Abstract
Accumulation of DNA damage deriving from exogenous and endogenous sources has significant consequences for cellular survival, and is implicated in aging, cancer, and neurological diseases. Different DNA repair pathways have evolved in order to maintain genomic stability. Genetic and environmental factors are likely to influence DNA repair capacity. In order to gain more insight into the genetic and environmental contribution to the molecular basis of DNA repair, we have performed a human twin study, where we focused on the consequences of some of the most abundant types of DNA damage (single-strand breaks), and some of the most hazardous lesions (DNA double-strand breaks). DNA damage signaling response (Gamma-H2AX signaling), relative amount of endogenous damage, and DNA-strand break repair capacities were studied in peripheral blood mononuclear cells from 198 twins (94 monozygotic and 104 dizygotic). We did not detect genetic effects on the DNA-strand break variables in our study.
Keywords: DNA repair, single-strand break repair, double-strand break repair, gamma-H2AX, heritability, twins
INTRODUCTION
Constant exposure to endogenous and exogenous agents induces damage to the cellular macromolecules including DNA. Many types of DNA lesions can cause genomic instability. Endogenous metabolic by-products frequently damage DNA but a broad range of DNA-damaging agents are also present in our environment including radiation from sunlight and radon, food mutagens, industrial chemicals, and cigarette smoke. The majority of DNA lesions are removed by the DNA repair mechanisms, and genomic stability is thereby maintained. Inefficient DNA repair may cause DNA damage accumulation, mutations, and cellular dysfunction, which is associated with cellular senescence, aging, cancer, and neurodegeneration [Jeppesen et al., 2011; Wolters and Schumacher, 2013].
DNA single-strand breaks (SSBs) are some of the most abundant DNA lesions, and DNA double-strand breaks (DSBs) are among the most lethal. Thus, effective repair mechanisms for these specific lesions are essential for genomic stability. DNA lesions derived from endogenous cellular metabolism are removed through the base excision repair (BER) pathway, including SSB repair (SSBR). Nucleotide excision repair (NER) is the pathway by which helix distorting DNA lesions are repaired.
DSBs are especially harmful, because they can lead to genome rearrangements. Immediately after DSB formation the histone protein H2AX is phosphorylated at serine 139 (γ-H2AX), which accumulates in the chromatin around the break as a DSB signaling response. Two major DSB repair (DSBR) mechanisms exist, they are nonhomologous end joining (NHEJ) and homologous recombination (HR). Briefly, NHEJ proteins recognize DSBs and form complexes on both sides of the DSB, and the DNA ends are processed and finally ligated. HR uses homologous sequences in sister chromatids to repair DSBs, especially those formed at collapsed replication forks. The error-free HR is active in the S and G2 phases of the cell cycle, whereas the error-prone NHEJ operates throughout the cell cycle [Kavanagh et al., 2013].
Monozygotic (MZ) twins derive from the same zygote and share all genetic material, whereas dizygotic (DZ) twins like ordinary siblings, share on average 50% of their segregating genes. In addition, twins also share early environmental factors. The classical twin study is based on the fact that MZ twins are genetically matched and relies on the assumption that intrapair MZ discordance in traits is attributed to environmental factors. In contrast, differences in both genetic and environmental factors influence DZ twins; hence, substantial influence from genetic factors on a trait is detectable as a greater MZ-compared to DZ similarity [Jinks and Fulker, 1970; van Dongen et al., 2012]. By twin studies, the variance of a trait can be divided into additive genetic- (A), dominance genetic- (D), shared environmental- (C), and nonshared environmental (E) effects. Additive genetic influence is the sum of effects of the individual alleles at all loci that influence the trait, whereas dominant genetic effects represent interaction between alleles at the same locus or different loci. Family members are exposed to shared environmental effects whereas nonshared environmental influences reflect differences among family members.
