Abstract
Accumulating evidence indicates that kappa-opioid receptors (KORs) and their endogenous ligand dynorphin (DYN) can play important roles in regulating the effects of stress. Here, we examined the role of KOR systems in the molecular and behavioral effects of acute (1-day) and chronic (10-day) social defeat stress (SDS) in mice. We found that acute SDS increased DYN mRNA levels within the nucleus accumbens (NAc), a key element of brain dopamine (DA) systems. In contrast, chronic SDS produced long-lasting decreases in DYN mRNA levels. We then examined if disruption of KOR function would affect development of SDS-induced depressive-like behaviors as measured in the intracranial self-stimulation (ICSS) and social interaction tests. Ablation of KORs from DA transporter (DAT)-expressing neurons delayed the development of SDS-induced anhedonia in the ICSS test, suggesting increased stress resilience. However, administration of the long-lasting KOR antagonist JDTic (30 mg/kg, intraperitoneal) before the SDS regimen did not affect anhedonia, suggesting that disruption of KOR function outside DA systems can oppose stress resilience. Social avoidance behavior measured after the 10-day SDS regimen was not altered by ablation of KORs in DAT-expressing neurons or by JDTic administration before testing. Our findings indicate that KORs expressed in DA systems regulate the effects of acute, but not chronic, social stress.
Keywords: social defeat stress, KOR, ICSS, mouse
INTRODUCTION
Exposure to stress can trigger debilitating psychiatric illnesses including depressive disorders (Kessler et al., 1997). Accumulating evidence suggests that dynorphin (DYN) and its cognate kappa-opioid receptor (KOR) play an important role in regulating stress responsiveness, motivation, and emotion (Bruchas et al., 2010; Knoll and Carlezon, 2010; Van’t Veer and Carlezon, 2013). KOR agonists produce dysphoria in humans (Pfeiffer et al., 1986) and depressive-like effects in rodents (Carlezon et al., 2006; Bruchas et al., 2010; Muschamp et al., 2012), whereas KOR antagonists can reduce the behavioral effects of stress (Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003; McLaughlin et al., 2003). There is considerable evidence that KOR antagonists are most effective when given before a stressor (Van’t Veer and Carlezon, 2013), as opposed to after stress-induced behavioral adaptations have already developed (e.g., drug withdrawal-related behaviors) (Chartoff et al., 2012). In rodents, acute stress exposure increases DYN levels within the striatum (Nabeshina et al., 1992; Shirayama et al., 2004), suggesting that molecular adaptations within basal forebrain regions can contribute to the development of depressive-like behaviors (Carlezon et al., 1998; Pliakas et al., 2001; Muschamp et al., 2011). However, the effects of more chronic stressors on brain KOR systems have not been thoroughly characterized.
Although the ways in which KOR systems influence mood and anxiety states is not fully understood, their effects are mediated, at least in part, by the mesolimbic dopamine (DA) reward circuit. The DA system is implicated in motivated behavior (Wise and Rompré, 1989) and the manifestation of prominent symptoms of depression, including anhedonia (reduced sensitivity to reward) and impairments in social interaction (Nestler and Carlezon, 2006). KORs within this circuitry can directly regulate DA function and behavior. For example, infusion of KOR agonists directly into the nucleus accumbens (NAc) reduces extracellular concentrations of DA (Donzanti et al., 1992; Maisonneuve et al., 1994) and induces depressive-like behaviors, including anhedonia, as indicated by increases in intracranial self-stimulation (ICSS) reward thresholds (Muschamp et al., 2011), and dysphoria, as indicated by conditioned place aversions (Bals-Kubik et al., 1993; Muschamp et al., 2011). In contrast, infusion of the KOR antagonist nor-binaltorphimine (norBNI) into the NAc elevates extracellular concentrations of DA (Maisonneuve et al., 1994) and has antidepressant-like effects in the learned helplessness paradigm (Newton et al., 2002). The effects of KOR agonists and antagonists within the NAc itself are likely mediated by KORs expressed on the terminals of DA inputs (Svingos et al., 1999) that regulate (inhibit) transmitter release. Consequently, alterations in the expression of DYN or KORs within the NAc may be sufficient to cause long-term changes in the function of the mesolimbic system and, by extension, motivated behavior.
The present studies were designed to examine the role of the KOR system in regulating behavioral and molecular adaptations to social defeat stress (SDS) in mice. SDS is an ethologically relevant model of stress-related illness that produces robust and long-lasting anhedonia and social avoidance (Krishnan et al., 2007; Donahue et al., 2014), key signs of psychiatric illness. Molecular adaptations within the mesolimbic DA circuit, such as increased expression of brain-derived neurotrophic factor (BDNF), are associated with susceptibility to SDS (Berton et al., 2006; Krishnan et al., 2007). KOR systems have also been implicated in mediating SDS-induced behavioral and molecular outcomes. Specifically, SDS-induced analgesia, defensive posturing, cocaine place preference, and avoidance behavior are prevented by pretreatment with norBNI and prodynorphin (pDYN) gene disruption (McLaughlin et al., 2006; Land et al., 2008; Bruchas et al., 2011). However, the time course of the onset of SDS-induced plasticity in KOR systems is currently unknown.
