight and obesity through improved eating and physical activity behaviors129 and to engage in sufficient aerobic and muscle-strengthening activity each week (with sufficient defined for adults as at least 150 minutes of moderate-intensity activity [or 75 minutes of vigorous-intensity activity or an equivalent combination of both] and muscle-strengthening activities at least 2 days each week involving all major muscle groups).34 These guidelines are consistent with those for cancer prevention recently updated by the ACS, which recommend that, throughout life, individuals should be as lean as possible without being underweight and that adults should engage in at least 150 minutes of moderate-intensity activity or 75 minutes of vigorous-intensity activity each week or an equivalent combination of both.130 The World Cancer Research Fund, together with the American Institute for Cancer Research, similarly recommends that individuals can reduce their risk of cancer by being as lean as possible within the normal body weight range and by staying physically active (at least 30 minutes every day) as part of everyday life.24 Recommendations for a healthy diet include maintaining appropriate calorie balance throughout life, consuming nutrient-dense foods and beverages, increasing the intake of colorful fruits and vegetables, and limiting the intake of refined grains, added sugars, cholesterol, saturated fats, trans fats, and sodium (salt).129,130
However, maintaining a healthy weight and engaging in sufficient physical activity cannot be promoted solely at the level of the individual. Supporting recommended healthy lifestyle behaviors will require concerted actions from individuals; communities; the media; federal, state, and local governments; food industries; international agencies; and sectors beyond what is usually considered public health, such as transportation and agriculture.84,89,131–134 Policy and environmental changes can help make healthy choices more accessible, available, and affordable.
Geographically specific information that can be linked across the individual, social, and physical environments is recognized increasingly as a key element in the framework for cancer surveillance.135 For example, enhancing data resources for monitoring and evaluating progress in diet, physical activity, and weight is a major focus of the National Collaboration of Childhood Obesity Research (NCCOR). The NCCOR, a collaborative effort with the CDC, the National Institutes of Health, the US Department of Agriculture, and the Robert Wood Johnson Foundation, is focused on policy and environmental research relevant to obesity prevention for children. One product of this initiative is the Catalogue of Surveillance Systems (available at: http://www.nccor.org/css/index.html [January 27, 2012]), which is an online resource of more than 80 health monitoring systems relevant to physical activity, diet, and obesity. These types of data resources are critical to efforts in further understanding the quantitative effects of different risk factors and effective interventions across multiple levels that may result in beneficial changes.
To plan for the future, it is imperative to know where we are and from whence we have come. Thus, it is important to monitor and evaluate the prevalence of and trends in health behaviors and disease incidence. Quality, population-based risk factor and cancer surveillance data can be used to identify areas and populations with unhealthy behaviors and high cancer rates that could benefit from targeted, effective strategies and interventions to improve health behaviors and support healthy environments.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contributions of the state and regional cancer registry staffs for their work in collecting the data used in this study. In addition, we thank Andrew Lake, Martin Krapcho, and Rick Firth of Information Management Services, Inc. and Susan A. Carlson, Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention, Atlanta, GA, for assistance with statistical analyses.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FUNDING SOURCES
This work was supported by the American Cancer Society, the Centers for Disease Control and Prevention, the National Cancer Institute of the National Institutes of Health, and the North American Association of Central Cancer Registries.
Footnotes
Additional Supporting Information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973–1995: a report card for the US. Cancer. 1998;82:1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 3.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. J Natl Cancer Inst. 2001;93:824–842. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Howe HL, Ries LAG, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on US cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 6.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 9.Howe HL, Wu X, Ries LAG, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer. 2006;107:1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 10.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Thun MJ, Ries LAG, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Third Edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 15.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 16.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. [Accessed January 27, 2012];Population Estimates Used in the NCI SEER*Stat Software. Available at: http://seer.cancer.gov/popdata/methods.html.
- 17.Minino A, Murphy S, Xu J, Kochanek K. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–136. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. [Accessed January 27, 2012];National Vital Statistics System. US Census Populations With Bridged Race Categories. Available at: http://www.cdc.gov/nchs/about/major/dvs/popbridge/popbridge.htm.