Several reports have investigated DNA repair in human populations; however, only few in twin populations. Twin studies reporting heritability estimates on DNA repair-related parameters have mostly indicated high genetic contribution for measures like micronucleus frequency, mutagenic sensitivity, and apoptotic- and cell cycle response to ionizing radiation [Camplejohn et al., 2006; Wu et al., 2006; Finnon et al., 2008; Surowy et al., 2011].
Twin studies estimating heritability of the capacity to repair specific DNA lesions are lacking. This may be due to limited availability of high-throughput methods for functional studies on DNA repair. Investigations of DNA repair factors in population studies have often included relatively few study participants resulting in low statistical power. Thus, we aimed to increase the statistical power by investigating a total of 198 twins (94 MZ and 104 DZ) from the Danish Twin Registry [Skytthe et al., 2013]. In this twin study, we investigated the influence of environmental and genetic factors on the DNA-strand break repair parameters, namely, endogenous SSB level, SSBR capacity, γ-H2AX response, and DSBR capacity in human peripheral blood mononuclear cells (PBMCs).
MATERIALS AND METHODS
Study Population
The study sample (n = 198) comprised participants from the Danish Twin Registry, which is a nationwide, population-based registry [Skytthe et al., 2013]. Only complete twin pairs were included in this analysis. Zygosity of the twin pairs was established through a questionnaire on the degree of similarity between twins in a pair [Christiansen et al., 2003]. Since the 1960s, selected cohorts from the registry have participated in questionnaire and survey studies. In the period 2008–2011, whole blood from randomly selected twins was collected. PBMCs were isolated according to the manufacturer’s protocol (BD Vacutainer® CPT™, REF 362761) and cryopreserved. The Science Ethics Committee of Southern Denmark has approved the study (Project number S-VF-19980072).
Analysis of DNA Damage and Repair Parameters in PBMCs
The methodology for the analysis of DNA damage and repair parameters was described in detail and graphically explained previously [Garm et al., 2013].
In short, Fluorimetric Detection of Alkaline DNA Unwinding (FADU) was performed in order to measure the level of endogenous strand breaks and DNA repair capacity in PBMCs 40 min after DNA damage induction by 3.8 Gy X-ray [Moreno-Villanueva et al., 2009; Garm et al., 2013]. By using a modified neutral comet assay γ-irradiated cells (6 Gy, Cs-137 source, 3 hr recovery) were analyzed for the capacity of repairing primarily induced DSBs [Garm et al., 2013]. The γ-H2AX response to induced DSBs (6 Gy, Cs-137 source, 1 hr incubation) was measured by flow cytometry [Muslimovic et al., 2008; Garm et al., 2013].
In order to minimize experimental variability, the same investigator performed all analyses. The only selection criterion was zygosity status, in order to include similar numbers of MZ and DZ twin pairs. We aimed for similar batch effects among MZ and DZ twins by analyzing approximately equal numbers of MZ and DZ twin pairs in each batch. Both twins in the pairs were analyzed in the same batches. The FADU data was presented as mean of four replicates, comet data as mean of 60–100 cells, and flow cytometric data was based on a minimum of 50,000 cells. The sample material did not allow additional replicates. Reported coefficients of variance (CV) were based on minimum 10 replicates of an internal control sample, which was used for standardization of data from different batches.
Analysis of Twin Similarity
A classical twin study approach was performed by analyzing MZ and DZ twins. Intraclass correlations (ICCs) were estimated using a Random ML regression approach (using Stata version 11.2). We included age and gender as covariates. To estimate the relative contribution of genetic and environmental factors to the DNA repair parameters, we performed a biometrical genetic analysis using model-fitting heritability [Rijsdijk and Sham, 2002]. The total variance was assumed to comprise A, D, C, and E effects. The effects of D and C cannot be simultaneously estimated because they are confounded. Therefore, ACE, ADE, AE, CE, and E models were fitted and compared by Akaike’s Information Criterion (AIC) and by likelihood ratio testing. Lowest AIC was used to determine which of the nonnested models had the best fit, whereas chi-squared likelihood ratio testing was used to select the best-fitting nested model, that is, the most parsimonious model was chosen if the P-value of the χ2-test was greater than the significance limit; 0.05. The variance components of the different DNA repair parameters were derived from the best-fitting model using the statistical program R (Version 2.14.2) and the mets (Analysis of Mulitvariate Event Times) package (Version 0.1–8).
