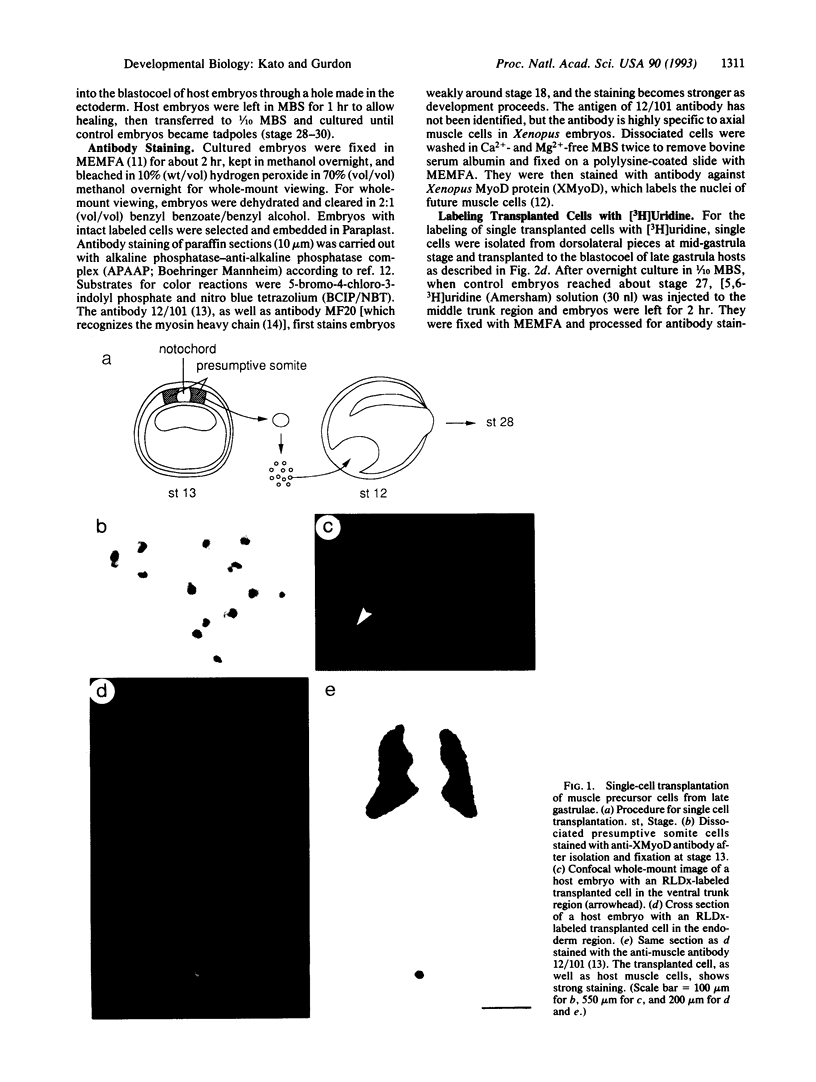
Abstract
We have used a single-cell transplantation technique to find out whether there is a stage in development when a single cell can reach and maintain its differentiated state in the absence of its neighbors. Muscle precursor cells from early, mid-, and late gastrula stages of Xenopus laevis embryos were isolated and transplanted singly into the ventral region of late gastrula hosts. Single cells from late gastrulae differentiated into muscle when surrounded by nonmuscle cells. Similar cells from early or mid-gastrulae did not, unless they were transplanted as a group of adjacent cells taken from the same region of an embryo. These results show that single embryonic cells in a tissue can complete their differentiation without interacting with their normal neighbors and that, in the case of Xenopus muscle precursor cells, they acquire this capacity at the late gastrula stage. Our results also suggest that, in addition to mesoderm induction, further cell interactions during gastrulation are required for Xenopus muscle cell differentiation.