- 19.Anderson R, Rosenberg H. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47:1–16. [PubMed] [Google Scholar]
- 20.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 21.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 24.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 25.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005–2008. NCHS Data Brief. 2010;51:1–8. [PubMed] [Google Scholar]
- 28.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States, 2005–2008. NCHS Data Brief. 2010;50:1–8. [PubMed] [Google Scholar]
- 29.National Center for Health Statistics (NCHS) Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: NCHS; 2011. [PubMed] [Google Scholar]
- 30.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 31.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance—United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services. Healthy People 2020. Washington, DC: US Department of Health and Human Services; 2010. [Google Scholar]
- 33.Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med. 2010;39:305–313. doi: 10.1016/j.amepre.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 35.Centers for Disease Control and Prevention. US Physical Activity Statistics. [Accessed January 27, 2012]; Available at: http://apps.nccd.cdc.gov/PASurveillance/StateSumV.asp#box.
- 36.Centers for Disease Control and Prevention. 2010 Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 37.Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11:19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 38.World Cancer Research Fund/American Institute for Cancer Research (WCFR/AICR) The Associations between Food, Nutrition and Physical Activity and the Risk of Breast Cancer: WCRF/AICR Systematic Literature Review Continuous Update Report. Washington, DC: American Institute for Cancer Research; 2008. [Google Scholar]
- 39.Crosbie EJ, Zwahlen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:3119–3130. doi: 10.1158/1055-9965.EPI-10-0832. [DOI] [PubMed] [Google Scholar]
- 40.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 42.Moore SC, Gierach GL, Schatzkin A, Matthews CE. Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer. 2010;103:933–938. doi: 10.1038/sj.bjc.6605902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–1980 through 2007–2008 [serial online] [Accessed January 27, 2012];NCHS Health E-Stats. 2010 Available at: http://www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.htm.
- 44.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening, incidence, and mortality—United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–889. [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- 46.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 47.Shavers VL, Jackson MC, Sheppard VB. Racial/ethnic patterns of uptake of colorectal screening, National Health Interview Survey 2000–2008. J Natl Med Assoc. 2010;102:621–635. doi: 10.1016/s0027-9684(15)30640-4. [DOI] [PubMed] [Google Scholar]
- 48.Paskett ED, Tatum C, Rushing J, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004;101:2650–2659. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. State-specific trends in lung cancer incidence and smoking—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1243–1247. [PubMed] [Google Scholar]
- 50.Etzioni R, Gulati R, Mariotto AB. Overview of prostate cancer trends in the era of PSA screening. In: Ankerst D, Tangen CM, Thompson IM, editors. Prostate Cancer Screening. Totowa, NJ: Humana Press; 2009. pp. 3–14. [Google Scholar]
- 51.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006;36:19–25. doi: 10.1093/jncimonographs/lgj016. [DOI] [PubMed] [Google Scholar]
- 52.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in US women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–739. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 53.Breen N, Gentleman JF, Schiller JS. Update on mammography trends. Cancer. 2011;117:2209–2218. doi: 10.1002/cncr.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1296–1302. doi: 10.1158/1055-9965.EPI-11-0250. [DOI] [PubMed] [Google Scholar]
- 55.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- 57.Cutler DM, Glaeser EL, Rosen AB. NBER Working Paper No. 13013. Cambridge, MA: National Bureau of Economic Research; 2007. Is the US Population Behaving Healthier? Available at: http://www.nber.org/papers/w13013.pdf. [Access date.] [Google Scholar]
- 58.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors [serial online] PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010;38:138–144. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 60.US Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, The President’s Council on Physical Fitness and Sports; 1996. [Google Scholar]