RESULTS
Descriptive Statistics
Similar numbers of MZ and DZ twin pairs were included in this study and characteristics of the study participants were summarized in Table I. The mean age was similar for MZ and DZ twins at the time of sampling. Females were overrepresented in both MZ and DZ twins because of different pairwise participation rates. The total study sample comprised 198 twins. For practical reasons it was not possible to include all study individuals for all analyses.
TABLE I.
Characteristics of Study Participants
| Group | Monozygotic | Dizygotic | |
|---|---|---|---|
| Individuals | N | 94 | 104 |
| Complete pairs | N | 47 | 52 |
| Mean age (Yr) | x̄ (SD) | 55.2 (8.37) | 56.7 (7.87) |
| Males | N | 32 | 30 |
| Females | N | 62 | 74 |
N, number; SD, standard deviation; x̄, mean value.
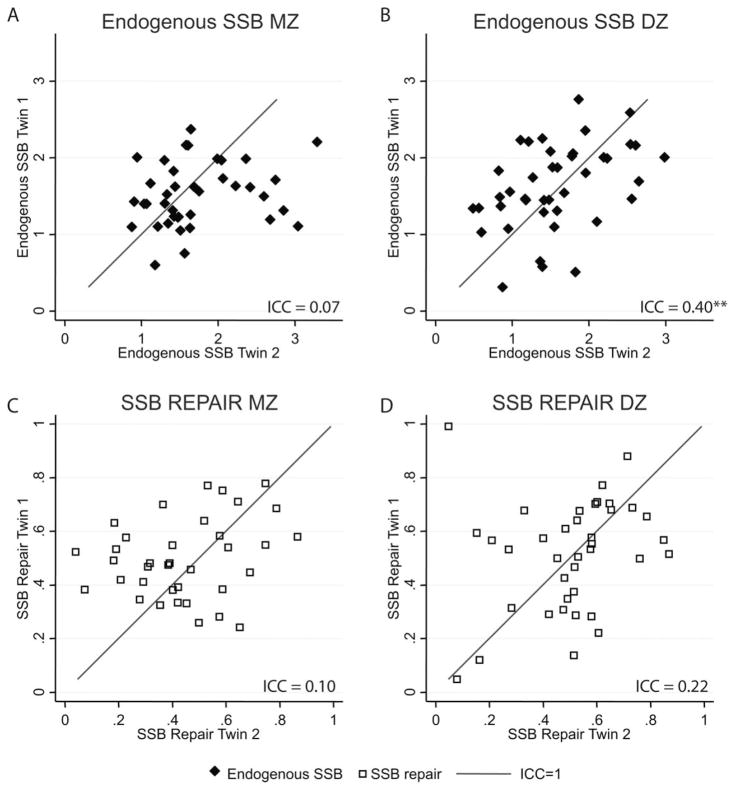
Correlation plots stratified by zygosity were used in order to compare twin similarity between MZ and DZ twins for endogenous SSB levels (Fig. 1A and B) and SSBR capacity (Fig. 1C and D). The within-twin pair similarity for MZ and DZ pairs separately is reported as age- and gender-adjusted ICC coefficients in Table II. The MZ ICC for the SSB parameters [endogenous SSBs: MZ ICC = 0.07 (P = 0.34); SSBR: MZ ICC = 0.10 (P = 0.27)] did not exceed the DZ correlations [endogenous SSB level: DZ ICC = 0.40 (P <0.01); SSBR: DZ ICC = 0.22 (P = 0.08)], suggesting no genetic component in the SSB parameters.
Fig. 1.