In the present study, we first used quantitative-PCR (q-PCR) to examine the effects of acute and chronic SDS on adaptations in KOR and pDYN (the precursor of DYN) mRNA levels within the NAc. Upon finding evidence of upregulation of pDYN mRNA levels, we then examined if disruption of KOR function can affect behavioral adaptations to SDS, as measured by the ICSS and social interaction tests. We have previously shown that both of these tests can quantify the depressive-like consequences of SDS, and both are sensitive to pharmacological interventions and genetic manipulations (Vialou et al., 2010; Donahue et al., 2014). We examined these behaviors following SDS in genetically engineered mice in which we disrupted KOR function by ablating KORs specifically within DA transporter (DAT)-expressing neurons (Van’t Veer et al., 2013a), and in wild-type mice after administration of the long-lasting KOR antagonist JDTic (Knoll et al., 2007).
METHODS
Subjects
Three lines of male mice were used for these studies: CD1 mice (Charles River) (retired breeders) were used as aggressors in all SDS studies, C57BL/6J mice (Jackson Laboratories) (25–35 g) were used as target mice in the molecular studies and some of the behavioral studies, and mutant mice with conditional knockout of KORs in DA neurons (or littermate controls) (25–35 g) were used as target mice in some of the behavioral studies. Mutant (MUT; DATCre/wtKORloxp/loxp) and control (CON; DATCre/wt KORwt/wt) mice were generated by breeding mice that express Cre in DAT-containing cells (DAT-Cre line 9075; Parlato et al., 2006) with KORloxp mice. KORloxp mice were generated by flanking Exon 3 of the murine KOR gene (Oprk1) with loxP sites, as previously described (Van’t Veer et al., 2013a). MUT and CON mice were littermates (in light of potential genetic differences between these mice and wild-type C57BL/6J mice) and had been backcrossed to wild-type C57BL/6J mice for at least 7 generations. Mice were maintained on a 12 h light (7:00 A.M. to 7:00 P.M.) cycle with free access to food and water. Procedures were approved by the McLean Hospital Institutional Animal Care and Use Committee in accordance with National Institutes of Health guidelines.
Social Defeat Stress (SDS)
The SDS procedure was performed as previously described (Golden et al., 2011; Donahue et al., 2014). CD1 mice were pre-screened for aggressive behavior (attack latencies <30 s on 3 consecutive days). Experimental (target) mice (C57BL/6, MUT, CON) were exposed to a novel CD1 aggressor mouse for 10 min on 1 day (acute SDS) or 10 consecutive days (chronic SDS). For chronic SDS q-PCR studies, defeated mice were segregated into susceptible and resilient subpopulations as described (Krishnan et al., 2007): mice with social interaction (SI) scores <1 were defined as “susceptible” and those with scores >1 were defined as “resilient.” For the acute SDS studies, defeated mice were not subdivided because multiple defeat sessions are required before the susceptible and resilient subtypes emerge (Krishnan et al., 2007).
Quantitative PCR
Levels of KOR and pDYN mRNA in the NAc were assessed in separate groups of C57BL6/J mice 48 hr after the following endpoints: acute SDS (1 day), chronic SDS (10 days), and chronic SDS followed by chronic treatment (35 days) with the standard antidepressant drug imipramine or vehicle in a cohort classified as susceptible (see Fig. 1). Because a 35-day regimen of imipramine has been shown to reverse some of the behavioral and molecular effects of chronic SDS in mice (Berton et al., 2006), the present studies were designed to determine if the effects of chronic SDS on KOR and pDYN mRNA expression are also sensitive to chronic treatment with this drug. Briefly, total RNA was collected from bilateral 14-gauge NAc punches as described previously (Golden et al., 2013), and analyzed for each individual mouse (n=6–8/group). Primer pairs have been reported previously (mOprk1, Van’t Veer et al., 2013a; mpDYN, Chartoff et al., 2009). Samples were normalized to GAPDH (glyceraldehyde-6-phosphate dehydrogenase). Data are expressed as fold differences of mRNA relative to GAPDH.
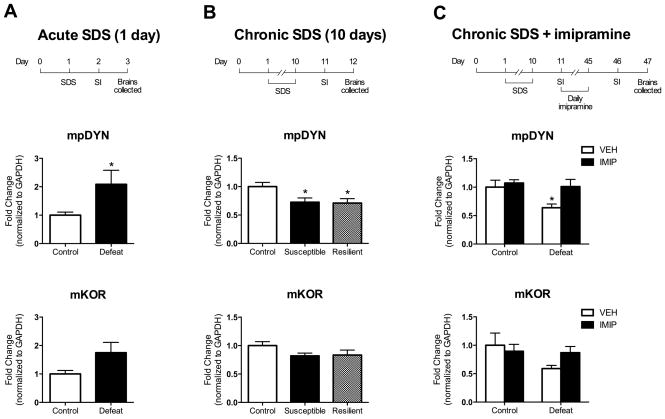
Figure 1.
