Abstract
Werner syndrome (WS) is an adult onset segmental progeroid syndrome caused by mutations in the WRN gene. The WRN gene encodes a 180 kDa nuclear protein that possesses helicase and exonuclease activities. The absence of WRN protein leads to abnormalities in various DNA metabolic pathways such as DNA repair, replication and telomere maintenance. Individuals with WS generally develop normally until the third decade of life, when premature aging phenotypes and a series of age-related disorders begin to manifest. In Japan, where a founder effect has been described, the frequency of Werner heterozygotes appears to be as high as 1/180 in the general population. Due to the relatively non-specific nature of the symptoms and the lack of awareness of the condition, this disease may be under-diagnosed in other parts of the world. Genetic counseling of WS patients follows the path of other autosomal recessive disorders, with special attention needed for cancer surveillance in relatives. Molecular diagnosis of WS is made by nucleotide sequencing and, in some cases, protein analysis. It is also of potential interest to measure WRN activities in WS patients. More than 50 different disease-causing mutations in the WRN gene have been identified in WS patients from all over the world. All but one of these cases has mutations that result in the premature termination of the protein. Here we describe the clinical, molecular and biochemical characteristics of WS for use by medical professionals in a health care setting. Additional information is available through the International Registry of WS (http://www.wernersyndrome.org).
Introduction
Clinical characteristics of Werner syndrome
The clinical phenotype of Werner syndrome (WS) is best summarized as the early onset of an aged-appearance and age-related common disorders (Fig. 1) (Epstein et al. 1966; Goto 1997). WS patients usually develop normally until they reach the third decade of life. Generally, the first clinical sign is a lack of the pubertal growth spurt during the teen years. Patients frequently recall that they were of average height when they entered grade school, but were the shortest ones in their class when they graduated from high school. A clinical study of Japanese WS reported the median height of affected individuals was 142 cm (range 122–161 cm) which was 13 cm shorter than the general population (Goto 1997). Median body weight was 36 kg (range 19–52 kg) that was 20 kg less than the general population. In our study, 95% of the WS patients were reported to have short stature, assessed by the primary care physician taking the height of family members into consideration (Huang et al. 2006). In their 20s and 30s, patients begin to manifest skin atrophy, loss of hair and graying hair. Subcutaneous fat tends to deposit on the trunk and combined with osteoporosis of the limbs, patients exhibit a stocky appearance. Some patients may present with a high-pitched voice and flat feet.
Fig. 1.
Werner syndrome patients. Japanese-American WS patient at ages 15 (top left) and 48 (top right) was originally reported in Epstein et al. (1966) showed early graying and thinning of hair, skin atrophy, loss of cutaneous fat and general aged feature. She carried a common Japanese WRN mutation that skips exon 26. A Caucasian WS patients, at age 8 (bottom left) and 36 (bottom right) developed bilateral cataracts, thinning of hair, atrophic skin and thin limbs (Hisama et al. 2006). She carried a homozygous deletion mutation in the helicase domain of WRN gene
Subsequently, WS patients develop common age-related disorders. Our recent survey of WS patients with a molecularly confirmed diagnosis revealed that the prevalence of cataracts was 100% (87/87) (Huang et al. 2006). The prevalence of osteoporosis at the time of diagnosis was 91%, hypogonadism 80%, diabetes mellitus 71%, neoplasms 43% and atherosclerosis 40% (Huang et al. 2006). These numbers depend on the age of the patient when clinical reports are made and how rigorously patients were examined. The chronological order of the onset of these complications is similar among Caucasian and Japanese WS patients (Epstein et al. 1966; Goto 1997).
Median age of death in the most recent study was 54 years (Huang et al. 2006). In previous studies, the most common cause of death was malignancy and myocardial infarction (Epstein et al. 1966; Goto 1997). Although these are also two common causes of death in the general population, unique characteristics of the cancers are observed in WS patients. Strikingly, the ratio of cancers of epithelial origin and sarcomas of mesenchymal origins is 1:1 in WS patients, whereas this ratio is approximately 10:1 in general population (Goto et al. 1996). Review of pathological studies of these malignancies revealed unusual primary sites for cancers in WS patients. For example, melanomas in WS patients are of the acral lentigenous type in the mucosas, and are unrelated to sun exposure (Goto et al. 1996). The primary sites of osteosarcomas in WS patients are more likely to be in the lower extremities, whereas these are more common in the upper extremities in the general population (Ishikawa et al. 2000).
There are other differences that have been noted between WS patients and normal elders. Atherosclerosis exhibits unique characteristics in WS patients. Atherosclerotic lesions are more extensive in arterioles. Skin ulcers around the ankles and elbows that are far more severe than those expected from diabetes mellitus are common in WS. The incidence of dementia of Alzheimer type is believed to be not increased in WS subjects (Postiglione et al. 1996; Mori et al. 2003; Sumi 1985). While in the general population, osteoporosis has a more pronounced effect on vertebrae in the general population, long bones, particularly those of the legs, tend to be more affected by osteoporosis in WS patients (Epstein et al. 1966; Rubin et al. 1992) although more studies are needed to be conclusive.