Correlation plots for endogenous SSB (solid diamonds) and SSB repair capacity (open squares) stratified by zygosity. The level of endogenous SSB was normalized to an internal control. The specific DNA-strand break repair data for one twin of a pair (Twin 1) was plotted against the co-twin (Twin 2); hence, one symbol represents a twin pair. The lines represent perfect correlation. Interclass coefficients (ICC) are presented for each plot (significance level: *P <0.05; **P <0.01). A: endogenous SSB MZ; B: endogenous SSB DZ; C, SSB repair MZ; D, SSB repair DZ.
TABLE II.
Twin Similarity: Intraclass Correlation Coefficients for DNA Strand Break Repair Variables
| Monozygotic | Dizygotic | ||
|---|---|---|---|
| Endogenous SSB | N | 78 | 82 |
| ICC | 0.07 | 0.40 | |
| P-value | 0.34 | <0.01 | |
| SSB Repair | N | 72 | 76 |
| ICC | 0.10 | 0.22 | |
| P-value | 0.27 | 0.08 | |
| DSB Repair | N | 86 | 100 |
| ICC | 0.41 | 0.34 | |
| P-value | <0.01 | <0.01 | |
| γ-H2AX response | N | 94 | 104 |
| ICC | 0.68 | 0.58 | |
| P-value | <0.01 | <0.01 |
DSB, double strand break; ICC, intraclass correlation; N, number; SSB, single strand break.
Intraclass correlations (ICC) were calculated using the random-effects ML regression model (adjusting for age and gender).
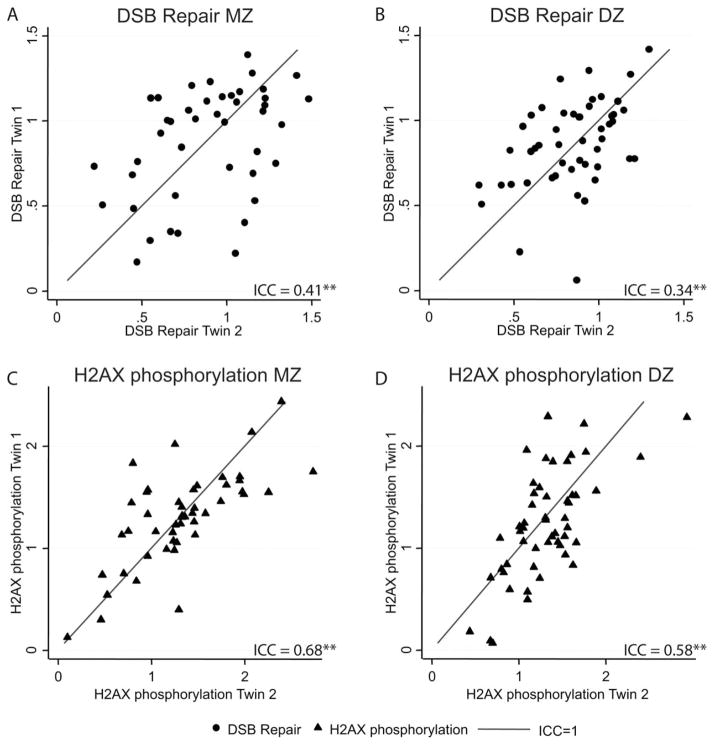
Similarly, zygosity stratified correlation plots were presented for DSBR capacity in Figure 2A and B and for the γ-H2AX response in Figure 2C and D. We identified moderate and high ICCs, respectively, for these two parameters. Both DSBR capacity and γ-H2AX response showed slightly higher MZ ICCs [DSBR capacity: MZ ICC = 0.41 (P <0.01); γ-H2AX response: MZ ICC = 0.68 (P <0.01)] compared to DZ correlations [DSBR capacity: DZ ICC = 0.34 (P <0.01); γ-H2AX response DZ ICC = 0.58 (P <0.01)] (Table II), which might indicate a moderate genetic effect on the DSB parameters.
Fig. 2.