Effects of acute SDS (A), chronic SDS (B), and chronic SDS with 35 days of imipramine (IMIP) treatment or saline (VEH) treatment on pDYN and KOR mRNA levels in the NAc. Top row: Experimental design depicting the time course of the SDS regimen and social interaction (SI) for each qPCR experiment. Middle row: pDYN mRNA data. Bottom row: KOR mRNA data. (A) Acute SDS elevated pDYN mRNA, and a similar trend was observed for KOR mRNA (n’s=5–6). (B) Chronic SDS decreased pDYN mRNA in both susceptible and resilient mice. A similar trend was observed for KOR mRNA levels (n’s=6–10). (C) Chronic SDS-induced decreases in proDYN were reversed by chronic (35 days) daily IMIP treatment, with a similar trend observed in KOR mRNA (n’s=6–8). *P<0.05 compared to controls; data are expressed as -fold differences in mRNA relative to GAPDH.
Intracranial self-stimulation (ICSS)
Mice (6–8 weeks of age) were implanted with monopolar electrodes aimed at the lateral hypothalamus (LH) as described previously (Donahue et al., 2014). After 1 week of recovery, mice were trained on a fixed-ratio schedule (FR1) to respond for brain stimulation. The stimulation current was adjusted to the lowest value (“minimum current”) that would sustain reliable responding (>40 rewards per min) and held constant throughout the duration of the experiment. Mice were then trained to respond to a series (“pass”) of 15 descending frequencies (158–32 Hz, at 0.05 log10 unit steps). Daily training sessions, which spanned 6–8 weeks, consisted of 4 consecutive passes. ICSS thresholds (Theta-0) were calculated using a least-squares line of best-fit analysis (Carlezon and Chartoff, 2007). The SDS regimen began after baseline thresholds were established (+/−15% for 5 days). Mice were tested in the ICSS test ~16 h after each defeat session, an interval in which SDS-induced anhedonia can be detected (Donahue et al., 2014). For KOR antagonist studies, JDTic was administered following the 5-day baseline period, and an additional ICSS session (~16 h post-injection) was run before the onset of defeat to ensure that JDTic alone did not alter ICSS thresholds. We have previously demonstrated that a 10-day regimen of SDS causes progressive increases in ICSS thresholds, reflecting the onset of anhedonia, but that control conditions over the same period of time have no effect (Donahue et al., 2014). Accordingly, the present studies focused on mice exposed to SDS only.
Social interaction (SI)
SI tests were conducted 24 h after the final defeat session, in both MUT and CON mice, and C57BL/6J mice pretreated with JDTic or VEH. Mice were placed in a social interaction arena and time spent in the interaction zone was tracked (Ethovision 3.0 software) for 2.5 min in the absence and presence of an unfamiliar CD1 mouse enclosed in a wire mesh cage. Social interaction scores were calculated as described previously (interaction time, target present)/(interaction time, target absent) (Krishnan et al., 2007; Donahue et al., 2014).
Drugs
Imipramine (20 mg/kg; Eli Lilly and Company, Indianapolis IN, USA) was dissolved in PBS and administered by i.p. injections for 35 days in chronic SDS and control mice. JDTic (30 mg/kg, IP; Research Triangle Institute, Research Triangle Park NC, USA) was dissolved in 0.9% saline and administered 24 h before the first day of chronic SDS. In preliminary studies, pretreatment with 30 mg/kg JDTic significantly reduced corticotrophin-releasing factor (CRF)-enhanced startle and blocked the ability of the KOR agonist U50,488 to increase tail withdrawal latencies (Van’t Veer et al., 2013b). A 24-h pretreatment period was used to ensure optimal KOR selectivity (Carroll et al., 2004). Control mice received identical treatments to defeated mice.
Statistical analysis
Data are expressed as mean ± SEM. Mean differences between groups were analyzed using Student’s t-tests or one- or two-way analyses of variance (ANOVAs), with repeated measures when endpoints were examined repeatedly over time. Significant (at p<0.05) effects were followed by Bonferoni post hoc tests. Statistical analyses were performed using Prism 5.0 (GraphPad Software).
RESULTS
Q-PCR analysis of NAc dissections demonstrated that acute and chronic SDS differentially regulate pDYN mRNA levels. Acute defeat increased pDYN mRNA levels compared to controls (t(9)=2.35, P<0.05); although KOR mRNA levels were marginally increased, this effect did not reach significance (t(9)=2.19, p=0.065) (Fig. 1A). Chronic SDS affected pDYN mRNA levels (F(2,20)=4.88, P<0.05), producing significant decreases in both susceptible and resilient defeat populations compared to controls (p’s<0.05). Chronic SDS also produced nominal decreases in KOR mRNA levels but the effect was not significant (F(2,20)=2.23, NS) (Fig. 1B). A 35-day regimen of imipramine treatment, which reverses chronic SDS-induced social avoidance (Berton et al., 2006), affected pDYN mRNA levels: there was a significant main effect of drug treatment (F(1,22)=13.59, p<0.05), a marginal effect of defeat (F(1,22)=12.36, p=0.057), but no significant interaction (F(1,22)=6.21, NS) (Fig. 1C). Post hoc analyses revealed a significant reduction in pDYN mRNA levels only in defeated mice treated with vehicle (P<0.05). Although a similar pattern was observed in KOR mRNA levels following the 35-day regimen of imipramine, there were no significant effects of drug treatment (F(1,23)=0.42, NS) or defeat (F(1,23)=2.61, NS), and no significant interaction (F(1,23)=2.07, NS) (Fig. 1C).