WRN gene product and WRN mutations
WS is caused by the mutations at the WRN locus on chromosome 8. The WRN gene spans more than 250 kb and consists of 35 exons, 34 of which are coding exons (Yu et al. 1997). WRN gene encodes one of the RecQ helicase family proteins, WRN, which has ATPase, helicase, exonuclease and single-stranded DNA annealing activities. WRN is ubiquitously expressed in tissues. Helicases separate complementary strands of nucleic acids in a reaction coupled to NTP hydrolysis. RecQ helicases have a common helicase domain with seven conserved motifs, which bind and hydrolyze ATP. WRN helicase activity is structure-specific and requires energy from ATP hydrolysis to unwind complementary strands of DNA with a 3’–’5 polarity. Unlike other RecQ helicases, WRN also has intrinsic 3’–5’ exonuclease activity. The preferred DNA substrates of WRN helicase activity, which resemble DNA metabolic intermediates include forked and Xap structures (intermediates in DNA replication and repair), bubble structures (intermediates in DNA repair and transcription), D-loop and Holliday junction structures (intermediates in DNA recombination) and G-quadruplex DNA and D-loop structures (associated with telomere DNA) (Opresko et al. 2003). Interestingly, the WRN helicase and exonuclease activities can also work in a coordinated manner on the same substrate. These two catalytic activities of WRN collectively provide access for proteins to the template during replication, recombination, and repair. Consistent with this notion, biochemical and genetic evidence suggest that WRN plays important roles in DNA repair, homologous recombination, replication, and in telomere maintenance (Bohr 2005).
A number of the interacting proteins of the WRN helicase have recently been identiWed. Considering the biochemical activities of WRN and of WRN-associated proteins, it is conceivable that in vivo WRN participates in many aspects of DNA metabolism, including DNA replication, recombination, and repair pathways or in a combination of these pathways, such as recombination during replication (Bohr 2005).
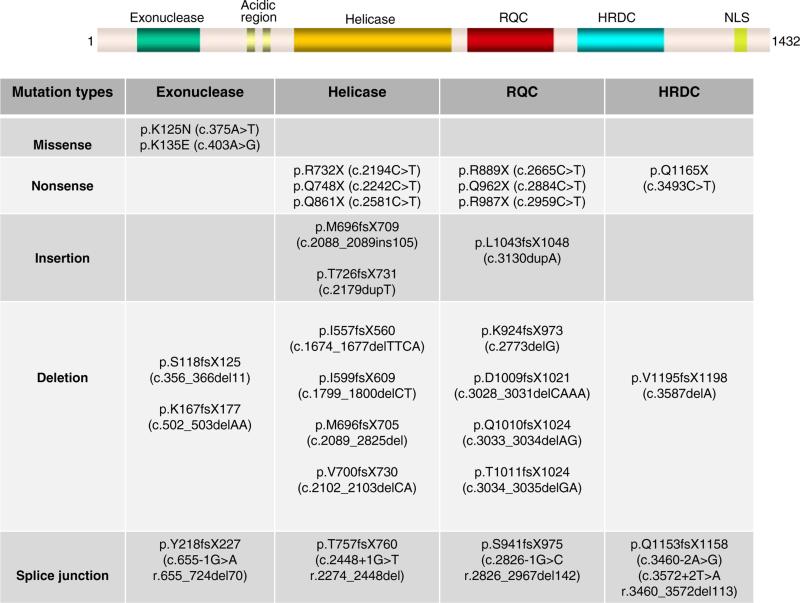
More than 50 different WRN disease mutations have been reported thus far (Goto et al. 1997; Oshima et al. 1996; Uhrhammer et al. 2006; Yu et al. 1997). The types of mutations observed in WRN patients are; (a) nonsense mutations that change an amino acid codon to a stop codon and cause the termination of protein translation; (b) insertions and/or deletions, which lead to reading frameshift and subsequent termination of protein translation; (c) substitutions at splice junctions that cause the skipping of exons and a subsequent frameshift; (d) missense mutations that cause the amino acid change in the protein (Huang et al. 2006). The mutations found in WS patients are summarized in Fig. 2. The truncation of WRN due to the nonsense mutations, insertions and/or deletions and splice mutations described above, cause the elimination of the nuclear localization signal (NLS) at the C-terminal end of the protein (Suzuki et al. 2001; Yamabe et al. 1997). Although almost all mutations reported so far in WS patients have generated a truncated protein, recently, two missense mutations (double missense mutations in a single subject) have been identified in exonuclease domain (Huang et al. 2006) (Fig. 2). These mutations appear to affect the stability of the protein rather than enzymatic activities.
Fig. 2.
Summary of WRN mutations found in WS patients. WRN protein is shown schematically. RQC, RecQ helicase conserved region; HRDC, helicase, RNaseD, C-terminal conserved region; NLS, nuclear localization signal. The amino acid notations of the mutations are located throughout the functional motifs of the WRN gene. Nucleotide notations are given in the parentheses. fs frame shift, del deletion, ins insertion, X stop codon
Disease mutations are found across the WRN gene. The most frequent mutation is the nonsense mutation at amino acid 369 in exon 9, Arg (CGA)-to-stop codon (TGA). This change accounts for approximately 25% of the mutations in non-Japanese and in Japanese WS cases (Goto et al. 1997; Huang et al. 2006). The most frequent Japanese WRN mutation is c.3139-1G>C that causes the skipping of exon 26. This change accounts for approximately 67% of the Japanese WRN mutations and is thought to be due to a founder effect (Goto et al. 1997; Satoh et al. 1999).
Genetic counseling
WS is an autosomal recessive genetic disease. Therefore patients with an established diagnosis of WS, along with their parents and siblings, should be referred for genetic counseling to ensure early identification and treatment of syndrome-associated manifestations. Construction and evaluation of a pedigree will allow the identification of family members who may be affected or at risk of being carriers of a WRN mutation.