Correlation plots for DSB repair (circles) and γ-H2AX response (triangles) stratified by zygosity. The DSB repair and γ-H2AX response data was normalized as described to an internal control. Data for one twin of a pair (Twin 1) was plotted against the co-twin (Twin 2) as in Figure 1. Interclass coefficients (ICC) are presented for each plot (significance level: *P <0.05, **P <0.01). A, DSB repair MZ; B, DSB repair DZ; C, H2AX phosphorylation MZ; D, H2AX phosphorylation DZ.
Heritability Estimates
In order to test causal relations between the investigated DNA-strand break parameters and genetic and environmental factors structural equation analysis was performed. Age- and gender-adjusted estimates for the proportion of the variance explained by shared environment and nonshared environment are presented in Table III. For all DNA repair parameters, except for SSBR, the best-fitting biometrical model was the CE model, indicating the presence of common environmental factors and nonshared environmental factors in the variance in the DNA-strand break parameters. The best-fitting model for SSBR was the E model indicating that nonshared environmental factors account for the observed variance. These findings indicated no heritability in the analyzed DNA-strand break parameters, because A or D were not included in the best-fitting biometrical model. The estimates for shared environment (C) were in the range of 0.21–0.57, and the estimates for nonshared environment (E) were between 0.32 and 0.79.
TABLE III.
Biometrical Modeling Analysis of DNA Strand Break Repair Parameters (ACE Model)
| A | C | E | ||
|---|---|---|---|---|
|
|
||||
| (95% CI) | (95% CI) | (95% CI) | Best fitting model | |
| Endogenous SSB | 0.00 (n/a)a | 0.29 (0.13; 0.51) | 0.71 (0.49; 0.87) | CE |
| SSB repair | 0.00 (n/a)a | 0.21 (0; 0.93) | 0.79 (0.40; 0.97) | E |
| DSB repair | 0.00 (n/a)a | 0.40 (0.24; 0.57) | 0.60 (0.43; 0.76) | CE |
| γ-H2AX response | 0.11 (0; 0.79) | 0.57 (0.26; 0.84) | 0.32 (0.20; 0.47) | CE |
A, additive genetic; C, shared environmental; CI, confidence interval; D, dominance genetic; DSB, double strand break; E, nonshared environmental; SSB, single strand break.
Estimates were adjusted for age and gender.
D was estimated to 0 for all DNA strand break parameters according to the ADE models.
CI was not applicable for the variance component A = 0 because the ACE and CE models were identical (χ2 = 0, P = 1).
DISCUSSION
In this twin study, we analyzed DNA repair mechanisms important for maintenance of genomic stability in order to better understand the individual variance in DNA repair. Heritability studies of DNA repair parameters estimate to which extent DNA repair is under genetic and/or environmental control. This information is central for future studies focusing on functional heritable genetic variation or environmental factors that cause differences in DNA repair between individuals. A better understanding of the heritability of DNA repair has the potential to increase our knowledge of the molecular processes of aging and a number of diseases, expected to be associated with DNA repair.
The best-fitting biometric models indicate that the variance of SSBR was because of nonshared environmental (E) factors, whereas the level of endogenous SSBs, γ-H2AX response, and DSBR capacity can be accounted for by both common environmental (C) factors and nonshared environmental (E) factors. Inevitably, all experimental data are affected by sources of error. Thus, experimental variability of the methods used for analyzing DNA repair parameters may have influenced the heritability estimate and is a major concern in these kinds of studies. The interassay CV were generally acceptable (CV <15%) for endogenous SSB detection, SSBR-, and DSBR capacity; however, the H2AX phosphorylation assay resulted in higher interassay variability (CV = 28.3%). This variability was corrected for by normalizing to internal control samples. Experimental variation observed across multiple batches complicated the task of combining data from different batches [Leek et al., 2010; Garm et al., 2013]. Unexpectedly, MZ and DZ twins showed high ICC for DSB repair and γ-H2AX response. Both twins within the twin pairs were analyzed in the same batches, which may inflate the twin correlations [Bischoff et al., 2005], and hence overestimate the C estimates that were considerable for γ-H2AX response and DSBR capacity. Similarly, co-twins were analyzed pairwise in the FADU assay, but the twin correlation was not inflated because of considerably smaller batch effects compared to the DSB parameters.