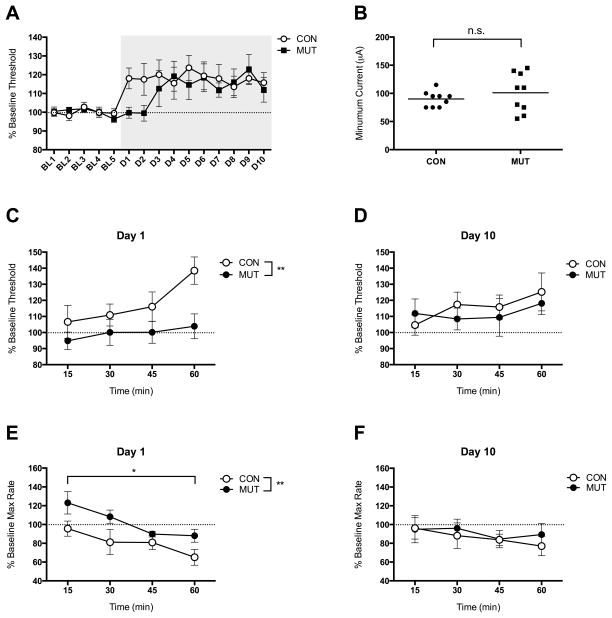
The ability of acute SDS to upregulate pDYN mRNA levels in the NAc raised the possibility that KOR systems in this region play an important role in the development of depressive-like behaviors. Consistent with this hypothesis, MUT mice lacking KORs in DA-expressing neurons show a delay in the onset of SDS-induced increases in ICSS thresholds (Fig. 2A). Importantly, group differences in the threshold-elevating effects of SDS could not be explained by baseline differences in sensitivity to the rewarding impact of the brain stimulation: the minimum current required to sustain reliable responding did not differ between MUT and CON mice (t(16)=0.89, NS (Fig. 2B). We restricted our analysis to the two endpoints used in q-PCR studies: Day 1 and Day 10 of SDS. A two-way ANOVA on mean ICSS thresholds over time on Day 1 revealed a significant main effect of genotype (F(1,16)=8.56, P<0.01) (Fig. 2C). There were no significant differences between MUT and CON mice on Day 10 (F(1,16)=0.20, NS) (Fig. 2D); both genotypes mice show increased thresholds. A two-way ANOVA on ICSS maximum response rates on Day 1 revealed a significant main effect of genotype (F(1,8)=16.21, p<0.01) and time (F(3,24)=4.61, p<0.05) (Fig. 2E), with rates decreasing over time in both genotypes. There were no significant differences in maximum response rates between genotypes on Day 10 (Fig. 2F).
Figure 2.
Effects of KOR ablation in DAT-containing neurons on ICSS behavior following SDS. (A) Average thresholds for baseline days (BL1-5) and defeat days (D1-10) in MUT (DAT-KORlox/lox) and CON (KORlox/lox) mice. Gray background depicts the 10-day SDS regimen; n=9 per group. (B) There were no significant group effects on the minimum current required to support reliable ICSS. (C) ICSS thresholds were higher in the MUT mice than in CON mice on Day 1 of SDS. (D) Group differences were no longer evident by Day 10. (E) Maximum (Max) rates of responding were lower in CON mice than MUT mice on Day 1. (F) Group differences in maximum rates were no longer evident by Day 10. *P<0.05, **P<0.01; data are expressed as mean (+/− SEM) percent change from baseline (pre-SDS) values.