Clinical assessment and diagnostic criteria
Diagnostic criteria for WS were originally proposed in 1994 (Nakura et al. 1994) to establish a “definite,” “probable,” or “possible” diagnosis of WS (Table 1). With the advent and increased availability of molecular diagnosis, sequencing of the WRN gene has replaced urinary hyaluronic acid testing for diagnostic purposes. Parental consanguinity or affected siblings are no longer used for diagnosis. Clinical diagnosis is currently based on the remaining four cardinal signs (cataracts, skin change, short stature, and graying or loss of hair) and additional signs. These cardinal signs are seen in more than 95% of the molecularly diagnosed cases (Huang et al. 2006). Additional signs are seen in 90% (osteroporosis, voice change) to 40% (atherosclerosis).
Table 1.
Clinical diagnostic criteria for Werner syndrome
| Major signs and symptoms (onset over 10 years old) |
| Cataracts (bilateral) |
| Characteristic dermatological pathology (tight skin, atrophic skin, pigmentary alterations, ulceration, hyperkeratosis, regional subcutaneous atrophy) and characteristic facies (‘bird-like’ face) |
| Short stature |
| Premature greying and/or thinning of scalp hair |
| [Parental consanguinity (third cousin or closer) or aVected sibling] |
| [Positive 24-h urinary hyaluronic acid test when available] |
| Additional signs and symptoms |
| Type 2 diabetes mellitus |
| Hypogonadism (secondary sexual underdevelopment, diminished fertility, testicular or ovarian atrophy) |
| Osteoporosis |
| Osteosclerosis of distal phalanges of fingers and/or toes (x-ray diagnosis) |
| Soft tissue calcification |
| Evidence of premature atherosclerosis (e.g., history of myocardial infarction) |
| Neoplasms: mesenchymal (i.e. sarcomas), rare (unusual), or multiple |
| Abnormal voice (high-pitched, squeaky, or hoarse) |
| Flat feet |
| Definite diagnosis |
| All the major signs and two additional signs |
| Probable diagnosis |
| The first three major signs and any two others |
| Possible diagnosis |
| Either cataracts or dermatological alterations and any four others |
| Exclusion of diagnosis |
| Onset of signs and symptoms before adolescence (except short stature) |
Differential diagnosis
The differential diagnosis of WS includes other progeroid syndromes with and without increased cancer susceptibility. It should be emphasized that WS is an adult onset disease and, except for the short stature, the aged appearance and age-related common disorders start after sexual maturity.
Laminopathies are a group of disorders caused by mutations of the type V nuclear intermediate Wlaments, lamin A/C, encoded by the LMNA gene (Broers et al. 2006). Increasing numbers of LMNA mutations have been reported in various types of muscular dystrophies, cardiomyopathies, lipodystrophies and progeroid syndromes. Overlapping phenotypes of these conditions are not uncommon. A subset of WS patients do not show mutations at the WRN locus, but show heterozygous amino acid substitutions in the heptad repeat region of LMNA that is called atypical WS. In the International Registry of WS, (http://www.wernersyndrome.org; it is established at the Department of Pathology, University of Washington, Seattle, WA in 1988, and has been providing diagnostic criteria for WRN and contact information for a central repository of WS data, research and materials), approximately 15% of clinically diagnosed WS with no WRN mutation (patients with “atypical WS”) carried heterozygous missense LMNA mutations that have dominant effect (Chen et al. 2003). Patients affected with atypical WS seem to have early onset (early 20s) of aging phenotypes and show an accelerated rate of progression than those who are affected by classic WS (WRN gene mutant). Absence of bilateral ocular cataracts and diabetes is also common.
Mandibuloacral dysplasia (MAD) is a rare autosomal recessive disorder characterized by short stature, beaked nose, small recessed chin, short fingers, thin, slanted shoulders and hyperpigmented skin. MAD is part of the differential diagnosis of WS as some cases of MAD are caused by LMNA mutations (Novelli et al. 2002). Characteristic facial features and the absence of bilateral cataracts would differentiate MAD from WS.
Hutchinson–Gilford progeria syndrome (HGPS) is a childhood onset progeria caused by a unique splicing mutation of the LMNA gene (Eriksson et al. 2003). HGPS patients develop an aged appearance (characteristic facial features, alopecia, loss of subcutaneous fat, and short stature) by their second to third year of life. Motor and mental development is normal. Individuals with HGPS develop severe generalized atherosclerosis. Complications of cardiovascular disease usually lead to death between the first and second decade of life with the median age of death being 13 years. Progeria patients generally do not develop cancers by the time of their death. HGPS can be distinguished from WS by the age of onset, characteristic facial features and general appearance.
Rothmund–Thomson syndrome (RTS) is another cancer predisposition syndrome caused by RecQ helicase genes, RECQL4 (Mohaghegh and Hickson 2002). Although RTS patients may display progeroid features, it is a childhood onset disorder. Additionally, RTS patients may display distinctive cutaneous features such as telangiectasias, scaling and dyschromia.
Finally, type 2 diabetes mellitus with vascular complications may resemble WS. Scleroderma and mixed connective tissue disorders may present skin features similar to WS. Scleroderma does not seem to involve other accelerated aging features, and has specific gastrointestinal, pulmonary, renal and cardiac manifestations. Isolated juvenile cataracts and myotonic dystrophy do not have other features of WS.