Genetic factors were not identified to contribute to the interindividual variance observed in the DNA-strand break parameters. However, we had expected some influence of genetic components on the DNA repair measures because we previously found SNPs in genes coding for DNA repair gene products to be associated with longevity [Soerensen et al., 2012]. Individual differences in DNA repair may be affected by genetic factors in the whole population, but we may not have been able to detect it in this twin study because of the effect of environmental factors, measurement errors, and batch effects. Gene–environment interactions may complicate the determination of specific genetic and environmental effects and are of concern in twin studies [Hoover, 2000; Kaprio, 2012]. However, gene–environment interactions are beyond the scope of this study because of the observation of no genetic component.
Genetic differences might only affect specific DNA repair pathways other than strand break repair. Nevertheless, our data indicated that environmental components contribute more to DNA-strand break parameters than genetic components.
Whereas no genetic component was identified for these specific DNA-strand break repair parameters within the investigated age span, genetic factors could potentially affect DNA-strand break repair at old age. This scenario was observed for longevity where minimal genetic influence on longevity was identified prior to age 60, but genetic factors for survival were demonstrated to be increasingly important at old age [Hjelmborg et al., 2006]. Similarly, genetic factors may contribute to DNA-strand break repair at old age. However, it is beyond the limits of this study to determine this because of a limited number of 60-plus year old study participants.
A recent review, focusing on stress-induced effects on genomic instability, highlighted that the genome is more sensitive to the environment than previously anticipated [Fonville et al., 2011]. Environmental stressors such as toxins, irradiation, inflammation, and nutritional factors are suggested to induce changes to the genome. DSBs are particularly mutagenic and a balance between the error-free HR and the error-prone NHEJ has been suggested to exist [Kavanagh et al., 2013]. If environmental factors regulate the activity of DSBR capacity or the balance between the DSBR sub-pathways, it could lead to increased mutations, and genomic instability.
The radiation dose used in this study was chosen to allow functionality of the molecular assays and was similar to what has been used in other studies [Muslimovic et al., 2008; Trzeciak et al., 2008; Moreno-Villanueva et al., 2009]. While the doses used may be considered high in relation to physiological and environmental exposures this level of damage is efficiently repaired in mammalian cells [Torudd et al., 2005] and thus are suitable for our study.
Twin studies on the repair of specific DNA lesions have not previously been performed, but primarily high heritability estimates have been reported for mutagen sensitivity, micronucleus frequencies [Surowy et al., 2011], cell cycle and apoptotic response [Camplejohn et al., 2006; Finnon et al., 2008], chromosomal radiosensitivity [Borgmann et al., 2007; Curwen et al., 2010], and chromosomal damage [Tedeschi et al., 2004]. Mutagen sensitivity, an indirect measure of the repair capacity of various types of DNA damages have been suggested to be highly heritable [Wu et al., 2006]. Our study deviated from the study of Wu and co-workers as we performed direct analysis of DNA-strand break repair parameters. This may explain the discrepant findings. Several of the above-mentioned studies included few study subjects, with less than 20 twin pairs in either MZ or DZ groups, which could result in amplified random effects. In contrast, our current study included a high number of twins in order to obtain heritability estimates by biometric modeling of variance components. Notably, in addition to the limited statistical power in these related studies, other critical aspects should be mentioned, especially high ICCs above 0.9 [Borgmann et al., 2007] and CVs exceeding 30% [Curwen et al., 2010].
In conclusion, we were not able to detect any influence of genetic factors on the DNA-strand break parameters in the age span investigated. Our findings suggest that future studies on DNA-strand break repair modulation should further explore the impact of various environmental factors. Importantly, heritability of other DNA repair pathways (e.g., NER and BER) should also be explored.