- 61.US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Decrease Overweight and Obesity. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 62.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 64.Polednak AP. Estimating the number of US incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev. 2008;32:190–199. doi: 10.1016/j.cdp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Ewertz M, Jensen M-B, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 66.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 67.Protani M, Coory M, Martin J. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 68.Meyerhardt JA, Ma J, Courneya KS. Energetics in colorectal and prostate cancer. J Clin Oncol. 2010;28:4066–4073. doi: 10.1200/JCO.2009.26.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res. 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Littman AJ, Koepsell TD, Forsberg CW, Boyko EJ, Yancy WS. Jr. Preventive care in relation to obesity: an analysis of a large, national survey. Am J Prev Med. 2011;41:465–472. doi: 10.1016/j.amepre.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 73.Cohen SS, Palmieri RT, Nyante SJ, et al. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer. 2008;112:1892–1904. doi: 10.1002/cncr.23408. [DOI] [PubMed] [Google Scholar]
- 74.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kvaavik E, Tell GS, Klepp K-I. Predictors and tracking of body mass index from adolescence into adulthood: follow-up of 18 to 20 years in the Oslo Youth Study. Arch Pediatr Adolesc Med. 2003;157:1212–1218. doi: 10.1001/archpedi.157.12.1212. [DOI] [PubMed] [Google Scholar]
- 76.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 77.Aaron DJ, Jekal YS, LaPorte RE. Epidemiology of physical activity from adolescence to young adulthood. World Rev Nutr Diet. 2005;94:36–41. doi: 10.1159/000088204. [DOI] [PubMed] [Google Scholar]
- 78.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daniels SR. Complications of obesity in children and adolescents. Int J Obes. 2009;33:S60–S65. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 80.Dwyer T, Magnussen CG, Schmidt MD, et al. Decline in physical fitness from childhood to adulthood associated with increased obesity and insulin resistance in adults. Diabetes Care. 2009;32:683–687. doi: 10.2337/dc08-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 82.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nimptsch K, Giovannucci E, Willett WC, Fuchs CS, Wei EK, Wu K. Body fatness during childhood and adolescence, adult height, and risk of colorectal adenoma in women. Cancer Prev Res. 2011;4:1710–1718. doi: 10.1158/1940-6207.CAPR-11-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.International Agency for Research on Cancer (IARC) Weight Control and Physical Activity. IARC Handbook on Cancer Prevention. Vol. 6. Lyon, France: IARC; 2002. [Google Scholar]
- 85.World Cancer Research Fund/American Institute for Cancer Research. Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2009. [Google Scholar]
- 86.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126:692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 88.Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.US Department of Health and Human Services. The Surgeon General’s Vision for a Healthy and Fit Nation. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2010. [Google Scholar]
- 90.Larsson SC, Wolk A. Excess body fatness: an important cause of most cancers. Lancet. 2008;371:536–537. doi: 10.1016/S0140-6736(08)60247-0. [DOI] [PubMed] [Google Scholar]
- 91.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 92.McTiernan A. Physical Activity, Dietary Calorie Restriction, and Cancer Energy Balance and Cancer. New York: Springer; 2010. [Google Scholar]
- 93.Endogenous Hormones Breast Cancer Collaborative Group. Key TJ, Appelby PN, Reeves GK, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk [serial online] Diabetol Metab Syndr. 2011;3:12. doi: 10.1186/1758-5996-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152:2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 96.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 97.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 98.Coyle YM. Lifestyle, genes, and cancer. Methods Mol Biol. 2009;472:25–56. doi: 10.1007/978-1-60327-492-0_2. [DOI] [PubMed] [Google Scholar]
- 99.Hursting SD, Lashinger LM, Colbert LH, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Targets. 2007;7:484–491. doi: 10.2174/156800907781386623. [DOI] [PubMed] [Google Scholar]
- 100.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41:207–215. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clinic Proc. 2010;85:1138–1141. doi: 10.4065/mcp.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 103.Brawer R, Brisbon N, Plumb J. Obesity and cancer. Prim Care. 2009;36:509–531. doi: 10.1016/j.pop.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 104.US Department of Health and Human Services. The Surgeon General’s Call to Action to Support Breastfeeding. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2011. [Google Scholar]