Counseling
Since WS is inherited in an autosomal recessive manner, the proband's parents are obligate heterozygotes for a disease-causing mutation. As with all recessive conditions, the siblings of an affected individual have a 25% chance of being affected, a 50% chance of being asymptomatic carriers, and a 25% chance of being non-carriers. Heterozygotes of a WRN mutation are asymptomatic and do not appear to be at increased risk for WS-speciWc symptoms.
The risk for the offspring of an individual with WS to develop the disease is negligible unless the affected individual and his/her reproductive partner are consanguineous. In Japan, where the frequency of heterozygotes may be as high as 1/150, the risk of WS in the offspring of an affected patient is still lower than 1/500. Siblings of individuals with WS may be affected and may wish to be tested. Siblings old enough to be clearly asymptomatic may also choose to be tested for carrier status.
We have not been able to quantitatively assess the degree of hypogonadism of WS. There is a pedigree in which compound heterozygous status of a deceased female subject was inferred from the analysis of 2 apparently normal children (PCH pedigree in Huang et al. 2006), suggesting that some WS patients could have a near-normal level of reproduction.
Clinical management and surveillance
Patients should be managed by a multidisciplinary team, as symptoms of WS span several disciplines. All practitioners should encourage lifestyles that include smoking avoidance, regular exercise, and weight control to reduce the risk of atherosclerosis.
Initial diagnosis
Recommendations for the clinical diagnosis of WS include screening for type 2 diabetes mellitus, lipid profiles, a physical examination for cancers common in WS, an ophthalmologic examination (including slit lamp examination), and an extensive skin examination for typical skin findings, especially early ulcerations of the feet (with careful attention to nail beds and soles of feet for acral lentiginous melanoma). Additional screening includes an MRI if neurologic symptoms, such as chronic headaches suggesting meningioma, are present. Finally, an assessment of coping and psychological fitness in light of prognosis and reproductive advice regarding the rapid decline of fertility is suggested.
Treatment of manifestations
There is no cure for WS, only treatment of symptoms as they occur. Primary recommendations include aggressive treatment of skin ulcers with standard or novel techniques and strict control of type 2 diabetes mellitus. With regards to diabetes, favorable results have been reported with the use of pioglitazone (Yeong and Yang 2004; Yokote et al. 2004). The use of cholesterol-lowering drugs may be considered if the patient's lipid profile is abnormal, but muscle atrophy may complicate this treatment. Other recommendations include surgical treatment of ocular cataracts and treatment of malignancies in a standard fashion.
Surveillance
Annual screening for type 2 diabetes mellitus, an annual lipid profile, and regular physical exams for malignancies common in WS and other skin manifestations are recommended for Werner patients. Also recommended are annual ophthalmologic examinations for cataracts and the monitor for the cardiovascular diseases with particular attention to signs of angina.
Molecular diagnosis
The molecular diagnosis of WS combines nucleotide sequencing with Western blot (WB) analysis (Huang et al. 2006). RT-PCR sequencing is used for the initial screening as it detects splicing and other unique mutations common in WS. We identified a homozygous mutation in intron 18 that creates a new exon, resulting in a 105 bp insertion between exons 18 and 19 at the mRNA level, which would not have been detected by standard genomic PCR sequencing (Oshima et al. 1996). There is also an extensive deletion that spans from exon 19 to 23 identified in 3 European cases which would be easily missed if it were to present as a heterozygous mutation (Oshima et al. 1996). The mutations identified by RT-PCR are confirmed with genomic PCR sequencing. Thus, it is useful to use both RT-PCR and genomic PCR sequencing methods, which have previously been described along with the primer sequencing and PCR conditions, for molecular diagnosis of WS (Oshima et al. 1996; Yu et al. 1997). These methods are currently used by the International Registry of WS.
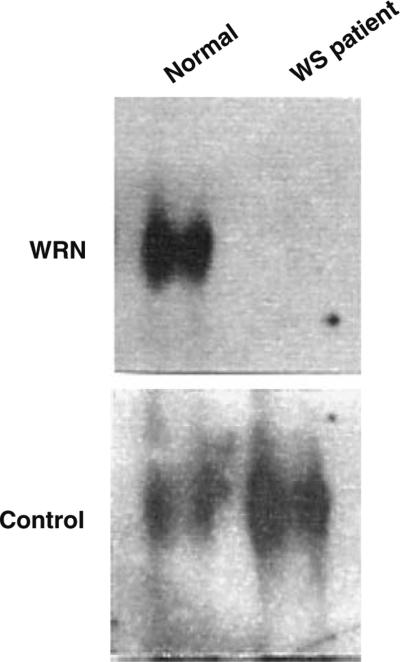
The absence of normal WRN protein is further confirmed by WB analysis. This method examines the WRN gene at the protein level. Conditions for the WB analysis methods have been described before (Gray et al. 1998; Wang et al. 1999). Since mutant mRNAs coding for the truncated proteins usually undergo rapid degradation, the normal WRN protein is not detectable in the samples from the WS patients by WB analysis (Goto et al. 1999) (Fig. 3). To date, 58 patient cell samples that carry WRN mutations were also examined for WRN protein, using the antibody against C-terminal region of the protein. In all cases, WRN proteins were not detectable. Depending on the site of mutations and location of the epitope of the antibody, the mutant WRN protein may be detected with a different size. In Japan, where WS is relatively common and one type of mutation predominates, WB analysis were reported to have been employed as an effective screening methods (Goto et al. 1999). RT-PCR, and WB analysis require live cell materials from patients, typically fibroblasts or lymphoblastoid cell lines, which may not be practical in some cases. When only DNA is available, genomic PCR sequencing is employed as a sole method, with the caution that it is not as sensitive as the other methodologies.