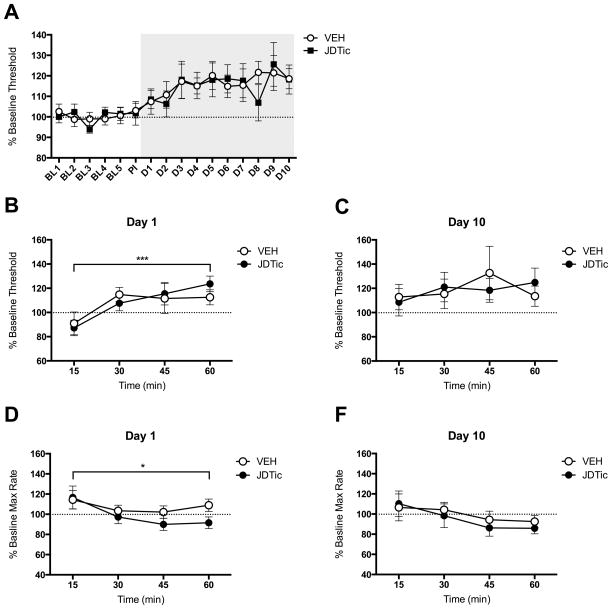
Considering the putative role of KORs within the DA system in stress resilience, we evaluated ICSS thresholds after each defeat episode in mice pretreated with JDTic or VEH to further examine the temporal involvement of KOR function in stress-induced anhedonia. Interestingly, administration of JDTic before the start of the SDS regimen did not affect the onset of anhedonia (Fig. 3A). A two-way ANOVA on mean ICSS thresholds over time on Day 1 revealed a significant main effect of time (F(3,39)=6.96, p<0.001), but not of treatment (F(1,13)=0.02, NS) (Fig. 3B); both treatment groups exhibited increases in ICSS thresholds over the 1-h test session. There were no significant differences between treatment groups on Day 10 (F(1,13)=0.002, NS) (Fig. 3C). A two-way ANOVA on mean maximum response rates on Day 1 revealed a significant main effect of time (F(4,52)=3.69, p<0.05), but not of treatment (F(1,13)=1.38, NS) (Fig. 3D). There were no significant effects of time or treatment on ICSS maximum response rates on Day 10 (Fig. 3E). There were no systematic (between-experiment or among-group) differences in ICSS electrode placements, which were indistinguishable from those presented previously (Muschamp et al., 2012).
Figure 3.
Effects of pre-treatment with the KOR antagonist JDTic (30 mg/kg,i.p.) on ICSS behavior following SDS. (A) Average thresholds for baseline days (BL1-5), post-injection day (PI) and defeat days (D1-10) in mice pre-treated with VEH and JDTic 24 h before the SDS regimen. JDTic pre-treatment had no effect on ICSS thresholds on its own (PI day). SDS increased reward thresholds in both VEH and JDTic pre-treated mice; n=7–8 per group. (B) SDS increased ICSS thresholds over time in both groups. (C) There were no group differences on Day 10. (D) Maximum (Max) rates of responding decreased over time on Day 1, but there were no group differences. (E) There were no group differences in maximum rates on Day 10. *P<0.05, ***P<0.001; data are expressed as mean (+/− SEM) percent change from baseline (pre-SDS) values.
Because KOR disruption did not mitigate the consequences of chronic SDS in the ICSS test, we hypothesized that KOR ablation in DAT-expressing neurons or KOR antagonism would not be sufficient to reverse social avoidance produced by chronic SDS. A two-way ANOVA on social interaction scores revealed that defeated MUT and CON mice both displayed social avoidance compared to controls (Fig. 4A): there was a significant main effect of group (F(1,28)=20.22, Pp<0.001), but not of genotype (F(1,28)=0.01, NS). In the cohort treated with the KOR antagonist, a two-way ANOVA revealed that both VEH- and JDTic-pretreated defeated mice showed reduced social interaction compared to controls (Fig. 4B): there was a significant main effect of group (F(1,18)=17.15 p<0.001), but not of treatment (F(1,18)=0.003, NS).
Figure 4.
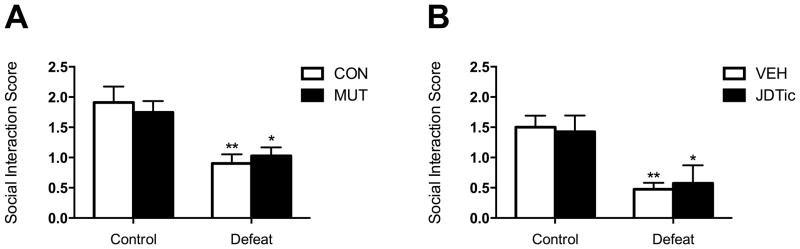
Effects of KOR ablation and KOR antagonism in SDS-induced social avoidance. (A) There were no differences in social avoidance behavior between MUT and CON mice following a 10-day regimen of SDS (n’s=8). (B) There were no differences in social avoidance behavior between mice that received pretreatment with the long-lasting KOR antagonist JDTic (30 mg/kg, i.p.) or VEH following chronic SDS (n’s=6). *P<0.05 and **P<0.01 compared to control condition.
DISCUSSION
We report that endogenous KOR systems within the mesolimbic DA circuitry are involved in mediating the effects of acute but not chronic (repeated) SDS. Mice subjected to acute SDS showed rapid and significant increases in pDYN mRNA levels and nominal increases in KOR mRNA levels within the NAc, whereas mice subjected to chronic (10 day) SDS showed significant decreases in pDYN mRNA levels regardless of whether they were “stress-susceptible” or “stress-resilient” in SI tests. This down-regulation was long-lasting and reversed by chronic treatment with imipramine. The findings with imipramine are important because they demonstrate that like some of the behavioral effects of chronic SDS (Berton et al., 2006), some of the molecular effects of chronic SDS are sensitive to long-term treatment with a standard antidepressant drug. Mutant mice with KOR gene disruption specifically within DA neurons were resilient to the pro-depressive effects of acute SDS, as indicated by a delay in the onset of SDS-induced increases in ICSS thresholds (a sign of anhedonia). However, while this mutation delayed the onset of threshold elevations, it did not prevent them; thresholds in these mice were similar to those of controls by the test following the fourth SDS session. Administration of JDTic before the start of the SDS regimen did not affect the onset of anhedonia, which was unexpected considering our previous observations that pretreatment with KOR antagonists can block the development of stress-related behavioral adaptations, including drug withdrawal and CRF-enhanced startle (Knoll et al., 2007; Chartoff et al., 2012; Van’t Veer et al., 2013a). Consistent with these findings, chronic SDS-induced social avoidance was not reversed by KOR gene disruption in DAT-containing neurons or KOR antagonism. These studies suggest that stimulation of KORs by DYN within the mesolimbic DA system is an important component of acute stress responses, but KOR systems may be subject to counter-adaptations or become desensitized following repeated stress.