Full text
PDF

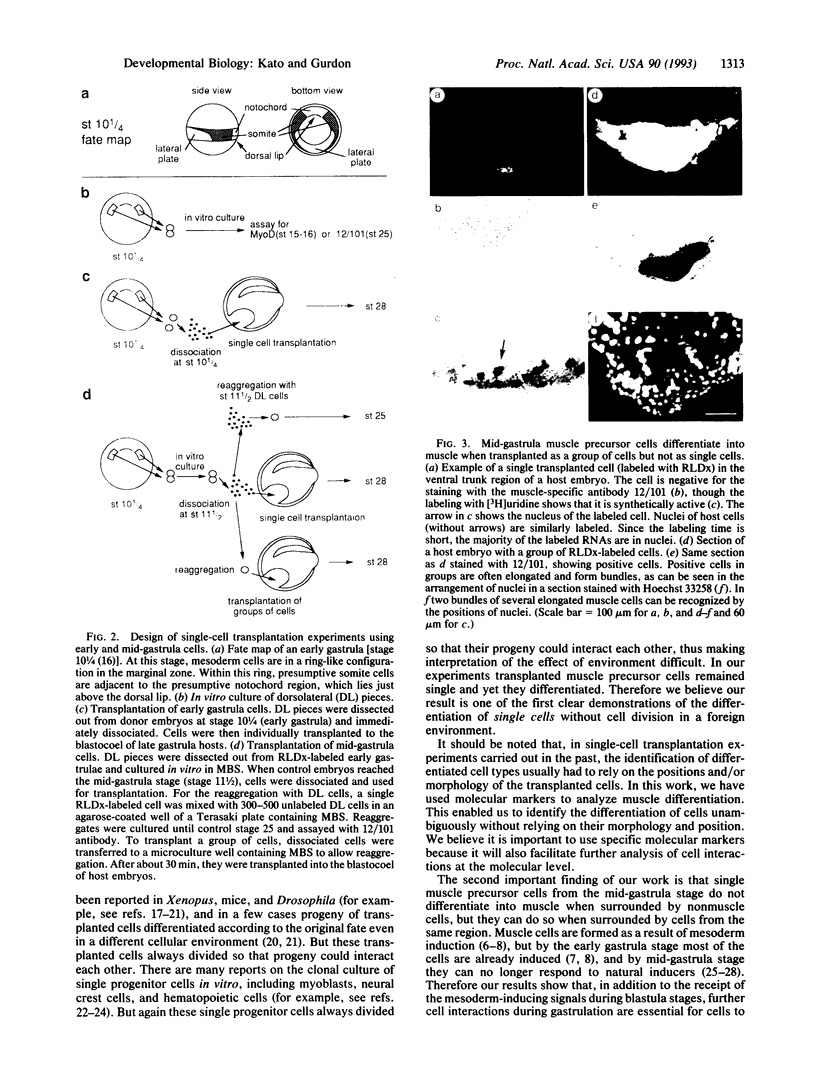

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroffio A., Dupin E., Le Douarin N. M. Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development. 1991 May;112(1):301–305. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Hughes S. M. Cell lineage in vertebrate development. Curr Opin Cell Biol. 1990 Dec;2(6):981–985. doi: 10.1016/0955-0674(90)90145-5. [DOI] [PubMed] [Google Scholar]
- Brivanlou A. H., Harland R. M. Expression of an engrailed-related protein is induced in the anterior neural ectoderm of early Xenopus embryos. Development. 1989 Jul;106(3):611–617. doi: 10.1242/dev.106.3.611. [DOI] [PubMed] [Google Scholar]
- Dale L., Slack J. M. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987 Jun;100(2):279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Frank D., Harland R. M. Transient expression of XMyoD in non-somitic mesoderm of Xenopus gastrulae. Development. 1991 Dec;113(4):1387–1393. doi: 10.1242/dev.113.4.1387. [DOI] [PubMed] [Google Scholar]
- Gillespie L. L., Paterno G. D., Slack J. M. Analysis of competence: receptors for fibroblast growth factor in early Xenopus embryos. Development. 1989 May;106(1):203–208. doi: 10.1242/dev.106.1.203. [DOI] [PubMed] [Google Scholar]
- Godsave S. F., Slack J. M. Clonal analysis of mesoderm induction in Xenopus laevis. Dev Biol. 1989 Aug;134(2):486–490. doi: 10.1016/0012-1606(89)90122-x. [DOI] [PubMed] [Google Scholar]
- Grainger R. M., Gurdon J. B. Loss of competence in amphibian induction can take place in single nondividing cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1900–1904. doi: 10.1073/pnas.86.6.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. A community effect in animal development. Nature. 1988 Dec 22;336(6201):772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Fairman S., Mohun T. J., Brennan S. Activation of muscle-specific actin genes in Xenopus development by an induction between animal and vegetal cells of a blastula. Cell. 1985 Jul;41(3):913–922. doi: 10.1016/s0092-8674(85)80072-6. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. The generation of diversity and pattern in animal development. Cell. 1992 Jan 24;68(2):185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Tiller E., Roberts J., Kato K. A community effect in muscle development. Curr Biol. 1993 Jan;3(1):1–11. doi: 10.1016/0960-9822(93)90139-f. [DOI] [PubMed] [Google Scholar]
- Heasman J., Wylie C. C., Hausen P., Smith J. C. Fates and states of determination of single vegetal pole blastomeres of X. laevis. Cell. 1984 May;37(1):185–194. doi: 10.1016/0092-8674(84)90314-3. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Gurdon J. B. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990 Sep 13;347(6289):197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B., Dilworth S. M. Expression of XMyoD protein in early Xenopus laevis embryos. Development. 1992 Jan;114(1):31–38. doi: 10.1242/dev.114.1.31. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989 Nov;8(11):3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development. 1991 Feb;111(2):551–560. doi: 10.1242/dev.111.2.551. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Xu W. L. States of determination of single cells transplanted between 512-cell Xenopus embryos. Dev Biol. 1989 Jan;131(1):119–125. doi: 10.1016/s0012-1606(89)80043-0. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Melton D. A. Diffusible factors in vertebrate embryonic induction. Cell. 1992 Jan 24;68(2):257–270. doi: 10.1016/0092-8674(92)90469-s. [DOI] [PubMed] [Google Scholar]
- Keller R. E. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev Biol. 1976 Jul 1;51(1):118–137. doi: 10.1016/0012-1606(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Kintner C. R., Brockes J. P. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984 Mar 1;308(5954):67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Muntz L. Myogenesis in the trunk and leg during development of the tadpole of Xenopus laevis (Daudin 1802). J Embryol Exp Morphol. 1975 Jun;33(3):757–774. [PubMed] [Google Scholar]
- New H. V., Howes G., Smith J. C. Inductive interactions in early embryonic development. Curr Opin Genet Dev. 1991 Aug;1(2):196–203. doi: 10.1016/s0959-437x(05)80070-x. [DOI] [PubMed] [Google Scholar]
- Schnabel R. Early determinative events in Caenorhabditis elegans. Curr Opin Genet Dev. 1991 Aug;1(2):179–184. doi: 10.1016/s0959-437x(05)80067-x. [DOI] [PubMed] [Google Scholar]
- Singh L., Matsukuma S., Jones K. W. The use of Y-chromosome-specific repeated DNA sequences in the analysis of testis development in an XX/XY mouse. Development. 1987;101 (Suppl):143–149. doi: 10.1242/dev.101.Supplement.143. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Dale L., Slack J. M. Cell lineage labels and region-specific markers in the analysis of inductive interactions. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):317–331. [PubMed] [Google Scholar]
- Stüttem I., Campos-Ortega J. A. Cell commitment and cell interactions in the ectoderm of Drosophila melanogaster. Dev Suppl. 1991;Suppl 2:39–46. [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Single-cell origin of mouse hemopoietic colonies expressing multiple lineages in variable combinations. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6689–6693. doi: 10.1073/pnas.80.21.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes K., Yaqoob M., Smith J. C. Mesoderm induction in Xenopus laevis: responding cells must be in contact for mesoderm formation but suppression of epidermal differentiation can occur in single cells. Development. 1988 Dec;104(4):609–618. doi: 10.1242/dev.104.4.609. [DOI] [PubMed] [Google Scholar]
- Technau G. M. A single cell approach to problems of cell lineage and commitment during embryogenesis of Drosophila melanogaster. Development. 1987 May;100(1):1–12. doi: 10.1242/dev.100.1.1. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]