Fig. 3.
Western blot analysis of WRN protein. Cells derived from the WS patient expressed no detectable WRN protein of the correct size observed in the cells from the normal individual. Control bands are shown here to ensure the equal loading of the protein
At the present time, it may be possible for highly skilled researchers to demonstrate the feasibility of enzyme assays in Werner LCLs with known mutations, using, for example, immunoprecipitated lysates (Moser et al. 2000). However, unlike lysosomal enzymes, there are no substrates that are specific only to the WRN helicase or exonuclease. There are no commercially available anti-WRN antibodies known to be specific enough for this purpose. Moreover, WRN protein is highly unstable outside of the nuclei. We therefore do not recommend using enzyme assays of LCLs for diagnostic purposes without extensive validation.
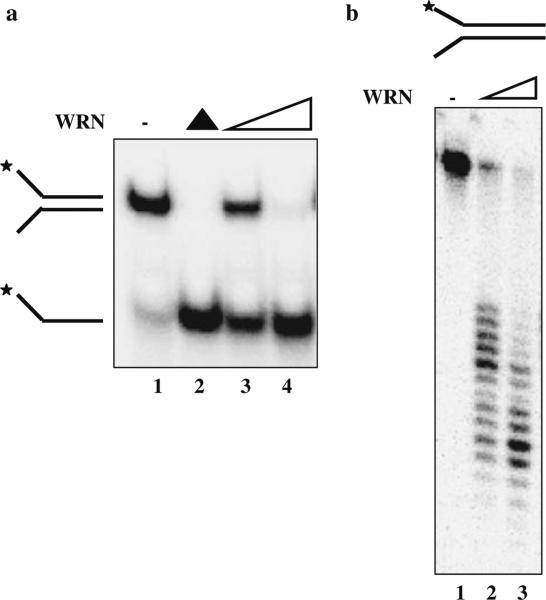
On the other hand, enzyme activities can be reliably measured in vitro using recombinant proteins (Fig. 4a, b). This requires recombinant protein production in eukaryotic cells followed by affinity purification and radiolabeled biochemical assays. There also are a number of polymorphisms in the coding region whose significance is not yet understood. Therefore it may not be suited for the routine clinical molecular diagnosis at this time. When we identify an alteration with unknown significance, however, recombinant protein assays become necessary to conclude whether the changes are pathogenic. The International Registry of WS has been conducting these studies as reflex tests on a research basis. The approach depends on the location and type of the mutation. Computational structural analysis, although not diagnostic, has been helpful to guide us to the most likely mechanism of missense mutations (Huang et al. 2006).
Fig. 4.
a WRN helicase activity. WRN protein (3 nM, lane 2 and 6 nM, lane 3) was incubated with a forked DNA substrate for 15 min at 37°C. Reaction products were analyzed on a 12% native polyacrylamide gel. Lane 1 forked DNA substrate, lane 4 heat-denatured substrate. b WRN exonuclease activity on a 34 bp forked substrate. Reactions contained 4 or 8 nM WRN (lanes 2 or 3, respectively) and were incubated with 0.5 nM DNA substrate for 15 min at 37°C. Lane 1 DNA substrate. Products were heat-denatured for 5 min at 95°C, analyzed on a 14% denaturing polyacrylamide gel and visualized using a PhosphorImager
Development of enzyme assays is particularly important because the possibility exists that a single amino acid substitution in WRN may cause the disease without a change in the WB analysis. While missense disease mutations that abolish enzymatic activities mutations have not yet been reported, there are experimentally generated mutations that abolish helicase or exonuclease activities (Gray et al. 1998; Wang et al. 1999). Double missense mutations identified in one case did alter WRN protein stability, but not enzymatic activity. Reasons why we have not identified such diseases mutations could be because (1) WS is rare and we simply have not come across them yet, (2) missense mutations that abolish enzyme activities may be more toxic than null mutations and the patients may not survive beyond childhood, or (3) such mutations may cause phenotypes different from the WS and therefore those cases are not screened for the WRN gene. We recently showed that site directed mutation of lysine at codon 1016 in the RQC motif significantly reduces helicase activity and the binding activities of various DNA substrates (Lee et al. 2005). Therefore, single mutations in genes encoding WRN itself or its interacting partners such as replication protein A, PCNA, Ku, and DNA topoisomerase I could be responsible for some of the patients who meet the clinical and diagnostic criteria for WRN or those who present with atypical features. Analysis of helicase and exonuclease activities in recombinant proteins will continue to be instrumental to confirm the clinical diagnosis of WS (Fig. 4a, b). As such, developing reliable biochemical analyses using white blood cells appears to be an interesting approach of clinical potential.
WS and cancer
WS is also characterized by a high predisposition to various cancer types, in particular mesenchymal sarcomas, such as soft tissue sarcoma and malignant melanoma, and epithelial tumors (Futami et al. 2008). To our knowledge, the specific somatic mutation in the WRN gene, which causes the specific cancer type, has not yet been identified. Several epidemiologic studies have been done searching the single nucleotide polymorphisms (SNPs) of WRN. It was shown that WRN Cys1367Arg SNP is associated with increased breast cancer risk in German familial breast cancer patients (Wirtenberger et al. 2006). However, this SNP did not contribute to the breast cancer development in Taiwanese population, indicating the racial differences in SNPs in different populations (Ding et al. 2007). The finding of both WRN Cys1367Arg and p53 Pro72 in individuals increased the breast cancer risk compared to the single polymorphisms (Wirtenberger et al. 2006). WRN Cys1367Arg is located near the NLS in the C-terminal of WRN gene, which is also p53 binding site. Wirtenberger et al. (2006) suggested that WRN Cys1367Arg might affect the binding of p53 to WRN and that interferes with the apoptotic function of p53 and that might the cause of the increased breast cancer phenotype.