We used qPCR to determine if SDS induces plasticity in KOR or pDYN mRNA levels in the NAc, a brain region shown to play a critical role in mediating susceptibility to SDS (Berton et al., 2006; Krishnan et al., 2007). We found a biphasic relationship between the time-course of stress exposure and pDYN mRNA levels: a single SDS session caused increases in levels, whereas chronic SDS caused a long-lasting down-regulation of levels, with similar trends observed in KOR mRNA levels. The acute stress effects are consistent with reports of increased KOR system-related markers following acute fear conditioning (Knoll et al., 2011) and immobilization stress (Shirayama et al., 2004). However, the long-lasting down-regulation of pDYN levels was surprising in light of previous reports indicating that 7 days of SDS increases DYN levels in the NAc of rats (Bérubé et al., 2013), and that repeated swim stress increases KOR immunoreactivity in the NAc of mice (Bruchas et al., 2008). The discrepancy likely reflects differences among the specific types and durations of stress and highlights a complex role for brain KOR systems in regulating responsiveness to stress. It also highlights the fact that different types of stressors may engage these systems in fundamentally different ways. While the present experiments focus on the NAc because of its well-established role in the regulation of motivation (Nestler and Carlezon, 2006), it is possible that dynamic changes in KOR signaling in other brain regions engaged by stress also contribute to the effects of SDS. For instance, there is evidence that KORs on DA neurons in the ventral tegmental area (VTA) are necessary for KOR-mediated aversive behavior (Bals-Kubik et al., 1993; Chefer et al., 2013) and can regulate DA function in the NAc (Chefer et al., 2013; Nestler and Carlezon, 2006). Clearly, more work is necessary to thoroughly characterize the neural circuits by which KOR systems can regulate the effects of acute and chronic stressors.
Daily chronic imipramine treatment reverses chronic SDS-induced social avoidance (Berton et al., 2006) and, as we report here, down-regulates pDYN levels. While it may seem counterintuitive that treatment with an antidepressant elevates DYN/KOR function, which is typically associated with the expression of depressive behaviors in humans (Pfieffer et al., 1986) and depressive-like behaviors in laboratory animals (Land et al., 2008; Van’t Veer and Carlezon, 2013), this may indicate that antidepressants act by restoring homeostasis in dysregulated KOR systems. KOR system plays an important role in mediating responses to pain, and activation of KORs has been shown to mediate analgesia by inhibiting pain circuits (see Ribeiro et al., 2005). Thus, restoring pDYN and KOR mRNA levels to baseline may normalize responses to stress or threat.
Our finding that SDS regulates mRNA levels within KOR systems in a time-dependent manner is corroborated by our behavioral findings using ICSS and SI. ICSS is advantageous for investigating time-sensitive changes in response to SDS because it is an exceptionally reliable behavior in sufficiently trained mice, and the ability to perform repeated testing enables day-to-day analysis of the accumulated behavioral effects of repeated exposure to stress (Chartoff and Carlezon, 2007; Donahue et al., 2014). These characteristics have enabled us to use ICSS to track with the development of anhedonia in mice over the course of a 10-day SDS regimen (Donahue et al., 2014). In the present studies, we found that disruption of KOR function in DA-containing neurons attenuated the anhedonic effects of acute SDS (Day 1) in the ICSS test, but did not prevent the anhedonic effects of chronic SDS (Day 10). Surprisingly, pre-treatment with the KOR antagonist JDTic did not delay the onset of SDS-induced anhedonia. It is important to note that we also observed minor alterations in ICSS maximum rates. We use the “curve-shift variant” of the ICSS procedure, in which alterations in reward threshold and maximum response rates can vary independently (Carlezon and Chartoff, 2007). That is, it is possible to see shifts in thresholds without changes in maximum rates (often seen with low/intermediate doses of stimulant drugs), and likewise possible to see changes in maximum rates without shifts in thresholds (which can be seen by manipulations such as tightening the response lever/wheel). Alterations in maximum response rates can be indicative of alterations in task difficulty, performance ability, motivation, or fatigue (Carlezon and Chartoff, 2007; Do Carmo et al., 2009; John et al., 2013). In the studies involving KOR ablation, the mutant mice had generally higher ICSS rates than controls on Day 1. Since the reductions in maximum response rates in the control mice accompanied SDS-induced elevations in ICSS thresholds, they may reflect a depressive-like effect. Accordingly, the lack of a corresponding effect in the mutants may represent an additional indicator of resilience. Consistent with this interpretation, there were no differences in maximum response rates by Day 10, at which time the ICSS thresholds seen in the mutant mice had become elevated to a degree where they were indistinguishable from those seen in the controls. In the studies involving JDTic, there was a significant main effect of time, indicating that maximum response rates were higher at the beginning of the session than at the end, regardless of treatment condition.