Acknowledgments
Grant sponsors: Danish Agency for Science, Technology and Innovation; Grant number: 09–063256. The VELUX Foundation. Ausschuss für Forschungsfragen, University of Konstanz.
We thank all members of The Danish Aging Research Center for contributions and discussions related to this project. Facilities for performing improved comet assay were kindly offered by Christiane Beer. Inge Petersen assisted with statistical expertise, whereas Jakob Mortensen and Axel Skytthe contributed with managing data from the Danish Twin Registry, and their contributions are greatly acknowledged. Ulla Birk Henriksen is greatly acknowledged for technical support. Gudrun von Scheven, Monika Schulz, and Barbara Bausch are thanked for technical support to the FADU assay. The VELUX foundation and the AFF (Ausschuss für Forschungsfragen), University of Konstanz, supported this work. The Danish Twin Registry is supported by a grant from The National Program for Research Infrastructure 2007 from the Danish Agency for Science, Technology and Innovation.
Footnotes
AUTHOR CONTRIBUTIONS
CG, TS, KC, and VAB contributed by generating the study concept and study design. CG performed the sampling and all molecular biological assays, conducted data cleanup and data analysis, interpreted data, and drafted the manuscript. TS, KC, and VAB contributed by supervising the research and contributing to writing of the manuscript. LAL contributed with statistical support. MMV and AB contributed by helping with acquisition and interpretation of the FADU data.
References
- Bischoff C, Graakjaer J, Petersen HC, Hjelmborg JB, Vaupel JW, Bohr V, Koelvraa S, Christensen K. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8(5):433–439. doi: 10.1375/183242705774310141. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Haeberle D, Doerk T, Busjahn A, Stephan G, Dikomey E. Genetic determination of chromosomal radiosensitivities in G0- and G2-phase human lymphocytes. Radiother Oncol. 2007;83(2):196–202. doi: 10.1016/j.radonc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Camplejohn RS, Hodgson S, Carter N, Kato BS, Spector TD. Heritability of DNA-damage-induced apoptosis and its relationship with age in lymphocytes from female twins. Br J Cancer. 2006;95(4):520–524. doi: 10.1038/sj.bjc.6603257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K, Kyvik K. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2003;6(4):275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- Curwen GB, Cadwell KK, Winther JF, Tawn EJ, Rees GS, Olsen JH, Rechnitzer C, Schroeder H, Guldberg P, Cordell HJ, Boice JD., Jr The heritability of G2 chromosomal radiosensitivity and its association with cancer in Danish cancer survivors and their offspring. Int J Radiat Biol. 2010;86(11):986–995. doi: 10.3109/09553002.2010.496027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnon P, Robertson N, Dziwura S, Raffy C, Zhang W, Ainsbury L, Kaprio J, Badie C, Bouffler S. Evidence for significant heritability of apoptotic and cell cycle responses to ionising radiation. Hum Genet. 2008;123(5):485–493. doi: 10.1007/s00439-008-0500-1. [DOI] [PubMed] [Google Scholar]
- Fonville NC, Ward RM, Mittelman D. Stress-induced modulators of repeat instability and genome evolution. J Mol Microbiol Biotechnol. 2011;21(1–2):36–44. doi: 10.1159/000332748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garm C, Moreno-Villanueva M, Burkle A, Petersen I, Bohr VA, Christensen K, Stevnsner T. Age and gender effects on DNA-strand break repair in peripheral blood mononuclear cells. Aging Cell. 2013;12(1):58–66. doi: 10.1111/acel.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119(3):312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Hoover RN. Cancer-nature, nurture, or both. N Engl J Med. 2000;343(2):135–136. doi: 10.1056/NEJM200007133430210. [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog Neurobiol. 2011;94(2):166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks JL, Fulker DW. Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior. Psychol Bull. 1970;73(5):311–349. doi: 10.1037/h0029135. [DOI] [PubMed] [Google Scholar]