WRN Cys1367Arg SNP is previously described as a protective factor of some diseases associated with WS like myocardial infarction and type2 diabetes mellitus (Ye et al. 1997; Hirai et al. 2005). Recently, WRN Cys1367Arg has been shown to be a protective in soft tissue sarcomas, sarcomas with reciprocal chromosomal translocations and malignant fibrous histiocytoma (Nakayama et al. 2008). In addition, 1367Arg showed a significant protective affect against non-Hodgkin lymphoma in two patients (Shen et al. 2006). The location of WRN Cys1367Arg does not affect the localization of WRN to the nucleus although it finds near the NLS (Bohr et al. 2004). It has also been shown that the presence of this SNP exhibits little or no effect in the helicase and exonuclease activities of WRN (Bohr et al. 2004; Kamath-Loeb et al. 2004).
In various tumor types, the WRN gene is inactivated by CpG island promoter methylation (Agrelo et al. 2006). Promotor methylation and gene silencing of WRN have been shown in colon cancer, breast cancer, non-small cell lung, gastric tumors, and leukemia. The epigenetic inactivation of the WRN gene has important clinical relevance. Cancer cells where WRN is epigenetically silenced are very sensitive towards topoisomerase inhibitors and DNA-damaging agents. Importantly, this observation has been translated to colon cancer patients treated with the chemotherapeutic drug irinotecan (CPT-11), which has topoisomerase-inhibitor activity (Agrelo et al. 2006). The comparative survival results from WRN-hypermethylated (n = 45) versus unmethylated (n = 43) colorectal tumor samples, indicated that the median time for death of patients was 39.4 months for WRN methylated colon tumors (no expression of WRN) in contrast to 20.7 months for WRN unmethylated samples (Agrelo et al. 2006). Thus, WRN promoter methylation represents a predictor of increased survival in colon cancer patients treated with the topoisomerase inhibitor irinotecan. WS cells are also hypersensitive to camptothecin (CPT), an analog of irinotecan (Harrigan et al. 2007). The correlation between WRN methylation status and irinotecan response clearly needs to be independently confirmed by other investigators, as numerous genes undergo methylation-associated changes in the tumors (Esteller 2007). Future studies with different tumors types and different DNA damaging agents in epigenetically silenced WRN cells would be necessary to support these findings.
Conclusion
WS is a rare autosomal recessive disorder, characterized by early onset of numerous aging symptoms, including atherosclerosis, osteoporosis, type 2 diabetes mellitus and enhanced risk of rare cancers. Because of the relatively non-specific nature of the symptoms and several clinical features of the disease, understanding the clinical, molecular and biochemical characteristics of WS thoroughly, as we discussed in this review, will help to diagnose WS accurately. In addition, rather than using one technique for the diagnosis of WS, combining different methods mentioned in this review is also important for its diagnosis.
Acknowledgments
This work was partially supported by the Intramural Research Program of the NIH, National Institute on Aging, and by the NIH grant CA78088 (to J.O).
Contributor Information
Meltem Muftuoglu, Laboratory of Molecular Gerontology, National Institute on Aging, NIH, 5600 Nathan Shock Dr, Baltimore, MD 21224, USA.
Junko Oshima, Department of Pathology, University of Washington, Seattle, WA 98195, USA.
Cayetano von Kobbe, Cancer Vaccines Group, Chimera Pharma (Bionostra Group), 28760 Tres Cantos, Madrid, Spain.
Wen-Hsing Cheng, Department of Nutrition and Food Science, University of Maryland, College Park, MD 20742, USA.