It is unclear why the mice with ablation of KORs in DA systems showed initial resilience to SDS, whereas mice treated with the KOR antagonist did not. There are at least four possible explanations, all of which would require considerably more effort to investigate. First, it is possible that KOR receptors within brain DA systems are indeed crucial for regulating resilience-like behavior, but the consequences of KOR ablation are more efficacious than those of KOR antagonism. It is important to note that the dose of JDTic used here (30 mg/kg, IP) has been shown in preliminary studies to block CRF-induced elevations in startle in the same strain of mice (C57BL/6J) (Van’t Veer et al., 2013b). A second possibility is that KOR antagonism by JDTic causes fundamentally different intracellular consequences than KOR ablation. As one example, prototypical KOR antagonists JDTic and norBNI appear to have “biased agonist” or “ligand-directed signaling” properties (Melief et al., 2011), a process by which a ligand can simultaneously act as an agonist and an antagonist at different functions mediated by the same receptor (Urban et al., 2007). Although JDTic does appear to have anti-stress effects in many behavioral tests (Van’t Veer and Carlezon, 2013), it is possible that its biased agonist effects on the c-Jun-kinase (JNK) signaling cascade (Melief et al., 2011) detract from its effects on SDS. A third possibility is that KOR antagonists have pro-depressive effects that are mediated outside of brain DA systems. We have shown that intra-amygdala microinjections of KOR antagonists have anxiolytic-like effects in rats, but infusions dorsal to this region produce, if anything, anxiogenic-like effects (Knoll et al., 2007). Fourth, it is possible that there is an interaction between pain and KOR antagonism that obscures the anti-stress effect of JDTic. KOR agonists have analgesic effects, and it is well documented that SDS elicits antinociception in mice (Tramullas et al., 2012; Van’t Veer and Carlezon 2013). KOR antagonism blocks both psychological and physical stress-induced antinociception (Takahashi et al.,1990; McLaughlin et al., 2003; McLaughlin et al., 2006). As such, JDTic may block the analgesic effects of endogenous KOR stimulation that result from the types of physical encounters that are unique to the SDS procedure, unmasking pain-related suppression of ICSS (DoCarmo et al., 2009). This effect would be absent in the KOR-mutant mice if the antinociceptive effects of KOR activation are mediated outside of brain DA systems. Indeed, we have previously shown that CON and MUT mice exhibit equivalent increases in tail withdrawal latency in response to KOR activation, suggesting that DAT-expressing cells do not mediate the antinociceptive effects of KOR agonists (Van’t Veer et al., 2013a). The acute stress resilience observed in MUT mice may be attributed to both KOR antagonism within the DA system and the uninhibited analgesic effects of endogenous KOR activation outside of the DA system. These findings provide a rationale for resource-intensive future work that may differentiate among these various possibilities, such as use of viral vectors to reinstate KOR expression in select brain areas or JNK inhibitors to block the biased agonist effects of JDTic. Selective blockade of KORs in specific brain regions implicated in both SDS and KOR-mediated stress effects, including the NAc and other regions such as the VTA (Krishnan et al., 2007) and dorsal raphe nucleus (Bruchas et al., 2011), may help to elucidate the neural circuitry underlying KOR-mediated regulation of stress responses.
Consistent with the ICSS findings, neither KOR gene disruption in DAT-containing cells nor JDTic treatment was effective in reversing social avoidance following chronic SDS. This finding is not surprising considering that any sign of initial resilience in the mutant mice in the ICSS test were absent by Day 10. When considered together, our results are consistent with previous reports showing that acute stress induces behavioral effects that are mediated by DYN/KOR signaling. For example KOR agonists produce immediate increases in ICSS thresholds (Todenkopf et al., 2004; Carlezon et al., 2006; Muschamp et al., 2013), whereas antagonists reverse the effects of a single SDS bout on cocaine conditioned place preference (Land et al., 2009) and analgesia (McLaughlin et al., 2006). While our behavioral finding that chronic SDS was impervious to disruptions in KOR signaling is consistent with our molecular findings, there are numerous reports indicating that KOR signaling mediates the effects of repeated stress (see Knoll and Carlezon, 2010). Of particular relevance, KOR antagonists and DYN gene ablation block the effects of repeated SDS on submissive posturing (McLaughlin et al., 2006). However, there are notable differences between that study and ours, including the specific SDS procedures used and the time-course of the paradigm, which may underlie this discrepancy. It is also possible that KOR signaling facilitates the expression of only some behaviors in response to repeated stress. Indeed, we recently reported that acute ketamine treatment blocks social avoidance, but not anhedonia, suggesting that various domains of depression may be regulated by distinct brain circuits (Donahue et al., 2014).