- Kaprio J. Twins and the mystery of missing heritability: The contribution of gene-environment interactions. J Intern Med. 2012;272(5):440–448. doi: 10.1111/j.1365-2796.2012.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JN, Redmond KM, Schettino G, Prise KM. DNA double strand break repair: A radiation perspective. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5151. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Leek JT, Scharpf RB, Bravo HC, Simcha D, Langmead B, Johnson WE, Geman D, Baggerly K, Irizarry RA. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet. 2010;11(10):733–739. doi: 10.1038/nrg2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Villanueva M, Pfeiffer R, Sindlinger T, Leake A, Muller M, Kirkwood TB, Burkle A. A modified and automated version of the “Fluorimetric Detection of Alkaline DNA Unwinding” method to quantify formation and repair of DNA strand breaks. BMC Biotechnol. 2009;9:39. doi: 10.1186/1472-6750-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic A, Ismail IH, Gao Y, Hammarsten O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protoc. 2008;3(7):1187–1193. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Christiansen L, Kyvik KO, Bodker FL, Hvidberg L, Petersen I, Nielsen MM, Bingley P, Hjelmborg J, Tan Q, Holm NV, Vaupel JW, McGue M, Christensen K. The danish twin registry: Linking surveys, national registers, and biological information. Twin Res Hum Genet. 2013;16(1):104–111. doi: 10.1017/thg.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen M, Dato S, Tan Q, Thinggaard M, Kleindorp R, Beekman M, Jacobsen R, Suchiman HE, de Craen AJ, Westendorp RG, Schreiber S, Stevnsner T, Bohr VA, Slagboom PE, Nebel A, Vaupel JW, Christensen K, McGue M, Christiansen L. Human longevity and variation in GH/IGF-1/insulin signaling, DNA damage signaling and repair and pro/antioxidant pathway genes: Cross sectional and longitudinal studies. Exp Gerontol. 2012;47(5):379–387. doi: 10.1016/j.exger.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowy H, Rinckleb A, Luedeke M, Stuber M, Wecker A, Varga D, Maier C, Hoegel J, Vogel W. Heritability of baseline and induced micronucleus frequencies. Mutagenesis. 2011;26(1):111–117. doi: 10.1093/mutage/geq059. [DOI] [PubMed] [Google Scholar]
- Tedeschi B, Cicchetti R, Argentin G, Caporossi D, Pittaluga M, Parisi P, Vernole P. Aphidicolin and bleomycin induced chromosome damage as biomarker of mutagen sensitivity: A twin study. Mutat Res. 2004;546(1–2):55–64. doi: 10.1016/j.mrfmmm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Torudd J, Protopopova M, Sarimov R, Nygren J, Eriksson S, Markova E, Chovanec M, Selivanova G, Belyaev IY. Dose-response for radiation-induced apoptosis, residual 53BP1 foci and DNA-loop relaxation in human lymphocytes. Int J Radiat Biol. 2005;81(2):125–138. doi: 10.1080/09553000500077211. [DOI] [PubMed] [Google Scholar]
- Trzeciak AR, Barnes J, Ejiogu N, Foster K, Brant LJ, Zonderman AB, Evans MK. Age, sex, and race influence single-strand break repair capacity in a human population. Free Radic Biol Med. 2008;45(12):1631–1641. doi: 10.1016/j.freeradbiomed.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nat Rev Genet. 2012;13(9):640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- Wolters S, Schumacher B. Genome maintenance and transcription integrity in aging and disease. Front Genet. 2013;4:19. doi: 10.3389/fgene.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Spitz MR, Amos CI, Lin J, Shao L, Gu J, de Andrade M, Benowitz NL, Shields PG, Swan GE. Mutagen sensitivity has high heritability: Evidence from a twin study. Cancer Res. 2006;66(12):5993–5996. doi: 10.1158/0008-5472.CAN-06-1007. [DOI] [PubMed] [Google Scholar]