Dru F. Leistritz, Department of Pathology, University of Washington, Seattle, WA 98195, USA
Vilhelm A. Bohr, Laboratory of Molecular Gerontology, National Institute on Aging, NIH, 5600 Nathan Shock Dr, Baltimore, MD 21224, USA
References
- Agrelo R, Cheng WH, Setien F, Ropero S, Espada J, Fraga MF, Herranz M, Paz MF, Sanchez-Cespedes M, Artiga MJ, Guerrero D, Castells A, von Kobbe C, Bohr VA, Esteller M. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc Natl Acad Sci USA. 2006;103:8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA. Deficient DNA repair in the human progeroid disorder, Werner syndrome. Mutat Res. 2005;577:252–259. doi: 10.1016/j.mrfmmm.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Bohr VA, Metter EJ, Harrigan JA, von Kobbe C, Liu JL, Gray MD, Majumdar A, Wilson DM, Seidman MM. Werner syndrome protein 1367 variants and disposition towards coronary artery disease in Caucasian patients. Mech Ageing Dev. 2004;125:491–496. doi: 10.1016/j.mad.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, Mian IS, Kennedy BK, Oshima J. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- Ding SL, Yu JC, Chen ST, Hsu GC, Shen CY. Genetic variation in the premature aging gene WRN: a case-control study on breast cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 2007;16:263–269. doi: 10.1158/1055-9965.EPI-06-0678. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966;45:177–221. doi: 10.1097/00005792-196605000-00001. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16:R50–R59. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- Futami K, Ishikawa Y, Goto M, Furuichi Y, Sugimoto M. Role of Werner syndrome gene product helicase in carcinogenesis and in resistance to genotoxins by cancer cells. Cancer Sci. 2008;99:843–848. doi: 10.1111/j.1349-7006.2008.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech Ageing Dev. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomarkers Prev. 1996;5:239–246. [PubMed] [Google Scholar]
- Goto M, Imamura O, Kuromitsu J, Matsumoto T, Yamabe Y, Tokutake Y, Suzuki N, Mason B, Drayna D, Sugawara M, Sugimoto M, Furuichi Y. Analysis of helicase gene mutations in Japanese Werner's syndrome patients. Hum Genet. 1997;99:191–193. doi: 10.1007/s004390050336. [DOI] [PubMed] [Google Scholar]
- Goto M, Yamabe Y, Shiratori M, Okada M, Kawabe T, Matsumoto T, Sugimoto M, Furuichi Y. Immunological diagnosis of Werner syndrome by down-regulated and truncated gene products. Hum Genet. 1999;105:301–307. doi: 10.1007/s004399900151. [DOI] [PubMed] [Google Scholar]
- Gray MD, Wang L, Youssoufian H, Martin GM, Oshima J. Werner helicase is localized to transcriptionally active nucleoli of cycling cells. Exp Cell Res. 1998;242:487–494. doi: 10.1006/excr.1998.4124. [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Fan J, Momand J, Perrino FW, Bohr VA, Wilson DM., III WRN exonuclease activity is blocked by DNA termini harboring 3′ obstructive groups. Mech Ageing Dev. 2007;128:259–266. doi: 10.1016/j.mad.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Suzu S, Hinokio Y, Yamada T, Yoshizumi S, Suzuki C, Satoh J, Oka Y. WRN gene 1367 Arg allele protects against development of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:287–292. doi: 10.1016/j.diabres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Hisama FM, Bohr VA, Oshima J. WRN's tenth anniversary. Sci Aging Knowledge Environ. 2006;2006:e18. doi: 10.1126/sageke.2006.10.pe18. [DOI] [PubMed] [Google Scholar]
- Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, Poot M, Rubin CD, Chen DF, Yang CC, Juch H, Dorn T, Spiegel R, Oral EA, Abid M, Battisti C, Lucci-Cordisco E, Neri G, Steed EH, Kidd A, Isley W, Showalter D, Vittone JL, Konstantinow A, Ring J, Meyer P, Wenger SL, von HA, Wollina U, Schuelke M, Huizenga CR, Leistritz DF, Martin GM, Mian IS, Oshima J. The spectrum of WRN mutations in Werner syndrome patients. Hum Mutat. 2006;27:558–567. doi: 10.1002/humu.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Miller RW, Machinami R, Sugano H, Goto M. Atypical osteosarcomas in Werner Syndrome (adult progeria). Jpn J Cancer Res. 2000;91:1345–1349. doi: 10.1111/j.1349-7006.2000.tb00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath-Loeb AS, Welcsh P, Waite M, Adman ET, Loeb LA. The enzymatic activities of the Werner syndrome protein are disabled by the amino acid polymorphism R834C. J Biol Chem. 2004;279:55499–55505. doi: 10.1074/jbc.M407128200. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kusumoto R, Doherty KM, Lin GX, Zeng W, Cheng WH, von Kobbe C, Brosh RM, Jr, Hu JS, Bohr VA. Modulation of Werner syndrome protein function by a single mutation in the conserved RecQ domain. J Biol Chem. 2005;280:39627–39636. doi: 10.1074/jbc.M506112200. [DOI] [PubMed] [Google Scholar]
- Mohaghegh P, Hickson ID. Premature aging in RecQ helicase-deficient human syndromes. Int J Biochem Cell Biol. 2002;34:1496–1501. doi: 10.1016/s1357-2725(02)00039-0. [DOI] [PubMed] [Google Scholar]