KOR antagonists seem most effective in attenuating the effects of stress when administered before the stressor (Knoll and Carlezon, 2010; Chartoff et al., 2012; Van’t Veer et al., 2013a). Thus, it is possible that the KOR system, at least within the mesolimbic DA pathway, is involved in initiating the response to stress, but is less involved in maintenance of depressive-like behaviors. This may be due to activation of other stress-responsive systems (Keeney et al., 2006), or receptor desensitization due to repeated activation of the endogenous KOR system (McLaughlin et al., 2006). It was recently reported that KOR activation potentiates cocaine preference-reinstatement following exposure to an acute stressor, but not following exposure to repeated SDS or chronic mild stress (Al-Hasani et al., 2013), suggesting that repeated or chronic stressors induce tolerance to subsequent KOR signaling. The mechanisms by which the KOR system temporally regulates stress responsiveness are not known, but differential effects of acute and chronic SDS on hypothalamic-pituitary-adrenal (HPA) axis function have also been reported (Keeney et al., 2006). Likewise, severe stress has been shown to produce a shift in the valence of CRF in the NAc, demonstrating that the type and duration of stress can play critical roles in determining the nature of changes in neural circuit function (Lemos et al., 2012). Clearly, additional work is necessary to determine the anatomical loci and mechanisms that underlie differences in acute versus chronic stress. A better understanding of the temporal relationship between neural systems affected by stress and behavioral outcomes may ultimately lead to the development of better therapeutics for stress-related illnesses in humans.
Acknowledgments
FUNDING
This research was funded by a National Institutes of Health grant MH063266 (to WC).
Footnotes
DISCLOSURES
Dr. Carlezon discloses that he is inventor on a patent that claims the use of kappa-opioid receptor antagonists for the treatment of depression (Assignee: McLean Hospital) and over the past two years has received compensation for editorial duties from the American College of Neuropsychopharmacology. Dr. Carroll is an inventor of US patents claiming the composition of JDTic owned by the Research Triangle Institute. All other authors report no biomedical financial interests.
References
- Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacol. 2013;4(96):1–10. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bérubé P, Laforest S, Bhatnagar S, Drolet G. Enkephalin and dynorphin mRNA expression are associated with resilience or vulnerability to chronic social defeat stress. Physiol and Behav. 2013;122:237–245. doi: 10.1016/j.physbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim-stress induces kappa-opioid mediated activation of ERK1/2 MAPK. Neuroreport. 2008;19(14):1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2(11):2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, et al. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphylation in NAc tissue. Mol Pharmacol. 2009;75(3):704–12. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacol. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Bäckman CM, Gigante ED, Shippenberg TS. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacol. 2013;38(13):2623–31. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA., Jr The selective non-peptide delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal ΔFos B overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76(7):550–8. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: side of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78(2):192–210. [PubMed] [Google Scholar]
- Golden SA, Covington HE, III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, Kennedy PJ, Robison AJ, Gonzalez-Maeso J, Neve RL, Turecki G, Ghose S, Tamminga CA, Russo SJ. Epigenetic reguation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19(3):337–44. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’t Veer A, Gompf HS, Carlezon WA, Jr, Cohen BM, et al. Blockade of astrocyctic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacol. 2012;37:2467–2475. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeny A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrin. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual review of psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Caroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323(3):838–45. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Fergeson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BAS, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PEM. Severe Stress switches CRF action in the nucleus accumbens from appetitive to aversive. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a κ-opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacol. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Jr, Cohen BM, et al. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Nemeth CL, Robison AJ, Nestler EJ, Carlezon WA., Jr Δ FosB enhances the rewarding effects of cocaine while reducing the pro-depressive effects of the kappa-opioid receptor agonist U50488. Biol Psychiatry. 2012;71:44–50. doi: 10.1016/j.biopsych.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Katoh A, Wada M, Kameyama T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sciences. 1992;51:211–217. doi: 10.1016/0024-3205(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato R, Rieker C, Turiault M, Tronche F, Schutz G. Survival of DA neurons is independent of CREM upregulation in absence of CREB. Genesis. 2006;44:454–464. doi: 10.1002/dvg.20236. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21(18):7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of κ-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Senda T, Tokuyama S, Kaneto H. Further evidence for the implication of a kappa-opioid receptor mechanism in the production of psychological stress-induced analgesia. Japan J Pharmacol. 1990;53:487–494. doi: 10.1254/jjp.53.487. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacol. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tramullas M, Dinan TG, Cryan JF. Chronic psychosocial strss induces visceral hyperalgesia in mice. Stress. 2012;15(3):281–92. doi: 10.3109/10253890.2011.622816. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacol. 2013;229(3):434–52. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY, et al. Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacol. 2013a;38:1585–1597. doi: 10.1038/npp.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer AV, Carroll FI, Cohen BM, Carlezon WA., Jr . Program No. 86.17/LLL42. Neuroscience 2013 Abstracts. San Diego, CA: Society for Neuroscience; 2013b. Disruption of kappa opioid receptor function reduces stress effects on anxiety-like behavior. Online. [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13(6):745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]