- 105.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 106.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in US occupation-related physical activity and their associations with obesity [serial online] PLoS One. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monasta L, Batty GD, Cattaneo A, et al. Early life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010;11:695–708. doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 108.National Prevention Council. National Prevention Strategy. Washington, DC: US Department of Health and Human Services, Office of the Surgeon General; 2011. [Google Scholar]
- 109.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Accessed January 27, 2012]. State Indicator Report on Fruits and Vegetables, 2009. Available at: http://www.fruitsandveggiesmatter.gov/indicatorreport. [Google Scholar]
- 110.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [Accessed January 27, 2012]. State Indicator Report on Physical Activity, 2010. Available at: http://www.cdc.gov/physicalactivity/downloads/PA_State_Indicator_Report_2010.pdf. [Google Scholar]
- 111.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Accessed January 27, 2012]. Children’s Food Environment State Indicator Report, 2011. Available at: www.cdc.gov/obesity/downloads/ChildrensFoodEnvironment.pdf. [Google Scholar]
- 112.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Accessed January 27, 2012]. Breastfeeding Report Card—United States, 2011. Available at: http://www.cdc.gov/breastfeeding/data/reportcard3.htm. [Google Scholar]
- 113.Mays GP, Smith SA. Evidence links increases in public health spending to declines in preventable deaths. Health Aff. 2011;30:1585–1593. doi: 10.1377/hlthaff.2011.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 115.Finkelstein EA, DiBonaventura M, Burgess SM, Hale BC. The costs of obesity in the workplace. J Occup Environ Med. 2010;52:971–976. doi: 10.1097/JOM.0b013e3181f274d2. [DOI] [PubMed] [Google Scholar]
- 116.Pratt M, Macera CA, Wang G. Higher direct medical costs associated with physical inactivity. Phys Sportsmed. 2000;28:63–70. doi: 10.3810/psm.2000.10.1237. [DOI] [PubMed] [Google Scholar]
- 117.Wang F, McDonald T, Champagne LJ, Edington DW. Relationship of body mass index and physical activity to health care costs among employees. J Occup Environ Med. 2004;46:428–436. doi: 10.1097/01.jom.0000126022.25149.bf. [DOI] [PubMed] [Google Scholar]
- 118.Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc. 1999;31:S663–S667. doi: 10.1097/00005768-199911001-00026. [DOI] [PubMed] [Google Scholar]
- 119.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2. 2008;148:1–23. [PubMed] [Google Scholar]
- 120.Howlader N, Ries LA, Stinchcomb DG, Edwards BK. The impact of underreported Veterans Affairs data on national cancer statistics: analysis using population-based SEER registries. J Natl Cancer Inst. 2009;101:533–536. doi: 10.1093/jnci/djn517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15:188–188. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 122.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 123.Stewart AW, Jackson RT, Ford MA, Beaglehole R. Underestimation of relative weight by use of self-reported height and weight. Am J Epidemiol. 1987;125:122–126. doi: 10.1093/oxfordjournals.aje.a114494. [DOI] [PubMed] [Google Scholar]
- 124.Rowland M. Self-reported weight and height. Am J Clin Nutr. 1990;52:1125–1133. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]
- 125.Merrill RM, Richardson JS. Validity of self-reported height, weight, and body mass index: findings from the National Health and Nutrition Examination Survey, 2001–2006 [serial online] Prev Chronic Dis. 2009;6:A121. [PMC free article] [PubMed] [Google Scholar]
- 126.Bowlin SJ, Morrill BD, Nafziger AN, Lewis C, Pearson TA. Reliability and changes in validity of self-reported cardiovascular disease risk factors using dual response: the Behavioral Risk Factor Survey. J Clin Epidemiol. 1996;49:511–517. doi: 10.1016/0895-4356(96)00010-8. [DOI] [PubMed] [Google Scholar]
- 127.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer [serial online] PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 129.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 130.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 131.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100:590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frieden TR, Dietz W, Collins J. Reducing childhood obesity through policy change: acting now to prevent obesity. Health Aff. 2010;29:357–363. doi: 10.1377/hlthaff.2010.0039. [DOI] [PubMed] [Google Scholar]
- 133.Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pate RR. A national physical activity plan for the United States. J Phys Act Health. 2009;6(suppl 2):S157–S158. [PubMed] [Google Scholar]
- 135.Wingo PA, Howe HL, Thun MJ, et al. A national framework for cancer surveillance in the United States. Cancer Causes Control. 2005;16:151–170. doi: 10.1007/s10552-004-3487-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.