- Mori H, Tomiyama T, Maeda N, Ozawa K, Wakasa K. Lack of amyloid plaque formation in the central nervous system of a patient with Werner syndrome. Neuropathology. 2003;23:51–56. doi: 10.1046/j.1440-1789.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- Moser MJ, Kamath-Loeb AS, Jacob JE, Bennett SE, Oshima J, Monnat RJ., Jr WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 2000;28:648–654. doi: 10.1093/nar/28.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama R, Sato Y, Masutani M, Ogino H, Nakatani F, Chuman H, Beppu Y, Morioka H, Yabe H, Hirose H, Sugimura H, Sakamoto H, Ohta T, Toyama Y, Yoshida T, Kawai A. Association of a missense single nucleotide polymorphism, Cys1367Arg of the WRN gene, with the risk of bone and soft tissue sarcomas in Japan. Cancer Sci. 2008;99:333–339. doi: 10.1111/j.1349-7006.2007.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakura J, Wijsman EM, Miki T, Kamino K, Yu CE, Oshima J, Fukuchi K, Weber JL, Piussan C, Melaragno MI. Homozygosity mapping of the Werner syndrome locus (WRN). Genomics. 1994;23:600–608. doi: 10.1006/geno.1994.1548. [DOI] [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D'Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, Tudisco C, Pallotta R, Scarano G, Dallapiccola B, Merlini L, Bonne G. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71:426–431. doi: 10.1086/341908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opresko PL, Cheng WH, von Kobbe C, Harrigan JA, Bohr VA. Werner syndrome and the function of the Werner protein; what they can teach us about the molecular aging process. Carcinogenesis. 2003;24:791–802. doi: 10.1093/carcin/bgg034. [DOI] [PubMed] [Google Scholar]
- Oshima J, Yu CE, Piussan C, Klein G, Jabkowski J, Balci S, Miki T, Nakura J, Ogihara T, Ells J, Smith M, Melaragno MI, Fraccaro M, Scappaticci S, Matthews J, Ouais S, Jarzebowicz A, Schellenberg GD, Martin GM. Homozygous and compound heterozygous mutations at the Werner syndrome locus. Hum Mol Genet. 1996;5:1909–1913. doi: 10.1093/hmg/5.12.1909. [DOI] [PubMed] [Google Scholar]
- Postiglione A, Soricelli A, Covelli EM, Iazzetta N, Ruocco A, Milan G, Santoro L, Alfano B, Brunetti A. Premature aging in Werner's syndrome spares the central nervous system. Neurobiol Aging. 1996;17:325–330. doi: 10.1016/0197-4580(96)00033-4. [DOI] [PubMed] [Google Scholar]
- Rubin CD, Zerwekh JE, Reed-Gitomer BY, Pak CY. Characterization of osteoporosis in a patient with Werner's syndrome. J Am Geriatr Soc. 1992;40:1161–1163. doi: 10.1111/j.1532-5415.1992.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Satoh M, Imai M, Sugimoto M, Goto M, Furuichi Y. Prevalence of Werner's syndrome heterozygotes in Japan. Lancet. 1999;353:1766. doi: 10.1016/S0140-6736(98)05869-3. [DOI] [PubMed] [Google Scholar]
- Shen M, Zheng T, Lan Q, Zhang Y, Zahm SH, Wang SS, Holford TR, Leaderer B, Yeager M, Welch R, Kang D, Boyle P, Zhang B, Zou K, Zhu Y, Chanock S, Rothman N. Polymorphisms in DNA repair genes and risk of non-Hodgkin lymphoma among women in Connecticut. Human Genet. 2006;119:659–668. doi: 10.1007/s00439-006-0177-2. [DOI] [PubMed] [Google Scholar]
- Sumi SM. Neuropathology of Werner syndrome. In: Salk D, Fujiwara Y, Martin GM, editors. Werner's syndrome and human aging. Plenum, New York: 1985. pp. 215–218. [Google Scholar]
- Suzuki T, Shiratori M, Furuichi Y, Matsumoto T. Diverged nuclear localization of Werner helicase in human and mouse cells. Oncogene. 2001;20:2551–2558. doi: 10.1038/sj.onc.1204344. [DOI] [PubMed] [Google Scholar]
- Uhrhammer NA, Lafarge L, Dos SL, Domaszewska A, Lange M, Yang Y, Aractingi S, Bessis D, Bignon YJ. Werner syndrome and mutations of the WRN and LMNA genes in France. Hum Mutat. 2006;27:718–719. doi: 10.1002/humu.9435. [DOI] [PubMed] [Google Scholar]
- Wang L, Evans AE, Ogburn CE, Youssoufian H, Martin GM, Oshima J. Werner helicase expression in human fetal and adult aortas. Exp Gerontol. 1999;34:935–941. doi: 10.1016/s0531-5565(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Wirtenberger M, Frank B, Hemminki K, Klaes R, Schmutzler RK, Wappenschmidt B, Meindl A, Kiechle M, Arnold N, Weber BH, Niederacher D, Bartram CR, Burwinkel B. Interaction of Werner and Bloom syndrome genes with p53 in familial breast cancer. Carcinogenesis. 2006;27:1655–1660. doi: 10.1093/carcin/bgi374. [DOI] [PubMed] [Google Scholar]
- Yamabe Y, Sugimoto M, Satoh M, Suzuki N, Sugawara M, Goto M, Furuichi Y. Down-regulation of the defective transcripts of the Werner's syndrome gene in the cells of patients. Biochem Biophys Res Commun. 1997;236:151–154. doi: 10.1006/bbrc.1997.6919. [DOI] [PubMed] [Google Scholar]
- Ye L, Miki T, Nakura J, Oshima J, Kamino K, Rakugi H, Ikegami H, Higaki J, Edland SD, Martin GM, Ogihara T. Association of a polymorphic variant of the Werner helicase gene with myocardial infarction in a Japanese population. Am J Med Genet. 1997;68:494–498. doi: 10.1002/(sici)1096-8628(19970211)68:4<494::aid-ajmg30>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Yeong EK, Yang CC. Chronic leg ulcers in Werner's syndrome. Br J Plast Surg. 2004;57:86–88. doi: 10.1016/j.bjps.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Yokote K, Honjo S, Kobayashi K, Fujimoto M, Kawamura H, Mori S, Saito Y. Metabolic improvement and abdominal fat redistribution in Werner syndrome by pioglitazone. J Am Geriatr Soc. 2004;52:1582–1583. doi: 10.1111/j.1532-5415.2004.52430_4.x. [DOI] [PubMed] [Google Scholar]
- Yu CE, Oshima J, Wijsman EM, Nakura J, Miki T, Piussan C, Matthews S, Fu YH, Mulligan J, Martin GM, Schellenberg GD. Mutations in the consensus helicase domains of the Werner syndrome gene. Werner's Syndrome Collaborative Group. Am J Hum Genet. 1997;60:330–341. [PMC free article] [PubMed] [Google Scholar]